Abstract
The RAS-RAF-MEK-ERK signaling pathway (MAPK signaling pathway) plays a significant role in multiple pathological behaviors and is most frequently dysregulated in more than 30% of human cancers. As key elements in this pathway, MEK1/2 play crucial roles in tumorigenesis and the inhibition of apoptosis, which makes their inhibition an attractive antitumor strategy. Dozens of potent non-ATP-competitive allosteric MEK1/2 inhibitors have been developed that have produced substantial improvement in clinical outcomes over the past decade. However, the efficacy of these agents is limited, and response rates are variable in a wide range of tumors that harbor RAS and RAF mutations due to the development of resistance, which is derived mainly from the persistence of MAPK signaling and increased activation of the mutual feedback networks. Both intrinsic and acquired resistance to MEK inhibitors necessitates the synergistic targeting of both pathways to restore the therapeutic effects of a single agent. In this review, the significant role of the MAPK pathway in carcinogenesis and its therapeutic potential are comprehensively examined with a focus on MEK inhibitors. Then, the activation of feedback networks accompanying MEK inhibition is briefly reviewed. Combination strategies that involve the simultaneous inhibition of the original and resistance pathways are highlighted and elaborately described on the basis of the latest research progress. Finally, the obstacles to the development of MEK-related combination systems are discussed in order to lay the groundwork for their clinical application as frontline treatments for individual patients with MAPK-hyperactivated malignancies.
Keywords: MAPK signaling pathway, MEK inhibitor, reciprocal feedback networks, combination therapy, malignancy
Introduction
The RAS-RAF-MEK-ERK pathway is the best characterized of the classical mitogen-activated protein kinase (MAPK) pathways1. It is an evolutionarily conserved transduction pathway that transmits signals from cell surface receptors and involves the sequential phosphorylation and activation of three protein kinases in a phosphoprotein relay system, and it regulates multiple key physiological processes, including cellular proliferation and survival programs2. Physiological MAPK activation is tightly controlled by feedback loops at multiple levels and is essential for regulating normal cell growth and division, gene expression, cell cycle progression, and homeostasis3. However, MAPK signaling is aberrantly activated in more than one-third of human tumors that primarily express constitutively mutant RAS and BRAF4. The probability of MEK and ERK mutation is very low; however, these enzymes play prominent roles in tumorigenesis and malignant transformation5. These findings have enabled the development of small molecule inhibitors that target components involved in MAPK signaling and that have become important cancer therapeutic agents. In particular, the unique structures, narrow substrate specificity, and minimal mutation rates of MEK1/2 render them ideal targets for therapeutic development6. In recent years, dozens of MEK inhibitors (MEKi) have achieved considerable clinical outcomes, and four have even been approved by the US Food and Drug Administration (FDA) for the first-line treatment of cancers induced by RAS/RAF dysfunction7. Although substantial improvements and promising clinical activity have been observed, response rates (RR) vary between individuals, and the drug efficacy is discounted because of the development of resistance mainly as a result of the emergence of mutual feedback networks8.
MEKi resistance has emerged as a critical issue; patients may not benefit from MEKi as a result of primary resistance or may encounter acquired resistance, during which an initial response is followed by tumor progression and decreased survival9. Both intrinsic and acquired resistance to MEKi are frequently associated with the persistence of ERK signaling, feedback loops, crosstalk with other pathways (mainly the PI3K-AKT-mTOR pathway), or a shift to a mesenchymal phenotype10. In addition, acquired resistance to MEKi involves an ERK-independent mechanism, in which MEK inhibition causes acute inactivation of ERK that results in c-Myc degradation and, in turn, the expression and activation of several tyrosine kinase receptors (TKRs)11. Identification of feedback networks and predictive biomarkers is essential to reveal the mechanisms involved in resistance to MEKi and reductions in efficiency; more importantly, this implies that innovative combination approaches with other targeted therapeutics may be needed to overcome these challenges.
Based on this reversal strategy, incremental synergistic treatments have been used in academic or clinical settings and achieved considerable results in improving MEKi resistance and expanding drug efficacy12. For instance, a combination of MEKi (MEK162) with cytotoxic chemotherapy (paclitaxel, PTX) was used for second-line treatment of relapsing ovarian tumors and increased antitumor activity without any additional toxicity13. Dual targeting of MEK and PI3K (phosphoinositide 3-kinase) was combined with radiotherapy, and this enhanced the response to radiation of K-RAS-mutant non-small cell lung cancer (NSCLC)14. When combined with nanoparticle (NP)-based photothermal therapy (PTT) agents, the antitumor activity of MEKi (PD-0325901) was greatly improved in neurofibromatosis type 1 (NF1)-associated malignant peripheral nerve sheath tumors (MPNSTs)15. Due to the increasing emphasis on immunotherapy, clinical trials of a combination of MEKi with immunotherapy agents for advanced-stage melanoma were systematically summarized in a recent study16. Based on the prevalence of mutual feedback networks in MEKi resistance, combinations of targeted therapeutics that included MEKi+BRAF inhibitor17, ERK inhibitor (ERKi)18, PI3K inhibitor19, AKT (protein kinase B, PKB) inhibitor20, HER (human epidermal growth factor receptor) inhibitor21, and PARP [poly (adenosine diphosphate-ribose) polymerase] inhibitor22, were used to overcome MEKi resistance and enhance antitumor response. Based on various cooperative schemes, the use of combinations of MEKi and targeted agents to inhibit feedback networks was the focus of this paper.
This review focuses on the important role of the MAPK pathway in tumorigenesis. First, the biological functions of MAPK signaling and MEK kinases are outlined, and the developmental status of MEKi is emphasized. Then, the feedback networks involved in MEKi resistance are summarized. Next, the synergistic strategies are enumerated and described in detail based on the most recent research progress. Finally, the obstacles faced by academics and clinicians during the development of MEKi and MEK-related combinations are considered, and future possibilities for the exploitation of efficient MEK inhibition systems are explored. In brief, the development of synergistic systems targeting MEK and reciprocal feedback networks will lay a scientific foundation for overcoming resistance to MEKi and improving the curative effects of treatment on malignancies.
The RAS-RAF-MEK-ERK pathway and MEK inhibitors
RAS-RAF-MEK-ERK signaling pathway
The MAPK signaling cascade, which plays a critical role in multiple physiological processes, is one of the best-studied signal transduction pathways, and it is the most frequently dysregulated signaling cascade in human cancer. MAPK signaling is initiated by the dimerization, activation, and transphosphorylation of receptor tyrosine kinases (RTKs), such as epidermal growth factor receptor (EGFR), HER kinase, mesenchymal to epithelial transition factor (MET), and fibroblast-growth factor receptor, on the cell surface via the binding of growth factors, cytokines, and extracellular mitogens23. The activated growth factor receptors interact with a series of adaptor proteins, such as growth factor receptor-bound protein 2 (GRB2), that then recruit guanine nucleotide exchange factors (GEFs) to the plasma membrane. These GEFs can activate membrane-bound RAS small guanosine triphosphate (GTP)ases (H-RAS, N-RAS, and K-RAS), which are intrinsically stagnant and function as a guanosine diphosphate (GDP)/GTP-regulated switch by catalyzing the conversion of inactive GDP-bound RAS to active GTP-bound RAS24. Activated RAS subsequently recruits RAF serine/threonine kinases to the plasma membrane and activates them via a complex series of events involving phosphorylation, dimerization, and protein-protein interactions25. As MAP kinase kinase kinases (MAPKKK), RAF family members (A-RAF, B-RAF, and C-RAF) utilize RAS proteins as common upstream activators and principally activate the kinase effectors MAP kinase kinases (MAPKKs), MEK1 and MEK2 through phosphorylation26. MEK1 and MEK2, which are tyrosine and serine/threonine dual-specificity kinases, subsequently catalyze the activation via phosphorylation of the effector MAP kinases ERK1 and ERK2, which are the only known physiological substrates of MEK1/227. Unlike RAF and MEK, which are very substrate-specific, activated ERK1/2 phosphorylate a panoply of nuclear and cytoplasmic targets (> 600) that includes transcription factors, kinases, phosphatases, and cytoskeletal proteins, all of which are involved in diverse cellular responses such as cell proliferation, survival, differentiation, motility, metabolism, programmed cell death, embryogenesis, and angiogenesis28.
In physiological conditions, MAPK signaling is evolutionarily conserved and tightly controlled by feedback loops at multiple levels, and it programmatically transmits signals from cell surface receptors to promote cell proliferation/survival and maintain homeostasis29. However, this pathway involves one of the most vigorous signaling cascades that dominate carcinogenesis. Indeed, the components of this signaling cascade are frequently mutant in human cancer; more than 30% of human tumors express gain-of-function mutations in RAS-encoding genes30, and the development of approximately 8% of all tumors, including 50% of melanomas, 45% of papillary thyroid cancers, and 36% of low-grade ovarian cancers, is triggered by a genetic mutation in one of the RAF family members4. The low incidence of mutations in MEK and ERK-encoding genes cannot obscure the important roles of these genes in malignant transformation and tumorigenesis5. Following the aberrant activation of the MAPK pathway, an autocrine/paracrine loop is established that supplies proliferative signals and stimulates cell growth31. The expression of cell cycle regulators is altered and leads to premature cell cycle arrest and halts progression32. The pro-apoptotic proteins are repressed, and the anti-apoptotic proteins are activated33. Senescence evasion is promoted by the upregulation of telomerase; epithelial-to-mesenchymal transition (EMT) is upregulated and cell invasiveness and motility are accelerated34. The interactions between cancerous and stromal cells are disturbed, which affects angiogenesis and hides cancer cells from the immune system35. These findings prompted the development of small-molecule inhibitors targeting the kinases of the MAPK pathway that could serve as promising cancer therapeutics.
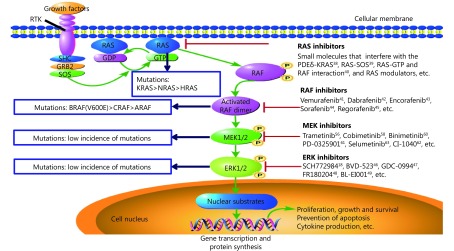
Among the members of the MAPK family, RAS is insensitive to currently available medications. Several RAF inhibitors (RAFi) have been developed and have shown considerable activity in clinical trials36. Limited progress and benefits have been obtained from the development and evaluation of ERK1/2 selective inhibitors, partly because ERK is the only known downstream target of MEK, and ERK regulates a number of cellular events37. The RAS-RAF-MEK-ERK pathway is represented diagrammatically in Figure 1, containing the mutation sites and frequencies of pivotal effectors in human tumors, and the corresponding targeted inhibitors. In comparison, the narrow substrate specificity and the unique structural characteristics of MEK1/2 make it a potential bottleneck and an ideal target for therapeutic development. A clear understanding of MEK kinase is of great importance for the development of inhibitors.
1.
Simplified schematic of the RAS-RAF-MEK-ERK signaling pathway, the mutation sites and frequencies of key effectors, and their representative targeted inhibitors.
MEK kinase
MEK1/2 are 45-50 kDa proteins that share 37%–44% amino acid identity in the kinase domain and 86% identity in the catalytic domain. Unlike other homologous proteins, MEK1/2 contain strong leucine-rich nuclear export signals (NESs) in their N-termini50. They are closely related dual-specificity protein kinases; when phosphorylated by activated RAF at the Ser218 and Ser222 residues, activated MEK1/2 in turn phosphorylate the threonine (Thr202) and tyrosine (Tyr204) residues51. Although they are considered to be functionally equivalent, MEK1/2 are regulated differentially and non-interchangeably during a variety of cellular events, including epidermal hyperplasia and tumorigenesis52. As MEK lies downstream of RAS/RAF and specifically activates ERK, it has become an attractive candidate for targeted therapy of cancers with RAS and RAF mutations. Several MEKi have been developed, and some have shown remarkable potency and selectivity during clinical evaluation7.
The current status of MEK inhibitors in cancer therapy
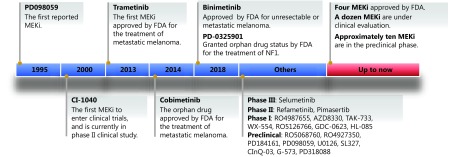
Unlike other kinase inhibitors, MEKi ensures high specificity by binding to a hydrophobic pocket adjacent to but not overlapping with the adenosine triphosphate (ATP)-binding site. This binding locks the conformation of MEK and prevents its interaction with ERK activation loops, which produces the high efficacy of MEKi that is directly proportional to the degree of activation of the MAPK pathway in RAS, RAF or EGFR-mutant tumors53. Therefore, since the first MEK inhibitor, PD098059, was reported in 199554, a series of selective, non-ATP-competitive and allosteric MEKi have been developed and achieved superior results in basic and clinical research, which are connected by a timeline in Figure 2.
2.
Timeline charting the development process and research status of MEK inhibitors.
CI-1040, which was developed by Lorusso et al.55 in 2000, was the first small-molecule MEKi to enter clinical trials. It exhibited encouraging tumor growth inhibition and antitumor activity in preclinical and phase I studies. Even though CL-1040 was well tolerated, the clinical trials of CI-1040 were halted because of insufficient antitumor activity and clinical efficacy during phase II evaluation that was mainly attributed to the poor pharmacokinetic properties, such as low bioavailability and rapid metabolism55. Since then, numerous clinical studies on various MEKi have been conducted to evaluate their therapeutic potential. To date, four MEKi have achieved clinical success and have been approved by FDA for the treatment of patients with RAS or RAF-mutant cancer. Beyond that, a dozen MEKi are in the process of clinical evaluation, and approximately ten compounds are in the preclinical phase. The current clinical experience of MEKi is described in this section depending on their clinical stage and briefly summarized in Table 1.
1.
The clinical study status and kinase activity of representative MEK inhibitors
| MEK inhibitor | Target | Kinase activity (IC50) | Clinical phase | Tumors | Obstacles | Ref |
| PA, pilocytic astrocytomas; CNS, central nervous system. | ||||||
| CI-1040 | MEK1/2 | 2.3 nmol/L | Phase II | Breast cancer, CRC, NSCLC, pancreatic cancer | Poor pharmacokinetic properties such as low bioavailability and rapid metabolism | 55 |
| Trametinib | MEK1/2 | 0.7/0.9 nmol/L | FDA approved | Melanoma, CRC, NSCLC, biliary cancer, papillary thyroid carcinoma | Rash, diarrhea, peripheral edema | 57 |
| Cobimetinib | MEK1 | 0.9 nmol/L | FDA approved | Melanoma, leukemia, CRC | Rash, pyrexia, chorioretinopathy, gastrointestinal disorders | 58 |
| Binimetinib | MEK1/2 | 12 nmol/L | FDA approved | Melanoma | Rash, nausea, vomiting, diarrhea, peripheral edema, fatigue | 60 |
| PD-0325901 | MEK1/2 | 0.33 nmol/L | FDA approved | NF1, NSCLC, CRC, melanoma, breast cancer | Musculoskeletal, neurological, ocular toxicity | 61 |
| Selumetinib (AZD6244) | MEK1 | 14 nmol/L | Phase III | NSCLC, melanoma, CRC, HCC, glioma, NF1, PA | Rash, diarrhea, nausea, fatigue, blurred vision | 63 |
| Refametinib | MEK1/2 | 19/47 nmol/L | Phase II | HCC, CRC, melanoma, pancreatic cancer | Dermatological, gastrointestinal and ocular toxicity, low tolerance | 64 |
| Pimasertib | MEK1/2 | 5-11 nmol/L | Phase II | Ovarian cancer, melanoma, breast cancer, NSCLC, HCC, CRC, pancreatic cancer | Diarrhea, fatigue, nausea, ocular toxicity | 65 |
| RO4987655 | MEK1/2 | 5.2 nmol/L | Phase I | Melanoma, NSCLC, CRC | Gastrointestinal and eye disorders, skin and CNS-related toxicity | 66 |
| AZD8330 | MEK1/2 | 7 nmol/L | Phase I | Melanoma | Mental status changes, acneiform dermatitis, fatigue, diarrhea, vomiting | 67 |
| TAK-733 | MEK1/2 | 3.2 nmol/L | Phase I | Melanoma, CRC, NSCLC, pancreatic cancer, breast cancer | Rash, diarrhea, increased blood CPK | 68 |
| WX-554 | MEK1/2 | 4.7/11 nmol/L | Phase I | Cervical cancer, ampullary cancer, CRC | Poor tolerability, drug toxicity | 69 |
| RO5126766 | MEK1/2 | 160 nmol/L | Phase I | Melanoma, CRC | Rash, diarrhea, acneiform dermatitis, elevated CPK, blurred vision | 70 |
| GDC-0623 | MEK1/2 | 0.13 nmol/L | Phase I | Melanoma, NSCLC, pancreatic cancer | – | 71 |
| HL-085 | MEK1 | 1.9-10 nmol/L | Phase I | Melanoma, CRC | – | 7 |
| RO5068760 | MEK1 | 25±12 nmol/L | Preclinical | Melanoma, CRC, lymphoma, pancreatic cancer | No apparent toxicity in tumor cells and xenografts. | 72 |
| RO4927350 | MEK1/2 | 23 nmol/L | Preclinical | A broad spectrum of RAS or BRAF-mutant cancers | No apparent toxicity in tumor cells and xenografts. | 73 |
| PD184161 | MEK | 10-100 nmol/L | Preclinical | HCC | No apparent toxicity in tumor cells and xenografts. | 74 |
| PD098059 | MEK1 | 2 mol/L | Preclinical | NSCLC, bronchoepithelial inflammation | No apparent toxicity in tumor cells and xenografts. | 75 |
| U0126 | MEK1/2 | 70/60 nmol/L | Preclinical | Cervical cancer, CRC, HCC, embryonal rhabdomyosarcoma, glioblastoma, pancreatic cancer | No apparent toxicity in tumor cells and xenografts. | 76 |
| SL327 | MEK1/2 | 0.18/0.22 mol/L | Preclinical | ATC | – | 77 |
| CInQ-03 | MEK1/2 | 5/10 mol/L | Preclinical | CRC | – | 78 |
| G-573 | MEK | 406 nmol/L | Preclinical | CRC, NSCLC | – | 79 |
| PD318088 | MEK1 | – | Preclinical | Leukemia | – | 80 |
An orally bioavailable allosteric MEKi, trametinib, was first approved by the FDA for the treatment of metastatic melanoma with the BRAF V600E/K mutation in May 201356. It is a potent MEK1/2 inhibitor [half-maximal inhibitory concentration (IC50) of 0.7/0.9 nmol/L] that preferentially binds to unphosphorylated MEK1/2 and prevents RAF-dependent MEK activation57. During clinical application, improved efficiency and progression-free survival (PFS) resulted in increased overall survival compared to that of standard-of-care chemotherapy56. The second approved MEKi was cobimetinib (GDC-0973), which is a potent and highly selective MEKi with an IC50 of 0.9 nmol/L against MEK158. It was awarded orphan drug status by the FDA in 2014 for BRAF V600-mutant melanoma and was then approved for the treatment of unresectable or metastatic melanoma with a BRAF V600E/K mutation in combination with vemurafenib in November 201559. In June 2018, an oral small molecule MEKi, binimetinib, with an IC50 of 12 nmol/L against MEK1/2 was approved as a new treatment option for unresectable or metastatic melanoma with a BRAF V600E/K mutation when synergistically administered with an oral small molecule BRAF inhibitor, encorafenib. This important decision was based on satisfactory phase III clinical results, particularly those showing that median progression-free survival (mPFS) was doubled by double-agent treatment compared to single-agent treatment60. In November 2018, another MEKi, PD-0325901, was granted orphan drug status by the FDA for the treatment of NF1. PD-0325901 is a selective small molecule MEKi with an IC50 of 0.33 nmol/L against MEK1/2 that has been proven to have great potential for treating NF161.
In the course of clinical trials, more than a dozen highly effective MEKi were screened for activity testing and performance evaluation. CI-1040 was the foremost MEKi (IC50 = 2.3 nmol/L) that was investigated in the clinical stage. The initial encouraging Phase I results allowed it to progress into clinical phase II for the treatment of breast cancer, lung cancer, colorectal cancer (CRC), and pancreatic cancer. Nevertheless, CI-1040 was eliminated due to poor bioavailability and insufficient efficacy, which was attributed to poor exposure and rapid clearance in phase II clinical trials62. Selumetinib (AZD6244) is a potent, highly selective, non-ATP-competitive MEK1 inhibitor with an IC50 of 14 nmol/L. It was included in a phase III clinical study of the treatment of KRAS-mutant NSCLC and BRAF-mutant melanoma via single or combination therapy because of its robust preclinical and phase I/II antitumor activity. Rash, diarrhea, nausea, fatigue, and blurred vision were the most common toxic side effects63. Refametinib is a highly selective allosteric MEK1/2 inhibitor that does not exhibit obvious brain or neural tissue accumulation. Its IC50 was 19 and 47 nmol/L against MEK1 and MEK2, respectively, and its potency and favorable pharmacokinetic profile urged its inclusion in phase I, I/II or phase II clinical trials involving single or combination therapy of RAS-mutant hepatocellular carcinoma (HCC), CRC, and metastatic pancreatic cancer. However, treatment-related dermatological, gastrointestinal and ocular toxicity were the main side effects of refametinib, and it resulted in especially low tolerance and serious side effects due to drug toxicity that necessitated dosing adjustment in combination cases64. Pimasertib is an orally bioavailable non-ATP-competitive MEK1/2 inhibitor (IC50 of 5-11 nmol/L) with potent antitumor activity in cell lines, xenograft models, and phase I/II studies that involve advanced or metastatic solid tumors with constitutive activation of the MAPK pathway, including ovarian cancer, melanoma, breast cancer, NSCLC, HCC, CRC, and pancreatic cancer, when used for treated alone or in combination with other targeted therapeutics65.
In addition, several other MEKi have been included in phase I clinical trials. RO4987655 is a selective and orally bioavailable MEK1/2 inhibitor with an IC50 of 5.2 nmol/L. A dose escalation phase I study was conducted on patients with advanced melanoma, KRAS-mutant NSCLC and CRC. Definite clinical effects were observed in combination with toxic effects such as skin-related toxicity, gastrointestinal disorders, and eye disorders66. AZD8330 is a selective, orally active, and non-ATP-competitive MEK1/2 inhibitor with an IC50 of 7 nmol/L. It was in the midst of a clinical phase I trial and was employed as a single agent in patients with advanced solid tumors, such as melanoma. The most commonly experienced toxic events included dose-limiting mental status changes, acneiform dermatitis, fatigue, diarrhea, and vomiting67. TAK-733 is a potent, selective, and non-ATP-competitive small-molecule allosteric MEK1/2 inhibitor with an IC50 of 3.2 nmol/L. It showed broad preclinical antitumor activity in mouse xenograft models of human melanoma, CRC, NSCLC, pancreatic cancer and breast cancer. A dose-escalation phase I trial was carried out in patients with advanced solid tumors, which revealed the maximum tolerance and a generally manageable toxicity profile that included rash, diarrhea, and increased blood creatine phosphokinase (CPK)68. WX-554 is a selective, orally available, noncompetitive, and allosteric MEK1/2 inhibitor with an IC50 of 4.7/11 nmol/L. Preliminary phase I pharmacokinetic and pharmacodynamic results revealed that WX-554 was well tolerated in patients with advanced cervical cancer and ampullary cancer69. RO5126766 is a novel, potent first-in-class dual MEK/RAF inhibitor that binds to MEK1/2 to form a stable RAF-MEK-RO5126766 complex (IC50 of 160 nmol/L) to arrest the cell cycle and inhibit tumor growth. An open-label, dose-escalation phase I study was undertaken in patients with BRAF or NRAS mutant melanoma to clarify the tolerability and toxic effects, such as rash, diarrhea, and elevated CPK, of RO512676670. Two other MEKi, GDC-0623 and HL-085, with IC50 values of 0.13 and 1.9-10 nmol/L against MEK1/2 and MEK1, respectively, are currently being evaluated in phase I clinical trials involving patients with advanced and metastatic solid tumors71.
As potential candidates that could be used for clinical applications, several novel MEKi have been reported to be in preclinical development. For example, RO5068760 could potently inhibit MEK1 and had an IC50 of 0.025 ± 0.012 μmol/L. It also showed significant efficacy in tumors with the BRAF V600E mutation72. RO4927350 is a potent and highly selective non-ATP-competitive MEK1/2 inhibitor with an IC50 of 23 nmol/L. It exhibited significant antitumor efficacy in a wide variety of tumors by inhibiting ERK and MEK phosphorylation simultaneously, prevented a feedback-induced increase in MEK phosphorylation, and reduced drug resistance73. PD184161 is an orally active MEKi with an IC50 of 10-100 nmol/L, and it exerts time- and concentration-dependent antitumor effects on HCC in vitro and in vivo74. PD098059 (IC50 of 2 μmol/L) highly inhibited MEK in a selective manner and prevented ERK phosphorylation75. U0126 inhibited MEK1/2 (IC50 of 70/60 nmol/L), caused profound depletion of ATP, and suppressed tumor growth in cell culture and a mouse model76. SL327 is a homolog of U0126 with an IC50 of 0.18/0.22 μmol/L against MEK1/2, and it showed significant combined activity in a doxorubicin (DOX)-resistant anaplastic thyroid carcinoma (ATC) tumor model77. CInQ-03 is a novel and specific MEK1/2 inhibitor with an IC50 of 5/10 μmol/L; it strongly recognized the binding pocket and inhibited ERK phosphorylation in vitro and in vivo78. In addition, G-573 (estimated IC50 of 406 nmol/L during the inhibition of ERK phosphorylation)79 and PD318088 (a non-ATP-competitive MEKi)80 have also been undergoing systematic preclinical evaluation.
Despite promising effects, most investigational MEKi encountered bottlenecks during development due to depressed antitumor activity. Several factors might contribute to the decreased clinical activity of MEKi. First, the correlation between the degree of MEK1/2 suppression and its necessity and adequacy for antitumor responses and toxicity production is unclear. Second, alternative substrates of RAS/RAF besides MEK potentially compensate for the effect of MEK inhibition and eliminate the antitumor activity of MEKi. Third, the absence of predictive biomarkers results in the inefficiency of MEKi in unselected patients with tumors that are supposed to be sensitive to MEK inhibition. Last but not least, the loss of autoregulatory negative feedback to RAF from ERK may result in the activation of non-MAPK effectors of RAF and, more extensively, the presence of crosstalk among the complex signaling feedback networks that contribute to tumorigenesis and that coexist with MEK suppression. Both mechanisms will protect cancer cells from apoptosis induced by MEK inhibition and eventually lead to MEKi resistance and weakened drug activity81. Therefore, it is necessary to improve the efficacy of MEKi by clarifying the resistance-related feedback networks and proposing targeted reversal strategies.
MEK inhibition-related feedback and crosstalk networks
Although the MAPK pathway appears linear, several feedback loops within the pathway and cross-talk between the RAS-RAF-MEK-ERK pathway and other signaling cascades, such as PI3K-AKT-mTOR, p38 and c-Jun N-terminal kinase JNK, nuclear factor (NF)-κB, wingless/integrated (Wnt)-β-catenin, Hedgehog, Notch, transforming growth factor β (TGFβ)-SMAD, are important factors that lead to resistance to MEKi10. Combined targeting of reactivated sites using anticancer inhibitors with MEKi will be a reasonable strategy to reverse drug resistance based on the premise of clarifying the resistance mechanism.
Resistance to MEKi is mainly divided into primary resistance and acquired resistance, both of which are influenced by the host microenvironment and disease state, including genetic typing, disease stage, and treatment history. In the case of primary resistance, patients do not benefit from MEKi, which makes patient screening an important issue. However, during acquired resistance, the initial response is positive but as the disease progresses an appreciable but limited survival advantage is present82. Mechanistically, biochemical feedback loops and crosstalk with other pathways are responsible for MEKi resistance. ERK inhibition suppresses its phosphorylation and leads to the active repression of RAF and MEK. MEK inhibition weakens the negative feedback circuit, resulting in the excitation of the RAS-RAF-MEK-ERK pathway and the accumulation of activated MEK and ERK. Reduced expression of the ERK-phosphatase DUSP6 may also lead to sustained activation of ERK despite MEK inhibition83. Moreover, ERK-dependent resistance is also modulated by growth factors or TKR deregulation. The hepatocyte growth factor (HGF)/c-MET pathway is important in primary resistance to MEKi84. The expression of EMT is also a predictive factor for MEKi resistance85.
In addition to the mechanisms that underlie primary resistance, acquired resistance is also regulated by other ERK-dependent or independent mechanisms. For example, a point mutation of MAP2K1 interferes with MEKi binding and leads to resistance toward MEKi86. In BRAF-mutant melanoma and CRC, MEK activity was restored by high levels of COT/Tpl2 and resulted in acquired resistance to MEKi87. These ERK-dependent processes suggest that the activation of the RAS pathway contributes greatly to MEKi resistance. During the typical ERK-independent mode, MEK inhibition causes prompt ERK inactivation and rapid c-Myc degradation and promotes the expression and activation of several TKRs, mainly platelet-derived growth factor receptor β (PDGFRβ), insulin-like growth factor 1 receptor (IGF1R), and human epidermal receptor 3 (HER3)88,89.
The revelation of the MEKi mechanism has led to two enlightening conclusions. First, we searched for predictive biomarkers that could predict response to MEKi in cells, tissues, and clinical models. In addition, BRAF-, NRAS-, and KRAS-activating mutations, ERK, PI3K, AKT, PTEN, and DUSP6, have been explored as predictive biomarkers of MEKi sensitivity90. Furthermore, fluctuations in the expression of genes involved in the RAS-ERK pathway were superior to pathway mutations and preferable in predicting dependence on signaling cascades and responses to MEKi in preclinical and clinical specimens91. Second, the cotargeting of MEK and resistance pathways by combining MEKi with partner TKR inhibitors and other targeted therapeutics involved in MEK inhibition-related feedback or crosstalk networks, EMT, interactions with the extracellular matrix, and apoptosis has generally been accepted. Moreover, ERKi have also been used for preclinical and clinical evaluations of tumors with ERK-dependent acquired resistance to MEKi18.
Targeting of mutual feedback and crosstalk networks to enhance the antitumor efficiency of MEK inhibitors
Coexisting oncogenic pathways attenuate the activity of MEKi and cause resistance, but they also suggest an important strategy for reversing resistance and improving efficacy that involves the cosuppression of MEK and feedback networks. In recent years, an increasing number of combinations of MEKi with other oncogenic inhibitors that have nonoverlapping toxicity profiles have gained intense research interest and potentiated the effectiveness of MEK inhibition. In this section, the representative and latest research progress is summarized in Table 2 and analyzed based on the combination order of MEKi with other targeted inhibitors involved in the RAS-RAF-MEK-ERK, PI3K-AKT-mTOR, p38, JNK, NF-κB, Wnt-β-catenin, Hedgehog, Notch, and TGFβ-SMAD pathways.
2.
Co-inhibition of MEK and mutual feedback/crosstalk networks for reversing MEKi resistance and improving therapeutic efficacy
| Strategy | Combination | Antitumor efficacy | Tumor model | Ref |
| ACT, adoptive cell transfer. | ||||
| MEK+BRAF | Binimetinib+
encorafenib |
Binimetinib: moderate TGI
Encorafenib: minimal TGI Combination: >80% of TGI, 10-fold enhancement in apoptosis, 7-12 fold increase in the expression of pro-apoptotic proteins |
NRAS or BRAF-mutant melanoma models: monolayer, spheroids, organotypic, and patient-derived tissue slice | 97 |
| MEK+BRAF | Cobimetinib+
vemurafenib |
mPFS: 9.9 months/combination, 6.2 months/control
ORR: 68%/combination, 45%/control CRR: 10%/combination, 4%/control 9-month survival rate: 81%/combination, 73%/control Decreased the morbidity of secondary cutaneous cancers. No apparent AEs of grade 3 or higher |
Advanced or metastatic BRAF V600-mutant melanoma | 98 |
| MEK+BRAF+
HER2 |
Selumetinib+
dabrafenib+ lapatinib |
Lapatinib markedly sensitized cancer cells to dose-dependent inhibition, improved the iodine and glucose-handling gene expression, radioiodine uptake, and prevented the MAPK rebound induced by the BRAF/MEK inhibitor | BRAF V600E-mutant papillary thyroid cancer | 21 |
| MEK+BRAF+
HER2 |
U0126/
selumitinib+ sorafenib+ lapatinib |
The combination induced distinguishable tumor inhibition, greater MAPK suppression and curative activity than alone | TNBC models | 99 |
| MEK+EGFR | Selumetinib/
cetuximab+ osimertinib |
RR: 80%/combination,
50%/alone mPFS: 28 weeks Inhibited proliferation, migration, and invasion of resistant cells |
EGFR-mutant NSCLC xenografts | 100 |
| MEK+BRAF+
immunotherapy |
BRAF/MEK
inhibitor+PD-1 inhibitor |
Two drugs are positively correlated at low doses,
while antagonistic at some high doses |
Animals, early and advanced clinical trials | 101 |
| MEK+BRAF+
immunotherapy |
Trametinib+
dabrafenib+ antigen- specific ACT |
The triple combination showed complete tumor regression, increased T cell infiltration into tumors, improved in vivo cytotoxicity, increased MHC expression, and global immune gene up-regulation | BRAF V600E-mutant melanoma | 102 |
| MEK+ERK | PD0325901/
G-573+ERK inhibitor |
MEK resistant KRAS mutant cells retain sensitivity to ERK inhibition.
Downstream blockade of ERK overcome multiple resistance mechanisms of MEKi |
KRAS mutant breast cancer and CRC | 87 |
| MEK+ERK | GSK1120212+
SCH772984 |
The combination showed significant tumor regression potency (98% regression), and relieved the resistance to MEKi, BRAFi, and MEK/BRAF inhibitors | RAS or BRAF-mutant CRC, melanoma, and pancreatic cancer | 18 |
| MEK+ERK | Cobimetinib+
GDC-0994 |
In PDAC model, combination reduced tumor volume in 5/8 of animals, repressed p90RSK, and improved PFS (18.5 vs. 7 days in vehicle).
In NSCLC model, combination resulted in overall tumor burden decrease (10/10), strong suppression of p90RSK, and improved PFS (102 vs. 58 days in vehicle) |
KRAS or BRAF-mutant NSCLC, melanoma, and PDAC | 104 |
| MEK+CDK1 | Cobimetinib+
R0-3306/ dinaciclib |
The combination greatly inhibited cell proliferation, suppressed tumor growth, and promoted apoptosis by cleavage of PARP and caspase-3 | BRAF-mutant CRC murine xenografts | 105 |
| MEK+PI3K+
mTOR |
Selumetinib+
ZSTK474+ BEZ235 |
The combination synergistically inhibited the phosphorylation of ERK, AKT, S6 and the tumor growth, with statistically significant TGI of (21.8±6.6)%, (19.9±8.3)%, (37.9±6.9)%, (75.8±3.1)%, and (59.0±7.4)% corresponding to ZSTK474, BEZ235, selumetinib, BEZ235+selumetinib, and ZSTK474+selumetinib at day 14 after administration | BRAF-mutant metastatic melanoma | 110 |
| MEK+PI3K+
PDGFR |
Selumetinib+
buparlisib+ pazopanib |
The combination decreased MAPK and PI3K signaling, changed the kinome in MAPK pathway, altered the resistance drivers, and managed TNBC brain metastasis | TNBC brain metastases model | 112 |
| MEK+PI3K+
HDAC |
GSK1120212+
BEZ235+TSA |
The combination inhibited cell proliferation by >99%, no observable lung metastatic foci, compared with the average of 8.1±1.7 foci per mouse in BEZ/GSK combination and 10±2 foci in vehicle | Highly aggressive and metastatic PDAC mouse model | 113 |
| MEK+AKT | CH5126766/
trametinib+ statins |
The combination enhanced the cell sensitivity, reversed the apoptotic resistance to MEKi by up-regulating the TRAIL, and improved the antitumor efficacy of MEKi | Human breast cancer MDA-MB-231 apoptotic resistant model | 115 |
| MEK+AKT+
mTOR |
AZD6244+
MK-2206+ AZD8055 |
The combination synergistically enhanced the effect of MEKi on cell proliferation and survival with combination index below 0.3 | Advanced CCA model | 116 |
| MEK+HSP90 | Trametinib+
AUY922 |
The combination suppressed MAPK and AKT pathways, sensitized NSCLC cells to MEKi, and increased apoptosis through cleaved PARP and caspase-3/7 pathway with sub-therapeutic doses | NSCLC model | 117 |
| MEK+Wnt | Selumetinib+
CsA/TNP-470 |
The combination recovered the cell responsiveness to selumetinib, showed synergistic anti-proliferative effect in CRC cells, effectively regressed tumor growth and promoted apoptosis of the PDTX models of CRC | Clinically relevant PDTX models of CRC | 118 |
| MEK+
β-catenin |
Trametinib+
RNAi trigger of β-catenin |
The combination synergistically inhibited tumor growth with >90% of TGI vs. 60% of single dosage, overcame the resistance to trametinib, and improved the mice survival in all selected tumor models | Xenografts of CRC, melanoma, and HCC | 119 |
| MEK+
HGF/cMET |
Trametinib+
LY2875358+ LY2801653 |
The combination reversed the resistance to trametinib, suppressed AKT activation, and promoted the pro-apoptotic PARP cleavage | Primary hepatic stellate cells, metastatic uveal melanoma explants | 120 |
| MEK+RIP1 | Selumetinib+
Nec-1 |
The combination overcame the resistance to selumetinib caused by the CYLD-relied activation of NF-κB pathway, and enhanced efficacy in cancer treatment | Melanoma cells | 121 |
Combination of MEK inhibitor with RAS-RAF-MEK-ERK pathway inhibitors
In some cases involving RAS- or RAF-mutant tumors, MEKi activate the RAS-RAF-MEK-ERK pathway by relieving ERK-dependent negative feedback, which leads to the attenuation of MEK inhibition. During MEKi resistance, inhibitors of components of the RAS-RAF-MEK-ERK pathway are the most pertinent therapeutic targets and are combined with MEKi to enhance their therapeutic effect. In this pathway, direct inhibitors of NRAS are relatively rare, so the possibility of combined blocking of this oncogenic signaling is realized by inhibiting two signaling components downstream of RAS. The most widely developed synergetic system utilizes MEKi+RAFi, and MEKi+ERKi has also represented a great advance in terms of improving the treatment quality of monotherapy.
The amplification of the upstream oncogenic driver of ERK signaling has been identified as a mechanism involved in MEKi resistance that results in the increased abundance of the oncogenic driver and the restoration ERK activity to ensure continuous cell growth. For example, in patients with BRAF mutant tumors, the addition of RAFi to MEKi is a desirable approach to delay or overcome MEKi resistance92. In an exploratory study, to reveal the difference in the efficacy of MEKi and RAFi used to treat BRAF-mutant melanoma and CRC, Whittaker et al.93 performed a genome-scale pooled short-hairpin RNA (shRNA) enhancer screen and identified multiple genes involved in the RTK/MAPK signaling axis. This resulted in the identification of CRAF as a key resistance mediator that could be overcome by the combination of pan-RAFi (rather than selective RAFi) with MEKi, which also strongly synergized in RAS-activating melanoma and CRC. Compared with single-agent treatment, dual MEKi and pan-RAFi treatment greatly induced apoptosis, enhanced efficacy, overcame intrinsic and acquired resistance and was shown to be a novel therapeutic strategy for BRAF- and KRAS-mutant tumors93. Then, specific efficacy was observed by selecting fourteen NRAS-mutant human melanoma cell lines and treating sensitive and resistant cells with pan-RAFi (Amgen Compd A), MEKi (trametinib) or a combination of the two. It was shown that combination treatment induced apoptosis in sensitive cells due to the p-MEK-associated synergistic effect. In addition, cell proliferation was blocked by the inhibition of the MAPK pathway and cyclin D1 expression. In contrast to resistant cells, cell proliferation was blocked in sensitive cells by the combined inhibition of the MAPK pathway and cyclin D3, and the cells also showed higher p-GSK3β levels and less apoptotic perturbation. It was concluded that MEKi+pan-RAFi was an effective option for stemming proliferation and promoting apoptosis in NRAS-mutant melanomas94.
In previously reported studies, the combination of RAFi with MEKi has achieved remarkable results in overcoming RAFi resistance95,96. In addition, the combination system has also obtained significant progress in reversing MEKi resistance. Typically, in NRAS-mutant melanoma models, including monolayer, spheroid, organotypic, and patient-derived tissue slice models, the MEKi binimetinib was combined with the BRAFi encorafenib, which increased pERK and facilitated MEKi-induced tumor growth inhibition and apoptosis by inducing an endoplasmic reticulum (ER) stress response via the PERK pathway. After administration in NRAS-mutant melanoma cells (SKMel147, WM1366, MelJuso, and WM1346 cells) or patient-derived NRAS-mutant melanoma cells, binimetinib caused moderate growth inhibition, and encorafenib had a minimal effect on tumor growth, but their combination synergistically suppressed tumor growth by >80%. In BRAF-mutant melanoma cells (SKMel19, Mel1617, and 451Lu cells), both drugs alone inhibited tumor growth by 75%, which was enhanced by their combination. Furthermore, the proportion of apoptotic cells was almost 40% after treatment with the inhibitor combination, which was confirmed by an assay involving the nucleosomal enrichment of cytosolic fractions during apoptosis. Binimetinib treatment resulted in moderate apoptosis (4-fold enrichment), which was obviously enhanced by combination treatment (10-fold enrichment); however, relatively low rates of apoptosis and growth inhibition in normal skin cells (<40%) were observed. During ER stress, PERK was activated, and phosphorylated eIF2α upregulated ER stress-related factors and the proapoptotic protein PUMA. The phosphorylation of eIF2α was increased 1.5-2-fold in NRAS-mutant melanoma cells when treated with encorafenib alone or in combination, and it was increased 7-12-fold in BRAF-mutant melanoma cells. MEKi stimulated the expression of the proapoptotic protein BIM and activated the mitochondrial apoptotic pathway, which was mediated by caspase 9 and caspase 3, and this was enhanced by combination with encorafenib. These results underscored the therapeutic potential of MEKi+BRAFi combination treatment for patients with NRAS- and/or BRAF-mutant melanoma 97.
In clinical studies, the combined inhibition of BRAF and MEK has also achieved considerable outcomes in patients with ERK pathway-deregulated tumors by preventing or delaying resistance to a single inhibitor. In a randomized phase III clinical trial, 495 patients with untreated, locally advanced or metastatic BRAF V600 mutant melanoma were used to evaluate the combination of vemurafenib (BRAFi) and cobimetinib (MEKi), during which vemurafenib+placebo was used as a control. The mPFS of the combination group was 9.9 months, while that of the control group was 6.2 months. The overall response rate (ORR) in the combination group was 68% [complete response rate (CRR) of 10%] and was 45% in the control group (complete response rate of 4%). Interim analyses of overall survival indicated that the 9-month survival rate of patients in the combination group and the control group were 81% and 73%, respectively. Moreover, vemurafenib+cobimetinib decreased the morbidity of secondary cutaneous cancers and resulted in no apparent adverse events (AEs) of grade 3 or higher. These results revealed the success of MEKi+BRAFi in improving PFS in patients with BRAF V600-mutant metastatic melanoma and provided a reference for the design and evaluation of other synergistic systems98.
To overcome BRAF/MEK inhibitor resistance caused by MEK inhibition-induced HER2/HER3 activation and MAPK pathway rebound, a HER inhibitor (HERi), lapatinib, was added to the BRAF/MEK system (dabrafenib/selumetinib) to sensitize BRAF V600E-mutant thyroid cancer cells for redifferentiation therapy. The addition of lapatinib largely prevented MAPK rebound and improved the effect of dabrafenib/selumetinib on the expression of iodine (I)- and glucose-handling genes, the cell membrane localization the of sodium (Na)/I symporter, radioiodine uptake, toxicity, and the efficiency of redifferentiation therapy in BRAF V600E-positive papillary thyroid cancer cells. This prominent potentiation would be verified in vivo and via clinical evaluation21. Other combination systems, such as EGFR/HER2 (lapatinib), RAF (sorafenib), and MEK (U0126, selumitinib), were designed to anchor the components of the EGFR/HER2-RAS-RAF-MEK-ERK pathway. After screening, selumitinib+sorafenib cotargeting synergistically and clearly inhibited tumor growth in triple negative breast cancer (TNBC) models and represented a promising combination therapy for this aggressive tumor type99. A similar system was designed that involved dual vertical EGFR blockade with osimertinib plus MEKi selumetinib/cetuximab as a novel therapeutic option and effectively ablated tumors in EGFR-mutant NSCLC100.
In some clinical practices, the combination of MEKi with BRAFi has enhanced therapeutic efficiency, but the response is short-lived. Conversely, tumor treatment with an immune checkpoint inhibitor, such as anti-PD-1, has a lower response rate but a more persistent response period that extends for several years. A combination of these treatments will contribute to an overall improved survival time. Inspired by this idea, Lai X et al.101 developed a mathematical model that included cancer cells, immune cells, interleukins, TGF-β, PD-1, PD-L1, BRAF/MEK inhibitors (at concentration γB) and PD-1 inhibitors (at concentration γA) to determine the relationship between BRAF/MEK inhibitors and PD-1 inhibitors in tumor therapy. This model mainly explored the influence of the combined concentration on drug efficacy by using partial differential equations. The result indicated that the two drugs were positively correlated at low doses and that tumor growth decreased if either γB or γA increased. However, the two drugs are antagonistic at high doses, and the results indicated the presence of concentration ranges wherein tumor volume increased along with the concentration of either drug. It was necessary to clarify the antagonistic ranges and avoid them in animals and early and advanced clinical trials101. Utilizing this strategy, a triple combination system was developed by integrating the MEKi trametinib, the BRAFi dabrafenib and immunotherapy. The combination resulted in complete tumor regression, increased T cell infiltration into tumors, increased major histocompatibility complex (MHC) expression, global upregulation of immune-related genes, and superior antitumor effects in a mouse model of syngeneic BRAF V600E-driven melanoma (SM1)102.
Because the pathway from RAF to ERK is linear, the intrinsic resistance to RAFi or MEKi is mainly due to the relief of ERK-involved negative feedback and pathway reactivation, and long-term acquired resistance to RAFi or MEKi is controlled by multiple mechanisms that recover ERK activity. ERKi has been validated as an important inhibitor to combine with MEKi or RAFi to forestall acquired resistance103. To verify this strategy, Hatzivassiliou G et al.87 cultured three cell lines (breast cancer cell line MDA-MB-231 and colon cancer cell lines LoVo and HCT-116) in increasing concentrations of the MEKi PD0325901 until they grew normally in 10 mmol/L (MDA-MB-231) and 5 mmol/L of PD0325901 (LoVo and HCT-116). The MEKi-resistant cell lines were obtained and were consistently found to have acquired mutations in the allosteric binding pocket of MEK. These resistant cells maintained ERK activation and retained their dependence on the RAS-RAF-MEK-ERK axis for survival and proliferation, and they were sensitive to ERK inhibition. Furthermore, the mechanism involved in MEKi resistance and MAPK pathway activation was acting on or upstream of MEK (K-RAS, B-RAF, or MEK), as ERK is a direct downstream substrate of MEK. Importantly, synergistic downstream inhibition of ERK and MEK by small molecule inhibitors would overcome multiple resistance mechanisms, inhibit the emergence of resistance, overcome acquired resistance to MEKi, and finally provide a rationale for cotargeting multiple components of the MAPK signaling pathway to maximize therapeutic efficiency for patients with K-RAS-mutant tumors87.
To combat the instantaneous response and resistance of MEKi associated with MAPK pathway reactivation, Morris EJ et al.18 investigated a novel, selective and ATP-competitive ERKi, SCH772984, that inhibited ERK1 and ERK2 with IC50 values of 4 and 1 nmol/L, respectively. It exhibited IC50 values less than 500 nmol/L in 88% of experimental BRAF-mutant cells and 49% of RAS-mutant tumor cells, whereas less than 20% sensitivity was observed in RAS and BRAF wild-type cells. It induced significant tumor regression in BRAF-mutant LOX melanoma xenografts (98% regression) and in a KRAS-mutant pancreatic MiaPaCa model (36% regression) at a 50 mg/kg dosage administered twice daily. Furthermore, SCH772984 was efficacious in BRAF-mutant melanoma or KRAS-mutant CRC cells that were resistant to the BRAFi PLX4032 or the MEKi GSK1120212, and was also effective in cells resistant to combined BRAF/MEK inhibitors. These results suggested that resistance to BRAF or MEK inhibitor caused by the reactivation of MAPK signaling was ERK-dependent and, importantly, that the introduction of ERKi into the process of MEK or RAF inhibition would be beneficial for the clinical treatment of RAFi or MEKi refractory malignant tumors with ERK reactivation18.
To overcome the transient and insufficient inhibition of the MAPK pathway by MEKi due to feedback reactivation, Merchant M et al.104 screened a series of MEKi and ERKi and then combined them and evaluated tumor inhibition in RAS- or BRAF-mutant models. Their combination was synergistic in RAS-mutant tumors but additive in BRAF-mutant models in which the RAF complex was dissociated from RAS and feedback was inhibited. Typically, the combination of cobimetinib (MEKi) and GDC-0994 (ERKi) significantly suppressed MAPK pathway output and tumor growth more effectively than single agents administered at their maximum tolerated doses and exhibited improved anti-tumor activity in multiple KRAS-mutant xenografts. Administration of cobimetinib twice weekly combined with daily treatment with GDC-0994 resulted in significant combined activity in the KRAS-mutant A549 model accompanied by a decrease in transcripts encoding multiple MAPK target genes. Phospho-p90RSK was enduringly suppressed, and cyclin D1 and Ki-67 were reduced, while cleaved-caspase 3 was increased after 24 h of treatment. Similar results were observed in BRAF- and NRAS-mutant melanoma. In a KRAS-mutant, genetically engineered mouse (GEM) model of pancreatic ductal adenocarcinoma (PDAC), combination treatment reduced tumor volume in the majority (5/8) of animals, clearly repressed downstream p-p90RSK, and significantly improved PFS (18.5 days compared with 7 days in the vehicle). In addition, in the NSCLC GEM model, the combination resulted in an overall decrease in tumor burden (10/10), strong suppression of p90RSK, and improved PFS relative to the control (102vs. 58 days). These findings underscored the importance of combined MEK and ERK inhibition in effectively ablating MAPK-dependent cancers104.
In addition, cyclin-dependent kinase 1 (CDK1) was proven to be a novel mediator of apoptosis resistance in BRAF V600E CRC and a potential target that could be used to enhance the efficacy of MEK inhibition. The combination of R0-3306 (CDK1 inhibitor) or dinaciclib (CDK1, 2, 5, and 9 inhibitor) and cobimetinib (MEKi) suppressed clonogenic survival to a greater extent than monotherapy and enhanced apoptosis via cleavage of PARP and caspase-3, and it also increased pH2Ax and Annexin V labeling in RKO and HT29 cells. In BRAF-mutant RKO CRC murine xenografts, the combinatorial use of dinaciclib plus cobimetinib inhibited tumor growth to a significantly greater extent than a single-agent regimen. Apoptosis was also promoted by the combined increase in cleaved caspase-8, -3, PARP and pH2Ax proteins in tumor tissues, which was confirmed by the cleavage of caspase-8 and -3 and the inhibition of the cell proliferation marker Ki-67 in mice. It was concluded that dual targeting with a CDK inhibitor and MEKi represented an effective therapeutic strategy in BRAF V600E CRC based on mechanism involved in the cooperative induction of apoptosis105.
Importantly, feedback reactivation of the MAPK pathway was a pivotal factor that led to the attenuated activity of MEKi, but increasing evidence has suggested that this inefficiency could be revived by cosuppressing the reactivated nodes while inhibiting MEK. In addition to the representative studies listed above, many other related studies have been undertaken to target MEK and other signaling components involved in the reciprocal feedback cascade that have achieved synergistically enhanced activity in basic and clinical stage studies106-108.
Combination of MEK inhibitor with PI3K-AKT-mTOR pathway inhibitors
The clinical activity of a single MEKi is decreased rapidly over time, and acquired resistance seems to inevitably occur, except that caused by the feedback-induced reactivation of the MAPK pathway. The upregulation of the PI3K pathway has also been implicated in resistance to MEKi, and both pathways are known to interact with each other at several nodes. Therefore, dual targeting of MEK and PI3K pathway effectors (PI3K, AKT, mTOR, and IGF-1R) represents a potential strategy for overcoming MEKi resistance109. Several studies are underway that aim to evaluate the clinical efficacy of these combination systems, and only the most recent representative examples are listed here.
To weaken MEKi resistance mediated by the activation of the PI3K pathway, Sweetlove et al.110 investigated combination therapy involving pan-PI3K signaling plus MEKi in BRAF-mutant melanoma. The efficiency and sensitivity of selumetinib (MEKi) and vemurafenib (BRAFi) in combination with ZSTK474 (pan-PI3K inhibitor), BEZ235 (pan-PI3K/mTOR inhibitor), an individual PI3K isoform inhibitor, and KU-0063794 (mTORC1/2 inhibitor) were tested in a panel of nine low-passage human BRAF-mutant metastatic melanoma cells. ZSTK474 and BEZ235 enhanced the anti-proliferative activity of selumetinib and vemurafenib in the majority of the selected cells with low expression of phosphorylated AKT (pAKT) by synergistically or additively increasing drug potency or the magnitude of cell growth inhibition. The combination synergistically inhibited phosphorylation of ERK, AKT, and S6 and tumor growth in NZM20 xenografts, with statistically significant tumor growth inhibition (TGI) values of 21.8 ± 6.6%, 19.9 ± 8.3%, 37.9 ± 6.9%, 75.8 ± 3.1%, and 59.0 ± 7.4% observed for ZSTK474, BEZ235, selumetinib, BEZ235+selumetinib, and ZSTK474+selumetinib at day 14 after administration. These findings suggested that MEKi resistance was partly mediated by PI3K pathway activation and could be reversed via the combined inhibition of molecular nodes in both cascades110.
In a nongermline, genetically engineered mouse model of glioblastoma (GBM), the combination of MEKi with a PI3K inhibitor directly improved target inhibition, RTK effector activation, and anticancer activity in mutant murine astrocytes. In GBM patient-derived xenografts (PDX), MEK/PI3K cotreatment disabled alternate effector activation and had synergistic effects in subcutaneous murine allografts111. For the treatment of TNBC brain metastases, mice bearing intracranial TNBC tumors were treated with MEK, PI3K, or PDGFR inhibitors alone or in combination. It was revealed that dual treatment with selumetinib (MEKi) and buparlisib (PI3K inhibitor) or pazopanib (PDGFR inhibitor) was synergistic and improved survival in intracranial TNBC cells. After cotreatment, MAPK and PI3K signaling was decreased in sensitive models but not in resistant models, and extensive kinome changes occurred, especially in MAPK pathway components. This provided a rationale for the combined inhibition of MEK and PI3K or MEK and PDGFR to alter the potential targetable resistance drivers and to manage TNBC brain metastasis112. Furthermore, in a highly aggressive and metastatic PDAC mouse model, cotargeting of MEK and PI3K blocked both pathways and caused growth inhibition more effectively than when either was targeted alone but failed to induce extensive cell death; thus, the use of other treatment options was indicated. The addition of histone deacetylase (HDAC) inhibitor greatly improved therapeutic outcomes and inhibited cell proliferation by > 99% via massive induction of apoptosis compared with those of the MEK+PI3K system, which decreased cell proliferation by less than 90%via mild cell death. In a nude mouse model of PDAC lung metastasis, the combination of GSK1120212 (MEKi), BEZ235 (PI3K inhibitor) and TSA (HDAC inhibitor) did not cause observable lung metastatic foci during treatment. In contrast, the combination of BEZ/GSK produced an average of 8.1 ± 1.7 foci per mouse, and the vehicle group had an average of 10 ± 2 foci. Mechanistically, coinhibition of MEK/PI3K/HDAC prevented drug resistance and improved drug tolerance in cancer cells based on a dormancy mechanism113.
In a previous report, the mechanism underlying compensatory AKT activation and drug resistance following MEK inhibition was revealed to involve the suppression of negative ERK-mediated feedback that resulted in phosphorylation of HER2 at Thr701. This conclusion indicated the need for the combined inhibition of AKT to abolish the desensitization of cancer cells to MEKi114. For example, Iizuka-Ohashi M et al.115 utilized anti-lipidemic drug statins to block the mevalonate pathway and repress AKT activation following MEK inhibition. During combined treatment with statins, the sensitivity of human breast cancer MDA-MB-231 cells to MEKi CH5126766 or trametinib was enhanced, and the tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) was upregulated to promote the reversal of apoptotic resistance to MEKi; this was dependent upon the inhibition of geranylgeranylation. This study highlighted statins as promising therapeutic targets that could be used to sensitize apoptosis-resistant cancer cells and improve the efficacy of MEKi115.
For resistant advanced cholangiocarcinoma (CCA), for which there is still no systematic treatment, the failure of therapy due to the emergence of resistance to MEKi on account of AKT pathway activation could be partially rescued by properly combining inhibitors of these respective pathways. In a comparative study, the anticancer efficiencies of MK-2206 (AKT inhibitor), AZD6244 (MEKi), and AZD8055 (mTOR inhibitor) were evaluated via single or combination administration in three CCA cell lines. This demonstrated that cotargeting of the PI3K/AKT/mTOR pathway synergistically enhanced the effects of MEKi on cell proliferation and survival, as indicated by a combination index below 0.3. Importantly, in the AZD6244+AZD8055 system, combinatorial treatment with MK-2206 was essential because AKT activation was closely associated with mTOR inhibition. These results underscored the importance of the vertical targeting of AKT and mTOR and the simultaneous suppression of the respective nodes involved in the MEK and AKT pathways for efficiently coping with CCA116. In another study, to abolish MEKi resistance via the AKT-activated bypass pathway, Park et al.117 combined the heat shock protein (HSP90) inhibitor AUY922 and trametinib to suppress the PI3K-AKT-mTOR and RAF-MEK-ERK pathways, which sensitized NSCLC cells to MEKi and increased apoptosis via cleaved PARP and the caspase-3/7 pathway at subtherapeutic doses. This represented an effective treatment regimen that could be used to manage KRAS-mutant NSCLC with MEKi resistance and to reduce the clinical toxicity of the AKT+MEK combination at therapeutic doses117.
In summary, the reactivation of the PI3K-AKT-mTOR pathway and its complex crosstalk with the RAS-RAF-MEK-ERK signaling pathway have been proven to be important factors contributing to MEKi resistance and, simultaneously, they provide an effective coinhibition method to reverse drug resistance and improve efficacy. Considerable achievements have been achieved in both theoretical and clinical studies involving MEK-involved cancer treatment. However, inefficiency inevitably occurs in proportional MAPK pathway-activated tumors due to feedback and the reactivation of other signaling pathways, which should be considered when selecting strategies to reverse MEKi resistance.
Combination of MEK inhibitor with other signaling inhibitors
Upregulation of the noncanonical Wnt-β-catenin pathway has been associated with MEKi resistance in tumors harboring RAS or BRAF mutations. Thus, dual inhibition of MEK and Wnt pathway effectors presents a potential strategy that could be used to overcome resistance to MEKi. Spreafico A et al.118 identified the Wnt/calcium pathway as a potential mediator of resistance to the MEKi selumetinib and constructed a shRNA that could be used to silence the genes of relevant Wnt receptors and ligands to recover responsiveness to selumetinib in CRC cells. Furthermore, selumetinib was combined with immunosuppresant cyclosporin A and the Wnt inhibitor TNP-470 and showed synergistic antiproliferative effects in CRC cells by modulating the activity of nuclear factor of activated T-cells (NFAT), and it was also effective in inducing tumor regression in clinically relevant patient-derived tumor explant (PDTX) models of CRC, as indicated by the increase in apoptotic markers118. In another elaborately designed study, tumor-selective nanoparticles containing a β-catenin-targeting RNA interference (RNAi) triggers were combined with the MEKi trametinib to synergistically inhibit tumor growth in xenograft models of CRC, melanoma, and HCC. The combination of MEKi/RNAi yielded synergistic efficacy and substantial tumor growth inhibition in different genetic subtypes of MAPK-activated CRC, which was reflected in > 90% TGI (60% after a single dosage). Remarkably, β-catenin-targeted RNAi treatment dramatically abolished intrinsic and acquired resistance to trametinib and improved survival in CRC liver metastasis and melanoma mouse models 119.
HGF signaling has been reported to be a mechanism that contributes to MEKi resistance that is mediated by the expression of Bcl-2-interacting mediator of cell death-extra large (Bim-EL) and Bcl-2 modifying factor (Bmf). Hence, cotargeting of HGF/cMET signaling with LY2875358 (anti-cMET antibody) and LY2801653 (dual cMET/recepteur d'origine nantais (RON) inhibitor) significantly reversed resistance to trametinib in primary hepatic stellate cells. The combination of LY2801653 and trametinib also suppressed AKT activation but promoted proapoptotic PARP cleavage in metastatic uveal melanoma explants. These discoveries confirmed that HGF/cMET signaling may serve as a target and therapeutic option that can be used to abolish MEKi resistance in metastatic uveal melanoma120.
Receptor-interacting protein kinase 1 (RIP1) was identified as a determinant of MEKi resistance via activation of the NF-κB pathway in melanoma cells, which mainly relied on the Snail1-mediated cylindromatosis (CYLD) process. This allowed the combination of targeting RIP1 with MEKi to be a potential strategy that could be used to overcome resistance and enhance efficacy in cancer treatment121.
In addition, some novel and important targets, including but not limited to tankyrases (TNKS)122, PDGFR (Hedgehog)123, zinc finger protein GLI124, Notch/γ-secretase125, PARP22, Rho-associated kinase (ROCK)126, and the TGFβ-SMAD pathway127 have also been utilized by combination systems to suppress MEKi resistance and enhance anticancer efficacy.
Concluding remarks and future perspectives
The RAS-RAF-MEK-ERK signaling pathway is aberrantly activated in more than 30% of human cancers, which frequently harbor mutations of the KRAS, NRAS and BRAF genes. The crucial location of MEK1/2 in this pathway, their unique molecular structural characteristics and their irreplaceable roles in tumorigenesis, cell proliferation and apoptosis inhibition make MEK inhibition an attractive therapeutic option for MAPK-related cancers. An abundance of highly selective and potent small molecule MEKi have been developed that have shown clinical efficacy in RAS- or RAF-mutant tumors. Furthermore, a variety of innovative combinations have been used to overcome intrinsic or acquired MEKi resistance and have achieved considerable synergetic effects that have enhanced single-agent activity.
Despite unprecedented and enthusiastic research progress, the patient responses to medication have not been entirely satisfactory, and some conclusions remain theoretical. More accurate and comprehensive understanding of resistance mechanisms needs to be achieved to design rational drug synergies. Additionally, rational and sufficient in vivo studies are essential to confirm the therapeutic benefits of combinations in comparison to single drugs. Therefore, great efforts should be made to address the following challenges to promote the clinical success of MEKi and its combinations.
(1) Expansion of predictive biomarkers. New biomarker development is a prerequisite for understanding the reduced effectiveness of MEKi and identifying responding patients. Furthermore, the mechanism underlying resistance to MEKi should be precisely defined with emerging techniques aimed at the extension and evaluation of combination approaches with other therapeutics8.
(2) Optimization of the drug administration schedule. Recent preclinical studies have shown that intermittent and pulsatile dosing is beneficial to alleviate toxicity, delay resistance occurrence, and prolong patient sensitivity and responsiveness to MEKi in patient-derived xenografts of RAS- or RAF-mutant melanoma128. Therefore, an in-depth clinical study is required to determine the preferred dosing strategy (continuous or intermittent).
(3) Relevance of preclinical trial models. Most of the existing preclinical achievements were made in subcutaneous xenograft tumors, which do not accurately reflect tumor characteristics and progression. Future research should directly focus on actual preinvasive or metastatic tumors to reflect real medical concerns129.
(4) Reduction of drug toxicity and development of new MEKi. Designing novel small molecular MEKi and illuminating their biochemical complexities and action modes has led to substantive breakthroughs in cancer treatment. Combining these new MEKi with other therapies will help to minimize toxicity and increase efficacy and is being developed as a therapeutic option that could be used to ablate MAPK pathway-based cancers in the near future3.
Acknowledgments
This work was funded by the Startup Foundation for Doctors of Shanxi Province (Grant No. SD1827), Startup Foundation for Doctors of Shanxi Medical University (Grant No. XD1824) to Y. Li, National Natural Science Foundation of China (Grant No. 81872147, 81572588), and Guangdong Provincial Special Fund of Science Innovation Strategy (Grant No. 180918104960680) to Y. Cui.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Chang L, Karin M Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 2.Dhillon AS, Hagan S, Rath O, Kolch W MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 3.Samatar AA, Poulikakos PI Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13:928–42. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 4.Cantwell-Dorris ER, O'Leary JJ, Sheils OM BRAFV600E: Implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10:385–94. doi: 10.1158/1535-7163.MCT-10-0799. [DOI] [PubMed] [Google Scholar]
- 5.Marais R, Light Y, Mason C, Paterson H, Olson MF, Marshall CJ Requirement of RAS-GTP-RAF complexes for activation of RAF-1 by protein kinase C. Science. 1998;280:109–12. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- 6.Mahapatra DK, Asati V, Bharti SK MEK inhibitors in oncology: A patent review (2015-present) Expert Opin Ther Pat. 2017;27:887–906. doi: 10.1080/13543776.2017.1339688. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Y, Tian H Current development status of MEK inhibitors. Molecules. 2017;22:1551–70. doi: 10.3390/molecules22101551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brighton HE, Angus SP, Bo T, Roques J, Tagliatela AC, Darr DB, et al New mechanisms of resistance to MEK inhibitors in melanoma revealed by intravital imaging. Cancer Res. 2018;78:542–57. doi: 10.1158/0008-5472.CAN-17-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuzillet C, Tijeras-Raballand A, de Mestier L, Cros J, Faivre S, Raymond E MEK in cancer and cancer therapy. Pharmacol Ther. 2014;141:160–71. doi: 10.1016/j.pharmthera.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 10.De Luca A, Maiello MR, D'Alessio A, Pergameno M, Normanno N The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets. 2012;16(Suppl 2):S17–27. doi: 10.1517/14728222.2011.639361. [DOI] [PubMed] [Google Scholar]
- 11.Duncan JS, Whittle MC, Nakamura K, Abell AN, Midland AA, Zawistowski JS, et al Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012;149:307–21. doi: 10.1016/j.cell.2012.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim C, Giaccone G MEK inhibitors under development for treatment of non-small-cell lung cancer. Expert Opin Investig Drugs. 2018;27:17–30. doi: 10.1080/13543784.2018.1415324. [DOI] [PubMed] [Google Scholar]
- 13.Ricci F, Guffanti F, Damia G, Broggini M Combination of paclitaxel, bevacizumab and MEK162 in second line treatment in platinum-relapsing patient derived ovarian cancer xenografts. Mol Cancer. 2017;16:97–102. doi: 10.1186/s12943-017-0662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toulany M, Iida M, Keinath S, Iyi FF, Mueck K, Fehrenbacher B, et al Dual targeting of PI3K and MEK enhances the radiation response of K-RAS mutated non-small cell lung cancer. Oncotarget. 2016;7:43746–61. doi: 10.18632/oncotarget.9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweeney EE, Burga RA, Li C, Zhu Y, Fernandes R Photothermal therapy improves the efficacy of a MEK inhibitor in neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Sci Rep. 2016;6:37035–43. doi: 10.1038/srep37035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luke JJ, Flaherty KT, Ribas A, Long GV Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14:463–82. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 17.Mor JM, Heindl LM Systemic BRAF/MEK inhibitors as a potential treatment option in metastatic conjunctival melanoma. Ocul Oncol Pathol. 2017;3:133–41. doi: 10.1159/000452473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris EJ, Jha S, Restaino CR, Dayananth P, Zhu H, Cooper A, et al Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov. 2013;3:742–50. doi: 10.1158/2159-8290.CD-13-0070. [DOI] [PubMed] [Google Scholar]
- 19.Hoeflich KP, Merchant M, Orr C, Chan J, Den Otter D, Berry L, et al Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res. 2012;72:210–9. doi: 10.1158/0008-5472.CAN-11-1515. [DOI] [PubMed] [Google Scholar]
- 20.Stewart A, Thavasu P, de Bono JS, Banerji U Titration of signalling output: insights into clinical combinations of MEK and AKT inhibitors. Ann Oncol. 2015;26:1504–10. doi: 10.1093/annonc/mdv188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng L, Jin Y, Liu M, Ruan M, Chen L HER inhibitor promotes BRAF/MEK inhibitor-induced redifferentiation in papillary thyroid cancer harboring BRAFV600E. Oncotarget. 2017;8:19843–54. doi: 10.18632/oncotarget.15773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun C, Fang Y, Yin J, Chen J, Ju Z, Zhang D, et al Rational combination therapy with PARP and MEK inhibitors capitalizes on therapeutic liabilities in RAS mutant cancers . Sci Transl Med. 2017;9:5148–65. doi: 10.1126/scitranslmed.aal5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al Roles of the RAF/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajalingam K, Schreck R, Rapp UR, Albert S RAS oncogenes and their downstream targets. Biochim Biophys Acta. 2007;1773:1177–95. doi: 10.1016/j.bbamcr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Leevers SJ, Paterson HF, Marshall CJ Requirement for RAS in RAF activation is overcome by targeting RAF to the plasma membrane. Nature. 1994;369:411–4. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 26.Hou P, Liu D, Xing M Genome-wide alterations in gene methylation by the BRAF V600E mutation in papillary thyroid cancer cells. Endocr Relat Cancer. 2011;18:687–97. doi: 10.1530/ERC-11-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roskoski R, J r ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol Res. 2012;66:105–43. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Na J, Furue MK, Andrews PW Inhibition of ERK1/2 prevents neural and mesendodermal differentiation and promotes human embryonic stem cell self-renewal. Stem Cell Res. 2010;5:157–69. doi: 10.1016/j.scr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Matallanas D, Birtwistle M, Romano D, Zebisch A, Rauch J, von Kriegsheim A, et al RAF family kinases: old dogs have learned new tricks. Genes Cancer. 2011;2:232–60. doi: 10.1177/1947601911407323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts PJ, Der CJ Targeting the RAF-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 31.Maurer G, Tarkowski B, Baccarini M RAF kinases in cancer-roles and therapeutic opportunities. Oncogene. 2011;30:3477–88. doi: 10.1038/onc.2011.160. [DOI] [PubMed] [Google Scholar]
- 32.Gysin S, Lee SH, Dean NM, McMahon M Pharmacologic inhibition of RAF-->MEK-->ERK signaling elicits pancreatic cancer cell cycle arrest through induced expression of p27kip1. Cancer Res. 2005;65:4870–80. doi: 10.1158/0008-5472.CAN-04-2848. [DOI] [PubMed] [Google Scholar]
- 33.Sahu RP, Batra S, Kandala PK, Brown TL, Srivastava SK The role of K-ras gene mutation in TRAIL-induced apoptosis in pancreatic and lung cancer cell lines. Cancer Chemother Pharmacol. 2011;67:481–7. doi: 10.1007/s00280-010-1463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim EK, Choi EJ Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Schafer R, Sers C RAS oncogene-mediated deregulation of the transcriptome: from molecular signature to function. Adv Enzyme Regul. 2011;51:126–36. doi: 10.1016/j.advenzreg.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Karoulia Z, Gavathiotis E, Poulikakos PI New perspectives for targeting RAF kinase in human cancer. Nat Rev Cancer. 2017;17:676–91. doi: 10.1038/nrc.2017.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, et al ERKs: A family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–75. doi: 10.1016/0092-8674(91)90098-J. [DOI] [PubMed] [Google Scholar]
- 38.Zimmermann G, Papke B, Ismail S, Vartak N, Chandra A, Hoffmann M, et al Small molecule inhibition of the KRAS-PDEδ interaction impairs oncogenic KRAS signalling. Nature. 2013;497:638–42. doi: 10.1038/nature12205. [DOI] [PubMed] [Google Scholar]
- 39.Nickerson S, Joy ST, Arora PS, Bar-Sagi D An orthosteric inhibitor of the RAS-SOS interaction. Enzymes. 2013;34(Pt. B):25–39. doi: 10.1016/B978-0-12-420146-0.00002-0. [DOI] [PubMed] [Google Scholar]
- 40.Shima F, Yoshikawa Y, Ye M, Araki M, Matsumoto S, Liao J, et al In silico discovery of small-molecule Ras inhibitors that display antitumor activity by blocking the Ras-effector interaction. Proc Natl Acad Sci U S A. 2013;110:8182–7. doi: 10.1073/pnas.1217730110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garbe C, Eigentler TK Vemurafenib. Recent Results Cancer Res. 2018;211:77–89. doi: 10.1007/978-3-319-91442-8_6. [DOI] [PubMed] [Google Scholar]
- 42.Kainthla R, Kim KB, Falchook GS Dabrafenib. Recent Results Cancer Res. 2014;201:227–40. doi: 10.1007/978-3-642-54490-3. [DOI] [PubMed] [Google Scholar]
- 43.Sidaway P Encorafenib-a new agent for advanced-stage disease. Nat Rev Clin Oncol. 2018;15:344–5. doi: 10.1038/s41571-018-0019-x. [DOI] [PubMed] [Google Scholar]
- 44.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ettrich TJ, Seufferlein T Regorafenib. Recent Results Cancer Res. 2018;211:45–56. doi: 10.1007/978-3-319-91442-8_3. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan RJ, Infante JR, Janku F, Wong DJL, Sosman JA, Keedy V, et al First-in-class ERK1/2 inhibitor Ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: Results of a phase I dose-escalation and expansion study. Cancer Discov. 2018;8:184–95. doi: 10.1158/2159-8290.CD-17-1119. [DOI] [PubMed] [Google Scholar]
- 47.Blake JF, Burkard M, Chan J, Chen H, Chou KJ, Diaz D, et al Discovery of (s)-1-(1-(4-chloro-3-fluorophenyl)-2-hydroxyethyl)-4-(2-((1-methyl-1h-pyrazol-5-yl)amino)pyrimidin-4-yl)pyridin-2(1h)-one (GDC-0994), an extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor in early clinical development. J Med Chem. 2016;59:5650–60. doi: 10.1021/acs.jmedchem.6b00389. [DOI] [PubMed] [Google Scholar]
- 48.Ohori M, Kinoshita T, Okubo M, Sato K, Yamazaki A, Arakawa H, et al Identification of a selective ERK inhibitor and structural determination of the inhibitor-ERK2 complex. Biochem Biophys Res Commun. 2005;336:357–63. doi: 10.1016/j.bbrc.2005.08.082. [DOI] [PubMed] [Google Scholar]
- 49.Liu B, Fu L, Zhang C, Zhang L, Zhang Y, Ouyang L, et al Computational design, chemical synthesis, and biological evaluation of a novel ERK inhibitor (BL-EI001) with apoptosis-inducing mechanisms in breast cancer. Oncotarget. 2015;6:6762–75. doi: 10.18632/oncotarget.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukuda M, Gotoh I, Gotoh Y, Nishida E Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J Biol Chem. 1996;271:20024–8. doi: 10.1074/jbc.271.33.20024. [DOI] [PubMed] [Google Scholar]
- 51.Sebolt-Leopold JS MEK inhibitors: a therapeutic approach to targeting the RAS-MAP kinase pathway in tumors. Curr Pharm Des. 2004;10:1907–14. doi: 10.2174/1381612043384439. [DOI] [PubMed] [Google Scholar]
- 52.Scholl FA, Dumesic PA, Barragan DI, Charron J, Khavari PA MEK1/2 gene dosage determines tissue response to oncogenic RAS signaling in the skin. Oncogene. 2009;28:1485–95. doi: 10.1038/onc.2008.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, et al RAS/RAF/MEK/ERK and PI3K/PTEN/AKT/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–64. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo . J Biol Chem. 1995;270:27489–94. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 55.Lorusso PM, Adjei AA, Varterasian M, Gadgeel S, Reid J, Mitchell DY, et al Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol. 2005;23:5281–93. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 56.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–14. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 57.Gilmartin AG, Bleam MR, Groy A, Moss KG, Minthorn EA, Kulkarni SG, et al GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition . Clin Cancer Res. 2011;17:989–1000. doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- 58.Rice KD, Aay N, Anand NK, Blazey CM, Bowles OJ, Bussenius J, et al Novel carboxamide-based allosteric MEK inhibitors: discovery and optimization efforts toward XL518 (GDC-0973) ACS Med Chem Lett. 2012;3:416–21. doi: 10.1021/ml300049d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ascierto PA, McArthur GA, Dreno B, Atkinson V, Liszkay G, Di Giacomo AM, et al Cobimetinib combined with vemurafenib in advanced BRAF(v600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248–60. doi: 10.1016/S1470-2045(16)30122-X. [DOI] [PubMed] [Google Scholar]
- 60.Shirley M Encorafenib and Binimetinib: First Global Approvals. Drugs. 2018;78:1277–84. doi: 10.1007/s40265-018-0963-x. [DOI] [PubMed] [Google Scholar]
- 61.Barrett SD, Bridges AJ, Dudley DT, Saltiel AR, Fergus JH, Flamme CM, et al The discovery of the benzhydroxamate MEK inhibitors CI-1040 and PD0325901. Bioorg Med Chem Lett. 2008;18:6501–4. doi: 10.1016/j.bmcl.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 62.Haasbach E, Muller C, Ehrhardt C, Schreiber A, Pleschka S, Ludwig S, et al The MEK-inhibitor CI-1040 displays a broad anti-influenza virus activity in vitro and provides a prolonged treatment window compared to standard of care in vivo . Antiviral Res. 2017;142:178–84. doi: 10.1016/j.antiviral.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 63.Seto T, Hirai F, Saka H, Kogure Y, Yoh K, Niho S, et al Safety and tolerability of selumetinib as a monotherapy, or in combination with docetaxel as second-line therapy, in Japanese patients with advanced solid malignancies or non-small cell lung cancer. Jpn J Clin Oncol. 2018;48:31–42. doi: 10.1093/jjco/hyx144. [DOI] [PubMed] [Google Scholar]
- 64.Van Laethem JL, Riess H, Jassem J, Haas M, Martens UM, Weekes C, et al Phase I/II study of refametinib (BAY 86-9766) in combination with gemcitabine in advanced pancreatic cancer. Target Oncol. 2017;12:97–109. doi: 10.1007/s11523-016-0469-y. [DOI] [PubMed] [Google Scholar]
- 65.Srinivas NR Pharmacology of Pimasertib, A Selective MEK1/2 Inhibitor. Eur J Drug Metab Pharmacokinet. 2018;43:373–82. doi: 10.1007/s13318-018-0466-x. [DOI] [PubMed] [Google Scholar]
- 66.Isshiki Y, Kohchi Y, Iikura H, Matsubara Y, Asoh K, Murata T, et al Design and synthesis of novel allosteric MEK inhibitor CH4987655 as an orally available anticancer agent. Bioorg Med Chem Lett. 2011;21:1795–801. doi: 10.1016/j.bmcl.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 67.Cohen RB, Aamdal S, Nyakas M, Cavallin M, Green D, Learoyd M, et al A phase I dose-finding, safety and tolerability study of AZD8330 in patients with advanced malignancies. Eur J Cancer. 2013;49:1521–9. doi: 10.1016/j.ejca.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 68.Dong Q, Dougan DR, Gong X, Halkowycz P, Jin B, Kanouni T, et al Discovery of TAK-733, a potent and selective MEK allosteric site inhibitor for the treatment of cancer. Bioorg Med Chem Lett. 2011;21:1315–9. doi: 10.1016/j.bmcl.2011.01.071. [DOI] [PubMed] [Google Scholar]
- 69.Haagensen EJ, Thomas HD, Schmalix WA, Payne AC, Kevorkian L, Allen RA, et al Enhanced anti-tumour activity of the combination of the novel MEK inhibitor WX-554 and the novel PI3K inhibitor WX-037. Cancer Chemother Pharmacol. 2016;78:1269–81. doi: 10.1007/s00280-016-3186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martinez-Garcia M, Banerji U, Albanell J, Bahleda R, Dolly S, Kraeber-Bodere F, et al First-in-human, phase I dose-escalation study of the safety, pharmacokinetics, and pharmacodynamics of RO5126766, a first-in-class dual MEK/RAF inhibitor in patients with solid tumors. Clin Cancer Res. 2012;18:4806–19. doi: 10.1158/1078-0432.CCR-12-0742. [DOI] [PubMed] [Google Scholar]
- 71.Hatzivassiliou G, Haling JR, Chen H, Song K, Price S, Heald R, et al Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature. 2013;501:232–6. doi: 10.1038/nature12441. [DOI] [PubMed] [Google Scholar]
- 72.Daouti S, Higgins B, Kolinsky K, Packman K, Wang H, Rizzo C, et al Preclinical in vivo evaluation of efficacy, pharmacokinetics, and pharmacodynamics of a novel MEK1/2 kinase inhibitor RO5068760 in multiple tumor models . Mol Cancer Ther. 2010;9:134–44. doi: 10.1158/1535-7163.MCT-09-0601. [DOI] [PubMed] [Google Scholar]
- 73.Daouti S, Wang H, Li WH, Higgins B, Kolinsky K, Packman K, et al Characterization of a novel mitogen-activated protein kinase kinase 1/2 inhibitor with a unique mechanism of action for cancer therapy. Cancer Res. 2009;69:1924–32. doi: 10.1158/0008-5472.CAN-08-2627. [DOI] [PubMed] [Google Scholar]
- 74.Klein PJ, Schmidt CM, Wiesenauer CA, Choi JN, Gage EA, Yip-Schneider MT, et al The effects of a novel MEK inhibitor PD184161 on MEK-ERK signaling and growth in human liver cancer. Neoplasia. 2006;8:1–8. doi: 10.1593/neo.05373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao Y, Ge CC, Wang J, Wu XX, Li XM, Li W, et al MEK inhibitor, PD98059, promotes breast cancer cell migration by inducing beta-catenin nuclear accumulation. Oncol Rep. 2017;38:3055–63. doi: 10.3892/or.2017.5955. [DOI] [PubMed] [Google Scholar]
- 76.Ong Q, Guo S, Zhang K, Cui B U0126 protects cells against oxidative stress independent of its function as a MEK inhibitor. ACS Chem Neurosci. 2015;6:130–7. doi: 10.1021/cn500288n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang W, Zhou J, Zhao L, Chen S Combination of SL327 and sunitinib malate leads to an additive anti-cancer effect in doxorubicin resistant thyroid carcinoma cells. Biomed Pharmacother. 2017;88:985–90. doi: 10.1016/j.biopha.2017.01.135. [DOI] [PubMed] [Google Scholar]
- 78.Kim DJ, Lee MH, Reddy K, Li Y, Lim DY, Xie H, et al CInQ-03, a novel allosteric MEK inhibitor, suppresses cancer growth in vitro and in vivo . Carcinogenesis. 2013;34:1134–43. doi: 10.1093/carcin/bgt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choo EF, Belvin M, Chan J, Hoeflich K, Orr C, Robarge K, et al Preclinical disposition and pharmacokinetics-pharmacodynamic modeling of biomarker response and tumour growth inhibition in xenograft mouse models of G-573, a MEK inhibitor. Xenobiotica. 2010;40:751–62. doi: 10.3109/00498254.2010.514365. [DOI] [PubMed] [Google Scholar]
- 80.Han S, Zhou V, Pan S, Liu Y, Hornsby M, McMullan D, et al Identification of coumarin derivatives as a novel class of allosteric MEK1 inhibitors. Bioorg Med Chem Lett. 2005;15:5467–73. doi: 10.1016/j.bmcl.2005.08.097. [DOI] [PubMed] [Google Scholar]
- 81.Zhao Y, Adjei AA The clinical development of MEK inhibitors. Nat Rev Clin Oncol. 2014;11:385–400. doi: 10.1038/nrclinonc.2014.83. [DOI] [PubMed] [Google Scholar]
- 82.Tijeras-Raballand A, Neuzillet C, Couvelard A, Serova M, de Gramont A, Hammel P, et al Resistance to targeted therapies in pancreatic neuroendocrine tumors (PNETs): molecular basis, preclinical data, and counteracting strategies. Target Oncol. 2012;7:173–81. doi: 10.1007/s11523-012-0229-6. [DOI] [PubMed] [Google Scholar]
- 83.Furukawa T, Sunamura M, Motoi F, Matsuno S, Horii A Potential tumor suppressive pathway involving DUSP6/MKP-3 in pancreatic cancer. Am J Pathol. 2003;162:1807–15. doi: 10.1016/S0002-9440(10)64315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, et al Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–9. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jing J, Greshock J, Holbrook JD, Gilmartin A, Zhang X, McNeil E, et al Comprehensive predictive biomarker analysis for MEK inhibitor GSK1120212. Mol Cancer Ther. 2012;11:720–9. doi: 10.1158/1535-7163.MCT-11-0505. [DOI] [PubMed] [Google Scholar]
- 86.Corcoran RB, Settleman J, Engelman JA Potential therapeutic strategies to overcome acquired resistance to BRAF or MEK inhibitors in BRAF mutant cancers. Oncotarget. 2011;2:336–46. doi: 10.18632/oncotarget.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hatzivassiliou G, Liu B, O'Brien C, Spoerke JM, Hoeflich KP, Haverty PM, et al ERK inhibition overcomes acquired resistance to MEK inhibitors. Mol Cancer Ther. 2012;11:1143–54. doi: 10.1158/1535-7163.MCT-11-1010. [DOI] [PubMed] [Google Scholar]
- 88.Abel EV, Basile KJ, Kugel CH 3rd, Witkiewicz AK, Le K, Amaravadi RK, et al Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. J Clin Invest. 2013;123:2155–68. doi: 10.1172/JCI65780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sullivan RJ, Flaherty KT Resistance to BRAF-targeted therapy in melanoma. Eur J Cancer. 2013;49:1297–304. doi: 10.1016/j.ejca.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 90.Holt SV, Logie A, Davies BR, Alferez D, Runswick S, Fenton S, et al Enhanced apoptosis and tumor growth suppression elicited by combination of MEK (selumetinib) and mTOR kinase inhibitors (AZD8055) Cancer Res. 2012;72:1804–13. doi: 10.1158/0008-5472.CAN-11-1780. [DOI] [PubMed] [Google Scholar]
- 91.Dry JR, Pavey S, Pratilas CA, Harbron C, Runswick S, Hodgson D, et al Transcriptional pathway signatures predict MEK addiction and response to selumetinib (AZD6244) Cancer Res. 2010;70:2264–73. doi: 10.1158/0008-5472.CAN-09-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Poulikakos PI, Solit DB Resistance to MEK inhibitors: should we co-target upstream? Sci Signal. 2011;4:16–8. doi: 10.1126/scisignal.2001948. [DOI] [PubMed] [Google Scholar]
- 93.Whittaker SR, Cowley GS, Wagner S, Luo F, Root DE, Garraway LA Combined pan-RAF and MEK inhibition overcomes multiple resistance mechanisms to selective RAF inhibitors. Mol Cancer Ther. 2015;14:2700–11. doi: 10.1158/1535-7163.MCT-15-0136-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Atefi M, Titz B, Avramis E, Ng C, Wong DJ, Lassen A, et al Combination of pan-RAF and MEK inhibitors in NRAS mutant melanoma. Mol Cancer. 2015;14:27–38. doi: 10.1186/s12943-015-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martin CA, Cullinane C, Kirby L, Abuhammad S, Lelliott EJ, Waldeck K, et al Palbociclib synergizes with BRAF and MEK inhibitors in treatment naïve melanoma but not after the development of BRAF inhibitor resistance. Int J Cancer. 2018;142:2139–52. doi: 10.1002/ijc.v142.10. [DOI] [PubMed] [Google Scholar]
- 96.Ahronian LG, Sennott EM, Van Allen EM, Wagle N, Kwak EL, Faris JE, et al Clinical acquired resistance to RAF inhibitor combinations in BRAF-mutant colorectal cancer through MAPK pathway alterations. Cancer Discov. 2015;5:358–67. doi: 10.1158/2159-8290.CD-14-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Niessner H, Sinnberg T, Kosnopfel C, Smalley KSM, Beck D, Praetorius C, et al BRAF inhibitors amplify the proapoptotic activity of MEK inhibitors by inducing ER stress in NRAS-mutant melanoma. Clin Cancer Res. 2017;23:6203–14. doi: 10.1158/1078-0432.CCR-17-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–76. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 99.Nagaria TS, Shi C, Leduc C, Hoskin V, Sikdar S, Sangrar W, et al Combined targeting of RAF and MEK synergistically inhibits tumorigenesis in triple negative breast cancer model systems. Oncotarget. 2017;8:80804–19. doi: 10.18632/oncotarget.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Della Corte CM, Ciaramella V, Cardone C, La Monica S, Alfieri R, Petronini PG, et al Antitumor efficacy of dual blockade of EGFR signaling by osimertinib in combination with selumetinib or cetuximab in activated EGFR human NCLC tumor models. J Thorac Oncol. 2018;13:810–20. doi: 10.1016/j.jtho.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 101.Lai X, Friedman A Combination therapy for melanoma with BRAF/MEK inhibitor and immune checkpoint inhibitor: a mathematical model. BMC Syst Biol. 2017;11:70–87. doi: 10.1186/s12918-017-0446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, et al Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med. 2015;7:279ra41–51. doi: 10.1126/scitranslmed.aaa4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kidger AM, Sipthorp J, Cook SJ ERK1/2 inhibitors: new weapons to inhibit the RAS-regulated RAF-MEK1/2-ERK1/2 pathway. Pharmacol Ther. 2018;187:45–60. doi: 10.1016/j.pharmthera.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 104.Merchant M, Moffat J, Schaefer G, Chan J, Wang X, Orr C, et al Combined MEK and ERK inhibition overcomes therapy-mediated pathway reactivation in RAS mutant tumors. PLOS ONE. 2017;12:e0185862–83. doi: 10.1371/journal.pone.0185862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang P, Kawakami H, Liu W, Zeng X, Strebhardt K, Tao K, et al Targeting CDK1 and MEK/ERK overcomes apoptotic resistance in BRAF-mutant human colorectal cancer. Mol Cancer Res. 2018;16:378–89. doi: 10.1158/1541-7786.MCR-17-0404. [DOI] [PubMed] [Google Scholar]
- 106.Arafeh R, Flores K, Keren-Paz A, Maik-Rachline G, Gutkind N, Rosenberg S, et al Combined inhibition of MEK and nuclear ERK translocation has synergistic antitumor activity in melanoma cells. Sci Rep. 2017;7:16345–53. doi: 10.1038/s41598-017-16558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee MS, Helms TL, Feng N, Gay J, Chang QE, Tian F, et al Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models . Oncotarget. 2016;7:39595–608. doi: 10.18632/oncotarget.9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Molnár E, Rittler D, Baranyi M, Grusch M, Berger W, Döme B, et al Pan-RAF and MEK vertical inhibition enhances therapeutic response in non-V600 BRAF mutant cells. BMC Cancer. 2018;18:542–52. doi: 10.1186/s12885-018-4455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Temraz S, Mukherji D, Shamseddine A Dual inhibition of MEK and PI3K pathway in KRAS and BRAF mutated colorectal cancers. Int J Mol Sci. 2015;16:22976–88. doi: 10.3390/ijms160922976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sweetlove M, Wrightson E, Kolekar S, Rewcastle GW, Baguley BC, Shepherd PR, et al Inhibitors of pan-PI3K signaling synergize with BRAF or MEK inhibitors to prevent BRAF-mutant melanoma cell growth. Front Oncol. 2015;5:135–48. doi: 10.3389/fonc.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McNeill RS, Canoutas DA, Stuhlmiller TJ, Dhruv HD, Irvin DM, Bash RE, et al Combination therapy with potent PI3K and MAPK inhibitors overcomes adaptive kinome resistance to single agents in preclinical models of glioblastoma. Neuro Oncol. 2017;19:1469–80. doi: 10.1093/neuonc/nox044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Van Swearingen AED, Sambade MJ, Siegel MB, Sud S, McNeill RS, Bevill SM, et al Combined kinase inhibitors of MEK1/2 and either PI3K or PDGFR are efficacious in intracranial triple-negative breast cancer. Neuro Oncol. 2017;19:1481–93. doi: 10.1093/neuonc/nox052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ischenko I, Petrenko O, Hayman MJ A MEK/PI3K/HDAC inhibitor combination therapy for KRAS mutant pancreatic cancer cells. Oncotarget. 2015;6:15814–27. doi: 10.18632/oncotarget.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen CH, Hsia TC, Yeh MH, Chen TW, Chen YJ, Chen JT, et al MEK inhibitors induce AKT activation and drug resistance by suppressing negative feedback ERK-mediated HER2 phosphorylation at Thr701. Mol Oncol. 2017;11:1273–87. doi: 10.1002/mol2.2017.11.issue-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iizuka-Ohashi M, Watanabe M, Sukeno M, Morita M, Hoang NTH, Kuchimaru T, et al Blockage of the mevalonate pathway overcomes the apoptotic resistance to MEK inhibitors with suppressing the activation of AKT in cancer cells. Oncotarget. 2018;9:19597–612. doi: 10.18632/oncotarget.24696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ewald F, Norz D, Grottke A, Hofmann BT, Nashan B, Jucker M Dual inhibition of PI3K-AKT-mTOR- and RAF-MEK-ERK-signaling is synergistic in cholangiocarcinoma and reverses acquired resistance to MEK inhibitors. Invest New Drugs. 2014;32:1144–54. doi: 10.1007/s10637-014-0149-7. [DOI] [PubMed] [Google Scholar]
- 117.Park KS, Oh B, Lee MH, Nam KY, Jin HR, Yang H, et al The HSP90 inhibitor, NVP-AUY922, sensitizes KRAS-mutant non-small cell lung cancer with intrinsic resistance to MEK inhibitor, trametinib. Cancer Lett. 2016;372:75–81. doi: 10.1016/j.canlet.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 118.Spreafico A, Tentler JJ, Pitts TM, Tan AC, Gregory MA, Arcaroli JJ, et al Rational combination of a MEK inhibitor, selumetinib, and the Wnt/calcium pathway modulator, cyclosporin a, in preclinical models of colorectal cancer. Clin Cancer Res. 2013;19:4149–62. doi: 10.1158/1078-0432.CCR-12-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ganesh S, Shui X, Craig KP, Koser ML, Chopda GR, Cyr WA, et al Beta-catenin mRNA silencing and MEK inhibition display synergistic efficacy in preclinical tumor models. Mol Cancer Ther. 2018;17:544–53. doi: 10.1158/1535-7163.MCT-17-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheng H, Chua V, Liao C, Purwin TJ, Terai M, Kageyama K, et al Co-targeting HGF/cMET signaling with MEK inhibitors in metastatic uveal melanoma. Mol Cancer Ther. 2017;16:516–28. doi: 10.1158/1535-7163.MCT-16-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lei FX, Jin L, Liu XY, Lai F, Yan XG, Farrelly M, et al RIP1 protects melanoma cells from apoptosis induced by BRAF/MEK inhibitors. Cell Death Dis. 2018;9:679–92. doi: 10.1038/s41419-018-0714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schoumacher M, Hurov KE, Lehar J, Yan-Neale Y, Mishina Y, Sonkin D, et al Inhibiting tankyrases sensitizes KRAS-mutant cancer cells to MEK inhibitors via FGFR2 feedback signaling . Cancer Res. 2014;74:3294–305. doi: 10.1158/0008-5472.CAN-14-0138-T. [DOI] [PubMed] [Google Scholar]
- 123.Sabbatino F, Wang Y, Wang X, Flaherty KT, Yu L, Pepin D, et al PDGFRα up-regulation mediated by sonic hedgehog pathway activation leads to BRAF inhibitor resistance in melanoma cells with BRAF mutation. Oncotarget. 2014;5:1926–41. doi: 10.18632/oncotarget.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vlckova K, Reda J, Ondrusova L, Krayem M, Ghanem G, Vachtenheim J GLI inhibitor GANT61 kills melanoma cells and acts in synergy with obatoclax. Int J Oncol. 2016;49:953–60. doi: 10.3892/ijo.2016.3596. [DOI] [PubMed] [Google Scholar]
- 125.Yang X, Xia W, Chen L, Wu CX, Zhang CC, Olson P, et al Synergistic antitumor effect of a gamma-secretase inhibitor PF-03084014 and sorafenib in hepatocellular carcinoma. Oncotarget. 2018;9:34996–5007. doi: 10.18632/oncotarget.26209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vogel CJ, Smit MA, Maddalo G, Possik PA, Sparidans RW, van der Burg SH, et al Cooperative induction of apoptosis in NRAS mutant melanoma by inhibition of MEK and ROCK. Pigment Cell Melanoma Res. 2015;28:307–17. doi: 10.1111/pcmr.12364. [DOI] [PubMed] [Google Scholar]
- 127.Smith MP, Ferguson J, Arozarena I, Hayward R, Marais R, Chapman A, et al Effect of SMURF2 targeting on susceptibility to MEK inhibitors in melanoma. J Natl Cancer Inst. 2013;105:33–46. doi: 10.1093/jnci/djs471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Das Thakur M, Salangsang F, Landman AS, Sellers WR, Pryer NK, Levesque MP, et al Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013;494:251–5. doi: 10.1038/nature11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gómez-Cuadrado L, Tracey N, Ma R, Qian B, Brunton VG Mouse models of metastasis: progress and prospects. Dis Model Mech. 2017;10:1061–74. doi: 10.1242/dmm.030403. [DOI] [PMC free article] [PubMed] [Google Scholar]