Abstract
Mycotic pseudoaneurysms usually arise from an infectious arteritis or mycotic aneurysms secondary to weakening and destruction of the arterial wall resulting in a contained rupture. We report a case of a mycotic pseudoaneurysm affecting the aortic isthmus of the thoracic aorta which is an extremely rare infection. To our knowledge no case report of mycotic pseudoaneurysm of the aortic isthmus secondary to salmonella infection has thus far been described. The specific case we present is also unique in that it posed a diagnostic imaging dilemma where the initial imaging revealed a periaortic mass which could not be accurately characterized and only on subsequent imaging reveal itself to be a thrombosed mycotic pseudoaneurysm. We hope that our case report highlights to the medical community the high degree of suspicion one should have regarding pseudoaneurysms when dealing with a complex mass intimately related to a vascular structure.
Keywords: Mycotic pseudoaneurysm, salmonella, aortic arch, aortic isthmus, aneurysm, CT chest, infection, CT aortogram, endovascular repair
CASE REPORT
An 88-year old Chinese gentleman was admitted to respiratory medicine department for hemoptysis. He complained of having six episodes of hemoptysis (tablespoon amount) for the last three days not associated with fever, loss of weight or appetite. His past medical history includes COPD (Gold D), ischemic heart disease, and an esophageal mass of unknown etiology (FNA was inconclusive and patient deferred follow-up). Patient’s last admission was for salmonella gastroenteritis which was complicated by acute kidney injury and type II acute myocardial infarction.
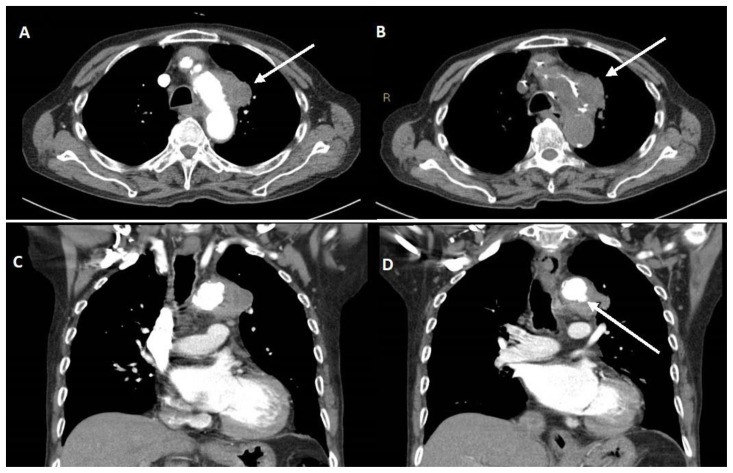
Upon admission, the patient’s initial chest radiograph was reported not to demonstrate any acute or suspicious findings and the patient was empirically started on IV trimethoprim / sulfamethoxazole for possible chest infection. Results from sputum AFB smears were negative, however sputum culture was positive for salmonella (sensitive to ceftriaxone, ciprofloxacin and trimethoprim / sulfamethoxazole). Initial contrast enhanced CT scan of the chest demonstrated a lobulated mass (55 HU), inseparable from the left wall of the aortic arch, measuring about 3.7 x 2.4 cm in axial dimensions (Fig. 1). Multiple borderline enlarged nodes were also seen scattered throughout the mediastinum. A follow-up non-contrast CT chest (Fig. 1B) showed that the periaortic mass was relatively hyperdense and the attenuation of the mass without contrast measured up to 53 HU. The primary consideration at this point was malignancy with mediastinal invasion or conglomerate / contiguous lymphadenopathy secondary to infection.
Figure 1.
An 89-year-old male with a peri-aortic mass.
Findings: Initial CT Thorax (A) contrast (B) non-contrast, demonstrating a lobulated mass (A and B, arrow), inseparable from the left lateral wall of aortic arch, measuring 3.7 x 2.4 cm in axial dimensions. Within the coronal image (C and D) there is a small ulceration in the inferior lateral wall of the aortic arch as evident by shallow pooling of contrast into the thickened wall of the aorta (D, arrow).
Technique: Contrast enhanced CT chest with axial (A and B) and coronal reformats (C and D). CT scan settings were 3.00 mm slice thickness at 120 kV and 40 mAs.
The patient then underwent transbronchial lung biopsy (TBLB) and bronchial brush cytology, both of which were negative for malignancy. However, TBLB did show evidence of bronchopneumonia. The bronchoalveolar lavage was negative for TB. At this point the patient no longer had hemoptysis but was started spiking fever. CT scan of the brain, abdomen and pelvis were negative for malignancy and source of infection.
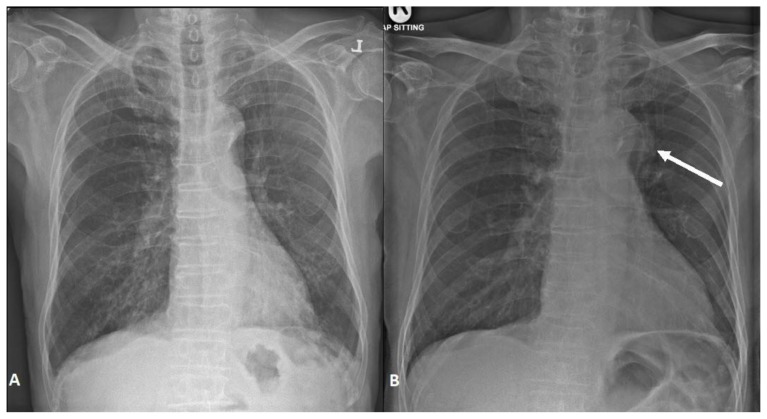
Subsequently, the blood and sputum cultures grew salmonella. This case was reviewed by infectious disease department and presented at the thoracic radiology grand round. In retrospect, it was noted at the thoracic radiology grand round that the initial chest radiograph was not unremarkable as previously thought and showed a subtle opacity along the left lateral wall of the aortic arch (Fig. 2B). It was highlighted that a previous chest radiograph performed just one-month ago (Fig. 2A) did not show a similar periaortic opacity and hence the periaortic mass had likely developed within the one-month interval. In view of negative histology for lung cancer, ongoing salmonella bacteremia and the rate of progression of the disease it was concluded that the lesion was likely of an infectious etiology. There was further discussion regarding the appearance of the aortic arch wall which appeared thickened, irregular and demonstrated shallow ulcerations at the inferior lateral aspect of the aortic arch (Fig. 1C). The patient was started on IV ceftriaxone and was advised to undergo CT aortogram to investigate for ulceration and pseudoaneurysm of the aorta.
Figure 2.
An 89-year-old male with a peri-aortic mass.
Findings: Posteroanterior chest x-ray 1-month prior to admission (A). Anteroposterior chest x-ray upon admission (B). Chest x-ray upon admission demonstrates a vague opacity along the left lateral wall of the aortic arch appearing inseparable from it (B, arrow). Chest radiograph performed 1-month earlier (A) did not show this peri-aortic opacity. This suggests the lesion must have developed in the interim suggesting a rapid rate of progression and making malignancy less likely.
Technique: Chest radiograph posteroanterior (A) and anteroposterior (B) views. Performed at 100 KV and 5 mAs.
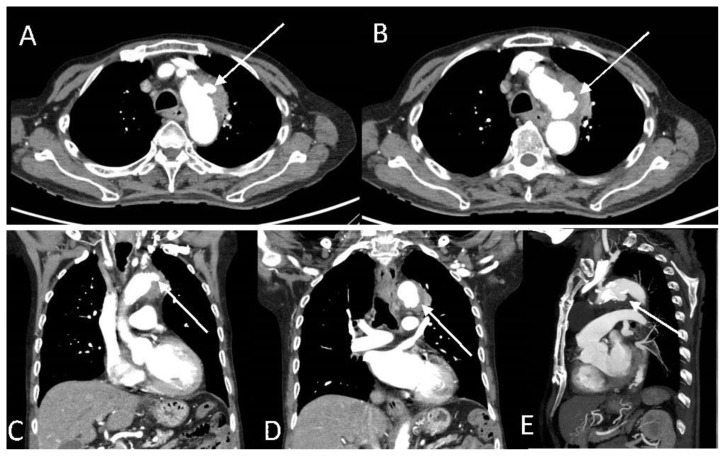
The CT aortogram showed that the periaortic mass epicentered at the lateral wall of the aortic isthmus appeared less prominent. However, two new contrast-filled focal out-pouching’s were seen along the lateral and inferior wall of the aortic isthmus consistent with salmonella related mycotic pseudoaneurysms (Fig. 3). The larger pseudoaneurysm at the inferior wall had a 0.8 cm neck and measured approximately 1.9 x 1.1 x 1.8 cm (Fig. 3 B, D and E) while the smaller pseudoaneurysm had a 0.4 cm neck and measured about 1.1 x 1.4 cm (Fig. 3 A and C). The patient was planned to continue IV ceftriaxone for six weeks and undergo stenting of the pseudoaneurysm towards the end of the antibiotic course.
Figure 3.
An 89-year-old male with peri-aortic pseudoaneurysms.
Findings: Initial CT aortogram showed that the periaortic soft tissue mass along the lateral wall of the aortic isthmus appeared less prominent. However, there were two new contrast-filled focal out-pouching's along the lateral and inferior wall of the aortic isthmus. Axial images of the two small pseudoaneurysms along the lateral (A, arrow) and inferior (B, arrow) wall of the aortic arch measuring 0.7 x 1.1 cm and 1.7 x 1.3 cm, respectively. Coronal images of the two small pseudoaneurysms along the lateral (C, arrow) and inferior (D, arrow) wall of the aortic arch. (D) Sagittal image shows the inferior pseudoaneurysm (arrow).
Technique: Contrast enhanced CT aortogram with axial, coronal and sagittal reformats. CT scan settings were 3.00 mm slice thickness at 100 kV and 115 mAs.
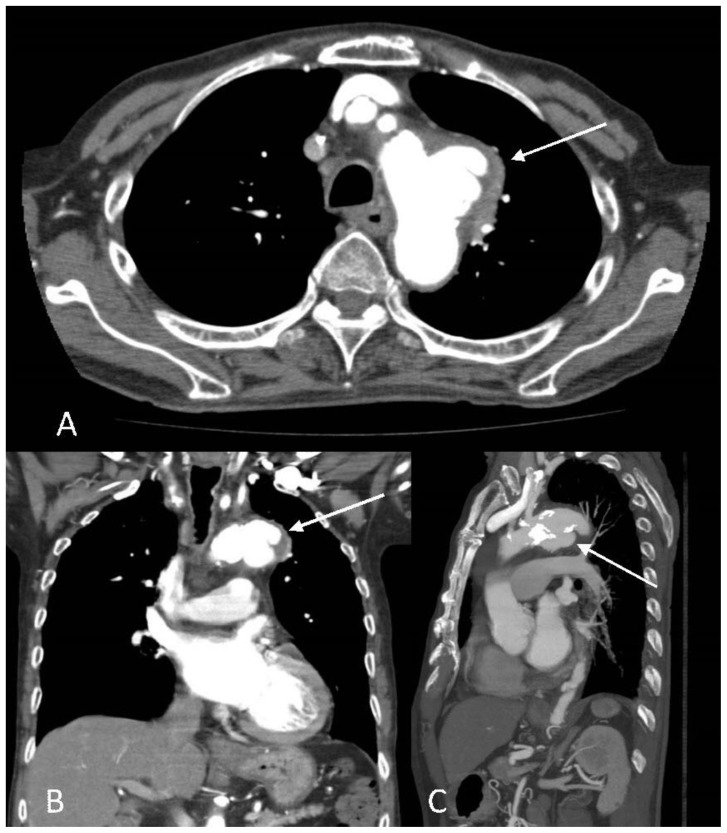
However, the follow-up CT aortogram demonstrated the previously described two small pseudoaneurysms now appeared to have coalesced into a large multilobulated pseudoaneurysm with a corresponding luminal defect extending from the anterior wall to the inferior margin of the aortic arch (Fig. 4). The pseudoaneurysm sac measured approximately 3.9 x 2.3 x 3.5 cm. No intramural hematoma or dissection was seen. In view of increasing size of the mycotic pseudoaneurysm the patient underwent an urgent thoracic endovascular aortic repair (TEVAR) in which a tapered stent graft was deployed across the aortic arch. Final aortogram did not show filling of pseudoaneurysm or endoleak (Fig. 5).
Figure 4.
An 89-year-old male with enlarging peri-aortic pseudoaneurysms.
Findings: The follow-up CT aortogram showed the previously described small pseudoaneurysms along the lateral and inferior wall had coalesced and developed into a larger lobulated pseudoaneurysm with a wide neck measuring 4.0 cm and pseudoaneurysm sac measuring 3.9 x 2.3 x 3.5 cm (A, Axial images, B, coronal images and (C) sagittal image arrows). In view of increasing size of the pseudoaneurysm the patient underwent urgent TEVAR.
Technique: Contrast enhanced CT aortogram with axial, coronal and sagittal reformats. CT scan settings were 3.00 mm slice thickness at 120 kV and 70 mAs.
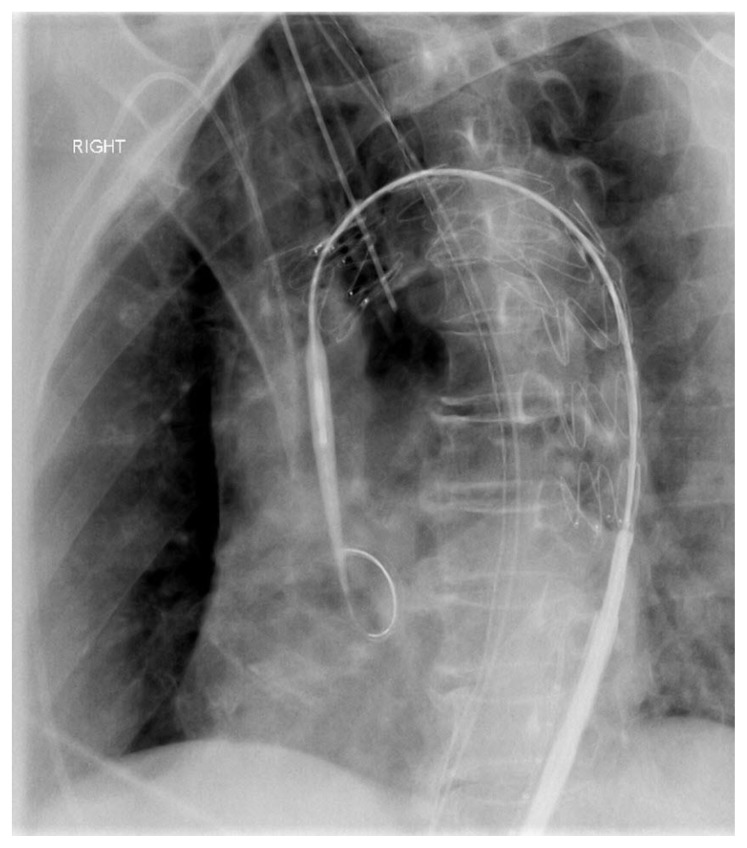
Figure 5.
An 89-year-old male underwent urgent TEVAR.
Findings: In view of increasing size of the mycotic pseudoaneurysm the patient underwent urgent thoracic endovascular aortic repair. A tapered stent graft was deployed across the aortic arch (Cook Alpha 34 x 30 x 161 mm was used).
Technique: Fluoroscopy guided TEVAR, left anterior oblique view is shown.
Follow-up imaging post TEVAR showed a small type II endoleak. However, the pseudoaneurysm did not show further increase in size. Unfortunately, the patient developed pneumonia and type II respiratory failure requiring intubation and passed away shortly thereafter.
DISCUSSION
Introduction
Pseudoaneurysms (also known as false aneurysms) are vascular abnormalities which lack a complete arterial wall and can result in catastrophic outcomes if not managed in a timely and accurate manner[1]. They arise from a disruption in the arterial wall continuity which allows blood to dissect into the tissues creating a perfused sac around the damaged artery (often eccentrically located) with a persistent communication with the arterial lumen. The perfused sac around the damaged artery is either contained by the media or adventitia or simply by the soft-tissue structures surrounding the injured vessel [2].
We report a case of a mycotic pseudoaneurysm secondary to salmonella infection involving the isthmus /ductus arteriosus of the thoracic aorta which is an extremely rare infection. To our knowledge no case report of aortic isthmus mycotic pseudoaneurysm secondary to salmonella infection has thus far been described. Although there has been a previous case report of an aortic isthmus mycotic pseudoaneurysm secondary to syphilis infection[3]. The specific case we present is also interesting because the periaortic mass seen on initial CT scan created an imaging dilemma and required multidisciplinary approach for further management.
Etiology & Demographics
Pseudoaneurysms can be caused by variety of etiologies, including trauma, inflammation, and various iatrogenic causes (e.g. surgery, percutaneous biopsy, drainage)[2]. Mycotic pseudoaneurysms usually originate from an infectious arteritis or mycotic aneurysms which result from the weakening and destruction of the arterial wall due to inflammation secondary to an infective source [4]. In particular, hematogenous spread of the microorganism into the vasa vasorum of the vessel wall results in inflammation that destroys the adventitia and muscularis which further weakens the artery to the extent that rupture occurs into the surrounding tissue resulting in a contained rupture and formation of a mycotic pseudoaneurysm[5], [6]. In our case report the patient had a history of salmonella gastroenteritis prior to his current admission. Upon admission blood cultures showed that the patient had salmonella bacteremia, which was likely the microbiological source for the mycotic pseudoaneurysm in the patient.
Majority of the pseudoaneurysms involving the thoracic aorta result from rapid deceleration or crush injuries associated with trauma i.e. motor vehicle accidents or falls[7]. The undersurface of the aorta near the insertion of the ductus arteriosum / aortic isthmus is the characteristic site of occurrence[7]. As such, atraumatic pseudoaneurysm at the aortic isthmus is a rare pathology.
Mycotic pseudoaneurysms may develop as a result of infection of a pre-existing aneurysm (i.e. mycotic aneurysm) or de novo. It has been previously reported that approximately 13.3% of the aneurysms have bacterial origin [8]–[10]. Approximately 55% of the bacterial organisms causing mycotic aneurysms belong to the gram-positive cocci, with Staphylococcus aureus accounting for around 45% of cases and Streptococci for 10%[11]. Salmonella infection is common, second only to Staphylococcus aureus[12]; approximately 30–40% are caused by enteric-derived organisms, of which half are non-typhi salmonella species[11]. Salmonella infections are particularly prevalent in Asian populations and older age groups (mainly because this group is more inclined to have atherosclerotic arteries), and carry high incidence for early rupture or possible mycotic pseudoaneurysm formation [11].
Clinical & Imaging Findings
The initial chest radiograph, although reported as normal, in retrospect demonstrated a vague opacity along the left lateral wall of the aortic arch (Fig. 2) which was not seen on prior chest radiograph done one month earlier (not available), suggesting a rapid progressing lesion. On the initial CT chest study, this corresponded to a poorly enhancing periaortic mass (relatively hyperdense on plain scan) which appeared inseparable from the aortic arch near the aortic isthmus (Fig. 2). No obvious contrast extravasation from the aorta was seen to suggest the possibility of a pseudoaneurysm at this point. This complex periaortic mass created a diagnostic imaging dilemma with wide differentials including both infectious and neoplastic etiology either arising from the mediastinum or periphery of the lung. The main reason for the difficulty in diagnosing the mycotic pseudoaneurysm on initial CT chest was mainly that it was completely thrombosed and did not show convincing contrast extravasation. Thrombosis of pseudoaneurysm is a known and common complication and there have been fair number of cases reported in literature that have shown that a thrombosed pseudoaneurysms can have complex appearance and mimic other non-vascular etiologies[13]–[17].
In an attempt to determine the etiology of the mass seen on initial CT chest the patient unfortunately underwent TBLB. Fortunately, this did not result in clinically significant hemorrhage or rupture; presumably because the TBLB only biopsied the clot/thrombosis portion of the pseudoaneurysm and kept a safe distance from the adjacent aorta. There have been cases published in literature in which thrombosed pseudoaneurysms have been mistaken for soft-tissue mass and undergone intervention. For example a case of complex solid-cystic mass within the breast which was aspirated but upon further investigation was discovered to be a thrombosed pseudoaneurysm [17]. This case study reminds us that thrombosed pseudoaneurysm can present a diagnostic challenge and may be confused with various etiologies with potential for devastating outcomes.
The results of the TBLB did not further clarify the etiology of the periaortic mass but in the multidisciplinary meeting it was brought to attention that the aortic arch wall around the mass appeared irregular and ulcerated possibly representing the site of mural defect and raising the possibility that the periaortic mass may represent a thrombosed pseudoaneurysm (Fig. 2). This was confirmed on subsequent imaging which showed a couple of contrast filled outpouchings into the periaortic mass clinching the diagnosis of mycotic pseudoaneurysm (Fig. 3). Follow-up images demonstrated that the couple of small mycotic pseudoaneurysms had increased in size and coalesced into one large multiloculated mycotic pseudoaneurysm (Fig. 4).
Treatment
It is well known that thoracic aortic pseudoaneurysms are seen as surgical challenge in view of the significant risks associated with any open thoracic surgical intervention [2]. It is reasonable to say that most surgeons/interventionalists would agree that symptomatic pseudoaneurysms should be treated [2]. However, whether or not to treat asymptomatic pseudoaneurysms is not straightforward and takes into account several factors which include patient comorbidity, clinical setting, type of pseudoaneurysm, anatomical location, etc. It should be noted that the risk of spontaneous rupture of ‘extraorganic visceral’ pseudoaneurysm (of any etiology) is very high and it is correlated with a mortality rate that may approach 100% [2], [18]. As such, it is the opinion of some authors that definitive treatment should be performed in all such cases.
The elderly patient in this case report had significant co-morbidities however in view of the increasing size of the mycotic pseudoaneurysm the decision to undergo an urgent TEVAR using a tapered stent graft was made (Fig. 5). The standard treatment has included surgery which entails wide debridement and resection of the infected aorta and the surrounding tissue[19]. This is followed by revascularization via in-situ or extra- anatomic grafting together with long-term antibiotics[19]. Mortality rates of up to 40 % has been previously described with this surgical treatment likely related to multiple factors including complexity of the surgery, severe co-existing medical co-morbidities and patient instability because of sepsis or rupture [19]. In the recent times endovascular stent graft repair has provided a feasible and less-invasive alternative in the management of aortic diseases. Favorable results with reduced perioperative mortality and morbidity have been reported in endovascular repair of abdominal aorta aneurysms [20], [21]. The endovascular approach has also been applied to the treatment of infected aortic pseudoaneurysms with relative success[19], [22]–[25]. Various authors have reported optimistic technical success with this procedure, with one study demonstrating a 100 % technical success rate with no hospital deaths after treating 7 patients with endovascular stent graft repair of infected thoracic pseudoaneurysms [19].
Differential Diagnosis
On the initial CT Chest scan the periaortic mass at the aortic arch/aortic isthmus created a diagnostic dilemma and was deemed indeterminate. It did not demonstrate typical features of a pseudoaneurysm which is characterized by contrast flow into a perivascular sac demonstrating density similar to that of blood pool on various contrast phases (arterial, venous and delayed). As such the differentials at this point included conglomerate nodal disease, pulmonary or mediastinal mass (i.e. lung carcinoma with infiltration into the mediastinum) and an infected collection. It was unfortunately overlooked that the mass was significantly dense on the non-contrast images (measuring up to 53 HU). Furthermore, there was significant atherosclerotic disease of the thoracic aorta. In retrospect given this additional information, specifically of a hyperdense mass adjacent to the aorta which demonstrates significant atherosclerotic disease, the differential of a thrombosed pseudoaneurysm is conceivable; albeit the overall appearance of the initial peri-aortic mass (Fig. 2) is not classic of a pseudoaneurysm.
On subsequent CT aortograms there was convincing outpouching of contrast from the aorta into the periaortic mass which was in keeping with pseudoaneurysm. Given the patient had salmonella bacteremia the diagnosis of mycotic pseudoaneurysm was confirmed. The differentials for this would include traumatic pseudoaneurysm and less likely a true aneurysm or an aortic rupture with surrounding hematoma. The clinical history did not reveal any significant recent trauma hence traumatic pseudoaneurysm was excluded. A true aneurysm should have a bulbous or fusiform dilatation of the aorta/artery and contain all 3 layers of the vessel wall. The pseudoaneurysm sac in our case was eccentric and the contrast filled outpouchings appeared to breach the aortic wall (i.e. did not contain all the layers of the aortic wall); these features are more compatible with pseudoaneurysm as opposed to a true aneurysm. It is particularly important when assessing a perivascular mass to ensure there is no vascular rupture which would be characterized by ‘free’ extravasation of contrast into the surrounding structures. In our case the contrast flowed into and was contained by a perivascular mass (i.e. pseudoaneurysm sac) making aortic rupture with surrounding hematoma unlikely.
TEACHING POINT
Mycotic pseudoaneurysm is a rare vascular pathology that can become thrombosed and mimic other pathologies creating a diagnostic dilemma which can lead to wrong management and result in devastating outcomes (e.g. rupture). Hence, we should always entertain the possibility of a vascular pathology within our differentials when confronted with a complex peri-aortic mass in order to avoid inappropriate management and reduce the risk of mortality.
Table 1.
Summary table of key aspects and imaging findings of mycotic pseudoaneurysms.
| Aetiology | Microorganisms, most often bacterial |
| Incidence | Insufficient information / unknown |
| Gender predilection | No convincing evidence of gender predilection |
| Age predilection | Older age group |
| Risk factors | Bacteraemia, infectious arteritis, atherosclerotic plaques / disease, pre-existing aneurysm. |
| Treatment | Treatment can involve arterial excision and in situ or extra-anatomical revascularization. Endovascular stenting has also become a valid alternative in selected cases. |
| Prognosis | Mycotic pseudoaneurysms of the aortic arch have a poor prognosis and high mortality. The risk of spontaneous rupture of extraorganic visceral pseudoaneurysm is very high regardless of their size and the mortality rate may approach 100% as cited previously. |
| Findings on Imaging |
|
Table 2.
Differential diagnoses table for mycotic pseudoaneurysm.
| US | CT | MRI | |
|---|---|---|---|
| Mycotic PSA |
|
|
|
| Traumatic PSA |
|
|
|
| True aneurysm |
|
|
|
| Ruptured aneurysm/PSA |
|
|
|
| Thrombosed (completely) PSA |
|
|
|
PSA = Pseudoaneurysm.
ABBREVIATIONS
- AFB
Acid-Fast Bacillus
- COPD
Chronic obstructive pulmonary disease
- CT
Computerized tomography
- FNA
Fine needle aspiration
- kV
Kilovolt
- mAs
Milliampere-seconds
- TB
Tuberculosis
- TBLB
Transbronchial lung biopsy
- TEVAR
Thoracic endovascular aortic repair
REFERENCES
- 1.Jesinger RA, Thoreson AA, Lamba R. Abdominal and Pelvic Aneurysms and Pseudoaneurysms: Imaging Review with Clinical, Radiologic, and Treatment Correlation. RadioGraphics. 2013;33(3):E71–E96. doi: 10.1148/rg.333115036. [DOI] [PubMed] [Google Scholar]
- 2.Saad NEA, Saad WEA, Davies MG, Waldman DL, Fultz PJ, Rubens DJ. Pseudoaneurysms and the Role of Minimally Invasive Techniques in Their Management. RadioGraphics. 2005;25(suppl_1):S173–S189. doi: 10.1148/rg.25si055503. [DOI] [PubMed] [Google Scholar]
- 3.Szabados SE, Varady E, Göbölös L. Luetic mycotic pseudoaneurysm of the aortic isthmus. Eur Heart J. 2009 Aug;30(16):1963–1963. doi: 10.1093/eurheartj/ehp202. [DOI] [PubMed] [Google Scholar]
- 4.Gonda RL, Gutierrez OH, Azodo MV. Mycotic aneurysms of the aorta: radiologic features. Radiology. 1988;168(2):343–346. doi: 10.1148/radiology.168.2.3260676. [DOI] [PubMed] [Google Scholar]
- 5.Bowden DJ, Hayes PD, Sadat U, Choon See T. Mycotic Pseudoaneurysm of the Superficial Femoral Artery in a Patient with Cushing Disease: Case Report and Literature Review. Vascular. 2009;17(3):163–167. doi: 10.2310/6670.2008.00060. [DOI] [PubMed] [Google Scholar]
- 6.Sueyoshi E, Sakamoto I, Nakashima K, Minami K, Hayashi K. Visceral and peripheral arterial pseudoaneurysms. Am J Roentgenol. 2005;185(3):741–749. doi: 10.2214/ajr.185.3.01850741. [DOI] [PubMed] [Google Scholar]
- 7.Stent VC, Warshauer DM, Archer RK, Selzman CH. Case 115?: Aortic Pseudoaneurysm from Penetrating Superior Vena Cava Stent. Radiology. 2007;243(3):3–6. doi: 10.1148/radiol.2433040944. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y, Bai Y, Yang C, Wang P, Gu L. Mycotic aneurysm due to Salmonella species: clinical experiences and review of the literature. Brazilian J Med Biol Res. 2018;51(9):e6864. doi: 10.1590/1414-431X20186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee WK, et al. Infected (Mycotic) Aneurysms: Spectrum of Imaging Appearances and Management. RadioGraphics. 2008;28(7):1853–1868. doi: 10.1148/rg.287085054. [DOI] [PubMed] [Google Scholar]
- 10.Bin Hsu R, Lin FY, Chen RJ, Hsueh PR, Wang SS. Antimicrobial drug resistance in salmonella-infected aortic aneurysms. Ann Thorac Surg. 2005;80(2):530–536. doi: 10.1016/j.athoracsur.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 11.Fisk M, et al. Mycotic aneurysms: A case report, clinical review and novel imaging strategy. QJM. 2012;105(2):181–188. doi: 10.1093/qjmed/hcq240. [DOI] [PubMed] [Google Scholar]
- 12.Pirvu A, Bouchet C, Garibotti FM, Haupert S, Sessa C. Mycotic aneurysm of the internal carotid artery. Ann Vasc Surg. 2013;27(6):826–830. doi: 10.1016/j.avsg.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ, et al. Pseudoaneurysm of the popliteal artery mimicking tumorous condition. J Korean Surg Soc. 2011 Dec;80 doi: 10.4174/jkss.2011.80.Suppl1.S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaczynski J. Superior mesenteric artery branch pseudoaneurysm mimicking an acute appendicitis. BMJ Case Rep. 2012;2012:2–3. doi: 10.1136/bcr.02.2012.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehmet A, Onur S, Mete G, Vedat B, Hacer B. Late popliteal artery pseudoaneurysm mimicking deep vein thrombosis?: Two case reports. Turkish Journal of Vasc Surgery. 2018;27(March):37–41. [Google Scholar]
- 16.Huang SW, Wei TS, Liu SY, Wang WT. Spontaneous totally thrombosed pseudoaneurysm mimicking a tendon tear of the wrist. Orthopedics. 2010;33(10):776. doi: 10.3928/01477447-20100826-23. [DOI] [PubMed] [Google Scholar]
- 17.Lee KH, Ko EY, Han BK, Shin JH, Kang SS, Hahn SY. Thrombosed pseudoaneurysm of the breast after blunt trauma. J Ultrasound Med. 2009;28(2):233–238. doi: 10.7863/jum.2009.28.2.233. [DOI] [PubMed] [Google Scholar]
- 18.Kapoor BS, Haddad HL, Saddekni S, Lockhart ME. Diagnosis and Management of Pseudoaneurysms: An Update. Curr Probl Diagn Radiol. 2009;38(4):170–188. doi: 10.1067/j.cpradiol.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Ting ACW, Cheng SWK, Ho P, Poon JTC, Kong H. Endovascular stent graft repair for infected thoracic aortic pseudoaneurysms - a durable option?? J Vasc Surg. 2005:701–705. doi: 10.1016/j.jvs.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 20.Greenhalgh PRM, Campus CC. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm ( EVAR trial 1 ), 30-day operative mortality results?: randomised controlled trial. Lancet. 2004:843–848. doi: 10.1016/S0140-6736(04)16979-1. [DOI] [PubMed] [Google Scholar]
- 21.Buskens E, DE Grobbee, Blankensteijn JD. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004:1607–1618. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- 22.Berchtold C, Eibl C, Seelig MH, Jakob P, Schönleben K. Endovascular treatment and complete regression of an infected abdominal aortic aneurysm. J Endovasc Ther. 2002;9(4):543–8. doi: 10.1177/152660280200900426. [DOI] [PubMed] [Google Scholar]
- 23.Stanley BM, Semmens JB, Lawrence-Brown MMD, Denton M, Grosser D. Endoluminal Repair of Mycotic Thoracic Aneurysms. J Endovasc Ther. 2003;10(3):511–515. doi: 10.1177/152660280301000316. [DOI] [PubMed] [Google Scholar]
- 24.Koeppel TA, Gahlen J, Diehl S, Prosst RL, Dueber C. Mycotic aneurysm of the abdominal aorta with retroperitoneal abscess: Successful endovascular repair. J Vasc Surg. 2004;40(1):164–166. doi: 10.1016/j.jvs.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 25.Beland MD, Soares GM, Dubel GJ, Forte MP, Murphy TP. Endovascular repair of a thoracic aorta mycotic pseudoaneurysm in a patient with history of Bacteroides Fragilis sepsis and leprosy. J Vasc Interv Radiol. 2005;16(2I):298–300. doi: 10.1097/01.RVI.0000148826.34331.A4. [DOI] [PubMed] [Google Scholar]