Abstract
Background
The plant-derived terpenoid, α-pinene is a bicyclic monoterpene potentially useful for the treatment of various diseases which also includes cancer and its types. The present investigation is about finding the anticancer activity of the α-pinene extracted from the leaves of Boswellia dalzielii over the PA-1 cancer cells of the human ovary.
Material/Methods
The cytotoxic activity of the α-pinene was evaluated using MTT and LDH assays which indicated that α-pinene could induce cytotoxicity in cancer-causing cells in the ovary. The consequences of α-pinene on the cell sequence regulation were determined by the staining technique using propidium iodide (PI) followed with flow cytometry.
Results
The cell cycle distribution analysis showed that α-pinene inhibit the cycle progression from G2 to M phase. In addition, apoptosis analysis is done through the double staining investigation using Annexin V-FITC/PI to analyze the controlled growth of α-pinene which is associated with the apoptosis. Caspase-3 a crucial enzyme involved in apoptosis was markedly increased in the α-pinene treated PA-1 cells. The apoptosis results reveal, that the cancer cells at the human ovary with α-pinene induces the significant populations of apoptotic cells.
Conclusions
Overall, α-pinene may exert anticancer effects in PA-1 cells by promoting cytotoxicity, suppression of cell sequence progression along with the programmed cell death.
MeSH Keywords: Apoptosis; Cytotoxicity Tests, Immunologic; Flow Cytometry; Ovarian Neoplasms
Background
Ovarian cancer is a fatal disease, with increasing death rates among women in developing countries. Most ovarian cancer patients are diagnosed at later or advanced stages because of absence in the early-stage symptoms and difficulty in detection at early stages. The paclitaxel-/platinum-based chemotherapy shows complete clinical response in most (70%) ovarian cancer patients, but the survival rate is only 20–25% due to reoccurrence [1,2]; therefore, it is crucial to develop novel therapeutic drugs as use in the final stage of cancer. Chemotherapy, radiotherapy, and immunotherapy are commonly employed to increase the survival rate of cancer patients. However, prognosis and diagnosis are crucial in many types of cancer, and the development of novel treatment methods is mandatory for certain cancers, such as malignant melanoma. More than 50% of drugs used in anticancer therapy are obtained from natural sources or their semisynthetic or synthetic derivatives [3]. The plant-derived compounds exhibit anticancer and other protective biological activities, with fewer adverse effects [4]. Thus, natural product research is important in discovery of novel drugs with efficient activity against various types of cancer.
Boswellia dalzielii is a tall (>13 m) aromatic tree allied to the Burseraceae family, also referred as “Frankincense tree” [5,6], which is wide-spread in African countries such as Togo, Burkina Faso, Benin, Ghana, Cameroon, Nigeria, and Ivory Coast. B. dalzielii is a traditional medicinal plant with useful medicinal properties; it has been used for treatment of fever, ulcers, leprosy, venereal diseases, gastrointestinal diseases, and inflammation [7,8]. The phytocomposition of Boswellia dalzielii includes tannins, saponins, cardiac glycosides, flavonoids, steroids, and terpenes, but does not contain alkaloids, and the composition varies in different parts of the tree (e.g., bark and leaves). B. dalzielii plants have been utilized to make high value-added products such as essential oils, extracts, and resins. The essential oils of plants such as coniferous trees, eucalyptus, rosemary, Psidium, and camphor contain the natural organic bicyclic monoterpene compound α-pinene (2,6,6-trimethylbicyclo[3.1.1]hept-2-ene). The phytochemistry of this plant varies. For example, the methanolic extract of B. dalzielii contains tannins, flavonoids, cardiac glycosides, and triterpenoids, whereas the bark possesses glycosides, steroids, carbohydrates, alkaloids, flavonoids, anthraquinones, and saponins [7,9]. The oil extracted from B. dalzielii leaves exhibits antimicrobial activity against B. subtilis, S. aureus, and C. albicans [7]. The aqueous extract from the stem bark is effective in treating ulcers, gastrointestinal disturbances, and diarrhoea [5,7].
Among the natural terpenoids, α-pinene is largely distributed in the plant world. It is a 4-membered ring structure alkene and its anticancer activity has been reported widely. The essential oils of Lavender angustifolia, Rosmarinus officinalis, Canarium tramdenanum, and Satureja myrtifolia were shown to contain α-pinene [10]. In the treatment of asthma, it acts as a bronchodilator at low concentrations. The antioxidant and antimicrobial potential of α-pinene has also been proven experimentally. The anticancer activities of α-pinene involve various molecular mechanisms such as regulation of cell cycle progression, antioxidant status, suppression of inflammation, and stimulation of apoptosis [11,12].
α-pinene from various natural resources was shown to stimulate mitochondrial-mediated apoptosis in various types of cancer. Apoptosis is a type programmed death of cancer cells, which is essential for the development of organs and tissue stability. It is characterized by condensation of chromatin, DNA fragmentation, development of apoptotic bodies, stimulation of caspases, and overproduction ROS. Several cancer drugs act on tumor cells by stimulating cell death through the apoptosis pathway. Defective apoptosis can cause cancer and cancer resistance to chemotherapy. Autophagic cell death is a type II programmed cell death characterized by autophagosomes engulfing the cytoplasm and organelles in the cytoplasm [13].
The α-pinene from Tanacetum gracile was proven to trigger mitochondrial-mediated apoptosis in human leukemia cells [14]. Similarly, α-pinene induced cell death and apoptosis in melanoma. In the murine melanoma cell line, α-pinene (150 μg/mL) extracted from the Schinus terebinthifolius Raddi induces apoptosis [12]. The growth of hepatocellular carcinoma cells (BEL-7402) was inhibited through cell cycle arrest (i.e., inhibition of the changeover from G2 to M phase), induction of apoptosis, and controlled cell expansion [15]. α-pinene has also been shown to inhibit human hepatoma tumor progression through suppression of miR221 and stimulation of oxidative stress and apoptosis of HelG2 cells [16]. α-pinene is one among the major compounds present in the essential oil extracted from B. dalzielii. In the present study, we performed cytotoxicity and apoptosis-mediated cell death assays to assess the ability of α-pinene extracted from Boswellia dalzielii leaves to protect against human ovarian cancer. Figure 1 provided a diagram of α-pinene extracted from the leaves of B. dalzielii. We also assessed the effect of α-pinene on cell cycle progression of cancer cells in the human ovary.
Figure 1.
The schematic representation of α-pinene extracted from the leaves of Boswellia dalzielii.
Material and Methods
Chemicals
Propidium iodide and Annexin V-fluorescein isothiocyanate (FITC) were procured from BD Biosciences (San Jose, CA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) was bought from Molecular Probes, Inc. (Eugene, USA). Annexin V-fluorescein isothiocyanate (FITC) was procured from Merck Millipore (Billerica, MA, USA). Fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s minimum essential medium (DMEM), were obtained from BD Biosciences (San Jose, CA, USA). Cisplatin was bought from Sigma Aldrich Co (St. Louis, MO, USA). Cisplatin was bought from Sigma Aldrich (St. Louis, MO, USA). All chemicals were of analytical grade.
Extraction of α-pinene from the leaves of βoswellia dalzielii
α-pinene was extracted from the leaves of Boswellia dalzielii [12]. The raw essential oil was extracted from the air-dried leaves of B. dalzielii using hydrodistillation in a Clevenger apparatus for about 5 h. The total oil yield extracted from the hydrodistillation was 1.30 g. Then, a portion of the oil was applied to an Si-gel column eluted with pentane in the flash chromatography technique, using CH2Cl2 and a gradient of CH2Cl2-MeOH 95: 5 and 9: 1 to produce 12 portions. Each of these fractions was analyzed individually through gas chromatography (GC) [12]. Fractions 1–3 corresponded to GC chromatograms containing a mixture of hydrocarbon derivatives purified using TLC on Si-gel coating with AgNO3 eluted with pentane: CH2Cl2 (1: 1) to give 14 mg of pure α-pinene. MS spectral analysis and NMR were done to determine the purified α-pinene and the comparison was made with evidence in the literature [17–19].
Determination of cytotoxic activity using MTT assay
The cytotoxic activity of the plant-derived α-pinene was analyzed in the cell lines of the human ovary (PA-1) using 3-(4, 5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. PA-1 cells were grown in Dulbecco’s modified Eagle’s medium/F12 supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere of 5% CO2. The logarithmic-phase cells were harvested and each well of a 96-well culture plate was filled with 100 μL and incubated for 24 h in a CO2 incubator at 37°C. Following this, the cells were processed with different concentrations (1, 5, 10, 20, 50, 75, and 100 μg/mL) of α-pinene and incubated for another 24 h. Then, MTT dye (5 mg/mL) (Molecular Probes, Inc., USA) was added and cells were incubated at 37°C in the absence of light for about 4 h. The supernatant was removed from the mixture and 75 μL of DMSO was mixed in, then the plates were agitated on a rotary shaker for 15 min. The absorptivity of the plate at 570 nm was recorded using a Bio-Rad iMark microplate reader (Bio-Rad, China). α-pinene processes the cells by reducing MTT to formazan product by mitochondrial dehydrogenase, and we assessed controlled cell multiplication.
Measurement of LDH activity
Lactate dehydrogenase (LDH) released from the damaged cells was measured using a Lactate Dehydrogenase Cytotoxicity Assay Kit (Cayman Chemicals, MI, USA) as per the manufacturer’s instructions. PA-1 cells were then processed with different concentrations of α-pinene (0, 10, 20, 50, 75, and 100 μg/mL) for 24 and 48 h. To the new 48-well plate, 100 μL of the medium and reaction mixture (100 μL) was mixed well and maintained at the optimum room temperature for 30 min. Lactate dehydrogenase catalyzed the conversion of pyruvate into lactate with the simultaneous production of nicotinamide adenine dinucleotide reduced (NADH) through the reduction of NAD+. The absorptivity rise at 490 nm was induced by the oxidation of NADH is absorbed to be proportional to the sample’s LDH activity. The results of LDH activity was expressed in terms of U/mL.
Apoptosis analysis through the double staining of Annexin V-FITC/PI
PA-1 cells were cultured in a 6-well plate at a density of 2×105 cells/2 mL and incubated in a CO2 incubator overnight at 37°C for 24 h. The first and final stages of apoptotic cells were determined through flow cytometry. The PA-1 cells were processed with α-pinene (20 μg/mL) and standard drug (cisplatin-15 μM) for 24 h. Then, the segregation of the cells was done by using trypsin-EDTA, and cells were washed and placed in cold 70% ethanol. Later, the cells were resuspended in binding buffer and FITC Annexin V (BD Biosciences, San Jose, CA, USA) for 15 min, following staining in propidium iodide solution. The cells were kept in the dark and assessed using a BD FACS Calibur device (BD Biosciences, USA) within 30 min. About 10 000 singlet cells were selected for analysis from all samples, including cell control.
Cell cycle analysis
Incubated human ovarian cells (PA-1) after processing with α-pinene and cisplatin for 24 h were collected and washed 2 times with ice-cold ethanol (70%) for 4 h followed by PBS wash. After washing, the cells were suspended back in the staining solution of PI (50 μg/mL) and RNase A (5 mg/mL). After the suspension, the cells were kept at room temperature in the absence of light for about 30 min. Then, the cell suspensions were subjected to flow cytometry (BD FACSCalibur) using about 10 000 cells per group.
Determination of caspase-3 activity
The ApoTarget™ Caspase Colorimetric Protease Assay Kit (Invitrogen, USA) was used to measure the activity of the α-pinene induced caspase-3 enzyme as per the manufacturer’s instructions. Human ovarian cells (PA-1) were treated with the α-pinene (20 μg/mL) and incubated separately for about 24 and 48 h. After incubation, the cells were collected and lysed with lysis buffer on ice for about 10 min, followed by centrifuging at 10 000 g for 1 min. The cell lysate (200 μg of protein) was added to the reaction buffer (50 μL) and substrate (5 μL) and incubated for 2 h at 37ºC. The absorptivity of the reaction mixture was observed at 405 nm using a spectrophotometer.
Statistical analysis
Results are given as mean ± standard error (SE). Statistical significance was assessed by one-way variance analysis using GraphPad Prism Software (GraphPad Software, San Diego, CA). P values less than 0.05 were considered to indicate a statistically significant difference.
Results
Identification of α-pinene
The oil was extracted from the dried leaves of B. dalzielli through hydrodistillation technique and the total yield of oil extract was 1.30%. The extracted raw oil was then subjected to FID/GC and GC/MS followed by fractionation on SiO2 and SiO2/AgNO3 chromatographic columns to obtain pure α-pinene.
Cell viability of α-pinene in human ovarian cancer cells
To estimate the anticancer effect of α-pinene, PA-1 cells were initially subjected to different concentrations of α-pinene (5, 10, 20, 50, 75, and 100 μg/mL) extracted from the leaves of B. dalzielli to elucidate the cell toxicity. Figure 2 shows the dose-dependent effect of α-pinene on the proliferation of PA-1 cells at different time intervals. A notable decrease in cell proliferation was identified in cells processed with α-pinene. The effect of α-pinene in the cultured cells was time-dependent. The cell toxicity assay showed 50% inhibition at the concentration of 20 μg/mL at 24 h of incubation, and this was used to validate the inhibition of α-pinene on cell cycle analysis.
Figure 2.
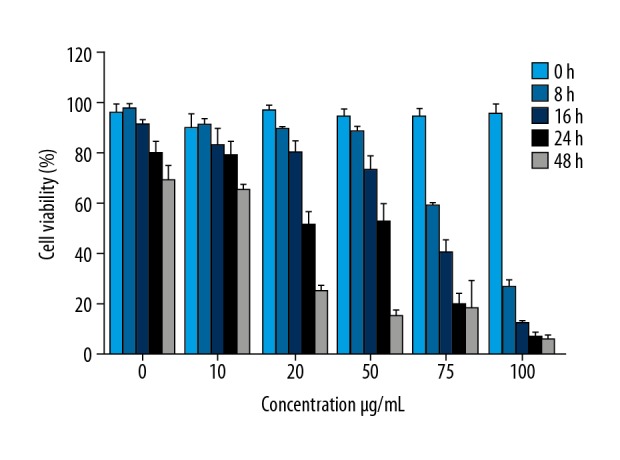
Effect of α-pinene extracted from the leaves of Boswellia dalzielii on cell proliferation in PA-1 cells using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. PA-1 cells were processed with different concentrations (0, 10, 20, 50, 75, and 100 μg/mL) of α-pinene at 0, 8, 16, 24, and 48 h. All values represent the mean ±SEM of 3 different investigations.
Effects of α-pinene on LDH activity
Cytotoxic studies were carried out based on the MTT reduction (mitochondrial dehydrogenase activity) and LDH activity. Therefore, we performed MTT and LDH assays to determine the cytotoxicity of α-pinene in PA-1 cells. LDH results revealed that concentration- and time-dependent cytotoxicity was observed in the PA-1 cells treated with α-pinene. A notable rise in the LDH level was noted in the α-pinene-treated PA-1 cells in a time- and concentration-dependent manner (Figure 3).
Figure 3.
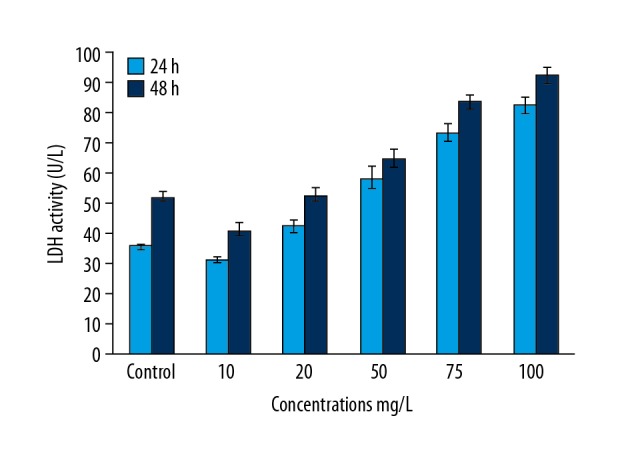
The effect of α-pinene extracted from the leaves of Boswellia dalzielii on cytotoxicity in PA-1 cells using lactate dehydrogenase (LDH) assay. PA-1 cells were then processed with different concentrations (0, 10, 20, 50, 75, and 100 mg/L) of α-pinene at 0, 8, 16, 24, and 48 h. All values represent the mean ± SEM of 3 different investigations.
Effects of α-pinene on cell cycle progression
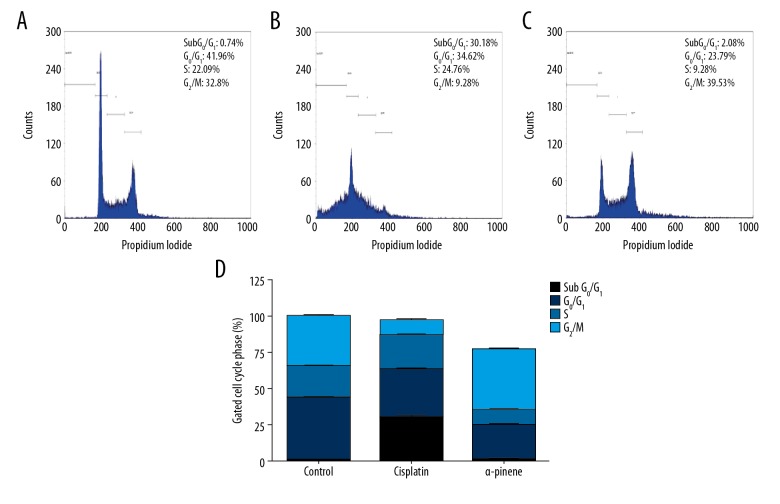
We assessed whether the growth suppression results of α-pinene in PA-1 cell is correlated with the stimulation of cell cycle arrest and apoptosis. The distribution of cells in the cell cycle was investigated through flow cytometry. Figure 4 shows the analysis of the PA-1 cell cycle treated with α-pinene (20 μg/mL). The results show that, in the G0/G1 phase, 41.96%, 34.62%, and 23.79% of cells were arrested in control, standard, and α-pinene groups, respectively. In S phase, 22.09%, 24.76%, and 9.28% of cells were arrested, and in G2/M phase, 32.8%, 9.28%, and 39.53% of cells were arrested in control, standard, and α-pinene groups, respectively.
Figure 4.
The effect of α-pinene extracted from the leaves of Boswellia dalzielii on PA-1 cell cycle distribution. Flow cytometry was used to analyze the dispersion profiles of PA-1 cells. (A) Control cells (untreated PA-1 cells), processed with PA-1 cells; (B) cisplatin (15 μM); (C) α-pinene (20 μg/mL) for 24 h; (D) graphical illustration showed the dispersion of cells in each phase of the cell sequence. Histograms represent the% of cells in the sub G1, G0/G1, S, and G2/M phase of the cell cycle. Each value represents the mean ±SEM of 3 different investigations.
Effects of α-pinene on caspase-3 activity
The extrinsic apoptotic pathway is described by the induction of caspase 8, whereas the intrinsic pathway is defined by the stimulation of caspase 9. The extrinsic and intrinsic pathways intersect at the induction of caspase 3. The presence of these caspases is equivalent to the amount of apoptosis. Caspases are cysteine proteases and are indispensable molecules in the apoptotic pathway. In the current analysis, the activity of the caspase-3 was enhanced in α-pinene-treated PA-1 cells by up to 10-fold and 14-fold as compared to untreated cells after 24 and 48 h of treatment (Figure 5).
Figure 5.
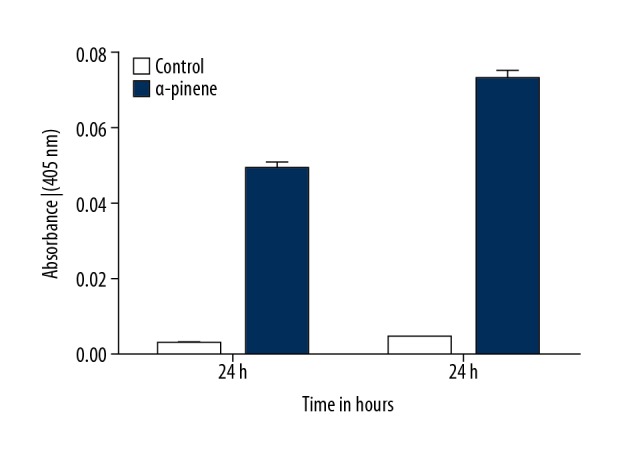
Induction of caspase-3 by the α-pinene-treated human ovarian cancer cells (PA-1). Caspase-3 activity was measured in the control (untreated PA-1 cells) and PA-1 cells treated with α-pinene (20 μg/mL) for 24 and 48 h. All values represent the mean ±SEM of 3 different investigations.
α-pinene induces apoptosis in PA-1 cells
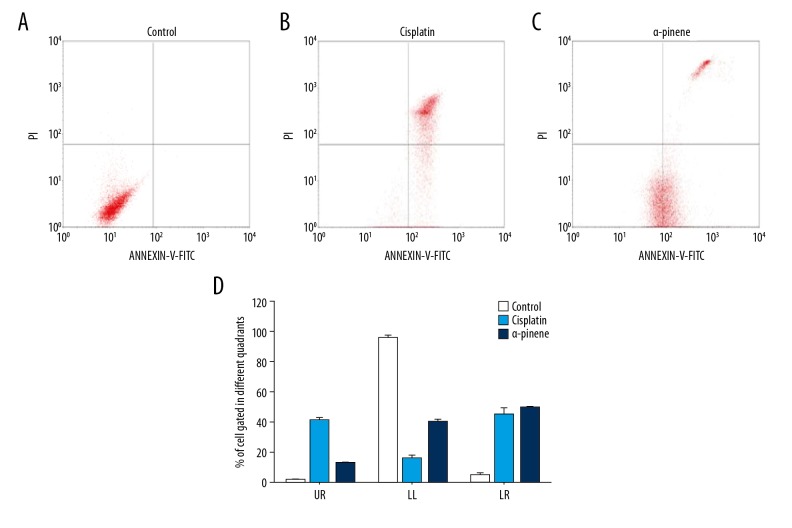
From the above findings, it is clear that α-pinene affects controlled cell growth in ovarian cancer cells. Hence, to further explore the anticancer effect of α-pinene, apoptosis assay was performed, as there was no necrotic effect observed under the microscope during the MTT and cell cycle assays. We stained experimental and control cells with FITC Annexin V and sorted them with a flow cytometer. A critical rise in the number of apoptotic cells (Annexin V positive cells) depending upon the dose at the right quadrants of flow cytometry graphs was observed in the α-pinene- and cisplatin-treated human ovarian cancer cells. Figure 6 illustrates the resulting percentage of apoptotic cells gated in control, standard (cisplatin), and α-pinene treatments. The lower left section population represents the viable cells demonstrated 94.95%, 15.45%, and 39.48%, while the upper left quadrant represents cells debris or necrotic cells as 0.09%, 0.2%, and 0.05%. The upper right section represents late apoptotic cells and the lower represents early apoptotic cells as 0.96%, 40.36%, and 11. 72%, respectively, and 4.08%, 44.18%, and 48.79% in control, standard, and α-pinene treatment groups, respectively. Thus, α-pinene exhibits 11.72% of late apoptotic cells and 48.79% of early apoptotic cells (LR) of Annexin V and PI compared to the standard drug cisplatin. It was previously reported that α-pinene effectively suppressed the growth of human prostate cancer cells and stimulated apoptosis and cell cycle arrest in PC-3 and DU145 cells. A notable increase in the proportion of total apoptotic and necrotic cells was observed in the PC-3 and DU145 cells when processed with α-pinene (2.5 μM) for 24 h.
Figure 6.
Effect of α-pinene extracted from the leaves of Boswellia dalzielii on apoptotic-mediated cell death induction in PA-1 cells. Quadrants showing the expression of: (A) Control (Untreated) PA- cells; (B) Illustration of the standard drug (Cisplatin, 15 μM)-treated PA-1 cells, (C) α-pinene (20 μg/mL)-treated PA-1 cells stained with PI/Annexin V-fluorescein isothiocyanate (V-FITC); and (D) Graphical representation of the percentage of cells gated in different quadrants. UR – % of late apoptotic cells; LL – % of viable cells; LR – % of early apoptotic cells. Each value represents the mean ±SEM of 3 different investigations.
Discussion
Recently, plant-based anticancer drugs have received attention due to awareness of the severe adverse effects, high cost, and emergence of drug resistance associated with modern synthetic drugs. Cytotoxic compounds are mostly used as anticancer drugs and the secondary metabolites such as alkaloids, flavonoids, phenolic compounds, glucosinolates, and terpenoids have cytotoxic activity. The different parts of B. dalzielli were shown to be effective in treating various diseases. In the present analysis, we evaluated the anticancer activity of the phytoprotective compound α-pinene extracted from the leaves of B. dalzielli on human ovarian cancer cells. α-pinene induced apoptosis, as shown by early disruption of mitochondrial potential, production of ROS, increased caspase-3 activity, heterochromatin aggregation, and DNA fragmentation [12]. NMR and MS spectral analysis was done to confirm the structure of α-pinene [20]. Several previous studies reported the concentration- and time-dependent effects of α-pinene on the proliferation of various types of cancer cells [15,21,22]. Higher cell growth inhibition was found in the hepatocellular carcinoma cell lines (BEL-7402) treated with α-pinene (8 mg/L) for 72 h [15]. These results demonstrated that α-pinene can effectively inhibit cancer cell growth. In line with our study, α-pinene was previously shown to enhance the LDH activity in a time-dependent fashion on human blood cells [23]. LDH can retain activity in cell viability investigations and is considered suitable for cytotoxicity assays, as well as to examine the noncytotoxic concentrations of novel phytotherapeutic agents [24]. The LDH released into the medium acts as a marker of cell death, and the elevated LDH activity is due to cell membrane disruption and leakage of the enzyme [25]. Several radical scavenging and antioxidant determination methods have shown that both α-pinene and β-pinene possess antioxidants [26].
Thus, the results show that considerable cell cycle (G2/M Phase) arrest is induced by α-pinene by decreasing the percentage of cells in the growth phase. Previous studies have shown that α-pinene induces cell cycle arrest in different types of cancer cells [15,21]. The treatment of α-pinene in BEL-7402 cells (a hepatocellular carcinoma cell line) inhibited the cell cycle transition from the G2 phase to the M phase in a time-dependent manner [15]. Furthermore, α-pinene induces cell cycle arrest and suppresses cell proliferation, leading to reduction in the size of tumor cells. Similarly, in the present analysis, α-pinene treatment of human ovarian cancer cells was proven to induce the arrest of the cell sequence in the transition from G2 to M phase. Similarly, prostate cancer cell growth and apoptosis were significantly suppressed by α-pinene in the human prostate cell line. The tumor progression was suppressed in mice administered α-pinene as compared to control mice [21]. The treatment of ovarian cancer cells with α-pinene resulted in inhibition of cell proliferation in MTT assay to reduce the size of ovarian cancer cells. Similarly, the dose-dependent cytotoxic activity of the polyphenolic compound butein was also seen in human ovarian cancer cells as determined by MTT assay [27]. The apoptotic phenotype increased after induction of several nucleases, while the activation of caspase-3 is crucial for heterochromatin aggregation and DNA fragmentation in apoptotic cells [28]. Therefore, to determine the involvement of caspase-3 in α-pinene-mediated apoptosis, caspase-3 activity was measured at the protein level. In eukaryotic cells, the fluidity of mitochondrial membranes is altered by some essential oils. Previous studies demonstrated that caspase-3 activity was increased in the malignant melanoma cells treated with plant-extracted α-pinene [12]. Furthermore, the results of TUNEL assay also showed that α-pinene induces apoptosis in xenograft tumors [21]. The apoptotic and antimetastatic activity of α-pinene extracted from Schinus terebinthifolius Raddi (Anacardiaceae) was demonstrated in a melanoma model [12]. α-pinene induces apoptosis through various processes such as the generation of ROS, interruption of mitochondrial potential, stimulation of caspase-3 activity, and DNA fragmentation. Similarly, the plant-origin polyphenolic compound butein induces apoptosis in human ovarian cancer cells (ES-2 and TOV-21G) through induction of extrinsic and intrinsic pathways. Butein treatment enhances caspase (caspase-3, -8, and -9) activity as well as increasing the concentration of cytosol cytochrome C. The anti-apoptotic proteins (Bcl-2 and Bcl-xL) and inhibitors of apoptotic (IAP) proteins (XIAP, survivin, CIAP-1, and CIAP-2) were downregulated while pro-apoptotic factors (Bax and Bad) were upregulated by butein treatment [27]. Thus, the observed responses suggest that α-pinene has an anticancer effect in PA-1 cells, possibly by inducing the apoptosis pathway.
Conclusions
The present study analyzed the anticancer effects of the plant-derived terpenoid α-pinene extracted from the leaves of B. dalzielli in the human ovarian cancer PA-1 cell line. Cytotoxic activity was examined by MTT and LDH assays, which indicated that α-pinene induces cytotoxicity in PA-1 cells. Assessment of cell sequence distribution showed that α-pinene decreased the growth of the cell sequence from the transition of the G2 to the M phase. Annexin FITC/PI staining assays showed that α-pinene induced apoptosis in PA-1 cells. The apoptotic enzyme caspase-3 was also increased in the α-pinene-treated PA-1 cells. Therefore, we conclude that α-pinene exhibits anticancer activity in ovarian cancer cells by controlled cell multiplication, cell sequence progression, and stimulation of apoptosis. These results suggest that α-pinene may be used as an anticancer agent for future treatment of ovarian cancer.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Gossner G, Choi M, Tan L, Fogoros S, et al. Genistein induced apoptosis and autophagocytosis in ovarian cancer cells. Gynecol Oncol. 2007;105:23–30. doi: 10.1016/j.ygyno.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF. Treatment goals in ovarian cancer. Int J Gynecol Cancer. 2005;15:3–11. doi: 10.1111/j.1525-1438.2005.15351.x. [DOI] [PubMed] [Google Scholar]
- 3.Cragg GM, Newman DJ. Antineoplastic agents from natural sources: Achievements and future directions. Expert Opin Invest Drugs. 2000;9:2783–97. doi: 10.1517/13543784.9.12.2783. [DOI] [PubMed] [Google Scholar]
- 4.Baskar V, Park SW, Nile S. An update on potential perspectives of glucosinolates on protection against microbial pathogens and endocrine dysfunctions in humans. Critical Rev Food Sci Nutrition. 2016;56(13):2231–49. doi: 10.1080/10408398.2014.910748. [DOI] [PubMed] [Google Scholar]
- 5.Etuk EU, Agaie BM, Onyeyili PA, Ottah CU. Anti-diarrhoea effect of Boswellia dalzielii stem bark extract in albino rats. J Pharmacol Toxicol. 2006;1:591–96. [Google Scholar]
- 6.Dimas K, Ishaka AO, Domingo AO, et al. Constituents of the essential oils of Boswellia dalzielii Hutch from Nigeria. J Essent Oil Res. 2006;18(2):119–20. [Google Scholar]
- 7.Nwinyi FC, Binda L, Ajoku GA, et al. Evaluation of the aqueous extract of Boswellia dalzielii stem bark for antimicrobial activities and gastrointestinal effects. Afr J Biotechnol. 2004;3(5):284–88. [Google Scholar]
- 8.Uzama D, Gbubele JD, Bwai MD, Kabir MG. Phytochemical, nutritional and antimicrobial screening of hexane, ethyl acetate and ethanolic extracts of Boswellia dalzielii leaves and bark. J Biosci Bioeng. 2015;3(5):76–79. [Google Scholar]
- 9.Alemika TE, Onawunmi GO, Olugbade TO. Antibacterial phenolics from Boswellia dalzielii. Niger J Nat Prod Med. 2006;10:108–10. [Google Scholar]
- 10.Begum A, Sandhya S, Shaffath Ali S, et al. An in-depth review on the medicinal flora Rosmarinus officinalis (Lamiaceae) Acta Sci Pol Technol Aliment. 2013;12:61–73. [PubMed] [Google Scholar]
- 11.Zhang Z, Guo S, Liu X, Gao X. Synergistic antitumor effect of α-pinene and β-pinene with paclitaxel against non-small-cell lung carcinoma (NSCLC) Drug Res (Stuttg) 2015;65:214–18. doi: 10.1055/s-0034-1377025. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo AL, Figueiredo CR, Arruda DC, et al. α-pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem Biophys Res Commun. 2011;411:449–54. doi: 10.1016/j.bbrc.2011.06.176. [DOI] [PubMed] [Google Scholar]
- 13.Cho YJ, Woo JH, Lee JS, et al. Eclalbasaponin II induces autophagic and apoptotic cell death in human ovarian cancer cells. J Pharmacol Sci. 2016;132(1):6–14. doi: 10.1016/j.jphs.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Verma M, Singh SK, Bhushan S, et al. Induction of mitochondrial-dependent apoptosis by essential oil from Tanacetum gracile. Planta Med. 2008;74:515–20. doi: 10.1055/s-2008-1074503. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Liu Y, Li M, et al. Anti-tumor effect of a-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J Pharmacol Sci. 2015;127(3):332–38. doi: 10.1016/j.jphs.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Xu Q, Li M, Yang M, et al. α-pinene regulates miR-221 and induces G2/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci Rep. 2018;38(6) doi: 10.1042/BSR20180980. BSR20180980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubmarawa D, Ogunwande IA, Okorie DA, et al. Constituents of the essential oils of Boswellia dalzielii Hutch. from Nigeria. J Essent Oil Res. 2006;18(2):119–20. [Google Scholar]
- 18.Adams RP. Identification of essential oils components by gas chromatography/Mass spectrometry. Allured Publ Corp; Carol Stream, IL: 1995. [Google Scholar]
- 19.Joulain D, Koenig WA. The Atlas of spectral data of sesquiterpene hydrocarbons by D. Joulain (Robertet S. A.) and W. A. König (University of Hamburg) Vol. 661. E. B. Verlag; Hamburg: 1998. pp. 21–29. [Google Scholar]
- 20.Kubeczka KH, Formácek V. Essential oils analysis by capillary gas chromatography and carbon-13 NMR spectroscopy. 2nd Edition. Wiley; 2002. [Google Scholar]
- 21.Zhao Y, Chen R, Wang Y, Yang Y. α-pinene inhibits human prostate cancer growth in a mouse xenograft model. Chemotherapy. 2018;63:1–7. doi: 10.1159/000479863. [DOI] [PubMed] [Google Scholar]
- 22.Aydin E, Türkez H, Geyikoglu F. Antioxidative, anticancer and genotoxic properties of α-pinene on N2a neuroblastoma cells. Biologia. 2013;68:1004–9. [Google Scholar]
- 23.Turkez H, Aydın E. In vitro assessment of cytogenetic and oxidative effects of α-pinene. Toxicol Ind Health. 2016;32(1):168–76. doi: 10.1177/0748233713498456. [DOI] [PubMed] [Google Scholar]
- 24.Specian AFL, Serpeloni JM, Tuttis K, et al. LDH, proliferation curves and cell cycle analysis are the most suitable assays to identify and characterize new phytotherapeutic compounds. Cytotechn. 2016;68(6):2729–44. doi: 10.1007/s10616-016-9998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokogawa K, Watanabe M, Takeshita H, et al. Serum aminotransferase activity as a predictor of clearance of drugs metabolized by CYP isoforms in rats with acute hepatic failure induced by carbon tetrachloride. Int J Pharmaceut. 2004;269:479–89. doi: 10.1016/j.ijpharm.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 26.Ho CL, Su YC. Composition, antioxidant and antimicrobial activities of the leaf essential oil of Machilus japonica from Taiwan. Nat Prod Commun. 2012;7:109–12. [PubMed] [Google Scholar]
- 27.Yang PY, Hu DN, Lin IC, Liu FS. Butein shows cytotoxic effects and induces apoptosis in human ovarian cancer cells. Am J Chin Med. 2015;43(4):769–82. doi: 10.1142/S0192415X15500482. [DOI] [PubMed] [Google Scholar]
- 28.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]