INTRODUCTION
Malignant brain tumors cultivate a variety of mechanisms to escape local and systemic immunity. Current treatments are restricted to neurosurgical procedures, chemotherapy, and radiotherapy.[34] The major limitation to effective therapeutic strategies lies in the impermissibility of the blood–brain barrier, which blocks the access of targeted drugs into tumor sites.[15] For long, it was believed that the lack of lymphatic drainage and antigen-presenting cells protects brain tumors from immunity; however, preclinical and translational studies in the past decade changed the perception on the role of immune cells in brain tumors.[12,32,37]
Brain tumor cells are equipped with the ability of secreting numerous chemokines, cytokines, and growth factors that stimulate the infiltration of various neural cells and a range of immune cells into the tumor. Altogether, these cells create a special niche called the tumor microenvironment, which is crucial for cancer proliferation, spread, and response to treatment. The tumor microenvironment has the ability of reprogramming attacking immune cells through local release of cytokines and chemokines,[17] which leads to protumor inflammatory or anti-inflammatory responses. Similar to other cancers, the brain tumor microenvironment has evolved multiple ways to inhibit the antitumor activity of immune cells.[29] Glioma research has shown that a strong link exists between the immune system response and disease progression.[18,31,35]
Natural killer (NK) cells are large granular lymphocytes that play an important role in antitumor immunity. On activation, NK cells induce target cell apoptosis through contact-dependent cytotoxicity primarily mediated by perforin and granzyme B[7,9,25] and secretion of pro-inflammatory cytokines such as tumor necrosis factor-α and interferon-γ.[6,27,36] In brain tumors, NK cells have proven to be effective in in vitro[2,4,8] and in vivo[1,16,30] settings. They can recognize and kill human glioblastoma cells that exhibit stem cell-like properties.[4,8] Therefore, in this paper, we explore the role of NK cells in the brain tumor microenvironment and discuss the challenges facing possible therapeutic regimens using NK cells against brain tumors.
NK CELLS AND THE BRAIN TUMOR MICROENVIRONMENT
NK cells constitute an integral part of the intratumoral immune cell population in the brain tumor microenvironment. Multiple studies have investigated NK cell numbers and activity in patients with brain tumors. Using suboptimal antibody techniques, Stevens et al.[33] showed that NK cells are present in metastatic brain tumors and craniopharyngiomas. Yang et al.[38] added that NK cells frequently infiltrated glioblastomas. Domingues et al.[11] confirmed their presence in meningiomas. Current advancements and the development of more precise methods offer the opportunity to study better the infiltration of NK cells in various brain tumors.
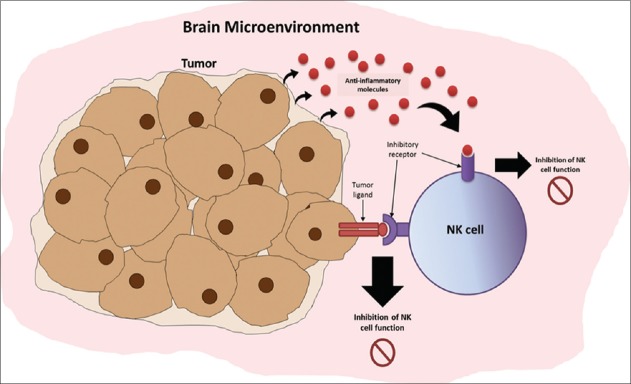
The functioning of NK cells is often affected in patients with brain tumors due to the immunosuppressive factors released by tumor cells. Glioma cells, for example, highly express a special form of major histocompatibility complex Class I molecules,[23,26] which act as ligands for inhibitory receptors expressed on NK cells [Figure 1]. Böttcher et al.[5] reported that tumors that secrete inflammatory mediators like cyclooxygenases (COX) and prostaglandin E2 suppress NK cell antitumor activity. Interestingly, COX-deficient tumors were extensively infiltrated by dendritic cells. The authors further uncovered a role for NK cells in recruiting dendritic cells to the tumor microenvironment by releasing CCL5 and XCL1 chemokines.[5] This NK cell-mediated activity governs dendritic cell antitumor function in various human cancers, like melanoma, breast cancer, lung cancer, and head and neck cancers. Amplifying the role of this axis was shown to impact survival positively.[5]
Figure 1:
Immune evasion mechanisms of brain tumors against natural killer (NK) cell surveillance and killing in the brain microenvironment. Tumor cells can secrete anti-inflammatory molecules (e.g., tumor necrosis factor-β) that bind and interact with NK cell receptors and eventually downregulate the frequency and cytotoxic activity of NK cells in the brain microenvironment. Tumor cells can also express ligands that bind to the inhibitory receptors of NK cells, which further downregulate their activity.
Impaired immune function and increased anti-inflammatory molecules are common in patients with glioma. In patients with glioblastoma multiforme, Fadul et al.[13] observed a decrease in the number of NK cells in isolated tumor specimens after being treated with radiation and temozolomide. The NK cell frequency and cytotoxic activity were downregulated as a result of anti-inflammatory molecules, like transforming growth factor-β (TGF-β). Moreover, the decrease in the level of expression of NK cell-activating receptor, NKG2D, in patients with glioblastoma further reflects the common immune evasion mechanisms exhibited by brain tumors.[22]
NK CELL-BASED THERAPIES IN NEURO-ONCOLOGY
Although studies show that NK cells account for a minority of infiltrating leukocytes in brain tumors,[24] novel therapies based on increasing NK cell recruitment and function are emerging. The major challenge facing NK cell therapies in neuro-oncology is the immunosuppressive environment of brain tumors that can downregulate NK cell activation and upregulate inhibition. Still, adoptive immunotherapy of brain tumors with NK cells holds promise if they are activated and expanded ex vivo and then injected into the tumor after local resection.
NK cells may play an important role in antitumor immune responses in patients with various brain tumors. Castriconi et al.[8] showed that NK cells can kill human glioblastoma cells that exhibit stem cell-like properties. Ishikawa et al.[21] demonstrated that pure autologous NK cells are safe and partially effective in patients with recurrent malignant gliomas; tumor regression was recorded in 4 of 9 patients. The partial efficacy of NK cells might be due to the tumor-induced immune suppression and immune escape mechanisms. A number of early phase trials are studying the dosage, safety, and side effects of NK cell therapy in the treatment of advanced cancers (NCT00823524 and NCT00909558). Choi et al.[10] reported that donor-derived NK cells are well tolerated at a median total dose of 2 × 108 cells/kg and can decrease the progression of acute leukemia.
In pediatric brain tumors, it has been suggested that NK cells may also be effective against medulloblastoma. A phase 1 clinical trial is currently undergoing whereby autologous expanded NK cells are injected into the brain of patients who have undergone resection of recurrent infratentorial tumors (NCT02271711). Phase 2 clinical trials are employing expanded autologous NK cells in combination with T cells to attack high-risk pediatric brain tumors (NCT01804634 and NCT02100891). It is believed that combination therapies involving NK cells might enhance the effectiveness and/or overcome brain tumor immune escape mechanisms.[14]
While these early phase trials are vital to realize the optimal dose, safety, and efficacy of NK cell therapies, combination tactics are likely compulsory to fully reap the benefits of adoptive cell therapy. Potentiating the immunogenicity of the brain tumor microenvironment through immunotherapy may be necessary to fully exploit the effects of NK cell therapy.[28] Immunotherapy can counteract the tumor-induced immunosuppression and synergize with NK cell therapy for maximal therapeutic benefit. Furthermore, combination therapies involving immunotherapeutic agents and NK cells can be vital in overcoming resistance to immune checkpoint inhibitors in some cancers. It has been shown that PD-1/PD-L1 blockade elicits a strong NK cell response that is indispensable for the full therapeutic effect of immunotherapy.[20] Ultimately, this can increase the portion of patients that can benefit from immunotherapy.
Although NK cell-based therapy exhibits limited neurological toxicity, it can produce immune reactions, such as graft versus host syndrome.[19] Another major challenge for this therapy is its cost. Expanding NK cells is very expensive, particularly when many purification and stimulation steps are essential.[3] In addition, the lack of a common large-scale clinical grade expansion method that yields uniform products is a hurdle. Moreover, the variability in NK cell number, phenotype, genotype, and function from individual donors are all obstacles that need to be overcome if NK cell therapy ought to make an impact in neuro-oncology in the future.
CONCLUSION
In general, NK cells play an important role in antitumor immunity and have been shown to be present in brain tumor settings. Initial preclinical and clinical studies based on autologous NK cell therapies against brain tumors have shown some promise. Further trials and research are needed to elucidate more the mechanisms through which tumor cells evade NK cell-mediated surveillance and attacks. Combination strategies with immune checkpoint inhibitors may be effective in antagonizing the tumor-induced immunosuppression and increasing the functionality of the NK cells in the brain tumor settings, among other cancers. Investigating pure NK cell fractions and/or stimulating endogenous NK cells could ease the development of efficient and cost-effective therapeutic approaches that exploit NK cells as anticancer agents. Achieving clinical success with NK cells will get us one step closer from removing the “terminal illness” tag associated with many brain tumors.
Contributor Information
Jawad Fares, Email: jawad.fares@northwestern.edu.
Mohamad Y. Fares, Email: myf04@mail.aub.edu.
Youssef Fares, Email: yfares@ul.edu.lb.
Disclaimer
The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Journal or its management.
REFERENCES
- 1.Alizadeh D, Zhang L, Brown CE, Farrukh O, Jensen MC, Badie B, et al. Induction of anti-glioma natural killer cell response following multiple low-dose intracerebral cpG therapy. Clin Cancer Res. 2010;16:3399–408. doi: 10.1158/1078-0432.CCR-09-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avril T, Vauleon E, Hamlat A, Saikali S, Etcheverry A, Delmas C, et al. Human glioblastoma stem-like cells are more sensitive to allogeneic NK and T cell-mediated killing compared with serum-cultured glioblastoma cells. Brain Pathol. 2012;22:159–74. doi: 10.1111/j.1750-3639.2011.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggio L, Laureano ÁM, Silla LM, Lee DA. Natural killer cell adoptive immunotherapy: Coming of age. Clin Immunol. 2017;177:3–11. doi: 10.1016/j.clim.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Blaylock RL. Cancer microenvironment, inflammation and cancer stem cells: A hypothesis for a paradigm change and new targets in cancer control. Surg Neurol Int. 2015;6:92. doi: 10.4103/2152-7806.157890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172:1022–37.e17. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell KS, Hasegawa J. Natural killer cell biology: An update and future directions. J Allergy Clin Immunol. 2013;132:536–44. doi: 10.1016/j.jaci.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182:3530–9. doi: 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 9.Cho D, Campana D. Expansion and activation of natural killer cells for cancer immunotherapy. Korean J Lab Med. 2009;29:89–96. doi: 10.3343/kjlm.2009.29.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi I, Yoon SR, Park SY, Kim H, Jung SJ, Jang YJ, et al. Donor-derived natural killer cells infused after human leukocyte antigen-haploidentical hematopoietic cell transplantation: A dose-escalation study. Biol Blood Marrow Transplant. 2014;20:696–704. doi: 10.1016/j.bbmt.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Domingues PH, Teodósio C, Ortiz J, Sousa P, Otero A, Maillo A, et al. Immunophenotypic identification and characterization of tumor cells and infiltrating cell populations in meningiomas. Am J Pathol. 2012;181:1749–61. doi: 10.1016/j.ajpath.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 12.Dunn GP, Dunn IF, Curry WT. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. 2007;7:12. [PMC free article] [PubMed] [Google Scholar]
- 13.Fadul CE, Fisher JL, Gui J, Hampton TH, Côté AL, Ernstoff MS, et al. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro Oncol. 2011;13:393–400. doi: 10.1093/neuonc/noq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fares J, Fares MY, Fares Y. Immune checkpoint inhibitors: Advances and impact in neuro-oncology. Surg Neurol Int. 2019;10:9. doi: 10.4103/sni.sni_366_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fares J, Kanojia D, Cordero A, Rashidi A, Miska J, Schwartz CW, et al. Current state of clinical trials in breast cancer brain metastases. Neurooncol Pract. 2019 doi: 10.1093/nop/npz003. DOI: 10.1093/nop/npz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friese MA, Wischhusen J, Wick W, Weiler M, Eisele G, Steinle A, et al. RNA interference targeting transforming growth factor-beta enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res. 2004;64:7596–603. doi: 10.1158/0008-5472.CAN-04-1627. [DOI] [PubMed] [Google Scholar]
- 17.Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska B. Immune microenvironment of gliomas. Lab Invest. 2017;97:498–518. doi: 10.1038/labinvest.2017.19. [DOI] [PubMed] [Google Scholar]
- 18.Godard S, Getz G, Delorenzi M, Farmer P, Kobayashi H, Desbaillets I, et al. Classification of human astrocytic gliomas on the basis of gene expression: A correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res. 2003;63:6613–25. [PubMed] [Google Scholar]
- 19.Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: A bench-to-bedside update. Blood. 2014;124:363–73. doi: 10.1182/blood-2014-01-514786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 2018;128:4654–68. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa E, Tsuboi K, Saijo K, Harada H, Takano S, Nose T, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24:1861–71. [PubMed] [Google Scholar]
- 22.Jachimowicz RD, Fracasso G, Yazaki PJ, Power BE, Borchmann P, Engert A, et al. Induction of in vitro and in vivo NK cell cytotoxicity using high-avidity immunoligands targeting prostate-specific membrane antigen in prostate carcinoma. Mol Cancer Ther. 2011;10:1036–45. doi: 10.1158/1535-7163.MCT-10-1093. [DOI] [PubMed] [Google Scholar]
- 23.Kmiecik J, Poli A, Brons NH, Waha A, Eide GE, Enger PØ, et al. Elevated CD3+and CD8+tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol. 2013;264:71–83. doi: 10.1016/j.jneuroim.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Kmiecik J, Zimmer J, Chekenya M. Natural killer cells in intracranial neoplasms: Presence and therapeutic efficacy against brain tumours. J Neurooncol. 2014;116:1–9. doi: 10.1007/s11060-013-1265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luetke-Eversloh M, Killig M, Romagnani C. Signatures of human NK cell development and terminal differentiation. Front Immunol. 2013;4:499. doi: 10.3389/fimmu.2013.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittelbronn M, Simon P, Löffler C, Capper D, Bunz B, Harter P, et al. Elevated HLA-E levels in human glioblastomas but not in grade I to III astrocytomas correlate with infiltrating CD8+cells. J Neuroimmunol. 2007;189:50–8. doi: 10.1016/j.jneuroim.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Moretta A, Locatelli F, Moretta L. Human NK cells: From HLA class I-specific killer ig-like receptors to the therapy of acute leukemias. Immunol Rev. 2008;224:58–69. doi: 10.1111/j.1600-065X.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 28.Ogbomo H, Cinatl J, Jr, Mody CH, Forsyth PA. Immunotherapy in gliomas: Limitations and potential of natural killer (NK) cell therapy. Trends Mol Med. 2011;17:433–41. doi: 10.1016/j.molmed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Perng P, Lim M. Immunosuppressive mechanisms of malignant gliomas: Parallels at non-CNS sites. Front Oncol. 2015;5:153. doi: 10.3389/fonc.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poli A, Wang J, Domingues O, Planagumà J, Yan T, Rygh CB, et al. Targeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survival. Oncotarget. 2013;4:1527–46. doi: 10.18632/oncotarget.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirahata M, Iwao-Koizumi K, Saito S, Ueno N, Oda M, Hashimoto N, et al. Gene expression-based molecular diagnostic system for malignant gliomas is superior to histological diagnosis. Clin Cancer Res. 2007;13:7341–56. doi: 10.1158/1078-0432.CCR-06-2789. [DOI] [PubMed] [Google Scholar]
- 32.Sonabend AM, Rolle CE, Lesniak MS. The role of regulatory T cells in malignant glioma. Anticancer Res. 2008;28:1143–50. [PubMed] [Google Scholar]
- 33.Stevens A, Klöter I, Roggendorf W. Inflammatory infiltrates and natural killer cell presence in human brain tumors. Cancer. 1988;61:738–43. doi: 10.1002/1097-0142(19880215)61:4<738::aid-cncr2820610417>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 34.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 35.Vauléon E, Tony A, Hamlat A, Etcheverry A, Chiforeanu DC, Menei P, et al. Immune genes are associated with human glioblastoma pathology and patient survival. BMC Med Genomics. 2012;5:41. doi: 10.1186/1755-8794-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vivier E, Ugolini S. Natural killer cells: From basic research to treatments. Front Immunol. 2011;2:18. doi: 10.3389/fimmu.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17:6–10. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang I, Han SJ, Sughrue ME, Tihan T, Parsa AT. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: Evidence of distinct immunological microenvironments that reflect tumor biology. J Neurosurg. 2011;115:505–11. doi: 10.3171/2011.4.JNS101172. [DOI] [PubMed] [Google Scholar]