Abstract
Background:
Progressive multifocal leukoencephalopathy (PML), a potentially fatal demyelinating disease caused by the John Cunningham virus (JCV), can occur as a complication of treatment with rituximab, fingolimod, and dimethyl fumarate. The primary objective of this study was to determine changes in anti-JCV antibody index values in multiple sclerosis (MS) patients treated with these three medications. Second, we explored the relationship between absolute lymphocyte count (ALC), anti-JCV antibody index values, and various patient characteristics.
Methods:
In this retrospective chart review, we evaluated changes in JCV serology and ALC in 172 MS patients treated with fingolimod, rituximab, or dimethyl fumarate (2013–2016). Only those with known anti-JCV antibody and ALC values before starting the study medications were included. Subsequent values were obtained on an ad hoc basis throughout the study.
Results:
There was a significant decrease in anti-JCV antibody index values in patients treated with fingolimod and rituximab (P = 0.03 and P = 0.014, respectively). A non-significant decreasing trend in anti-JCV antibody index values occurred in patients treated with dimethyl fumarate. Notably, there was no relationship between ALC and anti-JCV antibody index values for patients treated with rituximab, fingolimod, or dimethyl fumarate.
Conclusions:
Anti-JCV antibody index values significantly decreased in MS patients treated with fingolimod and rituximab; however, this did not occur with dimethyl fumarate. Fingolimod and rituximab may impair the humoral response to the JCV. Nevertheless, a declining anti-JCV antibody index in MS patients treated with fingolimod or rituximab should not necessarily be interpreted as correlating with a decreased risk for PML.
Keywords: Anti-John Cunningham virus antibody index value, dimethyl fumarate, fingolimod, John Cunningham virus, lymphocyte count, multiple sclerosis, progressive multifocal leukoencephalopathy, rituximab

INTRODUCTION
Progressive multifocal leukoencephalopathy (PML) is a complex demyelinating disease of the central nervous system. It is caused by the John Cunningham virus (JCV), a human polyomavirus resulting in infection of the oligodendrocytes. PML is rare and can occur in patients with underlying immunosuppressive conditions, as a complication of antineoplastic therapy and as complication of the treatment of rheumatoid arthritis or multiple sclerosis (MS).[1,5]
Therapies for MS and risk for PML
Patients with MS can be treated with any of 13 approved disease-modifying therapies (DMTs). In addition, they may receive high-dose corticosteroids, rituximab, azathioprine, or cyclophosphamide at different stages. The aim for treating MS is to start early and reduce disease activity to optimize neurologic reserve, cognition, and physical function while mitigating risks.[2] One such risk for MS patients is PML when treated with natalizumab, fingolimod, and dimethyl fumarate therapies. PML has also been reported with the use of rituximab when used in the treatment of other autoimmune conditions and malignancy.[2] Notably, there is no specific treatment for PML, and the prognosis is, therefore, poor.[5]
Biomarkers for PML and potential impact of lymphopenia
Biomarkers signaling the risk for PML exist for natalizumab. Data regarding anti-JCV antibody index and JCV status are available and include the duration of treatment and prior immunosuppressive therapies.[7] The identification of biomarkers for other agents used to treat MS is not complete. Prior reports of PML occurring in MS patients correlated with fingolimod. However, how lymphopenia and the duration of treatment with fingolimod contributed to PML remains unclear.[1] Further, there are case reports of PML with dimethyl fumarate and rituximab, indicating that affected patients were lymphopenic, at the time, PML was diagnosed.[8]
Study goal
The goal of this study was to evaluate changes in anti-JCV antibody index values in MS patients treated with fingolimod (Gilenya), rituximab (Rituxan), and dimethyl fumarate (Tecfidera), and to correlate both changes and outcomes with the absolute lymphocyte count (ALC).
METHODS
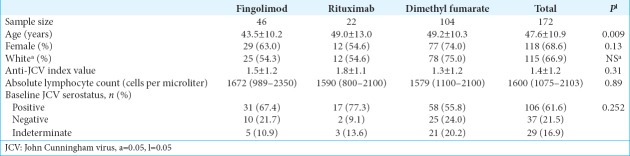
This retrospective study evaluated changes in JCV serology data and ALC in MS patients treated with fingolimod, rituximab, or dimethyl fumarate (2013–2016). MS patients met the 2010 revised McDonald criteria.[10] Only 172 of 244 patients with known JCV serology and ALC data before starting one of the study medications were included. JCV serology and ALC were evaluated at baseline and on an ad hoc basis throughout the study period. Additional data collected included: age, gender, race, JCV serology, and ALC [Table 1].
Table 1:
Baseline characteristics of the study population.
Primary outcomes looked at chronological changes in anti-JCV antibody index values. Secondary outcomes evaluated changes in serostatus, and ALC measurements correlated with time to lymphopenia (CTCAE 4.03; lymphopenia Grades 1–5).
Statistical analysis
JCV serology and ALC were obtained at baseline and on an ad hoc basis during clinical treatment. Initial anti-JCV antibody index values were obtained no >6 months before starting one of the three study medications. Anti-JCV antibody index values were analyzed using a linear mixed-effect model, with a random intercept and random slope for time, and a time by drug interaction main effect. Secondary changes in serostatus and time to lymphopenia were also analyzed using Pearson’s Chi-squared test (i.e., with exact P value computed, and Kaplan–Meier estimates of survival with log-rank test).
Demographic characteristics (age, gender, and race/ethnicity) and baseline laboratory data (anti-JCV antibody index, ALC, and serostatus) of the patients were compared among the three drugs using one-way analysis of variance, the Kruskal–Wallis test, and the Pearson Chi-squared test of association. When significant differences were noted, pairwise multiple comparisons were further explored with adjusted P value by the Tukey–Kramer’s method for one-way analysis of variance, the Bonferroni method for the Pearson’s Chi-squared test of association, and the Dwass-Steel-Critchlow-Fligner method for the Kruskal–Wallis test. All analyses were done using SAS 9.4Ò (SAS Institute, Inc., Cary, North Carolina, USA) with P ≤ 0.05 being statistically significant.
RESULTS
Anti-JCV antibody index values
There was a linear trend in anti-JCV antibody index values for patients treated with all three study medications [Figure 1]. Anti-JCV antibody indexes for patients treated with rituximab and fingolimod significantly decreased overtime, whereas those treated with dimethyl fumarate did not demonstrate a statistically significant decrease.
Figure 1:
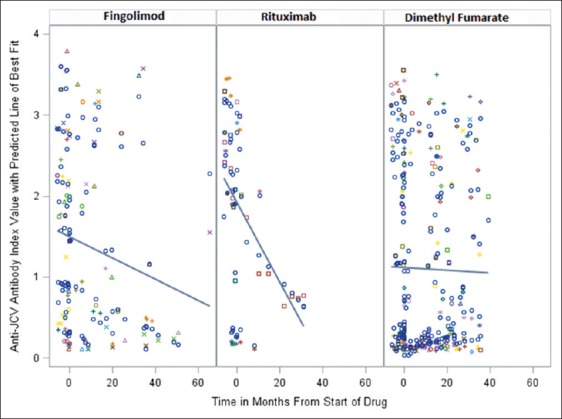
Changes in anti-JC index value with time.
Seroconversion
A total of five patients experienced seroconversion by the end of the study period from either indeterminate or negative to positive. The rate of seroconversion was not different among the three drugs.
ALC
A number of patients reached lymphopenia milestones. Lymphopenia, measured as cells/µL, was classified as Grade 1 (800–910), Grade 2 (500–799), Grade 3 (200–499), Grade 4 (<200), or Grade 5 (not detectable and patient death). No patient in this study manifested Grade 5 lymphopenia. Notably, 70% of fingolimod patients experienced drops in their ALC to <500 cells/µL, whereas none of the rituximab patients and only 1% of dimethyl fumarate patients achieved this milestone during the study [Figures 2-5].
Figure 2:
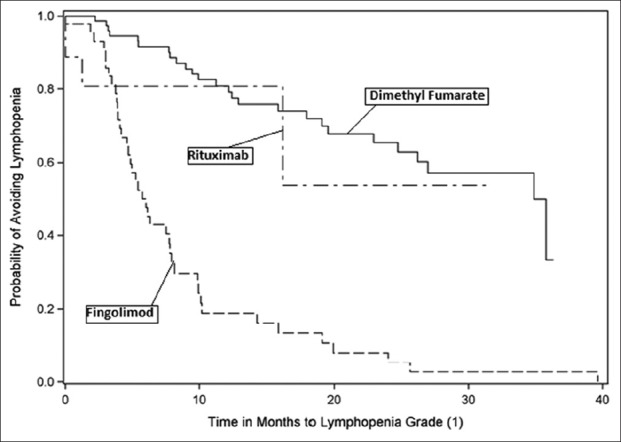
Kaplan–Meier curve - time to lymphopenia Grade 1.
Figure 3:
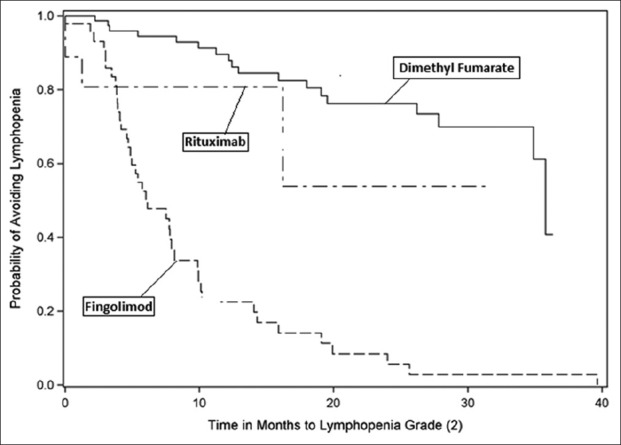
Kaplan–Meier curve - time to lymphopenia Grade 2.
Figure 4:
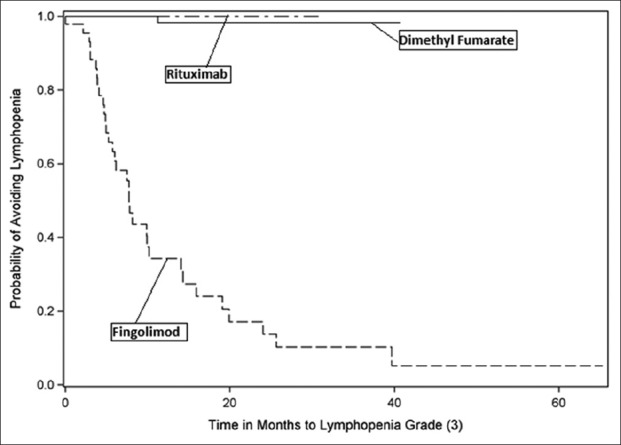
Kaplan–Meier curve - time to lymphopenia Grade 3.
Figure 5:
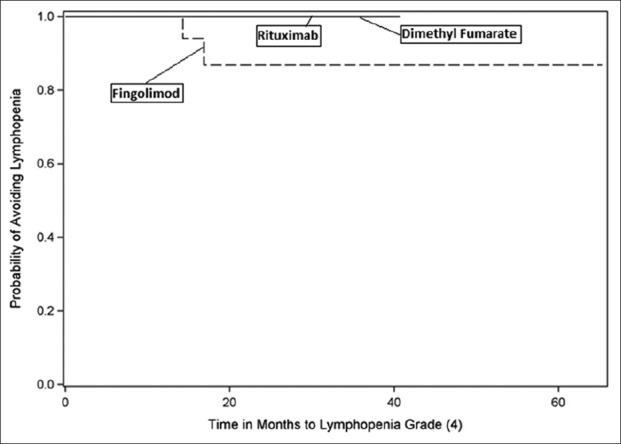
Kaplan–Meier curve - time to lymphopenia Grade 4.
DISCUSSION
Goals for the treatment of MS patients
Goals in the treatment of MS are to start early and reduce disease activity while optimizing neurologic reserve, cognition, and physical function.[6] Further, the aims are to minimize the risks associated with various therapies. All current agents affect immune function, and several are linked to new cases of PML. Here, we need biomarkers that can reliably guide us to mitigate the risk of PML associated with the various treatments available for patients with MS.
Natalizumab treatment for MS; PML correlated with biomarkers
Natalizumab, a highly effective therapy for MS, has provided the broadest set of biomarkers for stratifying the risk of PML. These biomarkers include time on therapy >2 years, prior immunosuppressants, JCV serostatus, and assessment of anti-JCV antibody index values. Published studies have quantified the risk of PML by any one or all four of these covariants.[7]
Revised guideposts for dimethyl fumarate therapy in MS patients
Six MS patients have had confirmed PML that correlated with dimethyl fumarate treatment; patients did not have prior natalizumab therapy. As all six instances of PML occurred in patients with Grade 2 lymphopenia or higher, the revised prescribing information now looks at the following guidepost for use: interruption of dimethyl fumarate treatment should be considered for patients whose ALC drops and persists <500 cells/µL for >6 months.[8]
13 Cases of PML in MS patients on fingolimod, no guidepost
In the post-marketing setting, there have been 13 cases of PML in MS patients on fingolimod not attributed to previous treatment with natalizumab.[3] 12 occurred after 2 years of treatment. The revised prescribing information for fingolimod acknowledges that the relationship between PML and treatment duration is unknown and that there is no specific cellular monitoring or testing that can mitigate risk.[4]
Current use of rituximab, ocrelizumab, and teriflunomide in JCV-positive MS patients
At present, rituximab, ocrelizumab, and teriflunomide are being used more routinely in MS patients who are JCV positive. Here, we reported our experience with fingolimod, rituximab, and dimethyl fumarate, as we tracked anti-JCV antibody index values and coincident changes in ALC during “clinical treatment of MS patients.”
Decreasing trend in anti-JCV antibody with fingolimod, rituximab, and dimethyl fumarate study medications
We identified a decreasing linear trend in anti-JCV antibody index values for patients treated with all three of these study medications. Those treated with fingolimod and rituximab had a statistically significant decrease; this might indicate that these drugs alter the reliability of the anti-JCV antibody index as a possible biomarker of PML risk overtime. Alternatively, patients treated with dimethyl fumarate did not show this statistically significant decrease in anti-JCV antibodies.
Furthermore, 70% of our fingolimod patients experienced Grade 3 and higher lymphopenia (65.9% with Grade 3 and another 4.3% with Grade 4, respectively), while for dimethyl fumarate, we saw Grade 3 lymphopenia in 1.2% of these patients (i.e., consistent with the findings of their pivotal studies).
Importance of declining anti-JCV antibody values
This study has shown that a declining anti-JCV antibody index value does not necessarily suggest a decreased risk of PML in patients treated with either fingolimod, rituximab, or dimethyl fumarate. While falling ALC counts did not correlate with declining anti-JCV antibody index values for any of the three agents used, we question whether and how lymphopenia impacts overall humoral immunity and immunologic memory.
Predictive value of anti-JVC antibody index in MS patients on study medications
MS patients treated with fingolimod or rituximab, before using natalizumab, may have the predictive value of their anti-JCV antibody index affected. Sequencing of DMT’s, and determining which agent is used first, may matter. Clinicians often make clinical decisions regarding the use of fingolimod and dimethyl fumarate based on anti-JCV antibody index value, assuming higher values are markers for increased risk of PML.[9] This is a hypothesis which is only proven for natalizumab.
Study limitations
Limitations of this study include the retrospective design, the small sample size, the failure to account for the use of steroids with rituximab, and failure to more accurately calculate seroconversion and seroreversion (e.g., incomplete documentation of baseline serostatus)
CONCLUSIONS
In MS patients, anti-JCV antibody index values significantly decreased in MS patients treated with fingolimod and rituximab (e.g., possible that fingolimod and rituximab impair the humoral response to the JCV). However, there was a non-significant decrease with dimethyl fumarate. A declining anti-JCV antibody index in fingolimod and rituximab-treated MS patients should not necessarily be interpreted as resulting in a declining risk for PML and may, paradoxically, be an indicator for increased PML risk.
Contributor Information
Stephen Farley, Email: stephen.farley@wchn.org.
Malcolm H. Gottesman, Email: Malcolm.Gottesman@nyulangone.org.
Sharon Friedman-Urevich, Email: sfriedman-urevich@nyuwinthrop.org.
Janin Ye, Email: jye@nyuwinthrop.org.
Mark Shen, Email: mshen@nyuwinthrop.org.
Denise Grueneberg, Email: dgrueneberg@nyuwinthrop.org.
Lorraine Martone, Email: lmartone@nyuwinthrop.org.
Rose Calixte, Email: rcalixte@med.cuny.edu.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Berger JR. Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord. 2017;12:59–63. doi: 10.1016/j.msard.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Clavel G, Moulignier A, Semerano L. Progressive multifocal leukoencephalopathy and rheumatoid arthritis treatments. Joint Bone Spine. 2017;84:671–5. doi: 10.1016/j.jbspin.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Druart C, El Sankari S, van Pesch V. Long-term safety and real-world effectiveness of fingolimod in relapsing multiple sclerosis. Patient Relat Outcome Meas. 2018;9:1–0. doi: 10.2147/PROM.S122401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EMA News and Press Releases:New recommendations to Minimize Risks of the Rare Brain Infection PML and a Type of Skin Cancer with Gilenya. 2015. [[Last accessed on 2018 Jan 25]]. Available from: http://www.ema.europa.eu/ema .
- 5.Ferenczy MW, Marshall LJ, Nelson CD, Atwood WJ, Nath A, Khalili K, et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 2012;25:471–506. doi: 10.1128/CMR.05031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannoni G, Butzkueven H, Dhib-Jalbut S, Hobart J, Kobelt G, Pepper G, et al. Brain health:Time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9(Suppl 1):S5–48. doi: 10.1016/j.msard.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Ho PR, Koendgen H, Campbell N, Haddock B, Richman S, Chang I, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis:A retrospective analysis of data from four clinical studies. Lancet Neurol. 2017;16:925–33. doi: 10.1016/S1474-4422(17)30282-X. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann-Horn K, Penkert H, Grein P, Leppmeier U, Teuber-Hanselmann S, Hemmer B, et al. PML during dimethyl fumarate treatment of multiple sclerosis:How does lymphopenia matter? Neurology. 2016;87:440–1. doi: 10.1212/WNL.0000000000002900. [DOI] [PubMed] [Google Scholar]
- 9.Pardo G, Jones DE. The sequence of disease-modifying therapies in relapsing multiple sclerosis:Safety and immunologic considerations. J Neurol. 2017;264:2351–74. doi: 10.1007/s00415-017-8594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis:2010 revisions to the mcDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]