Abstract
Background:
Our study shows that a membrane sealant/fiber fusogen polyethylene glycol (PEG) applied immediately on a sharp section of the spinal cord can mend the cord and lead to exceptional levels of motor recovery, with some animals almost normal.
Materials and Methods:
Before deploying such technology in man, long-term data in large mammals that exclude delayed complications (e.g., central pain), confirm the stability of motor recovery, and provide histological evidence of fiber regrowth are necessary. Here, we provide such evidence in dogs followed up over 6 months and in 2 cases up to 1 year along with imaging and histologic data.
Results:
We show that dogs whose dorsal cord has been fully transected recover locomotion after immediate treatment with a fusogen (PEG). No pain syndrome ensued over the long term. Diffusion tensor imaging magnetic resonance and histological, including immunohistochemical, data confirmed the re-establishment of anatomical continuity along with interfacial axonal sprouting.
Conclusions:
This study proves that a form of irreversible spinal cord injury (SCI) can effectively be treated and points out a way to treat SCI patients.
Keywords: Polyethylene glycol, spinal cord fusion, spinal cord injury

INTRODUCTION
We recently showed how polyethylene glycol (PEG), a fusogen[1,9] applied immediately on full transection of the spinal cord (so-called GEMINI protocol)[4] can lead to motor recovery in mice, rats, and dogs.[3,7,8,10] In this paper, we present the histological evaluation of the fusion process in dogs.[8]
MATERIALS AND METHODS
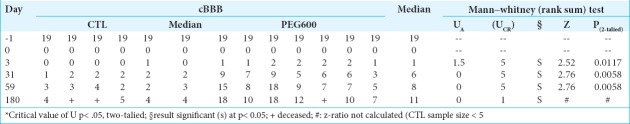
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Harbin Medical University (HMUIRB-2008-06) and the Institute of Laboratory Animal Science of China (A5655-01) and were in accordance with Directive 2010/63/EU of the European Parliament. Female beagles (8 kg) were used. The experimental group (PEG) consisted of 7 animals; 5 controls were treated with 0.9% NaCl. Spinal cord transection (SCT) at T10 was performed under general anesthesia. Animals were randomized to receive either 0.9% NaCl (2 mL) or PEG-600 (2 mL) applied topically to the site of SCT through a syringe and left in situ at the point of transection.[8] Over the next 6 months, all animals were assessed with the 20-point (0–19) canine Basso-Beattie-Bresnahan rating scale. At the end of 6 months, all animals except two from the PEG group were euthanized.
Neuroimaging
All animals were subjected to magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) using a 3.0 T MRI system (Achieva 3, Philips, Amsterdam, and The Netherlands) in the prone position. Sagittal, T2-weighted, fast spin-echo (TR = 1700 ms; TE = 100 ms; slice thickness = 3 mm; slice gap = 0.1; and NSA = 4), and axial single-shot echo-planar DTI (TR = 6100 ms; TE = 93 ms; voxel size = 2 mm × 2 mm; slice thickness = 2 mm; slice gap = 0; NSA = 2; and diffusion direction number = 15) sequences were acquired twice at 2 and 4 weeks postoperatively in all animals and then at 180 days.
Histologic assessment
After 6 months, all surviving beagles, except two from the PEG group, were anesthetized and perfused with 0.9% NaCl solution and 4% paraformaldehyde. Immediately thereafter, the T10 spinal cord was surgically accessed and the treated cord extracted en bloc by sectioning 2 cm above and below the point of fusion. The bloc was immersed in 4% paraformaldehyde for 24 h, followed by paraffin embedding.
Hematoxylin-Eosin (HE) and Chromotropic acid 2R-Brilliant Green (C-2R-G) stainings were carried out employing commercially available kits (Leagene, Beijing, PR China): C-2R-G stains myelin in red and the acellular extracellular matrix in green. All cords were studied on sagittal and transverse slices above, at and below the site of transection.
For HE staining, 7 µm-thick sagittal and coronal sections were dewaxed, hydrated and then placed directly into xylene twice for 10’, and then in alcohol at decreasing concentrations for 5’ each (100%, 95%, 85%, and 70%). The sections were rinsed in peroxidase blocking soluiton (PBS) 3 times for 5’, stained with hematoxylin for 10’ and then rinsed in distilled water; finally, the sections were differentiated in a 1% HCl solution and then dipped in distilled water. Sections were then stained with eosin for 3’ and then dehydrated in gradient ethanol; subsequently, the sections were placed into xylene twice for 5’. For C-2R-G staining, 7-μm thick sections were dewaxed and hydrated, then passage into xylene for 20’, 100% alcohol for 1’, and 95% alcohol for 5’. Afterward, the sections were stained with C-2R-G for 10’ followed by a triple rinse in 0.2% glacial acetic acid and counterstained in 0.5% brilliant green glacial acetic acid solution for 10’. Subsequently, sections were rinsed in distilled water for 2’, dehydrated in gradient ethanol, and treated with xylene twice for 5’.
All sections were visualized with an Olympus IX73 (Tokyo, Japan) optical microscope. The Image-Pro-Plus (Media Cybernetics, Rockville, MD, USA) software was employed for image analysis.
Immunohistochemistry
Longitudinal sections of spinal cord tissue cut at 10 µm were mounted onto silanized glass slides. Slides were air-dried for 15 min and washed in PBS before blocking in 10% normal bovine serum and 0.1% Triton X-100 in PBS at room temperature. Primary antibodies diluted in Dako antibody diluent (Dako, Denmark) were applied overnight at 4°C in a humidified chamber. Primary antibodies included anti-serotonin (5-HT; 1:1000, Immunostar, WI, USA) and anti-neurofilament 200 (NF200) (1:400; Sigma, USA). Slides were washed and secondary antibodies diluted in PBS applied for 1 h at room temperature – Alexa Fluor 488 or 594 conjugated IgG (1:2000, Molecular Probes, USA). Slides were washed in PBS and mounted with a cover-slip using VECTASHIELD (Vector Lab. the US). Images were acquired using a fluorescence microscope fitted with a digital camera system (Nikon, Japan) and routed to a Windows PC for quantitative analyses using Adobe Photoshop CS5 software. Attention was given to ensure identical settings for fluorescence exposure, amplifier gain and cut off across all images.
Statistical analysis
Datametrics for DTI were calculated by the machine software. Histologic indices (vacuolization, scarring, and myelin ratios) were calculated with GraphPad Prism 5 (LaJolla, CA, USA).
RESULTS
All 12 animals survived the operation. Initially, all animals had normal initial cBBB scores [Table 1]. Postoperatively, paraplegia was observed in all cases. PEG-treated animals showed the first sign of recovery within 3 days versus none in controls. At 6 months, all PEG-treated animals – but no controls – showed levels of behaviorally useful recovery. Results were significant at all-time points, with a gradual improvement over time (medians: 4 vs. 11; see ref. 8 and Table 1). Two controls were lost at follow-up; one treated beagle died accidentally. Urination required express voiding twice a day. Controls never showed signs of recovery at any time-point.
Table 1:
Post-op6 month cBBB.
Tractography
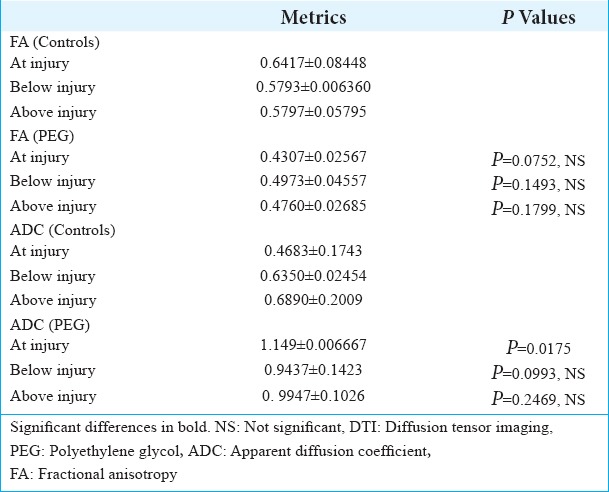
DTI showed tissue re-establishment of anatomical continuity in PEG-treated animals as opposed to controls [Figure 1]. Mean diffusivity (MD); a.k.a. Apparent diffusion coefficient (ADC), an index of cellularity, was significantly different at the injury level between treated animals and controls at 6 months [Table 2]. Fractional anisotropy, that measures axonal integrity and the degree of myelination, significantly differed at 2 months,[8] but only trended toward significance in the present study, likely due to the smaller sample at 6 months. On visual inspection (Tractography), the extent of cord reconnection correlated with behavioral recovery, with near-normal dogs with almost normal appearing cords versus no restitutio and integrum in untreated animals.
Figure 1:
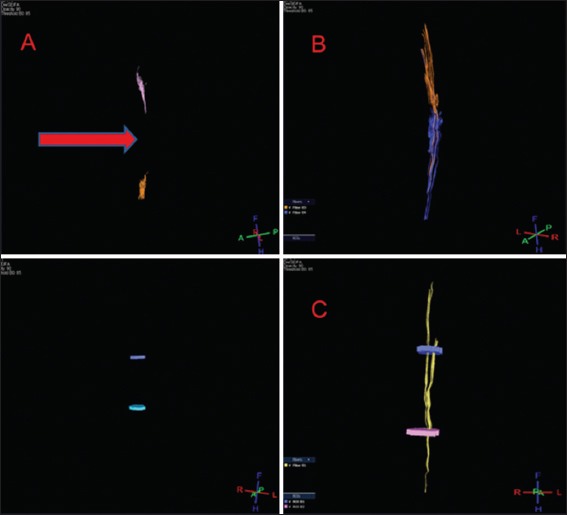
Representative diffusion tensor imaging scan after polyethylene glycol treatment (B and C) and placebo (A) at 6 months. The sectional gap in controls – unlike treatment animals – is still visible (A, arrow).
Table 2:
DTI: Datametrics and statistical analysis.
Histology and immunohistochemistry
On HE stained sections (sagittal and coronal), treated and untreated cords differed dramatically: vacuolization due to tissue injury (cysts) was minimal in treated cords [Figure 2; a1 and b1], with a highly significant difference with controls, a sign of the neuroprotective effects of PEG [Figure 3a; P = 0.0005]. On C-2R-B, myelin staining was abundant in PEG-treated animals versus very little in controls [Figure 2; a2 and a3, b2 and b3; Figure 3b; P < 0.0001]. In controls, both above and at injury site, axons clearly showed massive signs of Wallerian degeneration, including where corticospinal fibers course; on the contrary, in treated animals, fibers were spared to a large extent and shown crossing the fusional interface through the scar [Figure 2; a2 and a3, b2 and b3]. This was confirmed by immunolabeling NF protein (NF200), an integral component of the axonal cytoskeleton that evinced remarkable axonal sprouting across the transection site. Some of these fibers also stained for 5-HT [Figure 2; a4 and b4]. No significant difference was seen in the amount of scarring in both groups of animals [P = 0.7252; Figure 3c].
Figure 2:
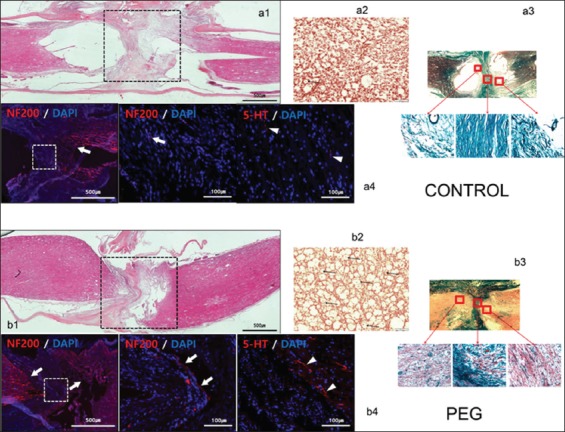
Histology and immunohistochemistry. Controls: a1: hematoxylin-eosin (HE) sagittal view of the cord: notice poor take-up of the stain by interface tissues. a2: chromotropic acid 2R-Brilliant Green (C-2R-G) staining of an axial slice showing widespread Wallerian degeneration. a3: C-2R-G stained sections above, at and below injury level: notice absence of axons across the interface. a4: neurofilament 200 (NF200) DAPI and 5HT DAPI sections. Notice near absence of regrowing fibers across the interface. Polyethylene glycol: b1: HE sagittal view of the cord: notice bright coloring as stains are taken up by treated tissues. b2: C-2R-G staining of an axial slice showing widely conserved axons at the interface (arrows). b3: C-2R-G stained sections above and below injury level: notice the abundance of axons across the interface. b4: NF200 DAPI and 5HT DAPI sections. Notice regrowing fibers across the interface.
Figure 3:
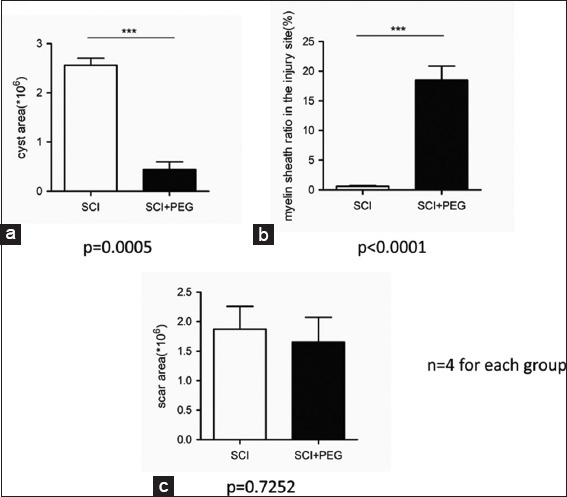
(a-c) Block statistics of histology assessment (GraphPad): notice the neuroprotective effects of polyethylene glycol as cavitation is largely avoided and axons spared. No effects on scarring were noticed.
DISCUSSION
This study shows that a membrane sealant/fiber fusogen (PEG) applied immediately on a sharp section of the spinal cord can re-establish anatomical continuity (and lead to behavioral recovery).
Sharpness of the transection is a key factor for successful axonal regeneration. An extremely sharp transection produces edema-free lesions, without cysts not scars, whereas a relatively blunt An extremely sharp transection followed by scars and cysts around the lesions.[13] Furthermore, axonal disintegration starts after about 10–20 min of transection.[2] In our study, PEG was applied immediately on severance and exerted its sealant/fusogen effects topically.
Both neuroimaging and histology confirm the neuroprotective effects on cells at the level of the section and regrowth of fibers across the fusion interface. This regrowth is focused on a cellular, short-fiber, and gray matter-based network of interneurons extending from the brainstem to the spinal cord -and inputed by fibers from cortical motor areas- that simultaneously conveys commands to motoneurons (Cortico-Truncoreticulo-Propriospinal Pathway – C-TRPS); it also embeds and links the Central Pattern Generators located in the cervical and lumbar cord responsible for the execution of motor programs, including gait.[4] The minimally disruptive (nanometers) section of the cord damages a very thin layer of these interneurons: PEG reseals their membranes and curbs cell death.[4] These same cells, along with others in proximity which were not damaged by the extra-sharp blade, can immediately regrow (sprout) their appendages and restablish contact between the apposed interfaces. Consequently, the gray matter neuropil is restored by spontaneous regrowth of the severed axons/dendrites at the point of contact between the apposed cords, as already suggested by a previous study by our group.[6] Present DTI and histological data clearly show the remarkable capacity of PEG to protect neural tissue (i.e., minimal cavitation, spared axons, and preserved cellularity on the at-injury MD/ADC index) from massive neuronal death and necrosis, contrary to what is generally seen – as explained above – after standard sections. The end result is a fast onset of initial clinical recovery (3 days) in treated animals; actually, the first effects can be evinced within 1 h of topical PEG application.[7] Part of the regrowing fibers are serotonergic: the descending serotonergic pathway, which originates in various populations of brainstem neurons, plays an important role in generating the rhythmic motor pattern associated with locomotor movement and establishes direct connections with lumbar motoneurons.[12]
Given the excellent recovery, the sprouting process in the TRPS does not appear to be haphazard, with disruptive misalignments. In any case, circuital readjustment in the spinal cord and within upper CNS stations is known to play a key role in recovery from spinal cord injuries (SCI).[5]
PEG does not appear to act via interfering with the scarring process, as shown by the nonsignificant difference between the two groups. PEG is applied immediately after SCT: no astrocytic scar is in place to hinder the process since a scar only becomes visible after about 1 week of injury. The astrocytic scar might actually promote axon regrowth in the early stages of SCI: it is only past the subacute stage that the scar slowly becomes nonpermissive.[11] PEG has no effects on scarring and thereby does not hinder any early beneficial effects: by the time the scar becomes nonpermissive, fibers will have already had time to cross the fusion interface and “bridge” the two stumps.
CONCLUSIONS
We have shown that the paralysis following full section of the spinal cord can be reversed by immediate topical application of a fusogen at the point of the section: recovery is due to axonal sprouting across the fusion interface.
Acknowledgements
The authors are supported by the National Natural Science Foundation of China (81470425), the Wu Liande Foundation of Harbin Medical University (Wld-qn1414), and the Harbin Science and Technology Bureau (2014RFXYJ023).
Contributor Information
Shuai Ren, Email: 18704600420@163.com.
Zehan Liu, Email: liuzehan0532@163.com.
C. Yoon Kim, Email: vivavets@gmail.com.
Kuang Fu, Email: fukuang4858637@163.com.
Qiong Wu, Email: wuqiong8651@sina.com.
Liting Hou, Email: liting_0222@163.com.
Linlin Sun, Email: zhiyaosll@163.com.
Jian Zhang, Email: 18846783612@163.com.
Qing Miao, Email: miaoqing20162@163.com.
Jin Kim, Email: jk208019@snu.ac.kr.
Vincenzo Bonicalzi, Email: sercan4@yahoo.it.
Xiangchen Guan, Email: 393694660@qq.com.
Mingzhe Zhang, Email: ahzhangmz@126.com.
Weihua Zhang, Email: 18530162296@163.com.
Junfeng Xu, Email: 1773211686@qq.com.
Sergio Canavero, Email: sercan@inwind.it.
Xiaoping Ren, Email: chinarenxg@126.com.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Abdou SA, Henderson PW. Fusogens: Chemical agents that can rapidly restore function after nerve injury. J Surg Res. 2019;233:36–40. doi: 10.1016/j.jss.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Canavero S, Ren X, Kim CY, Rosati E. Neurologic foundations of spinal cord fusion (GEMINI) Surgery. 2016;160:11–9. doi: 10.1016/j.surg.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Canavero S, Ren X, Kim CY. Reconstructing the severed spinal cord. Surg Neurol Int. 2017;8:285. doi: 10.4103/sni.sni_406_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canavero S, Ren X. Houston, GEMINI has landed: Spinal cord fusion achieved. Surg Neurol Int. 2016;7:S626–8. doi: 10.4103/2152-7806.190473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isa T. The brain is needed to cure spinal cord injury. Trends Neurosci. 2017;40:625–36. doi: 10.1016/j.tins.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Kim CY, Oh H, Ren X, Canavero S. Immunohistochemical evidence of axonal regrowth across polyethylene glycol-fused cervical cords in mice. Neural Regen Res. 2017;12:149–50. doi: 10.4103/1673-5374.199014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim CY. PEG-assisted reconstruction of the cervical spinal cord in rats: Effects on motor conduction at 1h. Spinal Cord. 2016;54:910–2. doi: 10.1038/sc.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Ren S, Fu K, Wu Q, Wu J, Hou L, et al. Restoration of motor function after operative reconstruction of the acutely transected spinal cord in the canine model. Surgery. 2018;163:976–83. doi: 10.1016/j.surg.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Lu X, Perera TH, Aria AB, Callahan LA. Polyethylene glycol in spinal cord injury repair: A critical review. J Exp Pharmacol. 2018;10:37–49. doi: 10.2147/JEP.S148944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren S, Liu ZH, Wu Q, Fu K, Wu J, Hou LT, et al. Polyethylene glycol-induced motor recovery after total spinal transection in rats. CNS Neurosci Ther. 2017;23:680–5. doi: 10.1111/cns.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–41. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- 12.Xia Y, Chen D, Xia H, Liao Z, Tang W, Yan Y, et al. Serotonergic projections to lumbar levels and its plasticity following spinal cord injury. Neurosci Lett. 2017;649:70–7. doi: 10.1016/j.neulet.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida Y, Kataoka H, Kanchiku T, Suzuki H, Imajyo Y, Kato H, et al. Transection method for shortening the rat spine and spinal cord. Exp Ther Med. 2013;5:384–8. doi: 10.3892/etm.2012.841. [DOI] [PMC free article] [PubMed] [Google Scholar]