Abstract
Objective:
We sought to estimate the incidence and predictors of all-cause mortality 6 months after heart failure hospitalization in Uganda.
Methods:
Mbarara Heart Failure Registry is a cohort of patients hospitalized with a clinical diagnosis of heart failure at Mbarara Regional Referral Hospital, Uganda. We measured serum electrolytes, cardiac markers, and echocardiograms. All participants were followed until death or end of 6 months. We used Fine and Gray models to estimate the incidence and predictors all-cause mortality.
Results:
A total of 215 participants were enrolled, 141 (66%) were women, and mean age 53 (standard deviation 22) years. Nineteen (9%) had diabetes, 40 (19%) had HIV, and 119 (55%) had hypertension. The overall incidence of all-cause mortality was 3.58 (95% CI 2.92, 4.38) per 1000 person-days. Men had higher incidence of death compared to women (4.02 vs 3.37 per 1000 person-days). The incidence of all-cause mortality during hospitalization was almost twice that of in the community (27.5 vs 14.77 per 1000 person-days). In adjusted analysis, increasing age, NYHA class IV, decreasing renal function, smoking, each unit increase in serum levels of Potassium, BNP, and Creatine kinase-MB predicted increased incidence of 6 months all-cause death whereas taking beta-blockers and having an index admission on a weekend compared to a week day predicted survival.
Conclusions and interpretation:
There is a high incidence of all-cause mortality occurring in-hospital among patients hospitalized with heart failure in rural Uganda. Heart failure directed therapies should be instituted to curb heart failure-related mortality.
Keywords: Acute heart failure, All-cause mortality, Sub-Saharan Africa
1. Introduction
Hospitalization with acute heart failure is an important cause of mortality in both resource rich and resource limited countries [1,2]. In USA, for example, in-hospital mortality following an acute heart failure hospitalization varies between 2.3 and 3.8% [3,4]. Though no data exists in resource limited countries for comparison, heart failure (HF) is a major cause of socioeconomic and public health burden worldwide [3], in US alone about 6 million people are living with HF [3,5,6] while in sub-Saharan Africa, HF accounts for up to 7% of all hospitalizations [7]. In resource rich settings, coronary artery disease, either alone or in combination with hypertension, is the leading cause of HF [8]. In contrast, resource poor settings are undergoing an epidemiological shift from predominately non-ischemic etiologies of HF e.g., rheumatic heart disease and endemic cardiomyopathies [9,10] to ischemic etiologies as seen in resource rich settings [4,11].
However, epidemiological studies estimating mortality rates attributable to heart failure in sub-Saharan Africa are from specialized urban centers which are not generalizable to populations from these countries as the majority live in rural areas [9,12,13]. As such reports such as those of the sub-Saharan Africa Survey of Heart Failure (THESUS-HF) [9] study that reported a 18% 6-month mortality grossly underestimate the all-cause mortality rate among heart failure patients in sub-Saharan African populations.
In consequence, surveillance of the incidence of heart failure related mortality and etiologies are important for public health and identification of patients for prioritized emergency therapies. We aimed to determine the incidence and predictors of all-cause mortality 6 months after an acute heart failure hospitalization event in southwestern Uganda.
2. Methods
2.1. Study population
Participants were from the ongoing Mbarara Heart Failure Registry (MAHFER), a longitudinal observational study on heart failure outcomes in southwestern Uganda (ClinicalTrials.gov Identifier: ). Briefly, patients aged 13 years or greater were consecutively enrolled into MAHFER from June 2015 to March 2017 within 24 h of hospitalization. The heart failure diagnosis for inclusion was based on the presence of clinical symptoms and signs of heart failure [14] to mirror routine care such our findings are generalizable to resource constraint settings where diagnostic tests are not readily available. Patients with an acute exacerbation of chronic kidney disease, chronic obstructive pulmonary disease, or acute liver disease with no features of heart failure were excluded. Participants were actively followed daily during hospitalization and every month post-hospital discharge until 6 months or death, whichever came first. Of note, participants were managed according to the discretion of the admitting team of physicians. Standardized protocols for management of heart failure are not currently in place at the Mbarara Regional Referral Hospital, as is the case in most resource poor settings where cardiology specialists are scarce.
The ethical review boards at the Mbarara University of Science and Technology, the University of Virginia Health System, and the Uganda National Council of Science and Technology approved the conduct of this study. All participants signed a written informed consent.
2.2. Data collection
We used standardized questionnaires to collect data on participant demographic information, past medical history (i.e., cardiovascular risk factors and co-morbid conditions), prior hospitalizations and discharge medications, New York heart association (NYHA) functional class, symptoms, vital signs and physical exam, acute cardiovascular-related and non-cardiovascular therapies, hospital course (i.e. in-hospital worsening HF and other adverse events) and outpatient course. The questionnaire also captured information on household asset ownership, smoking history (age of starting, duration and intensity of smoking and efforts to quit), history of diagnosis and/or management of cardiovascular disease or risk factors (hypertension, diabetes mellitus), and list of medications administered.
Pre-specified data collection was done during all days of the index hospital stay following study enrollment and monthly during outpatient visits following hospital discharge or until death within 6 months of enrollment. At each outpatient visit, study staff obtained updated medical and medication history, NYHA functional class, symptoms, vital signs, medication adherence, and interval events including hospitalizations.
Upon enrollment, a trained study nurse administered questionnaires to capture information on demographic data, socioeconomic status, history of chronic illnesses and smoking. We measured blood pressure (Panasonic EW3109), body temperature, respiratory rate, and performed clinical examinations daily during hospital stay. In addition, a trained internist performed echocardiography (Philips HD7 XE Diagnostic ultrasound system, China) in the course of participants hospital stay. We categorized Left ventricular ejection fractions (LVEF) as reduced (≤40%), midrange (41 to 49%), or preserved (≥50%) [14].
A study nurse screened all participants for HIV as per national guidelines and on every other day, performed bedside testing of Brain natriuretic peptide (BNP), Creatine kinase (MB isomer) (CK-MB), and cardiac Troponin I using a point of care i-STAT®1Abbott analyzer (Abbott Point of Care, Princeton, New Jersey, USA). In addition, a separate blood specimen was taken to laboratory to measure complete blood counts (Sysmex® XS-1000i, Japan), serum aminotransferases, and lipid profile (Humaster® 200, Germany). Laboratory tests were performed at Mbarara Regional Referral Hospital laboratory, which has standardized internal quality control protocols and participates in external quality control programs by the National Health Laboratory Service.
2.3. Statistical analysis
We described the population baseline demographic and clinical characteristics using descriptive statistics; proportions for categorical and binary variables, mean and standard deviation for normally distributed continuous variables, and median and interquartile range for non-normally distributed continuous variables.
We used principle component analysis to generate assets index scores based on household utilities and characteristics such as durable asset ownership, household characteristics, utilities, highest education attained, occupation, and goods sold, [15] to derive composite socioeconomic status measures with highest discriminatory capabilities [16]. Participants were divided into quintiles of assets index scores (poorest, poor, average, rich, and richest).
We used a-priori knowledge to select potential factors: age, gender, smoking, New York heart association (NYHA) functional class, Left ventricular ejection fractions, serum sodium, BUN, creatinine, BNP, systolic blood pressure, that were postulated to be predictors of all-cause mortality within 6 months of hospitalization with acute heart failure.
We used the Fine and Gray modeling to estimate the standardized hazard and predictors of 6 months all-cause mortality. The competing risk of death models accounted for loss to follow-up before death as a separate event so as to minimize overestimation of risk [17]. All analyses were performed with STATA® Statistical Software version 15 (StataCorp LP, College Station, Texas, USA).
3. Results
We screened 396 consecutive patients and enrolled 217 participants between June 1, 2015, and March 28, 2017. Of those enrolled, 2 participants were excluded after they were found to have chronic obstructive pulmonary disease and chronic kidney disease with no evidence of heart failure. Of those enrolled in the study, by end of 6 months of follow-up, 16 (7%) participants were lost to follow-up.
The mean age was 53 years (standard deviation of 21.71), females constituted 65.58% of total sample probably because of their better health seeking behavior [18,19]. Comorbid conditions were hypertension in 119 (55.35%), diabetes mellitus in 19 (8.84%), and human immunodeficiency virus (HIV) in 40 (18.60%). A total of 21 (9.77%) had self-reported current history of smoking (Table 1).
Table 1.
Baseline characteristics of participants, MAHFER study 2017.
| Characteristic | N = 215 |
|---|---|
| Demographics | |
| Age (in years), mean (±sd) | 53 (21.71) |
| Women, n (%) | 141 (65.58) |
| Risk factors | |
| Smoking, never, n (%) | 170 (79.07) |
| Former, n (%) | 24 (11.16) |
| Current, n (%) | 21 (9.77) |
| Hypertension, n (%) | 119 (55.35) |
| Diabetes, n (%) | 19 (8.84) |
| HIV infected, n (%) | 40 (18.60) |
| Clinical features | |
| De novo heart failure, n (%) | 83 (38.60) |
| Decompensated chronic heart failure, n (%) | 132 (61.40) |
| Acute heart failure with cardiogenic shock, n (%) | 40 (18.60) |
| Acute heart failure with congestion, n (%) | 195 (90.70) |
| Blood tests (at baseline i.e., time of admission) with normal ranges | |
| Hemoglobin (12, 17g/dL), mean (±sd) | 13.06 (8.98) |
| Total cholesterol (5, 220 mg/dL), mean (±sd) | 109.80 (43.96) |
| Low-density lipoprotein (50, 17 mg/dL), mean (±sd) | 74.67 (35.75) |
| High-density lipoprotein (35, 130 mg/dL), mean (±sd) | 28.61 (14.40) |
| Triglyceride (150, 250 mg/dL), mean (±sd) | 109.78 (62.37) |
| Creatinine (0.60, 1.10 mg/dL), mean (±sd) | 3.63 (6.40) |
| Sodium (135, 145 mmol/L), mean (±sd) | 131.95 (14.96) |
| Blood urea nitrogen (10, 50 mg/dL), mean (±sd) | 85.94 (139.4) |
| Potassium (3.5, 5.5 mmol/L), mean (±sd) | 4.61 (1.34) |
| Creatine kinase-MB (0, 4.9 ng/mL), mean (±sd) | 4.46 (10.32) |
| Troponin I (<0.01 ng/mL), mean (±sd) | 0.07 (0.42) |
| Brain natriuretic peptide (<100 pg/mL), mean (±sd) | 2191.20 (1769.30) |
| Vital signs | |
| Systolic blood pressure (mm Hg), mean (±sd) | 122 (29.37) |
| Diastolic blood pressure (mm Hg), mean (±sd) | 78.56 (21.26) |
| NYHA¥ class III, n (%) | 93 (43.26) |
| NYHA¥ class IV, n (%) | 112 (52.09) |
| Echocardiogram and electrocardiograph features | |
| LVEF*, mean (±sd) | 40.92 (15.30) |
| LVDdζ, mean (±sd) | 5.7 (4.97) |
| LAϕ, mean (±sd) | 4.2 (1.11) |
| Medication use prior to admission | |
| Self-reported poor adherence to medication | 128 (59.53) |
| ACEI/ARBs, n (%) | 50 (23.26) |
| Beta-blockers, n (%) | 39 (18.14) |
| Furosemide, n (%) | 85 (39.53) |
| HMG CoA inhibitors, n (%) | 4(1.86) |
| Digoxin, n (%) | 30 (13.95) |
| Antiplatelet agents, n (%) | 38 (17.67) |
| Medication use during hospital stay | |
| Oxygen therapy, n (%) | 29 (13.49) |
| Inotropic agents, n (%) | 22 (10.23) |
| Furosemide, n (%) | 195 (90.70) |
| ACEI/ARBs, n (%) | 59 (27.44) |
| Beta-blockers, n (%) | 41 (19.07) |
| HMG CoA inhibitors, n (%) | 7 (3.26) |
| Digoxin, n (%) | 52 (24.19) |
| Antiplatelet agents, n (%) | 74 (34.42) |
SD: standard deviation; NYHA¥: New York Heart Association; LVEF*: left ventricular Ejection fraction; LVDdζ: left ventricular diameter during diastole; LAϕ: left atrium; HMG: hydroxy-methylglutaryl-coenzyme reductase inhibitor; ACEI/ARB^: angiotensin converting enzyme inhibitor/angiotensin receptor blocker.
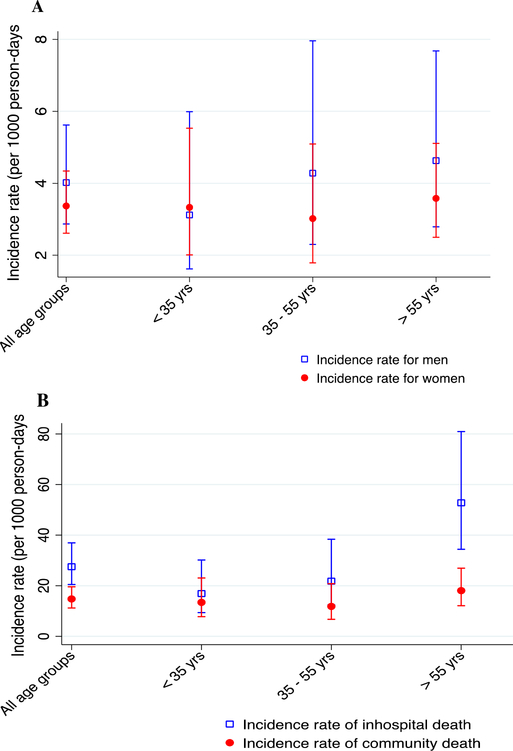
A total of 93 (43%) participants died within 6 months from time of enrollment representing an all-cause mortality incidence rate of 3.58 (95% CI 2.92, 4.38) per 1000 person-days. Among the deaths, 44 (47.3%) occurred in-hospital and 49 (52.7%) occurred in the community (Fig. 1). Among the 44 who died during hospitalization, 8 (18%) died within 48 h of hospitalization, 11 (25%) between 3 and 7 days, and 25 (57%) after a week of hospitalization.
Fig. 1.
Incidence rate of all-cause mortality (per 1000 person-days) by gender (panel A) and place of death (panel B).
Of those dead, 34 were men representing a gender-adjusted incidence rate of 4.02 (95% CI 2.87, 5.62) per 1000 person-days whereas 59 were women thus an incidence rate of 3.37 (95% CI 2.61, 4.34) per 1000 person-days. Among men, we observed an increase in incidence of mortality with increasing age i.e., in the age group <35 year the age- and gender-adjusted incidence of mortality was 3.12 (95% CI 1.62, 5.99) per 1000 person-days, in the age group 35 to 55 years the age- and gender-adjusted incidence of mortality was 4.28 (95% CI 2.30, 7.96) per 1000 person-days, and in the age group >55 years the age- and gender-adjusted incidence of mortality was 4.63 (95% CI 2.79, 7.68) per 1000 person-days.
The incidence of mortality was generally increased with age (Fig. 1), i.e., age-adjusted in-hospital incidence of mortality of 16.89 (95% CI 9.36, 30.51) per 1000 person-days for those in aged 35 years or less, 21.78 (95% CI 12.37, 38.35) per 1000 person-days for those aged between 35 and 55 years, and 52.76 (95% CI 34.40, 80.92) per 1000 person-days for those aged 55 years or greater. Incidence of mortality was lower in the community across age groups (incidence of morality of 13.39) (95% CI 7.77, 23.06) per 1000 person-days in age group 35 years or less, 11.81 (95% CI 6.71, 20.80) per 1000 person-days in ages between 35 and 55 year and 18.04 (95% CI 12.06, 26.92) per 1000 person-days for those aged 55 years or greater (Fig. 1).
In the adjusted analysis, the cumulative incidence of all-cause was higher among current smokers compared to never smokers (sub-distribution Hazard ratio (ASHR) 7.23, 95% CI 1.40, 37.26, p = 0.01), higher with decreasing estimated glomerular filtration rate (eGFR) (p for linear trend 0.001). We found the adjusted estimated sub-distribution Hazard Ratio and associated 95% confidence intervals were 2.66 (1.74 to 4.06, p < 0.0001) for a 1-mg/dL increase in serum potassium, 1.00 (1.00 to 1.00, p = 0.04) for a 1-pg/mL increase in BNP, higher for every 1-pg/mL increase in CKMB (ASHR 1.05, 95% CI 1.01, 1.10, p = 0.01). Also a 1-mg/dL increase in hemoglobin predicted an increase in the incidence of all-cause death (ASHR 1.01, 95% CI 1.00, 1.02, p = 0.01) (Table 2).
Table 2.
Standardized hazard ratios of all-cause mortality, MAHFER Study 2017.
| Variable | Unadjusted standardized hazard ratios (95% CI) | p-Value | Adjusted standardized hazard ratios (95% CI) | p-Value |
|---|---|---|---|---|
| Age, each year increase | 0.99 (0.99, 1.00) | 0.076 | 0.99 (0.99, 0.99) | 0.001 |
| Men | 1.16 (0.76, 1.76) | 0.494 | 0.35 (0.06, 2.03) | 0.245 |
| Women | Ref | Ref | Ref | Ref |
| Asset index | ||||
| Poorest | 0.74 (0.36, 1.50) | 0.403 | 0.14 (0.04, 0.55) | 0.005 |
| Poorer | 1.31 (0.67, 2.57) | 0.423 | 0.08 (0.03, 0.23) | 0.0001 |
| Average | Ref | Ref | Ref | Ref |
| Rich | 0.97 (0.48, 1.93) | 0.921 | 0.71 (0.11, 4.78) | 0.730 |
| Richest | 1.03 (0.51, 2.07) | 0.939 | 0.12 (0.02, 0.60) | 0.010 |
| History of smoking | ||||
| Never smoker | Ref | Ref | Ref | Ref |
| Former smoker | 0.92 (0.50, 1.69) | 0.782 | 0.99 (0.52, 1.90) | 0.999 |
| Current smoker | 1.87 (0.93, 3.76) | 0.080 | 7.23 (1.40, 37.26) | 0.018 |
| HIV infection | 1.09 (0.67, 1.76) | 0.738 | 1.16 (0.29, 4.55) | 0.831 |
| History of hypertension | 0.74 (0.49, 1.11) | 0.146 | 0.79 (0.38, 1.67) | 0.540 |
| History of diabetes mellitus | 0.83 (0.37, 1.83) | 0.640 | 0.94 (0.35, 2.54) | 0.901 |
| Functional status | ||||
| NYHA¥ class III | Ref | Ref | Ref | Ref |
| NYHA class IV | 2.11 (1.38, 3.23) | 0.001 | 2.52 (1.24, 5.12) | 0.011 |
| Six minute walk, (per meter) | 1.00 (0.99, 1.00) | 0.924 | 0.99 (0.99, 1.00) | 0.697 |
| Left ventricular ejection fraction (LVEF) | ||||
| Reduced LVEF (≤40%) | 1.04 (0.63, 1.72) | 0.885 | 1.10 (0.48, 2.51) | 0.813 |
| Midrange LVEF (41 to 49%) | Ref | Ref | Ref | Ref |
| Preserved LVEF (≥50%) | 0.75 (0.39, 1.44) | 0.385 | 0.66 (0.28, 1.57) | 0.347 |
| Estimated GFR£ stage | ||||
| GFR stage 1 | Ref | Ref | Ref | Ref |
| GFR stage 2 | 0.97 (0.53, 1.80) | 0.929 | 1.34 (0.41, 4.39) | 0.626 |
| GFR stage 3 | 1.86 (1.00, 3.47) | 0.051 | 2.79 (1.42, 5.47) | 0.003 |
| GFR stage 4 | 2.83 (1.01, 7.90) | 0.048 | 3.54 (1.04, 1.21) | 0.000 |
| GFR stage 5 | 2.53 (1.49, 4.29) | 0.001 | 3.12 (1.14, 8.55) | 0.027 |
| Timing ofindex admission | ||||
| Week day admission (Monday-Thursday) | Ref | Ref | Ref | Ref |
| Weekend admission (Friday-Sunday) | 0.89 (0.47, 1.71) | 0.734 | 0.17 (0.05, 0.49) | 0.001 |
| Baseline blood tests (each unit increase) | ||||
| Hemoglobin (g/dL) | 1.01 (0.98, 1.02) | 0.604 | 1.01 (1.00, 1.02) | 0.018 |
| Potassium (mmol/L) | 1.29 (1.10, 1.51) | 0.002 | 2.66 (1.74, 4.06) | 0.0001 |
| BUN€ (mg/dL) | 1.00 (1.00, 1.00) | 0.006 | 1.00 (0.99, 1.01) | 0.368 |
| Sodium (mmol/L) | 0.99 (0.98, 1.00) | 0.212 | 0.98 (0.97, 1.00) | 0.071 |
| BNP∞ (pg/mL) | 1.00 (1.00, 1.00) | 0.006 | 1.00 (1.00, 1.00) | 0.044 |
| Troponin I (ng/mL) | 1.60 (1.31, 1.94) | 0.0001 | 6.71 (1.25, 35.98) | 0.132 |
| Creatine-kinase (MB isomer) (ng/mL) | 1.05 (1.04, 1.06) | 0.0001 | 1.05 (1.01, 1.10) | 0.013 |
| Medications prior to admission | ||||
| ACEI or ARB^ | 1.00 (0.58, 1.70) | 0.988 | 0.38 (0.13, 1.08) | 0.069 |
| Beta-blocker | 1.01 (0.57, 1.78) | 0.985 | 0.23 (0.10, 0.56) | 0.001 |
| Frusemide | 0.95 (0.55, 1.63) | 0.841 | 3.45 (0.73, 16.26) | 0.117 |
| HMG$ CoA inhibitors | 0.52 (0.06, 4.21) | 0.541 | 1.72 (0.76, 3.85) | 0.190 |
| Digoxin | 0.84 (0.42, 1.67) | 0.623 | 1.03 (0.32, 3.29) | 0.954 |
| Antiplatelet agents | 0.65 (0.35, 1.18) | 0.159 | 0.54 (0.25, 1.16) | 0.113 |
| Self-reported medication adherence | ||||
| Good adherence | Ref | Ref | Ref | |
| Poor adherence | 0.84 (0.41, 1.73) | 0.645 | 0.83 (0.25, 2.74) | 0.758 |
NYHA¥: New York Heart Association; GFR£: estimated glomerular filtration rate; BUN€: blood urea nitrogen; BNP∞: brain natriuretic peptide; ACEI/ARB^: angiotensin converting enzyme inhibitor/angiotensin receptor blocker.
In contrast, taking beta-blocker medication compared to not and having an index admission on a weekend compared to those admitted on a week day predicted surviving until 6 months (end of follow up).
4. Discussion
To the best of our knowledge, this is the first quantification of the 6 months mortality among heart failure patients in a general hospital in sub-Saharan Africa. We found a substantially high cumulative incidence of all-cause mortality within 6 months following hospitalization for an acute heart failure event in a regional hospital in rural southwestern Uganda. More so, the incidence of mortality was higher in the community after discharge from hospital than during in hospital period. This highlights the underreporting of the actual mortality rates in resource constraint countries since enumeration of deaths occurring in adulthood stops in health care centers [12,20,21]. Also, compared to other prior studies from sub-Saharan Africa, we found a higher incidence of in-hospital mortality. This maybe attributable to the fact that prior studies were carried out in highly specialized cardiac centers with superior diagnostic, therapeutic, and human resources thus the lower in-hospital mortality rate while the current study was performed in general medicine ward of a regional hospital which is generalizable to the level of care where majority of heart failure patients seek care in low income countries such as Uganda [13,21]. Taken together, this finding expounds on the existing literature on all-cause mortality among heart failure patients in sub-Saharan Africa that, until now, reported only deaths occurring in hospitals, as such reported lower incidence of death associated with acute heart failure [12,13,20,21].
We found that for every 1-pg/mL increase in BNP and CKMB predicted all-cause mortality within 6 months, similarly BNP as a predictor of mortality has been established from several studies [22–24]. However, there are no similar studies from sub-Sahara Africa for comparison of these results. In addition, we found that the cumulative incidence death among participants with New York heart association (NYHA) functional class IV at time of hospitalization was higher than that of participants with NYHA class III as has been shown by others [13,25]. This could be explained by the late presentation with advanced heart failure class given the long distances travelled to reach health facilities as well as time delays in lower level centers where diagnostic and therapeutic expertise is limited [26,27]. Similarly, we found that deteriorating renal function (reduced estimated glomerular filtration) is an independent predictor of mortality among acute heart failure patients, as expected given the reducing systolic function [9,28–30]. As in other studies, taking beta-blocker medication compared to not predicted survival up to 6 months [31,32].
Surprisingly, there was a U-shaped relation between socioeconomic status (SES) and incidence of mortality i.e., those in the richest and poorest SES groups were associated with better prognosis. It is plausible that this is a reflection of the ability of the richest to buy lifesaving medications since therapy is mainly financed out of pocket [33]. However, those in the poorer SES group tended to be coming from the furthest locations and as such fewer made it to the hospital thereby their effect was diluted by the other more prevalent SES groups. Future studies evaluating these relationships are encouraged.
In contrast, having an index admission on a weekend compared to those admitted on a week day predicted survival up to 6 months, contrary to other reports that weekend admission maybe associated with high mortality [34,35] as a result of limited human resource.
The strength of the present study is that it is among the first general hospital-based study conducted in sub-Saharan Africa with follow-up of participants into the community. We were unable to adjudicate the immediate causes of death as this would have shade light on the heart failure related conditions directly leading to death. However, it is logistically difficult to identify these conditions in the study setting due to limitations with autopsy. Also, we are unable to elucidate the different underlying etiologies of heart failure or further characterize the participants with tests such as blood lactate, arterial oxygen saturation, D-dimer, and thyroid function tests among others.
In conclusion, there is a high incidence of 6 months all-cause mortality following hospitalization for an acute heart failure event in rural Uganda. Predictors of mortality include increasing age, NYHA class IV, decreasing renal function, smoking, each unit increase in serum levels of Potassium, BNP, and Creatine kinase-MB whereas taking beta-blockers and having an index admission on a weekend compared to a week day decreased mortality risk. More heart failure directed therapies such as beta-blockers should be instituted for heart failure management to reduce mortality.
Acknowledgments
The authors would like to thank the MAHFER study staff and patients at Mbarara Regional Referral Hospital.
Sources of funding
This study was supported by Abbott Point of Care, Inc. and Ruth C. and Henry F. Dunbar Cardiology Research endowment fund at the Cardiovascular Division University of Virginia Health System. The funders had no role in study design, conduct, data analysis, or production of manuscript.
Footnotes
Disclosure of previous presentations
None.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
References
- [1].Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. , Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF), J. Am. Coll. Cardiol 52 (2008) 347–356. [DOI] [PubMed] [Google Scholar]
- [2].Agbor VN, Essouma M, Ntusi NA, Nyaga UF, Bigna JJ, Noubiap JJ, Heart failure in sub-Saharan Africa: a contemporaneous systematic review and meta-analysis, Int. J. Cardiol 257 (2018) 207–215. [DOI] [PubMed] [Google Scholar]
- [3].Allen LA, O’Connor CM, Management of acute decompensated heart failure, Can. Med. Assoc. J 176 (2007) 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Choi D-J, Han S, Jeon E-S, Cho M-C, Kim J-J, Yoo B-S, et al. , Characteristics, outcomes and predictors of long-term mortality for patients hospitalized for acute heart failure: a report from the Korean heart failure registry, Kor. Circ. J 41 (2011) 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Allen LA, Tomic KES, Smith DM, Wilson KL, Agodoa I, Rates and predictors of 30-day readmission among commercially insured and Medicaid-enrolled patients hospitalized with systolic heart failure, Circ. Heart Fail 5 (2012) 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cuyjet Aloysius B., Akinboboye O, Acute heart failure in the African American patient, J. Card. Fail 20 (2014) 533–540. [DOI] [PubMed] [Google Scholar]
- [7].Damasceno A, Cotter G, Dzudie A, Sliwa K, Mayosi BM, Heart failure in sub-Saharan Africa: time for action, J. Am. Coll. Cardiol 50 (2007) 1688–1693. [DOI] [PubMed] [Google Scholar]
- [8].McMurray JJ, Stewart S, Epidemiology, aetiology, and prognosis of heart failure, Heart 83 (2000) 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Damasceno A, Mayosi BM, Sani M, Ogah OS, Mondo C, Ojji D, et al. , The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries: results of the sub-Saharan Africa survey of heart failure, Arch. Intern. Med 172 (2012) 1386–1394. [DOI] [PubMed] [Google Scholar]
- [10].Bloomfield GS, Barasa FA, Doll JA, Velazquez EJ, Heart failure in sub-Saharan Africa, Curr. Cardiol. Rev 9 (2013) 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fonarow Gregg C., Heywood J. Thomas, Heidenreich Paul A., Lopatin Margarita, Yancy CW, Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE), Am. Heart J. 153 (2007) 1021–1028. [DOI] [PubMed] [Google Scholar]
- [12].Makubi Abel, Hage Camilla, Lwakatare Johnson, Kisenge Peter, Makani Julie, Rydén Lars, et al. , Contemporary aetiology, clinical characteristics and prognosis of adults with heart failure observed in a tertiary hospital in Tanzania: the prospective Tanzania Heart Failure (TaHeF) study, Heart J 100 (2014. August) 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ogah OS, Stewart S, Falase AO, Akinyemi JO, Adegbite GD, Alabi AA, et al. , Short-term outcomes after hospital discharge in patients admitted with heart failure in Abeokuta, Nigeria: data from the Abeokuta Heart Failure Registry: cardiovascular topic, Cardiovasc. J. Afr 25 (2014) 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. , ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC, Eur. Heart J. 37 (2016) 2129–2200. [DOI] [PubMed] [Google Scholar]
- [15].Okello S, Ueda P, Kanyesigye M, Byaruhanga E, Kiyimba A, Amanyire G, et al. , Association between HIV and blood pressure in adults and role of body weight as a mediator: cross-sectional study in Uganda, J. Clin. Hypertens 19 (2017) 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Filmer D, Pritchett L, Estimating wealth effects without expenditure data—or tears, Policy Research Working Paper 1980, The World, Citeseer, 1998. [Google Scholar]
- [17].Austin PC, Lee DS, F JP., Introduction to the analysis of survival data in the presence of competing risks, Circ. Heart Fail. 133 (2016) 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Musoke David, Boynton Petra, Butler Ceri, Musoke MB, Health seeking behaviour and challenges in utilizing health facilities in Wakiso district, Uganda, Afr. Health Sci 14(2014) 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hutchinson Paul, Habte Demissie, Mulusa M, Health Care in Uganda: Selected Issues, World Bank Publication, 1999. [Google Scholar]
- [20].Ogah Okechukwu S., Sliwa M. Karen, Akinyemi Joshua O., Falase Ayodele O., Stewart Simon, Hypertensive heart failure in Nigerian Africans: insights from the Abeokuta Heart Failure Registry, J. Clin. Hypertens. (Greenwich) 17 (2015) 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sliwa Karen, D BA, M BM, et al. , Readmission and death after an acute heart failure event: predictors and outcomes in sub-Saharan Africa: results from the THESUS-HF registry, Eur. Heart J. (2013) 2–9. [DOI] [PubMed] [Google Scholar]
- [22].Maisel Alan, Hollander Judd E., Guss David, Peter McCullough Richard Nowak, Green Gary, et al. , Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT) a multicenter study of B-type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath, J. Am Coll. Cardiol 44 (2004) 1328–1333. [DOI] [PubMed] [Google Scholar]
- [23].Fonarow Gregg C., Peacock William F., Phillips Christopher O., Givertz Michael M., Lopatin Margarita, Admission B-type natriuretic peptide levels and in-hospital mortality in ccute decompensated heart failure, J. Am. Coll. Cardiol 49 (2007) 1943–1950. [DOI] [PubMed] [Google Scholar]
- [24].Masson Serge, Latini Roberto, Anand Inder S., Barlera Simona, Angelici Laura, Vago Tarcisio, et al. , Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial), J. Am. Coll. Cardiol 52 (2008) 997–1003. [DOI] [PubMed] [Google Scholar]
- [25].Chansa P, Lakhi S, Andrews B, Kalinchendo S, Sakr R, Factors associated with mortality in adults admitted with heart failure at the University Teaching Hospital in Lusaka, Zambia, Med. J. Zambia 41 (2014) 1–12. [Google Scholar]
- [26].Okello S, Rogers O, Byamugisha A, Rwebembera J, Buda AJ, Characteristics of acute heart failure hospitalizations in a general medical ward in Southwestern Uganda, Int. J. Cardiol 176 (2014) 1233–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tantchou Tchoumi Jacques Cabral, Ambassa Jean Claude, Kingue Samuel, Giamberti Alessandro, Frigiola Alessandro, Gianfranco B, Occurrence, aetiology and challenges in the management of congestive heart failure in sub-Saharan Africa: experience of the Cardiac Centre in Shisong, Cameroon, Pan. Afr. Med. J 8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Adams KirkwoodnF, Uddin N, P JH, Clinical predictors of in hospital mortality in acute decompensated heart failure: piecing together the outcome puzzle, Congest Heart Fail. 14(2008) 127–134. [DOI] [PubMed] [Google Scholar]
- [29].Belziti César A., Bagnati Rodrigo, Ledesma Paola, Vulcano Norberto, Fernández aS, Worsening renal function in patients admitted with acute decompensated heart failure: incidence, risk factors and prognostic implications, Rev. Esp. Cardiol 63 (2010) 294–302. [DOI] [PubMed] [Google Scholar]
- [30].Salah Khibar, Kok Wouter E., Eurlings Luc W., Bettencourt Paulo, Pimenta Joana M., Metra Marco, et al. , Competing risk of cardiac status and renal function during hospitalization for acute decompensated heart failure, J. Am. Coll. Cardiol 3 (2015) 751–761. [DOI] [PubMed] [Google Scholar]
- [31].Vikas Bhatia M, Navkaranbir S, Bajaj M, Kumar Sanam M, Taimoor Hashim M, Charity J, Morgan P, Sumanth D, Prabhu M, et al. , Beta-blocker use and 30-day all-cause readmission in Medicare beneficiaries with systolic heart failure, Am. J. Med 128 (2015) 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fonarow Gregg C., Stough Wendy Gattis, Abraham William T., Albert Nancy M., Gheorghiade Mihai, Greenberg Barry H., et al. , Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure. A report from the OPTIMIZE-HF registry, J. Am. Coll. Cardiol 50 (2007) 768–777. [DOI] [PubMed] [Google Scholar]
- [33].Okello S, Nasasira B, Muiru ANW, Muyingo A, Validity and reliability of a self-reported measure of antihypertensive medication adherence in Uganda, PLoS One 11 (2016), e0158499.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ansa Victor, Njideoffer Uchenna, Nworah Charles, Odigwe Clement, Otu Akaninyene, Otu A Patient outcomes following after-hours ans weekend admission for cardiovascular disease in a tertiary hospital in Calar, Nigeria, Cardiovasc. J. Afr 27 (2016) 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bell CM, R DA, Mortality among patients admitted to hospitals on weekends as compared with weekdays, N. Engl. J. Med 345 (2001) 663–668. [DOI] [PubMed] [Google Scholar]