Abstract
There is scant literature describing the effect of glomerular disease on health-related quality of life (HRQOL). The Cure Glomerulonephropathy study (CureGN) is an international longitudinal cohort study of children and adults with four primary glomerular diseases (minimal change disease, focal segmental glomerulosclerosis, membranous nephropathy, and IgA nephropathy). HRQOL is systematically assessed using items from the Patient-Reported Outcomes Measurement Informative System (PROMIS). We assessed the relationship between HRQOL and demographic and clinical variables in 478 children and 1115 adults at the time of enrollment into CureGN. Domains measured by PROMIS items included global assessments of health, mobility, anxiety, fatigue, and sleep impairment, as well as a derived composite measure incorporating all measured domains. Multivariable models were created that explained 7 to 32% of variance in HRQOL. Patient-reported edema consistently had the strongest and most robust association with each measured domain of HRQOL in multivariable analysis (adjusted β [95% CI] for composite PROMIS score in children, −5.2 [−7.1 to −3.4]; for composite PROMIS score in adults, −6.1 [−7.4 to −4.9]). Female sex, weight (particularly obesity), and estimated glomerular filtration rate were also associated with some, but not all, domains of HRQOL. Primary diagnosis, disease duration, and exposure to immunosuppression were not associated with HRQOL after adjustment. Sensitivity analyses and interaction testing demonstrated no significant association between disease duration or immunosuppression and any measured domain of HRQOL. Thus, patient-reported edema has a consistent negative association with HRQOL in patients with primary glomerular diseases, with substantially greater impact than other demographic and clinical variables.
Keywords: edema, health-related quality of life, patient-reported outcomes, primary glomerular disease
The 4 major primary glomerular diseases—minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN), and IgA nephropathy (IgAN)—represent some of the most common causes of chronic kidney disease managed by nephrologists. These diseases are a significant cause of end-stage kidney disease across all ages in the United States, accounting for 6.6% of all cases and up to 13.1% of cases in children.1 The treatment of glomerular diseases is associated with complications including infections, thromboembolism, and acute kidney injury, and result in frequent hospitalizations and significant burden on the health care system.2 Although the medical burden of glomerular diseases is well known, little has been published about their impact on the quality of life of affected patients. Understanding the perspective of the patient can help optimize care, education, and anticipatory counseling provided by health care teams.3
Both the National Institutes of Health and the Food and Drug Administration have set forth an imperative to examine the full disease experience of patients and patient-reported outcomes (PROs) in medical decision making, research, and clinical trials.4,5 Although research has advanced the understanding of health-related quality of life (HRQOL) in conditions such as advanced kidney failure,6 there is a paucity of data describing HRQOL in the primary glomerular diseases. Cross-sectional studies of children with nephrotic syndrome have included a description of HRQOL using instruments such as Patient-Reported Outcomes Measurement Information System (PROMIS) and PedsQL, finding substantial impairments in quality of life in areas such as social functioning and pain interference, with associations noted with disease duration and specific therapies.7,8 Quality-of-life impairments also have been shown in small studies of adults with nephrotic syndrome.9–11 Very little is known about HRQOL in patients with IgAN or MN, and scant effort has been made to comprehensively identify variables associated with HRQOL broadly in glomerular diseases.
The Cure Glomerulonephropathy study (CureGN) is a multicenter longitudinal cohort study of children and adults with the 4 major primary glomerular diseases (MCD, FSGS, MN, and IgAN). The aims of the current study are to describe the HRQOL and to determine the variables associated with HRQOL at enrollment in the CureGN cohort across age and disease groups.
RESULTS
Demographics
A Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) flow diagram of included patients from CureGN is shown in Figure 1. There were 478 children and 1115 adults with HRQOL data available at enrollment. These cohorts are described in Tables 1 and 2, respectively. An additional 42 children and 18 adults did not have HRQOL data available, representing only 8% and 2% of the available pediatric and adult CureGN participants (Supplementary Tables S1 and S2). The distribution of diseases differed between the pediatric and adult cohorts. From the IgAN cohort, IgA vasculitis (IgAV, formerly known as Henoch-Schonlein purpura) was analyzed separately. The proportion of children with MCD was greater than adults (28% vs. 13%, respectively), as was the proportion of children with IgAV (16% vs. 5%). The proportions of children and adults with FSGS (24% vs. 24%) and IgAN (26% vs. 31%) were similar. There were fewer females than males in both the pediatric and adult cohorts. Racial and ethnic distributions were similar for children and adults, two thirds of participants were white and nearly 90% were of non-Hispanic ethnicity. There were substantial differences in racial distribution among the diagnoses, with black participants more likely to have FSGS and less likely to have IgAN or IgAV. Thirty percent of children and 37% of adults were classified as obese.
Figure 1 |. STROBE flow diagram of included patients.
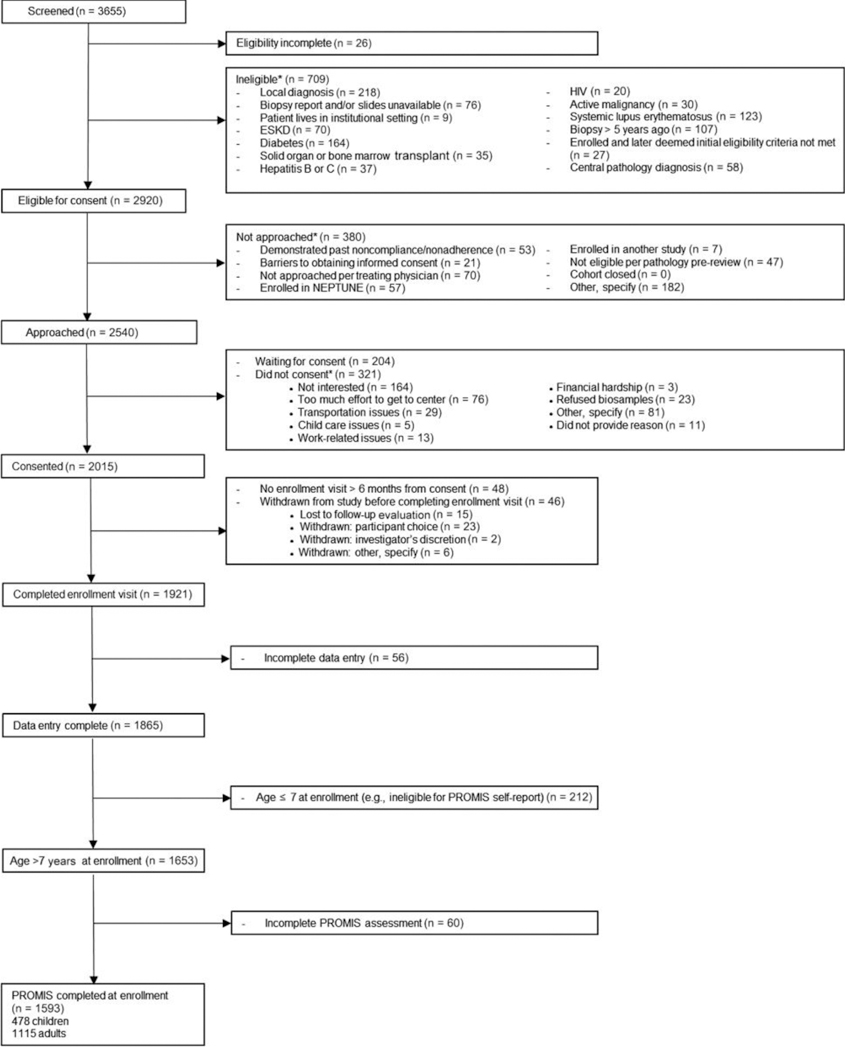
ESKD, end-stage kidney disease; NEPTUNE, NEPhrotic syndrome sTUdy NEtwork; PROMIS, Patient-Reported Outcomes Measurement Information System; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology
Table 1 |.
Description of pediatric CureGN patients with PROMIS data at enrollment
| Variables | All | MCD | FSGS | MN | IgAN | IgAV |
|---|---|---|---|---|---|---|
| N (%) | 478 (100) | 134 (28) | 115 (24) | 28 (6) | 126 (26) | 75 (16) |
| Median age, yr (IQR) | 13 (10–15) | 12 (9–14) | 14 (12–16) | 15 (13–16) | 14 (11–16) | 12 (9–14) |
| Female, n (%) | 202 (42) | 58 (43) | 55 (48) | 16 (57) | 48 (38) | 25 (33) |
| Race, n (%) | ||||||
| Black/African American | 92 (19) | 35 (26) | 43 (37) | 8 (29) | 4 (3) | 2 (3) |
| White/Caucasian | 319 (67) | 77 (57) | 59 (51) | 15 (54) | 103 (82) | 65 (87) |
| Other | 67 (14) | 22 (16) | 13 (11) | 5 (18) | 19 (15) | 8 (11) |
| Hispanic, n (%) | 49 (10) | 12 (9) | 12 (10) | 6 (21) | 14 (11) | 5 (7) |
| Obese, n (%) | 142 (30) | 33 (25) | 42 (37) | 13 (46) | 29 (23) | 25 (33) |
| Hematuria, n (%) | 137 (29) | 13 (10) | 6 (5) | 2 (7) | 89 (71) | 27 (36) |
| Any edema, n (%) | 152 (32) | 54 (40) | 40 (35) | 17 (61) | 22 (17) | 19 (25) |
| Median UP:C (IQR) | 0.5 (0.1–2.2) | 0.4 (0.1–2.7) | 1.2 (0.2–2.8) | 3.2 (0.9–7.4) | 0.3 (0.1–0.8) | 0.5 (0.2–1.4) |
| <0.3, n (%) | 170 (36) | 55 (41) | 31 (27) | 3 (11) | 55 (44) | 26 (35) |
| 0.3–1.5, n (%) | 129 (27) | 25 (19) | 27 (23) | 7 (25) | 41 (33) | 29 (39) |
| 1.5–3.5, n (%) | 55 (12) | 15 (11) | 19 (17) | 3 (11) | 12 (10) | 6 (8) |
| >3.5, n (%) | 76 (16) | 24 (18) | 20 (17) | 13 (46) | 9 (7) | 10 (13) |
| Missing, n (%) | 48 (10) | 15 (11) | 18 (16) | 2 (7) | 9 (7) | 4 (5) |
| Median eGFR (IQR) | 97 (82–116) | 111 (93–127) | 90 (60–108) | 97 (87–114) | 94 (81–109) | 101 (90–121) |
| <30, n (%) | 15 (3) | 1 (1) | 10 (9) | 1 (4) | 2 (2) | 1 (1) |
| 30–60, n (%) | 26 (5) | 6 (4) | 16 (14) | 1 (4) | 3 (2) | |
| 60–90, n (%) | 116 (24) | 19 (14) | 26 (23) | 7 (25) | 46 (37) | 18 (24) |
| >90, n (%) | 294 (62) | 98 (73) | 55 (48) | 18 (64) | 69 (55) | 54 (72) |
| Missing, n (%) | 25 (5) | 10 (7) | 6 (5) | 1 (4) | 6 (5) | 2 (3) |
| IST within 60 days, n (%) | ||||||
| None | 345 (72) | 90 (67) | 81 (70) | 16 (57) | 107 (85) | 51 (68) |
| Corticosteroids alone | 75 (16) | 22 (16) | 16 (14) | 5 (18) | 16 (13) | 16 (21) |
| Other IST | 58 (12) | 22 (16) | 18 (16) | 7 (25) | 3 (2) | 8 (11) |
| Past 60 days median prednisone dose, mg/kg/d (IQR) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.1 (0.1–0.3) | 0.2 (0.1–0.5) | 0.4 (0.1–0.7) | 0.3 (0.2–0.5) |
| IST type, n (%) | ||||||
| Corticosteroids | 105 (22) | 31 (23) | 25 (22) | 10 (36) | 17 (13) | 22 (29) |
| CNI | 35 (7) | 15 (11) | 11 (10) | 7 (25) | 1 (1) | 1 (1) |
| Cyclophosphamide | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| Mycophenolate | 19 (4) | 5 (4) | 6 (5) | 0 (0) | 2 (2) | 6 (8) |
| Rituximab | 8 (2) | 4 (3) | 4 (3) | 0 (0) | 0 (0) | 0 (0) |
| Median disease duration, mo (IQR) | 18 (5–42) | 34 (13–61) | 18 (6–45) | 6 (1–17) | 15 (4–34) | 9 (3–20) |
CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; IgAV, IgA vasculitis; IQR, interquartile range; IST, immunosuppressive therapy; MCD, minimal change disease; MN, membranous nephropathy; UP:C, urinary protein: creatinine ratio.
Table 2 |.
Description of adult CureGN patients with PROMIS data at enrollment
| Variables | All | MCD | FSGS | MN | IgAN | IgAV |
|---|---|---|---|---|---|---|
| N (%) | 1115 (100) | 141 (13) | 271 (24) | 305 (27) | 344 (31) | 54 (5) |
| Median age, yr (IQR) | 44 (31–58) | 43 (26–58) | 42 (29–56) | 55 (43–65) | 40 (30–51) | 35 (25–48) |
| Female, n (%) | 482 (43) | 77 (55) | 134 (49) | 110 (36) | 140 (41) | 21 (39) |
| Race, n (%) | ||||||
| Black/African American | 165 (15) | 21 (15) | 81 (30) | 45 (15) | 17 (5) | 1 (2) |
| White/Caucasian | 747 (67) | 95 (67) | 144 (53) | 218 (71) | 245 (71) | 45 (83) |
| Other | 203 (18) | 25 (18) | 46 (17) | 42 (14) | 82 (24) | 8 (15) |
| Hispanic, n (%) | 153 (14) | 11 (8) | 47 (17) | 27 (9) | 61 (18) | 7 (13) |
| Obese, n (%) | 415 (37) | 52 (37) | 109 (40) | 115 (38) | 113 (33) | 26 (48) |
| Hematuria, n (%) | 131 (12) | 3 (2) | 8 (3) | 10 (3) | 98 (28) | 12 (22) |
| Any edema, n (%) | 643 (58) | 91 (65) | 175 (65) | 212 (70) | 139 (40) | 26 (48) |
| Median UP:C (IQR) | 1.9 (0.6–4.4) | 1.8 (0.1–6.8) | 2.6 (0.9–4.9) | 3.3 (1.1–5.9) | 1.0 (0.4–2.2) | 1.0 (0.3–1.9) |
| <0.3, n (%) | 179 (16) | 42 (30) | 23 (8) | 31 (10) | 70 (20) | 13 (24) |
| 0.3–1.5, n (%) | 284 (25) | 19 (13) | 63 (23) | 49 (16) | 131 (38) | 22 (41) |
| 1.5–3.5, n (%) | 237 (21) | 14 (10) | 76 (28) | 65 (21) | 73 (21) | 9 (17) |
| >3.5, n (%) | 329 (30) | 51 (36) | 90 (33) | 137 (45) | 43 (13) | 8 (15) |
| Missing, n (%) | 86 (8) | 15 (11) | 19 (7) | 23 (8) | 27 (8) | 2 (4) |
| Median eGFR (IQR) | 68 (41–96) | 93 (63–114) | 57 (34–86) | 72 (46–94) | 57 (38–88) | 84 (51–112) |
| <30, n (%) | 151 (14) | 6 (4) | 54 (20) | 31 (10) | 55 (16) | 5 (9) |
| 30–60, n (%) | 328 (29) | 23 (16) | 89 (33) | 81 (27) | 125 (36) | 10 (19) |
| 60–90, n (%) | 290 (26) | 38 (27) | 63 (23) | 101 (33) | 75 (22) | 13 (24) |
| >90, n (%) | 323 (29) | 70 (50) | 60 (22) | 86 (28) | 83 (24) | 24 (44) |
| Missing, n (%) | 21 (2) | 4 (3) | 4 (1) | 6 (2) | 6 (2) | 1 (2) |
| IST within 60 days, n (%) | ||||||
| None | 860 (77) | 81 (57) | 204 (75) | 247 (81) | 296 (86) | 32 (59) |
| Corticosteroids alone | 119 (11) | 27 (19) | 28 (10) | 17 (6) | 31 (9) | 16 (30) |
| Other IST | 136 (12) | 33 (23) | 39 (14) | 41 (13) | 17 (5) | 6 (11) |
| Past 60 days median prednisone dose, mg/kg/d (IQR) | 0.1 (0.1–0.3) | 0.2 (0.1–0.5) | 0.1 (0.1–0.5) | 0.1 (0.1–0.1) | 0.1 (0.1–0.3) | 0.2 (0.1–0.3) |
| IST type, n (%) | ||||||
| Corticosteroids | 160 (14) | 35 (25) | 39 (14) | 31 (10) | 35 (10) | 20 (37) |
| CNI | 79 (7) | 21 (15) | 28 (10) | 25 (8) | 5 (1) | 0 (0) |
| Cyclophosphamide | 14 (1) | 2 (1) | 0 (0) | 11 (4) | 0 (0) | 1 (2) |
| Mycophenolate | 31 (3) | 7 (5) | 8 (3) | 0 (0) | 12 (3) | 4 (7) |
| ACTH | 3 (0) | 2 (1) | 1 (0) | 0 (0) | 0 (0) | |
| Rituximab | 13 (1) | 4 (3) | 3 (1) | 5 (2) | 0 (0) | 1 (2) |
| Median disease duration, mo (IQR) | 16 (5–40) | 20 (7–41) | 21 (6–45) | 14 (5–36) | 15 (4–41) | 10 (2–25) |
ACTH, adrenocorticotropic hormone gel; CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; IgAV, IgA vasculitis; IQR, interquartile range; 1ST, immunosuppressive therapy; MCD, minimal change disease; MN, membranous nephropathy; UP:C, urinary protein: creatinine ratio.
Clinical features
Only 32% of children reported any edema at presentation, as opposed to 58% of adults. Among both children and adults, edema was less common in IgAN or IgAV. The median urine protein:creatinine ratio (UP:C) was 0.5 (interquartile range [IQR], 0.1–2.2) for the pediatric cohort and 1.9 (IQR, 0.6–4.4) for the adult cohort. Thirty-six percent of the pediatric cohort, but only 16% of the adult cohort, had a UP:C less than 0.3, indicating relatively more pediatric participants were in complete disease remission at the time of assessment. Accordingly, the median estimated glomerular filtration rate (eGFR) was 97 ml/min per 1.73 m2 (IQR, 82–116 ml/min per 1.73 m2) for children but 68 ml/min per 1.73 m2 (IQR, 41–96 ml/min per 1.73 m2) for adults, and 91% of children with available data had an eGFR greater than 60 ml/min per 1.73 m2, while in adults this proportion was 56%. A minority of both children and adults had been exposed to immunosuppressive therapy (IST) within 60 days before enrollment (28% and 23%, respectively). Among those recently exposed to IST, corticosteroids were the most common agent used in both cohorts and among all diseases.
Baseline HRQOL assessed by PROMIS
Baseline HRQOL measures are summarized in Figure 2, which shows the distribution of scores across the 4 PROMIS domains measured in children and the 5 PROMIS domains measured in adults, as well as the calculated composite scores. Means and SDs are reported in the figures. The shapes of the distributions reflect the number of questionnaire items used for each domain. For example, anxiety and sleep impairment were each measured by a single 5-item Likert scale, thus those distributions have 5 discrete peaks and resulted in skewed values.
Figure 2 |. Distribution of PROMIS domains in (a) children and (b) adults.
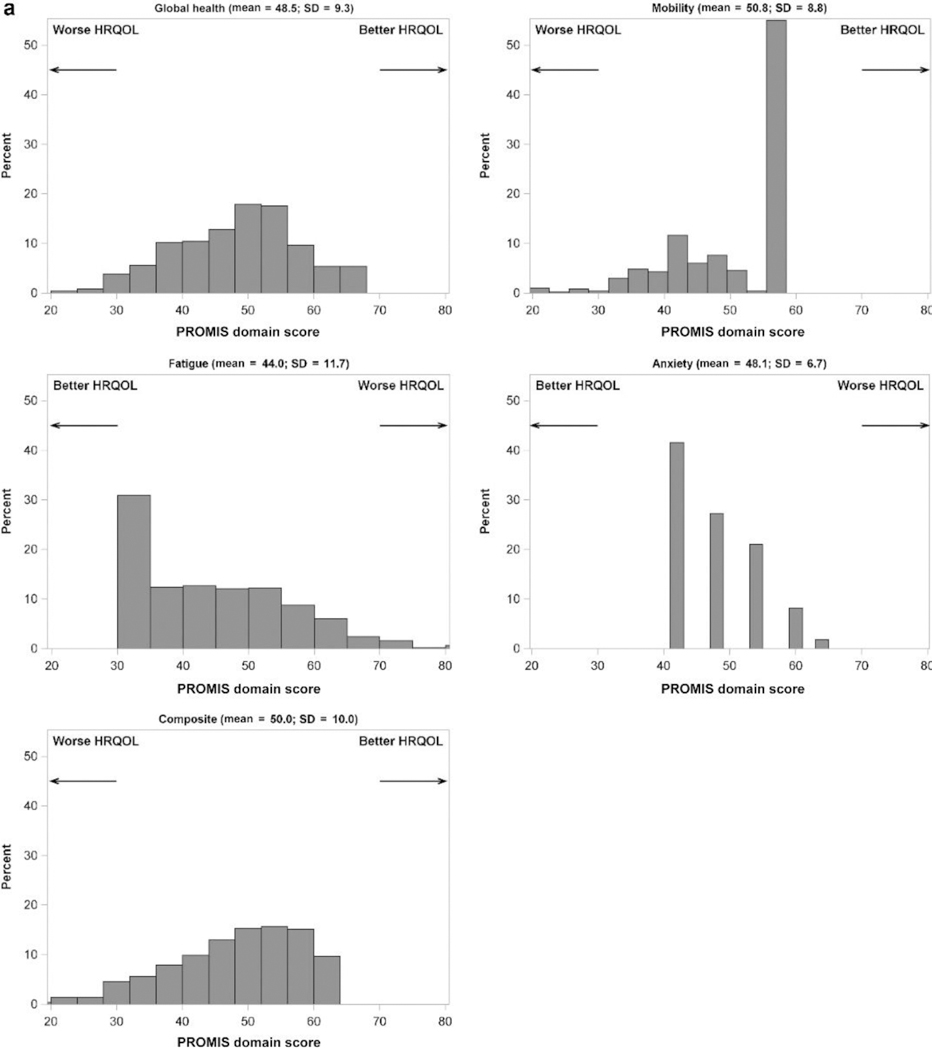
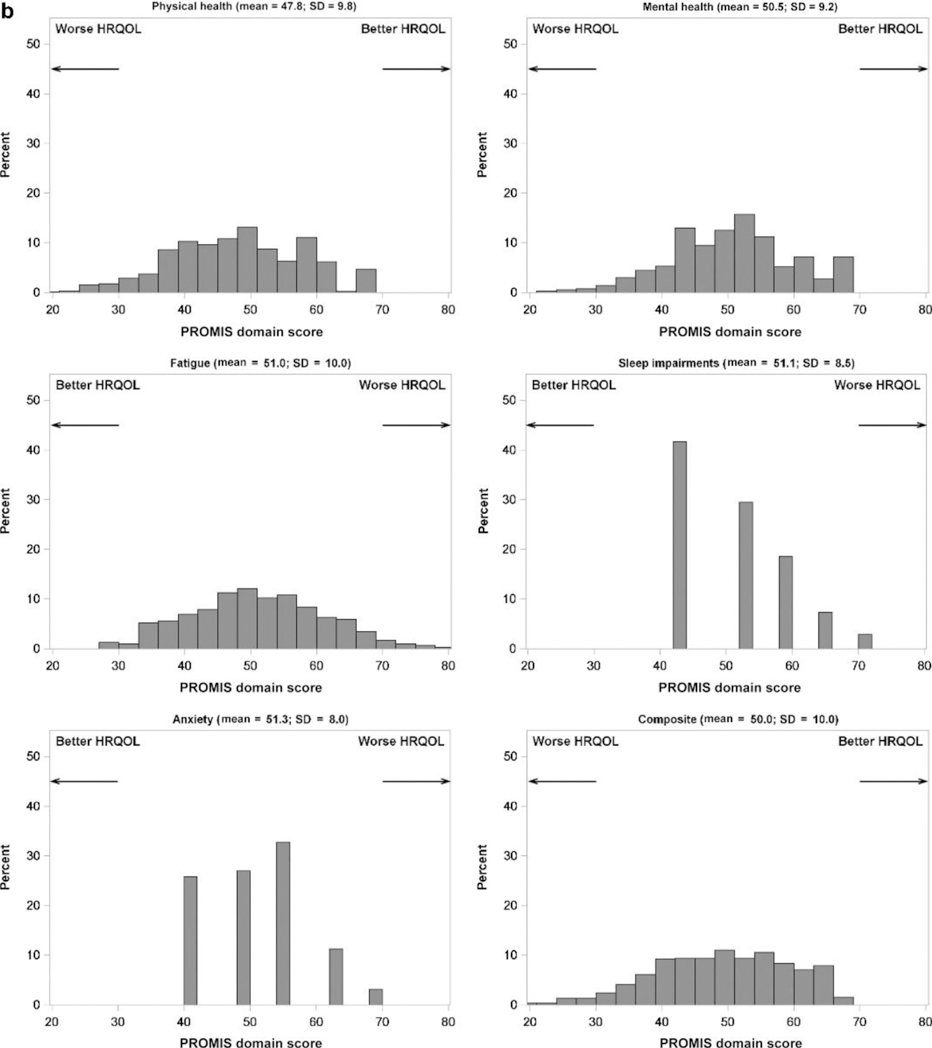
Note that the direction of better versus worse scores differs among domains and is indicated by arrows for each panel. HRQOL, health-related quality of life; PROMIS, Patient-Reported Outcomes Measurement Information System.
Edema and HRQOL
To identify potential predictors of HRQOL, multivariable linear regression models were created for each PROMIS domain. Initial analyses showed that the presence of any edema was associated strongly with worse HRQOL across both children and adults for every PROMIS domain. Using negative numbers to indicate worse HRQOL, adjusted linear regression coefficients (β) for the pediatric domains were as follows: −3.6 (95% confidence interval [CI], −5.4 to −1.8) for global health, −4.4 (95% CI, −6.1 to −2.7) for mobility, −6.4 (95% CI, −8.6 to −4.2) for fatigue, −2.1 (95% CI, −3.4 to −0.9) for anxiety, and −5.2 (95% CI, −7.1 to −3.4) for the composite score; and adult domains were as follows: −6.8 (95% CI, −8.0 to −5.6) for global physical health, −3.9 (95% CI, −5.0 to −2.8) for global mental health, −5.8 (95% CI, −7.0 to −4.5) for fatigue, −4.8 (95% CI, −5.8 to −3.8) for sleep impairment, −2.9 (95% CI, −3.9 to −1.9) for anxiety, and −6.1 (95% CI, −7.4 to −4.9) for the composite score (Figure 3). Because of the strength of this association, edema was characterized further by combining aspects of location and severity to create ordinal variables, and these variables were entered into multivariable models. The worst reported edema severity for each diagnostic group in children and adults is shown in Figure 4. Compared with children, adults reported more severe edema scores and were less likely to report no edema, even within diseases. The full distribution of edema scores by diagnosis and age group is available in Supplementary Tables S3 and S4.
Figure 3 |. The impact of any edema on PROMIS domain scores at enrollment in the CureGN study.
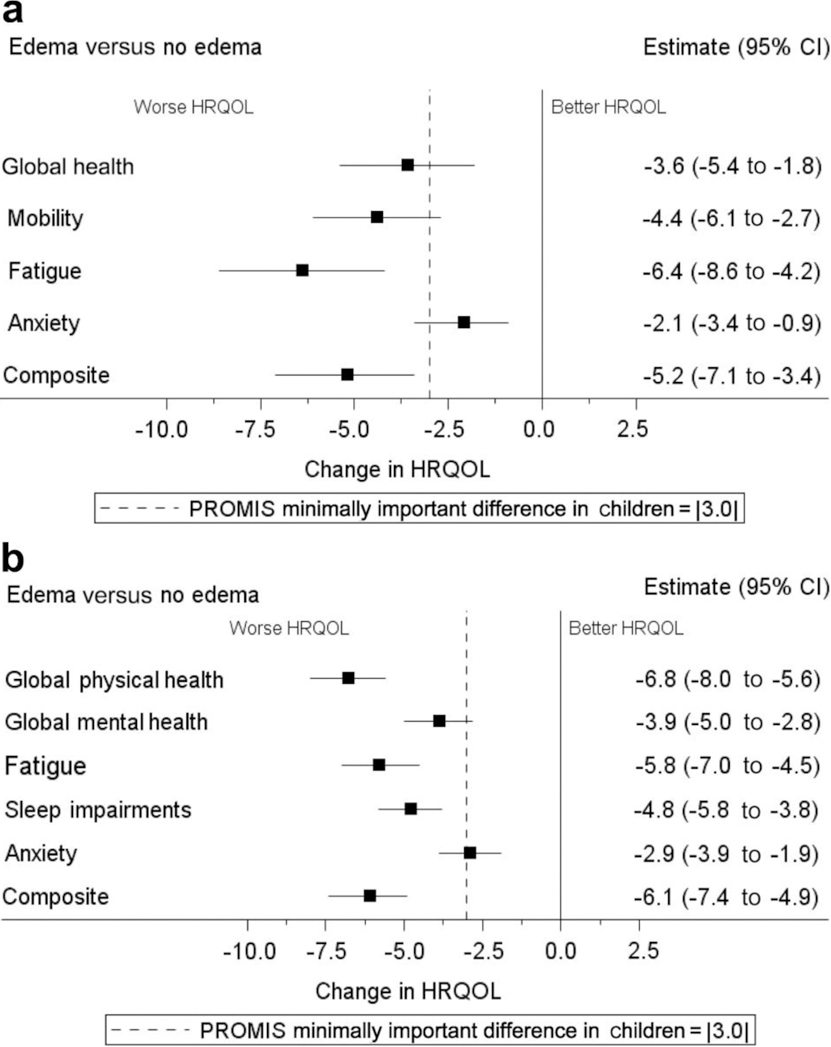
Plots of (a) children and (b) adults show the adjusted linear regression coefficients (β) from multivariable models, with tails indicating lower and upper confidence limits. For ease of comparison, each β estimate has been plotted as the negative of its absolute value, in which negative numbers indicate a worse patient-reported outcome. The dashed line denotes the minimally important difference from baseline. CI, confidence interval; HRqOl, health-related quality of life; PROMIS, Patient-Reported Outcomes Measurement Information System.
Figure 4 |. Worst edema severity by diagnosis in (a) children and (b) adults.
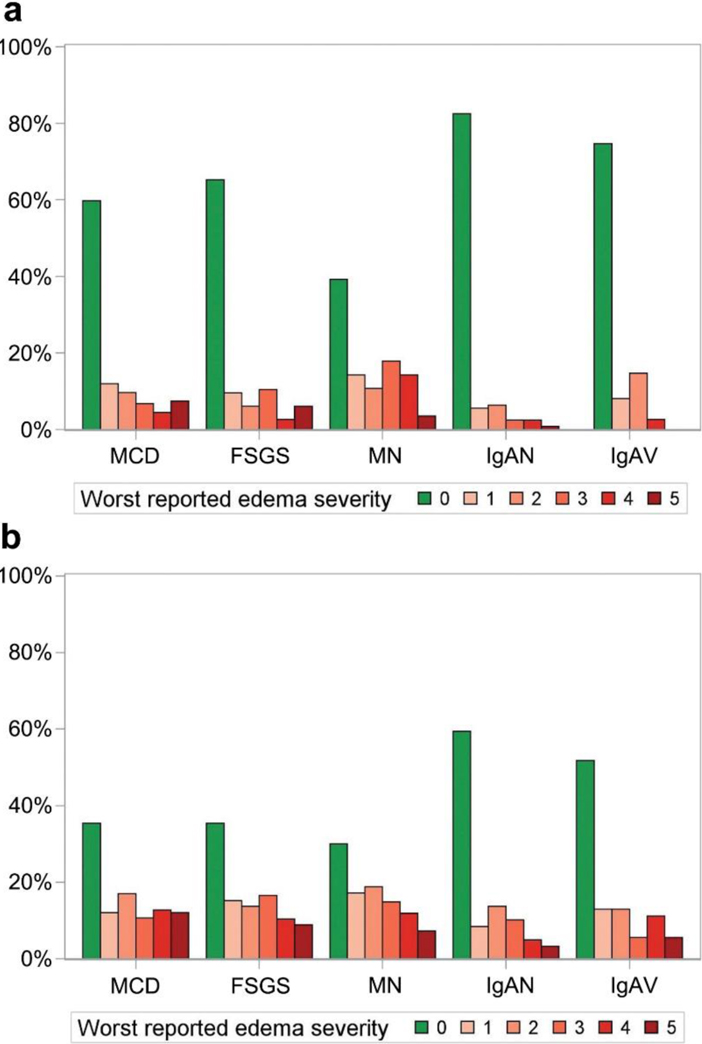
FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; IgAV, IgA vasculitis; MCD, minimal change disease; MN, membranous nephropathy.
The results of the final multivariable analyses are summarized in Tables 3 and 4 (unadjusted univariable analyses are shown in the Supplementary Tables S5−S15). In the final models, worst edema remained robustly associated with every HRQOL domain in adults: using negative numbers to indicate worse HRQOL, linear coefficients β were as follows: −1.6 (95% CI, −2.2 to −1.1) for global physical health, −0.7 (95% CI, −1.3 to −0.1) for global mental health, −0.6 (95% CI, −0.3 to −1.0) for anxiety, −2.1 (95% CI, −1.7 to −2.5) for fatigue, −1.1 (95% CI, −0.7 to −1.4) for sleep impairment, and −1.6 (95% CI, −2.0 to −1.2) for the composite score. Various edema variables were those most associated with HRQOL domains in children as well (Table 3). For certain domains, specific locations of edema (e.g., face, leg, genitals) had a residual association beyond worst edema, although the majority of the variance explained in most models was from worst edema. Increasing severity of edema was associated with stepwise worsening HRQOL in most domains for both children and adults (Figure 5), and η2 values were improved (indicating greater variance explained) by using the ordinal worst edema instead of the binary any edema. Despite the use of various edema terms, none of the final multivariable models showed evidence of significant multicollinearity (as indicated by the variance inflation factors in Tables 3 and 4, in which a variance inflation factor > 4 indicates potential collinearity and a variance inflation factor > 10 indicates multicollinearity requiring correction).
Table 3 |.
Final pediatric multivariable models for predicting HRQOL across measured domains
| β (95% CI) | P | Model η2 | Type I η2 | Type III η2 | VIF | |
|---|---|---|---|---|---|---|
| Global assessment of health | 0.09 | |||||
| Worst edema | −1.5 (−2.1 to −0.9) | <0.001 | 0.06 | 0.06 | 1.06 | |
| Weight | <0.001 | 0.03 | 0.03 | |||
| Underweight | −4.6 (−10.5 to 1.3) | 0.13 | 1.02 | |||
| Normal weight | Ref | Ref | Ref | |||
| Overweight | −0.7 (−3.0 to 1.6) | 0.54 | 1.18 | |||
| Obese | −3.8 (−5.7 to −1.9) | <0.001 | 1.15 | |||
| Anxiety | 0.07 | |||||
| Total edema | 0.3 (0.2−0.4) | <0.001 | 0.06 | 0.06 | 1.01 | |
| Sex (female vs. male) | 1.4 (0.2−2.6) | 0.02 | 0.01 | 0.01 | 1.01 | |
| Fatigue | 0.17 | |||||
| Face edema | 5.8 (3.2−8.4) | <0.001 | 0.09 | 0.03 | 1.24 | |
| Hand edema | 7.9 (4.2−11.7) | <0.001 | 0.04 | 0.03 | 1.27 | |
| Sex (female vs. male) | 3.1 (1.1−5.1) | 0.002 | 0.02 | 0.02 | 1.03 | |
| Weight | 0.01 | 0.02 | 0.02 | |||
| Underweight | −6.3 (−13.5 to 0.9) | 0.09 | 1.03 | |||
| Normal weight | Ref | Ref | ||||
| Overweight | 1.0 (−1.7 to 3.8) | 0.47 | 1.23 | |||
| Obese | 2.9 (0.6−5.2) | 0.01 | 1.15 | |||
| Mobility | 0.15 | |||||
| Whole-body edema | −4.9 (−8.6 to −1.2) | 0.01 | 0.09 | 0.01 | 2.10 | |
| Arm edema | −4.6 (−9.0 to −0.2) | 0.04 | 0.02 | 0.01 | 2.10 | |
| Leg edema | −3.7 (−6.1 to −1.4) | 0.002 | 0.02 | 0.02 | 1.37 | |
| Weight | 0.02 | 0.02 | 0.02 | |||
| Underweight | −1.5 (−7.3 to 4.2) | 0.60 | 1.02 | |||
| Normal weight | Ref | Ref | Ref | |||
| Overweight | −0.5 (−2.6 to 1.6) | 0.65 | 1.17 | |||
| Obese | −2.8 (−4.6 to −1.0) | 0.002 | 1.17 | |||
| Composite | 0.21 | |||||
| Worst edema | −1.9 (−2.6 to −1.2) | <0.001 | 0.15 | 0.05 | 1.46 | |
| Arm edema | −6.9 (−11.2 to −2.6) | 0.002 | 0.02 | 0.02 | 1.45 | |
| Sex (female vs. male) | −2.8(−4.2 to −0.8) | 0.003 | 0.01 | 0.02 | 1.03 | |
| Weight | 0.001 | 0.03 | 0.03 | |||
| Underweight | 0.8 (−5.6 to 7.1) | 0.81 | 1.02 | |||
| Normal weight | Ref | Ref | Ref | |||
| Overweight | −0.7 (−3.0 to 1.6) | 0.55 | 1.21 | |||
| Obese | −3.8 (−5.7 to −1.8) | 0.001 | 1.15 | |||
For each domain, a higher score means more of that item (e.g., higher anxiety scores imply worse anxiety, higher mobility scores imply better mobility). For the composite score, higher is better. Model η2 = overall η2 for the adjusted multivariable model; type I η2 = model η2 in an unadjusted model of the variable by itself (i.e., the various type I η2 values will sum to the model η2); type III η2 = the change in the total model η2 after removing that variable from the model.
CI, confidence interval; HRQOL, health-related quality of life; VIF, variance inflation factor.
Table 4 |.
Final adult multivariable models for predicting HRQOL across measured domains
| β (95% CI) | P | Model η2 | Type I η2 | Type III η2 | VIF | |
|---|---|---|---|---|---|---|
| Global assessment of physical health | 0.32 | |||||
| Worst edema | −1.6 (−2.2 to −1.1) | <0.001 | 0.26 | 0.02 | 3.52 | |
| Total edema | −0.3 (−0.4 to −0.2) | <0.001 | 0.01 | 0.02 | 3.48 | |
| eGFR (per 30-unit change) | 1.1 (0.7−1.6) | <0.001 | 0.02 | 0.02 | 1.02 | |
| Sex (female vs. male) | −1.6 (−2.6 to −0.5) | 0.002 | 0.01 | 0.01 | 1.02 | |
| Weight | <0.001 | 0.02 | 0.02 | |||
| Underweight | −2.0 (−7.0 to 2.9) | 0.43 | 1.02 | |||
| Normal weight | Ref | Ref | Ref | |||
| Overweight | −1.1 (−2.4 to 0.1) | 0.08 | 1.04 | |||
| Obese | −3.1 (−4.3 to −1.9) | 0.02 | 1.01 | |||
| Global assessment of mental health | 0.15 | |||||
| Worst edema | −0.7 (−1.3 to −0.1) | 0.02 | 0.12 | 0.01 | 3.48 | |
| Total edema | −0.3 (−0.5 to −0.2) | <0.001 | 0.02 | 0.02 | 3.47 | |
| eGFR (per 30-unit change) | 0.8 (0.3−1.3) | <0.001 | 0.01 | 0.01 | 1.01 | |
| Anxiety | 0.12 | |||||
| Worst edema | 0.6 (0.3−1.0) | 0.001 | 0.07 | 0.01 | 1.74 | |
| Hand edema | 2.1 (0.9−3.5) | 0.001 | 0.01 | 0.01 | 1.57 | |
| Genital edema | 2.2 (0.3−4.1) | 0.02 | 0.01 | 0.01 | 1.28 | |
| ln(UP:C) (per doubling) | 0.4 (0.1 −0.7) | 0.01 | 0.01 | 0.01 | 1.12 | |
| Hematuria (yes vs. no) | 2.5 (1.0−4.0) | 0.001 | 0.01 | 0.01 | 1.04 | |
| Sex (female vs. male) | 2.1 (1.1 −3.0) | <0.001 | 0.01 | 0.02 | 1.04 | |
| Fatigue | 0.23 | |||||
| Worst edema | 2.1 (1.7−2.5) | <0.001 | 0.20 | 0.08 | 1.49 | |
| Hand edema | 3.6 (2.1−5.1) | <0.001 | 0.02 | 0.02 | 1.49 | |
| eGFR (per 30-unit change) | −0.5 (−1.0 to −0.1) | 0.04 | <0.01 | <0.01 | 1.01 | |
| Sex (female vs. male) | 2.2 (1.1 −3.3) | <0.001 | 0.01 | 0.01 | 1.03 | |
| Sleep impairments | 0.15 | |||||
| Worst edema | 1.1 (0.7−1.4) | <0.001 | 0.11 | 0.03 | 1.61 | |
| Hand edema | 3.1 (1.7−4.4) | <0.001 | 0.02 | 0.02 | 1.52 | |
| Genital edema | 2.4 (0.6−4.3) | 0.01 | 0.01 | 0.01 | 1.27 | |
| eGFR (per 30-unit change) | −0.6 (−1.1 to −0.2) | 0.009 | <0.01 | 0.01 | 1.23 | |
| Age (per 10-unit change) | −0.6 (−0.9 to −0.3) | <0.001 | 0.01 | 0.01 | 1.24 | |
| Composite | 0.22 | |||||
| Worst edema | −1.6 (−2.0 to −1.2) | <0.001 | 0.17 | 0.04 | 1.62 | |
| Hand edema | −3.8 (−5.3 to −2.3) | <0.001 | 0.03 | 0.02 | 1.55 | |
| Genital edema | −3.4 (−5.5 to −1.2) | <0.001 | 0.01 | 0.01 | 1.28 | |
| Hematuria (yes vs. no) | −2.5 (−4.1 to −0.8) | 0.003 | <0.01 | 0.01 | 1.03 | |
| Sex (female vs. male) | −2.2 (−3.2 to −1.1) | <0.001 | 0.01 | 0.01 | 1.01 | |
For each domain, a higher score means more of that item (e.g., higher global assessment scores imply better health, higher anxiety scores imply worse anxiety). For the composite score, higher is better. Model η2 = overall η2 for the adjusted multivariable model; type I η2 = model η2 in an unadjusted model of the variable by itself (i.e., the various type I η2 values will sum to the model η2); type III η2 = the change in the total model η2 after removing that variable from the model.
CI, confidence interval; HRQOL, health-related quality of life; VIF, variance inflation factor.
Figure 5 |. PROMIS domain scores according to worst reported edema severity in (a) children and (b) adults.
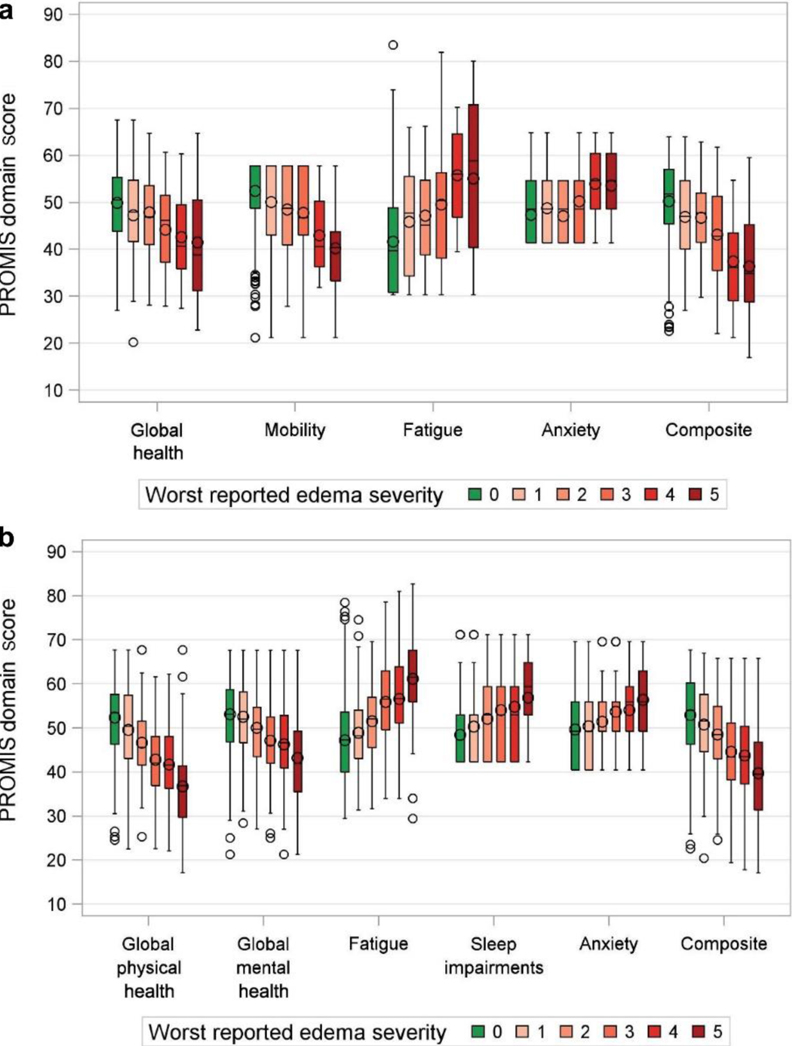
PROMIS, Patient-Reported Outcomes Measurement Information System.
Other associations with HRQOL
In both children and adults, female sex was associated with worse anxiety and fatigue, as well as worse composite HRQOL scores. In adults, female sex also was associated with worse global assessment of physical (but not mental) health. Weight status in children was associated significantly with global assessment of health, with a type I η2 comparable with that of worst edema (indicating those 2 variables explained a similar proportion of variance for that domain). Among children, weight status also was associated with fatigue, mobility, and composite HRQOL. In adults, weight status was associated significantly with global assessment of physical health only.
Typical biological measures of disease activity including eGFR and proteinuria seemed to have a minimal relationship to HRQOL in multivariable models. For pediatric HRQOL, neither eGFR nor UP:C contributed significantly to any of the multivariable models. For adult HRQOL, eGFR was associated significantly with global assessments of physical health and mental health, fatigue, and sleep impairment, whereas UP:C was associated with anxiety. The type I η2 values for these relationships were much smaller than those for edema, indicating that the experience of disease appears to have more impact on HRQOL than traditional quantitative laboratory biomarkers. Race, ethnicity, and diagnosis were not associated significantly with HRQOL in either children or adults in the multivariable models.
Disease duration and HRQOL
Disease duration had no relationship to HRQOL in either children or adults. We hypothesized that this could be because of an interaction with disease activity, in which those subjects with a short duration of disease were more likely to be symptomatic. To test this, we created models with interaction terms between worst edema severity and disease duration to explain HRQOL, however, we found no statistically significant interactions for any of the PROMIS scores. We then tested whether disease duration had a different effect on HRQOL across diagnostic categories, but even after stratifying by diagnosis no significant interactions were found.
Immunosuppression and HRQOL
Exposure to IST in the prior 60 days was not associated with HRQOL in the multivariable models. To explore this relationship further, we tested models with alternative IST variables including exposure limited to the prior 30 days or prior 7 days. Neither of those alternatives changed the results of the analysis compared with IST in the past 60 days. Furthermore, we tested for a dose-response relationship by operationalizing IST exposure as the number of days exposed to high-dose corticosteroids in the prior 60 days (defined as >40 mg/d prednisone equivalent in adults and >1 mg/kg/d in children) as well as the average daily corticosteroid dose in the prior 60 days (Supplementary Figure S1). Dose-dependent 1ST variables were not associated with any HRQOL domain in the multivariable model.
In a final sensitivity analysis, we tested if there was a difference in HRQOL by patients treated with corticosteroids alone compared with patients not on immunosuppression (Figure 6). Use of corticosteroids was not associated with differences in any of the PROMIS domains in either children or adults.
Figure 6 |. The impact of corticosteroids on PROMIS domain scores at enrollment in the CureGN study.
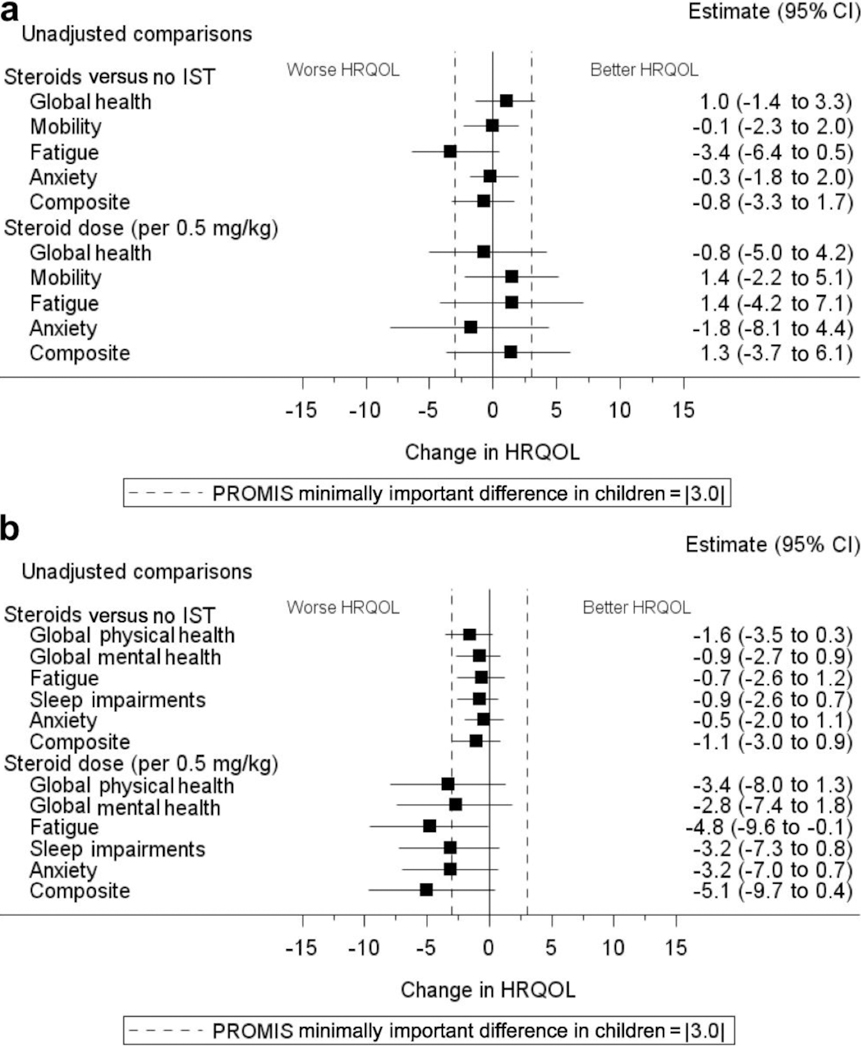
Plots of (a) children and (b) adults show the unadjusted linear regression coefficients (β), with tails indicating lower and upper confidence limits. For ease of comparison, each β estimate has been plotted as the negative of its absolute value, in which negative numbers indicate a worse patient-reported outcome. Analyses were based on 420 children (75 treated with corticosteroids vs. 345 not treated with any immunosuppression) and 979 adults (119 treated with corticosteroids vs. 860 not treated with any immunosuppression). CI, confidence interval; HRQOL, health-related quality of life; IST, immunosuppressive therapy; PROMIS, Patient-Reported Outcomes Measurement Information System.
DISCUSSION
This was a large study of HRQOL in primary glomerular disease, and included cross-disease comparisons as well as provided data in IgAN/IgAV and MN. In aggregate, adults reported relatively worse HRQOL scores in multiple domains compared with children. We found few differences in HRQOL between diseases. Edema had the strongest association with HRQOL in children and adults. It had a significant association with all domains measured, and accounted for the greatest amount of variance observed in HRQOL scores. This finding complements prior work that found edema was associated with poor PROMIS scores in children with chronic kidney disease.12 Edema is one of the major symptoms experienced by patients with glomerular disease and can affect patients adversely in a variety of ways. The severity of edema may be a correlate of disease severity, but we found that patient-reported edema severity explained more variance in HRQOL than traditional biological markers of disease severity such as eGFR or proteinuria. Furthermore, edema in this study was patient-reported, rather than clinician-reported, so correlation with worse HRQOL in other domains might be expected (i.e., a patient’s subjective report of edema may represent more than just simple interstitial fluid expansion, but a broader subjective impression of the disease experience).
Female sex was associated with worse anxiety and fatigue in children and adults, worse global assessment of physical health in adults, and worse composite HRQOL scores in both age groups. The overall variance explained was minimal, and dwarfed by the effect of edema. However, worse HRQOL experienced by females has been noted previously in studies of chronic kidney disease, rheumatologic disease, and a variety of other conditions.13–15 Our study extrapolated this relationship to primary glomerular diseases and highlights the importance of addressing gender disparities in disease outcomes both by personalizing treatment approaches as well as understanding systematic factors that might be at play.
A number of important variables were not associated significantly with HRQOL after multivariable adjustment. In children, diagnosis, disease duration, proteinuria, and eGFR did not have a significant effect on any of the HRQOL domains or with the composite score. In adults, eGFR had a minor impact on HRQOL, and diagnosis did not correlate with any HRQOL domain.
The use of IST did not affect HRQOL in our adjusted multivariable model. For our primary analyses, subjects were categorized as having used IST if they had received it within the past 60 days. We subsequently conducted sensitivity analyses using cut-off times of 30 and 7 days, as well as the number of days on high-dose corticosteroids in the past 60 days, and still found no effect on HRQOL. It may be that the average corticosteroid doses were too low in this population to elicit detectable adverse effects. Most subjects had less than 0.5 mg/kg/d of prednisone equivalent averaged over 60 days (Supplementary Figure S1). Another possible explanation for these findings is that IST can have positive and/or negative effects on HRQOL. There are well-known side effects to these medications that certainly could have an adverse effect on HRQOL, but they also have the potential to improve HRQOL by treating the underlying disease and/or by providing optimism of a treatment benefit. The effect of IST on HRQOL likely depends on timing of therapy relative to changes in disease status and is subject to confounding, making it challenging to elucidate a simple relationship.
We also examined whether disease duration was associated with HRQOL. Prior work found that disease duration negatively impacted some domains of HRQOL in children with active nephrotic syndrome.7 However, disease duration was not found to have a significant association with HRQOL in children or adults after adjusting for other factors, including after stratification by diagnosis.
Our finding that HRQOL was not related to diagnosis was unexpected but important. It suggests that among patients with the diseases studied here, the impact of glomerular disease on quality of life depends most on physical experience, exemplified by the presence of edema, rather than the underlying diagnosis. Our results, if confirmed, have implications for how we should evaluate and manage patients. Perhaps prioritizing control of edema (as reported by patients) will help us to have a more meaningful and positive effect on the disease experience. In addition, our results indicate that patient-reported edema may be an important outcome to assess systematically and routinely in both clinical practice and in trials of new therapies.
Ideally, when determining the domains most relevant and important to assess with a PRO instrument for a given disease, input from the target population is considered an essential step to establish content validity, and generally is elicited via qualitative research.16,17 Of note, a recently published study using qualitative methods to develop PRO measures in adults with FSGS18 was not available at the time that the PRO assessment for CureGN was created. However, concepts elicited and incorporated into this adult FSGS PRO development work, including fatigue, edema, and physical and emotional functioning, are included in the CureGN HRQOL assessment. Unfortunately, current literature does not exist for adults with the other primary glomerular diseases studied here (IgAN/IgAV, MN, and MCD) or in children with the biopsy-proven diagnoses included in CureGN. Future work of this type, including concept elicitation and other qualitative work to guide development of validated PRO measures, will be important in optimizing our instruments to better characterize patient experience specific to primary glomerular disease.
Our study had important limitations. As mentioned previously, this was a cross-sectional analysis because we only analyzed data collected at the time of enrollment. Prospectively obtaining serial longitudinal measures of HRQOL in the CureGN cohort should enrich our understanding of the determinants of patient experience, particularly as participants experience disease relapse and remission and are exposed to other treatments. This also will serve to diminish the variance inherent in data from a single point in time, as we present in the current study.
Any conclusions we can draw are limited by the instruments used. The HRQOL data collected in CureGN includes items selected from PROMIS, and the selection of domains and items relevant to nephrotic syndrome were based on scant available prior literature. For some populations within CureGN there were no published data to inform these decisions and in these cases selection was based on expert clinical knowledge of the diseases, their symptoms, and how we presumed they affect HRQOL. The creation of the assessment used in CureGN, including the relevant PROMIS items to evaluate HRQOL, was guided in part by studies that evaluated PRO in pediatric populations with nephrotic syndrome.7–9,19 However, there is less literature available for adults,10,11 and essentially none for MN or IgAN/IgAV. Furthermore, in an attempt to decrease the burden on study participants, the number of items included in the assessment was minimized. For these reasons, it is likely that we have not captured all of the pertinent domains and signals for these diseases. It bears emphasizing that although the PROMIS data in CureGN ideally reflect measures important to patients, these measures ultimately were chosen by the investigators during the study design and not by the patients themselves, and there may be discordance between the importance of symptoms captured in measures selected by clinician-scientists versus by patients. Since its initiation, CureGN has incorporated a patient advisory council, and the perspectives of this council may be useful in helping to optimize our collection of PRO going forward. However, formal study of the patient perspectives in this population likely also is necessary, as has been performed in hemodialysis and kidney transplant populations.20,21
PROMIS items are calibrated to a reference population, which is usually the US general population. We did not emphasize the absolute deficits in HRQOL in this study, although they can be implied from the score distributions (Figure 1). Although such comparisons are valuable from a public health perspective, exploring them was outside the scope of the study’s main focus of determining associations with HRQOL within glomerular disease patients. In addition, PROMIS domains are measured with separate items for children and adults, and thus are not directly comparable between those age groups. Establishing linkage methods to allow reliable comparison of domains between age groups is an important area of ongoing research.22
The interpretation of differences in PROMIS results is subject to consideration of clinically meaningful and statistical criteria for the determination of minimally important differences (MIDs). Clinically meaningful differences in scores have been assessed by patients, parent caregivers, and clinicians for children with nephrotic syndrome as well as other chronic pediatric conditions.23 In these populations, MIDs consistently were found to be clinically meaningful with a difference of 3 points in PROMIS scores. Statistical approaches have been used to estimate MIDs from studies of adults with health conditions, none of which included kidney disease. These statistical approaches generated MIDs in specific populations ranging from 4 to 8.5.24,25 Future use of PROMIS in clinical settings for adult patients with glomerular disease will benefit from the replication of the clinical MID work completed for children. Statistically significant differences in PROMIS are reported in this study as well as other scientific works. A statistically significant difference may be considered useful when devising approaches to populations rather than to individual patient management.
We did not identify a relationship between HRQOL and several purported associated variables, including disease duration and IST. We attempted to clarify this using several sensitivity analyses as described, but the possibility of failing to detect true relationships cannot be ruled out. Two other possible issues that may have contributed to the lack of association of immunosuppressive therapies with HRQOL include the low dose of corticosteroids in the majority of adults and the small number of patients receiving other (noncorticosteroid) therapies. It is worth highlighting the substantial variability in HRQOL that remained unexplained in the multivariable models. This emphasizes that much of a patient’s experience of how these diseases impact quality of life remains difficult to quantify. Whether it is because our instruments miss important aspects of disease, or whether they inadequately assess those that we chose to examine, is a critical area for future study. We speculate that the use of longitudinal data examining HRQOL associations with treatment exposures and response over time will be particularly informative.
In conclusion, patient-reported edema had the strongest association with HRQOL in children and adults with primary glomerular diseases, with clinically meaningful associations with all measured domains. Edema is one of the major symptoms experienced by patients with glomerular diseases and can affect patients adversely in a variety of ways. Our results indicate that patient-reported edema may be an important outcome to assess more systematically and routinely in both clinical practice and trials of new therapies. This foundation of knowledge will be built on with future analyses of HRQOL from longitudinal data in CureGN, as well as other glomerular disease cohorts. Future research is needed to more comprehensively identify and quantify the key variables impacting HRQOL in primary glomerular disease, optimize their assessment, and incorporate these measures into clinical trials.
METHODS
Patients
The CureGN study was designed to recruit children and adults with a diagnostic kidney biopsy within the past 5 years showing MCD, FSGS, MN, or IgAN (including IgAV) from more than 60 sites across the United States, as well as 2 sites in Canada and 1 in Italy (https://curegn.org). Approximately 600 each of MCD, FSGS, and MN participants and 650 IgAN/IgAV participants will be enrolled. Patients are ineligible if they have end-stage kidney disease, or any of the following before their first kidney biopsy: solid-organ or bone marrow transplant, active HIV infection, hepatitis B or C infection, diabetes mellitus, systemic lupus erythematosus, or active malignancy. Each participating site obtained approval from an institutional review board, and all patients and/or legal guardians of children provided informed consent and, where age appropriate, informed assent before enrollment in the study.
In the current study, we present enrollment data from all enrolled patients in CureGN with a completed enrollment visit at the time of analysis. During the in-person enrollment visit, demographics, clinical characteristics, laboratory test results, and patient- and/or parent proxy–reported outcomes measures were collected. Clinical characteristics included duration of disease, family history of kidney disease, medication history and current use, and comorbidities. Local laboratory test results, including serum creatinine, serum albumin, hematuria, and the UP:C, were abstracted from clinic records. Most recent laboratory values before enrollment as well as those from the closest date before kidney biopsy were recorded. Blood and urine samples also were collected at the enrollment visit and processed centrally by the CureGN laboratory to measure serum creatinine and protein in either 24-hour, morning void, or spot urine. Data were extracted on October 24, 2017.
HRQOL data
HRQOL was assessed using measures selected from PROMIS.26 Each PROMIS measure generates a T-score (mean, 50; SD, 10 in the calibration population), where a higher score indicates higher levels of the trait being measured (i.e., higher mobility score = better mobility; higher anxiety score = worse anxiety). We used an MID of 3.0 units, which is likely a conservative estimate based on available literature.23 Each question uses a common time frame of “the past 7 days”, and responses use a 5-item Likert scale from “never” to “almost always” for the majority of domains. PROMIS was administered as a paper form using a fixed number of items. These items were chosen a priori during the design of the CureGN study by a working group of clinician investigators, with the aim of measuring domains broadly relevant to patients with glomerular disease while balancing the time burden on study participants. Of note, pediatric and adult PROMIS items are separate, thus domains are not directly comparable and they are analyzed separately throughout. The choice of domains was informed by prior work in pediatric nephrotic syndrome; no prior work related to PROMIS in adult nephrotic syndrome was available.19
Pediatric domains included the following: global assessment of health (10-item short form), mobility (4-item custom form), fatigue (10-item short form), and anxiety (single-item custom form). Adult domains included the following: global assessment of physical and mental health (10-item short form), sleep impairments (single-item custom form), fatigue (7-item custom form), and anxiety (single-item custom form).
The actual items administered are available in the Supplementary Appendix.
Creation of composite HRQOL score
The composite child and adult HRQOL scores were created to summarize HRQOL from the various domains into a single summative measure. This was calculated by first reverse scoring PROMIS domains as needed so that all domains were oriented in the same direction (i.e., higher scores = better HRQOL). Next, scores were standardized to a mean of 0 and a SD of 1. These scores then were averaged, and rescaled back to a mean of 50 and SD of 10.
Although many clinicians and researchers may want to consider different aspects of HRQOL separately (given the context of a specific clinical question), there are also other clinicians and researchers who may want to consider a simple summary score that is representative of quality of life more generally. One common approach to the generation of composite scores is to combine scores that are conceptually related (e.g., mental HRQOL vs. physical HRQOL).27 Although domain-specific scores are readily interpretable, clinically meaningful composite scores have the advantage of being able to increase the power to detect subtle treatment-related differences that may be associated with improvements across multiple aspects of HRQOL.28,29 Therefore, an overall composite score that reflected HRQOL was generated for both children and adults. This was calculated by first reverse scoring PROMIS domains as needed so that all domains were oriented in the same direction (i.e., higher scores = better HRQOL). Next, scores were standardized to a mean of 0 and SD of 1. These scores then were averaged, and rescaled back to a mean of 50 and SD of 10. Each individual domain thus was equally weighted in the composite score.
Associations with HRQOL
Independent variables of interest included kidney biopsy diagnosis, age, sex, race, ethnicity, obesity (body mass index > 30 in adults; body mass index percentile >95 in children), UP:C, eGFR (as measured by Bedside Schwartz in children30 and Chronic Kidney Disease Epidemiology Collaboration formula (CKD-EPI) in adults31), hematuria, rash (for IgAV only), immunosuppressive therapy in the past 60 days (specifically corticosteroids, calcineurin inhibitors, mycophenolate, cyclophosphamide, rituximab, or corti-cotropin gel), average corticosteroid dose in the past 60 days (mg/kg/ d of prednisone or equivalent), and disease duration in months (measured as the time from the first diagnosis of kidney disease to the date of enrollment).
A particular variable of interest was the presence of edema at baseline. Edema was self-reported by the participant on the PRO form (Supplementary Appendix). Participants were directed to report the location of edema from 8 different areas (whole body, face, hands, arms, abdomen, genitalia, legs, feet), and to rate the severity of each on a scale of 0 to 5 points where 0 = absent and 5 = worst imaginable. Analyses considered edema characterized in several different ways: (i) any edema, (ii) location of edema, (iii) worst reported edema severity, and (iv) total edema severity (i.e., the sum of the 8 edema questions).
Statistical analysis
All analyses were stratified by age (<18 vs. ≥18 yr) given the different PRO instruments for pediatric and adult patients. Categoric variables are described using frequencies and percentages; continuous variables are described using medians and interquartile ranges. A series of linear regression models were fit to test for associations with each HRQOL domain separately, including the composite scores. Each independent variable listed earlier, including the various edema variables, was tested. We also included interaction terms a priori to test for subgroup variation in the effect of edema by therapy (edema*IST) and in the effect of diagnosis by disease duration and activity (disease*duration, disease activity*duration).
Any univariable association with HRQOL at a P value less than 0.20 entered a multivariable backward selection model. Variables were removed in order of descending P value until all remaining variables were statistically significant at a P value less than 0.05. η2 values were taken from each regression to describe the proportion of variance explained by the total model, as well as each individual covariate (type I η2). Type III η2 values also were calculated for each covariate, representing the reduction in the total model η2 after removal of that covariate. All analyses were conducted in SAS v9.4 (SAS Institute Inc., Cary, NC).
Supplementary Material
ACKNOWLEDGMENT
Funding for the CureGN Consortium is provided by UM1DK100845, UM1DK100846, UM1DK100876, UM1DK100866, and UM1 DK100867 from the National Institute of Diabetes and Digestive and Kidney Diseases. Patient recruitment is supported by NephCure Kidney International.
APPENDIX
Consortium collaborators
The CureGN Consortium members listed (from within the 4 Participating Clinical Center networks and the Data Coordinating Center) are collaborators on this article. CureGN Principal Investigators are noted by an asterisk.
The CureGN Consortium collaborators from Columbia University are as follows: Wooin Ahn, Columbia; Gerald B. Appel, Columbia; Revekka Babayev, Columbia; Ibrahim Batal, Columbia; Andrew S. Bomback, Columbia; Eric Brown, Columbia; Eric S. Campenot, Columbia; Pietro Canetta, Columbia; Lucrezia Carlassara, Columbia; Brenda Chan, Columbia; Debanjana Chatterjee, Columbia; Vivette D. D’Agati, Columbia; Elisa Delbarba, Columbia; Samriti Dogra, Columbia; Hilda Fernandez, Columbia; Bartosz Foroncewicz, University of Warsaw, Poland; Ali G. Gharavi, Columbia*; Gian Marco Ghiggeri, Gaslini Children’s Hospital, Italy; William H. Hines, Columbia; S. Ali Husain, Columbia; Namrata G. Jain, Columbia; Pascale Khairallah, Columbia; Byum Hee Kil, Columbia; Krzysztof Kiryluk, Columbia; Anushya Jeyabalan, Columbia; Wai L. Lau, Columbia; Fangming Lin, Columbia; Francesca Lugani, Gaslini Children’s Hospital, Italy; Maddalena Marasa, Columbia; Glen Markowitz, Columbia; Sumit Mohan, Columbia; Xueru Mu, Columbia; Krzysztof Mucha, University of Warsaw, Poland; Thomas L. Nickolas, Columbia; Stacy Piva, Columbia; Jai Radhakrishnan, Columbia; Maya K. Rao, Columbia; Regunathan-Shenk Renu, Columbia; Simone Sanna-Cherchi, Columbia; Dominick Santoriello, Columbia; Shayan Shirazian, Columbia; Michael B. Stokes, Columbia; Natalie Uy, Columbia; and Anthony M. Valeri, Columbia.
The CureGN Consortium collaborators from the Midwest Pediatric Nephrology Consortium are as follows: Larry A. Greenbaum,* Emory University; William E. Smoyer,* Nationwide Children’s; Amira Al-Uzri, Oregon Health and Science University; Josephine Ambruzs, Arkana Laboratories; Isa Ashoor, Louisiana State University Health Sciences Center; Diego Aviles, Louisiana State University Health Sciences Center; Rossana Baracco, Children’s Hospital of Michigan; John Barcia, University of Virginia; Sharon Bartosh, University of Wisconsin; Craig Belsha, Saint Louis University/Cardinal Glennon; Corinna Bowers, Nationwide Children’s Hospital; Michael C. Braun, Baylor College of Medicine/Texas Children’s Hospital; Yi Cai, Helen DeVos Children’s Hospital; Vladimir Chernitskiy, Mayo Clinic; Aftab Chishti, University of Kentucky; Donna Claes, Cincinnati Children’s Hospital; Kira Clark, Oregon Health and Science University; Carl Cramer, Mayo Clinic; Keefe Davis, Washington University in St. Louis; Elif Erkan, Cincinnati Children’s Hospital Medical Center; Daniel Feig, University of Alabama, Birmingham; Michael Freundlich, University of Miami/ Holtz Children’s Hospital; Joseph Gaut, Washington University in Saint Louis; Rasheed Gbadegesin, Duke University Medical Center; Melisha Hanna, Children’s Colorado/University of Colorado; Guillermo Hidalgo, East Carolina University; David Hooper, Cincinnati Children’s Hospital Medical Center; Tracy E. Hunley, Monroe Carell Jr Children’s Hospital at Vanderbilt University Medical Center; Amrish Jain, Children’s Hospital of Michigan; Mahmoud Kallash, Nationwide Children’s Hospital; Margo Kamel, Emory University; Myda Khalid, JW Riley Hospital for Children, Indiana University School of Medicine, Indianapolis; Jon B. Klein, The University of Louisville School of Medicine; Theresa Kump, Medical College of Wisconsin; Jerome C. Lane, Feinberg School of Medicine, Northwestern University; Helen Liapis, Arkana Laboratories; John Mahan, Nationwide Children’s; Nisha Mathews, University of Oklahoma Health Sciences Center; Carla Nester, University of Iowa Stead Family Children’s Hospital; Cynthia Pan, Medical College of Wisconsin; Larry Patterson, Children’s National Health System; Hiren Patel, Nationwide Children’s Hospital; Alice Raad, Ann and Robert H. Lurie Children’s Hospital of Chicago; Adelaide Revell, Nationwide Children’s Hospital; Michelle N. Rheault, University of Minnesota Masonic Children’s Hospital; Cynthia Silva, Connecticut Children’s Medical Center; Rajasree Sreedharan, Medical College of Wisconsin; Tarak Srivastava, Children’s Mercy Hospital; Julia Steinke, Helen DeVos Children’s Hospital; Susan Sumner, University of North Carolina; Katherine Twombley, Medical University of South Carolina; Scott E. Wenderfer, Baylor College of Medicine/Texas Children’s Hospital; Tetyana L. Vasylyeva, Texas Tech University Health Sciences Center; Chia-shi Wang, Emory University; Donald J. Weaver, Levine Children’s Hospital at Carolinas Medical Center; Craig S. Wong, University of New Mexico Health Sciences Center; and Hong Yin, Emory University.
The CureGN Consortium collaborators from the University of North Carolina are as follows: Anand Achanti, Medical University of South Carolina; Salem Almaani, The Ohio State University; Isabelle Ayoub, The Ohio State University; Milos Budisavljevic, Medical University of South Carolina; Maggie D’Angelo, University of North Carolina; Huma Fatima, The University of Alabama at Birmingham; Ronald Falk,* University of North Carolina; Agnes Fogo, Vanderbilt; Keisha Gibson, University of North Carolina; Dorey Glenn, University of North Carolina; Susan Hogan, University of North Carolina; J. Charles Jennette, University of North Carolina; Bruce Julian, University of Alabama at Birmingham; Jason Kidd, Virginia Commonwealth University; Louis-Philippe Laurin, Hôpital Maisonneuve-Rosemont Montreal; H. Davis Massey, Virginia Commonwealth University; Amy Mottl, University of North Carolina; Shannon Murphy, University of North Carolina; Patrick Nachman, University of North Carolina; Tibor Nadasdy, The Ohio State University; Jan Novak, University of Alabama at Birmingham; Samir Parikh, The Ohio State University; Caroline Poulton, University of North Carolina; Thomas Brian Powell, Columbia Nephrology Associates; Bryce Reeve, Duke University; Matthew Renfrow, University of Alabama at Birmingham; Monica Reynolds, University of North Carolina; Dana Rizk, University of Alabama at Birmingham; Brad Rovin, The Ohio State University; Virginie Royal, Hôpital Maisonneuve-Rosemont Montreal; Neil Sanghani, Vanderbilt; and Sally Self, Medical University of South Carolina.
The CureGN Consortium collaborators from the University of Pennsylvania are as follows: Sharon Adler, Los Angeles Biomedical Research Institute at Harbor, University of California Los Angeles; Nada Alachkar, Johns Hopkins University; Charles Alpers, University of Washington; Raed Bou Matar, Cleveland Clinic; Carmen Avila-Casado, University of Toronto/University Health Network; Serena Bagnasco, Johns Hopkins; Emily Brede, National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases; Elizabeth Brown, University of Texas Southwestern Medical Center; Daniel Cattran, University of Toronto; Michael Choi, Johns Hopkins; Katherine M. Dell, Case Western/Cleveland Clinic; Darren Dewalt, University of North Carolina; Michelle Denburg, Children’s Hospital of Philadelphia; Ram Dukkipati, Los Angeles Biomedical Research Institute at Harbor, University of California Los Angeles; Fernando C. Fervenza, Mayo Clinic; Alessia Fornoni, University of Miami; Crystal Gadegbeku, Temple University; Patrick Gipson, University of Michigan; Anny Gonzalez-Zea, Sunnybrook Health Sciences Centre; Leah Hasely, University ofWashington; Elizabeth Hendren, University of Toronto/University Health Network; Sangeeta Hingorani, Seattle Children’s Hospital; Michelle Hladunewich, University of Toronto/Sunnybrook; Jonathan Hogan, University of Pennsylvania; Lawrence B. Holzman,* University of Pennsylvania; Jean Hou, Cedars-Sinai Medical Center; J. Ashley Jefferson, University of Washington; Kenar Jhaveri, North Shore University Hospital; Duncan B. Johnstone, Temple University; Frederick Kaskel, Montefiore Medical Center; Amy Kogan, Children’s Hospital of Philadelphia; Jeffrey Kopp, National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program; Richard Lafayette, Stanford; Kevin V. Lemley, Children’s Hospital of Los Angeles; Laura Malaga-Dieguez, New York University; Kevin Meyers, Children’s Hospital of Pennsylvania; Alicia Neu, Johns Hopkins; Michelle Marie O’Shaughnessy, Stanford; John F. O’Toole, Case Western/Cleveland Clinic; Andrea Oliverio, University of Michigan; Matthew Palmer, University of Pennsylvania; Rulan Parekh, University Health Network, Hospital for Sick Children; Renee Pitter, University of Michigan; Heather Reich, University Health Network, University of Toronto, Toronto; Kimberly Reidy, Montefiore Medical Center; Helbert Rondon, University of Pittsburgh Medical Center; Kamalanathan K. Sambandam, University of Texas Southwestern; Matthew Sampson, University of Michigan; John R. Sedor, Case Western/Cleveland Clinic; David T. Selewski, University of Michigan; Christine B. Sethna, Cohen Children’s Medical Center- North Shore Long Island Jewish Health System; Jeffrey Schelling, Case Western; John C. Sperati, Johns Hopkins; Agnes Swiatecka-Urban, Children’s Hospital of Pittsburgh; Howard Trachtman, New York University; Katherine R. Tuttle, Spokane Providence Medical Center; Meryl Waldman, National Institutes of Health/ National Institute of Diabetes and Digestive and Kidney Diseases; Joseph Weisstuch, New York University; Roger Wiggins, University of Michigan; David Williams, University of Michigan; Cheryl Winkler, National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases; Suzanne Vento, New York University Langone Medical Center; Eric Young, University of Michigan; and Olga Zhdanova, New York University.
The CureGN Consortium collaborators from the Data Coordinating Center are as follows: Laura Barisoni, University of Miami; Charlotte Beil, Arbor Research Collaborative for Health; Richard Eikstadt, University of Michigan; Brenda Gillespie,* University of Michigan; Debbie S. Gipson,* University of Michigan; John Graff, Arbor Research Collaborative for Health; Stephen Hewitt, National Institutes of Health/National Cancer Institute; Peg Hill-Callahan, Arbor Research Collaborative for Health; Margaret Helmuth, Arbor Research Collaborative for Health; Emily Herreshoff, University of Michigan; Matthias Kretzler,* University of Michigan; Chrysta Lienczewski, University of Michigan; Sarah Mansfield, Arbor Research Collaborative for Health; Laura Mariani, University of Michigan; Keith McCullough, Arbor Research Collaborative for Health; Nicholas Moore, Arbor Research Collaborative for Health; Cynthia C. Nast, Cedars-Sinai Medical Center; Bruce M. Robinson, University of Michigan; Melissa Sexton, Arbor Research Collaborative for Health; Jonathan Troost, University of Michigan; Matthew Wladkowski, Arbor Research Collaborative for Health; Jarcy Zee, Arbor Research Collaborative for Health; and Dawn Zinsser, Arbor Research Collaborative for Health.
The CureGN Consortium Steering Committee Chair is Lisa M. Guay-Woodford, Children’s National Health System.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
Supplementary Appendix.
Supplementary material is linked to the online version of the paper at www.kidney-international.org.
Members of the CureGN Consortium are listed in the Appendix.
REFERENCES
- 1.United States Renal Data System. 2016 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016. [Google Scholar]
- 2.Rheault MN, Zhang L, Selewski DT, et al. AKI in children hospitalized with nephrotic syndrome. Clin J Am Soc Nephrol. 2015;10:2110–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selewski DT, Thompson A, Kovacs S, et al. Patient-reported outcomes in glomerular disease. Clin J Am Soc Nephrol. 2017;12:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choudhry S, Bagga A, Hari P, et al. Efficacy and safety of tacrolimus versus cyclosporine in children with steroid-resistant nephrotic syndrome: a randomized controlled trial. Am J Kidney Dis. 2009;53:760–769. [DOI] [PubMed] [Google Scholar]
- 5.Perrone RD, Coons SJ, Cavanaugh K, et al. Patient-reported outcomes in clinical trials of CKD-related therapies: report of a symposium sponsored by the National Kidney Foundation and the U.S. Food and Drug Administration. Am J Kidney Dis. 2013;62:1046–1057. [DOI] [PubMed] [Google Scholar]
- 6.Da Silva-Gane M, Wellsted D, Greenshields H, et al. Quality of life and survival in patients with advanced kidney failure managed conservatively or by dialysis. Clin J Am Soc Nephrol. 2012;7:2002–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selewski DT, Troost JP, Massengill SF, et al. The impact of disease duration on quality of life in children with nephrotic syndrome: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol. 2015;30:1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruth EM, Landolt MA, Neuhaus TJ, et al. Health-related quality of life and psychosocial adjustment in steroid-sensitive nephrotic syndrome. J Pediatr. 2004;145:778–783. [DOI] [PubMed] [Google Scholar]
- 9.Gipson DS, Trachtman H, Kaskel FJ, et al. Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney Int. 2011;79:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liborio AB, Santos JP, Minete NF, et al. Proteinuria is associated with quality of life and depression in adults with primary glomerulopathy and preserved renal function. PLoS One. 2012;7:e37763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shutto Y, Yamabe H, Shimada M, et al. Quality of life in patients with minimal change nephrotic syndrome. ScientificWorldJournal. 2013;2013, 124315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selewski DT, Massengill SF, Troost JP, et al. Gaining the Patient Reported Outcomes Measurement Information System (PROMIS) perspective in chronic kidney disease: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol. 2014;29:2347–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimbudzi E, Lo C, Ranasinha S, et al. Predictors of health-related quality of life in patients with comorbid diabetes and chronic kidney disease. PLoS One. 2016;11:e0168491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krasselt M, Baerwald C. Sex, symptom severity, and quality of life in rheumatology [e-pub ahead of print]. Clin Rev Allergy Immunol 10.1007/s12016-017-8631-6. [DOI] [PubMed] [Google Scholar]
- 15.Cherepanov D, Palta M, Fryback DG, et al. Gender differences in health- related quality-of-life are partly explained by sociodemographic and socioeconomic variation between adult men and women in the US: evidence from four US nationally representative data sets. Qual Life Res. 2010;19:1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity-establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2-assessing respondent understanding. Value Health. 2011;14: 978–988. [DOI] [PubMed] [Google Scholar]
- 17.Lasch KE, Marquis P, Vigneux M, et al. PRO development: rigorous. qualitative research as the crucial foundation. Qual Life Res. 2010;19: 1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 30. 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 19.Gipson DS, Selewski DT, Massengill SF, et al. Gaining the PROMIS. perspective from children with nephrotic syndrome: a Midwest pediatric nephrology consortium study. Health Qual Life Outcomes. 2013;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evangelidis N, Tong A, Manns B, et al. Developing a set of core outcomes for trials in hemodialysis: an international Delphi survey. Am J Kidney Dis. 2017;70:464–475. [DOI] [PubMed] [Google Scholar]
- 21.Sautenet B, Tong A, Manera KE, et al. Developing consensus-based priority outcome domains for trials in kidney transplantation: a multinational Delphi survey with patients, caregivers, and health professionals. Transplantation. 2017;101:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeve BB, Thissen D, DeWalt DA, et al. Linkage between the PROMIS(R) pediatric and adult emotional distress measures. Qual Life Res. 2016;25: 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thissen D, Liu Y, Magnus B, et al. Estimating minimally important difference (MID) in PROMIS pediatric measures using the scale-judgment method. Qual Life Res. 2016;25:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purvis TE, Andreou E, Neuman BJ, et al. Concurrent validity and responsiveness of PROMIS health domains among patients presenting for anterior cervical spine surgery. Spine (Phila Pa 1976). 2017;42:E1357–E1365. [DOI] [PubMed] [Google Scholar]
- 25.Purvis TE, Neuman BJ, Riley LH 3rd, et al. Discriminant ability, concurrent validity, and responsiveness of PROMIS health domains among patients with lumbar degenerative disease undergoing decompression with or without arthrodesis. Spine (Phila Pa 1976). 2018;43:1512–1520. [DOI] [PubMed] [Google Scholar]
- 26.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45: S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akshoomoff N, Beaumont JL, Bauer PJ, et al. VIII. NIH Toolbox Cognition Battery (CB): composite scores of crystallized, fluid, and overall cognition. Monogr Soc Res Child Dev. 2013;78:119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairclough DL. Summary measures and statistics for comparison of quality of life in a clinical trial of cancer therapy. Stat Med. 1997;16:1197–1209. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz CE, Patrick DL. Composite scores in comparative effectiveness research: counterbalancing parsimony and dimensionality in patient-reported outcomes. J Comp Eff Res. 2014;3:423–433. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


