Abstract
Glutathione (GSH) is the most abundant non-protein thiol, attaining cellular concentrations in the millimolar range. GSH functions to protect cells against endogenous and exogenous electrophiles. In addition, GSH serves as a cofactor for the GSH peroxidase family of enzymes which metabolize H2O2 as well as lipid peroxides. Through the action of glutathione S-transferase family of enzymes, GSH is conjugated to a variety of electrophilic endogenous compounds and exogenous chemicals, and thereby facilitates their efficient and safe elimination. Through the transsulfuration pathway, GSH biosynthesis is metabolically linked with cellular methylation, which is pivotal for epigenetic gene regulation. Accumulating evidence suggests that the underlying mechanisms of alcohol-associated tissue injury and carcinogenesis involve: (i) generation of the electrophilic metabolite acetaldehyde, (ii) induction of CYP2E1 leading to the formation of reactive oxygen species and pro-carcinogen activation, and (iii) nutritional deficiencies, such as methyl groups, resulting in enhanced susceptibility to cancer development. In this context, clinical and experimental investigations suggest an intimate involvement of GSH and related enzymes in the development of alcohol-induced pathological conditions. The aim of this review is to provide an overview of the GSH biosynthesis, cellular transsulfuration/transmethylation pathways, and their implications in the pathogenesis and treatment of alcohol-related disease and cancer.
Keywords: Alcoholic, Cancer, Oxidative stress, Glutathione, Transsulfuration, Methylation
3.1. Introduction
Glutathione (GSH) is a ubiquitous tripeptide composed of glutamate, cysteine and glycine. It presents as the most prevalent non-protein thiol in mammalian cells. Extensive research has revealed numerous and diverse cellular functions of GSH [1]. It detoxifies xenobiotics and endogenous metabolites through non-enzymatic or enzymatic mechanisms. It functions as a major antioxidant to protect cells against oxidative damage caused by reactive oxygen species (ROS). As such, it is essential in maintaining the intracellular redox balance and the thiol moieties of proteins. Through such processes, GSH can modulate protein function via redox post-translational modification. It also plays a role in the regulation of nitric oxide homeostasis. Through the transsulfuration pathway, GSH participates in cellular shuttling of other sulfur amino acids [2]. Given the diversity and importance of these functions of GSH, it should come as no surprise that alterations in GSH levels have been found to be associated with numerous human pathological conditions, including cancer, liver disease, cardiovascular disease, neurological disorders, diabetes, and other disease conditions [3].
Oxidative stress occurs when ROS are produced at levels exceeding those capable of being sequestered by normal cellular antioxidant processes. Chronic ethanol consumption induces oxidative stress in organs via cellular pathways that promote the overproduction of reactive molecules (including ROS and electrophilic products, such as acetaldehyde and lipid peroxidation-derived products) and/or the diminution of antioxidant defenses, such as GSH [4]. Studies in human subjects and animal models have implicated an important mechanistic role for disrupted GSH homeostasis in the pathogenesis of alcohol-related non-cancerous diseases, particularly alcoholic liver disease [5]. The involvement of changes in the GSH redox homeostasis in alcohol-associated cancers, however, appears more complex and remains to be elucidated. This review focuses on the links between GSH, the transsulfuration pathway, and alcohol-induced tissue injury, and their involvement in the development and therapy of alcohol-related cancers.
3.2. GSH Biosynthesis, Metabolism and Function
GSH is synthesized by two successive enzymatic reactions (Fig. 3.1) [6]. The first reaction, catalyzed by glutamate-cysteine ligase (GCL), couples glutamate and cysteine to form γ-glutamylcysteine (γ-GC). The second reaction couples γ-GC with glycine and is catalyzed by GSH synthase (GSS). Each of these enzymatic reactions consumes one molecule of ATP per catalytic cycle. The formation of γ-GC by GCL is considered the rate-limiting enzymatic step in GSH biosynthesis. For this reason, GCL rather than GSS has been the principal target of drugs designed to inhibit GSH biosynthesis [7] and to generate animal models with GSH deficiency [8]. GCL of higher eukaryotic organisms, in its most catalytically efficient form, is a heterodimer composed of a catalytic (GCLC) and a modifier (GCLM) subunit, each of which is encoded by separate genes. As its name implies, GCLC possesses all of the catalytic activity of GCL, and GCLM serves to optimize the kinetic properties of GCLC [9]. Both the GCLC and GCLM genes are up-regulated by electrophiles or agents that cause oxidant stress [10] via transcriptional mechanisms reminiscent of phase II drug metabolizing-enzyme genes. While GCLC and GCLM genes are commonly found up-regulated together, cell type-specific differential expression of GCLC and GCLM transcripts suggest independent regulation of these subunits [8]. Current evidence indicates that most, if not all, of the GSH biosynthetic activity resides in the cytoplasm [11]. The GSH, thus produced, is further distributed into intracellular organelles including the mitochondria, endoplasmic reticulum (ER) and nuclei [12].
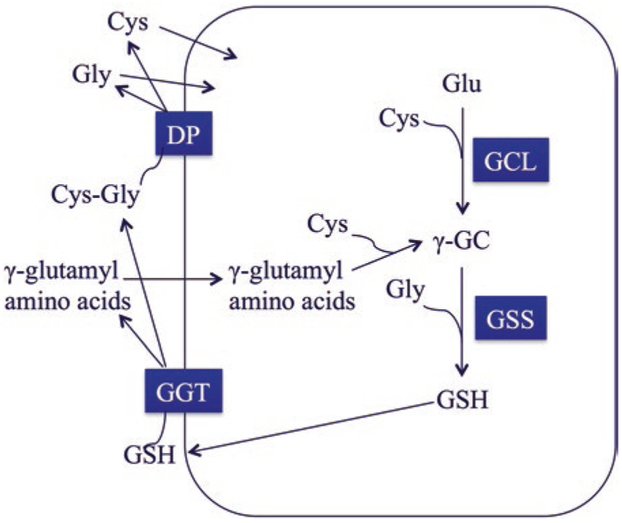
Fig. 3.1. Scheme of γ–glutamyl cycle for glutathione (GSH) biosynthesis and catabolism.
GSH is synthesized by two successive enzymatic reactions. Glutamate-cysteine ligase (GCL) couples glutamate (Glu) and cysteine (Cys) to form γ-glutamylcysteine (γ-GC), which is the rate-limiting step in GSH synthesis. GSH synthase (GSS) then couples γ-GC with glycine to form GSH. GSH can be transported out of the cell where it is catabolized by γ-glutamyl transferase (GGT). GGT cleaves the γ-glutamyl amide bond between Glu and Cys releasing cysteinylglycine (Cys-Gly) and γ–glutamyl amino acids. Cys-Gly can be further cleaved by an extracellular dipeptidase (DP), producing free Cys and Gly for reuse by the cell. γ–glutamyl amino acids can be taken up by the cell to form γ-GC, essentially bypassing the need for catalysis by GCL
Due to the presence of a unique γ-glutamyl amide bond between the γ-carbon of the glutamate side chain and the amino group of cysteine, GSH cannot be broken down by peptidases inside the cell. Rather, GSH must be transported through the plasma membrane and out of the cell, where it is metabolized by γ-glutamyl transferases [GGTs] [6]. These enzymes catalyze the ATP-dependent cleavage of the γ-glutamyl amide bond between glutamate and cysteine, and generates cysteinylglycine that can be further cleaved by an extracellular dipeptidase (DP). This reaction produces free cysteine and glycine, which can then be used by cells. These reactions for synthesis and degradation of GSH form a metabolic pathway known as the γ-glutamyl cycle [13] (Fig. 3.1). By way of this cycle, GSH participates in amino acid transport for cellular re-synthesis of GSH and other proteins. In addition, it represents a salvage pathway by which GSH can be produced independently of GCL [14].
GSH is the most abundant cellular thiol, attaining concentrations from 1 to 10 mM depending on the cell type [11, 15, 16]. The oxidized form of GSH is glutathione disulfide (GSSG). The cellular GSH/GSSG ratio has been used as an index of cellular redox status. Under normal circumstances, this ratio exceeds 10:1; a decrease in GSH/GSSG ratio is commonly associated with increased cellular oxidative stress [17]. GSH serves to protect cells against toxicity arising from exposure to excessive amounts of endogenous and exogenous electrophiles [7]. It scavenges hydroxyl radical and superoxide directly, and serves as a cofactor for the glutathione peroxidase (GPX) enzymes in metabolizing H2O2, as well as lipid peroxides [18]. Through the action of the glutathione S-transferase (GST) family of enzymes, GSH may be conjugated to a variety of electrophilic endogenous compounds and exogenous chemicals, and thereby facilitates their efficient and safe elimination [19]. Together, GSH and GSSG function as an important cellular redox buffering system that has been suggested to be involved in determining cell fate decisions, such as proliferation and apoptosis [20].
In subcellular compartments, GSH plays a pivotal role in the normal functioning of mitochondria, where oxygen consumption and generation of ROS occurs. GSH in the nucleus maintains the redox status of critical protein sulfhydryl groups that are necessary for expression, transcription activity, and DNA repair [21]. In contrast to other organelles, GSH in the endoplasmic reticulum exists more in the oxidized state (GSSG), which is believed to be necessary for providing the appropriate environment for assembly and secretory pathways for proteins [22].
3.3. The Transsulfuration Pathway
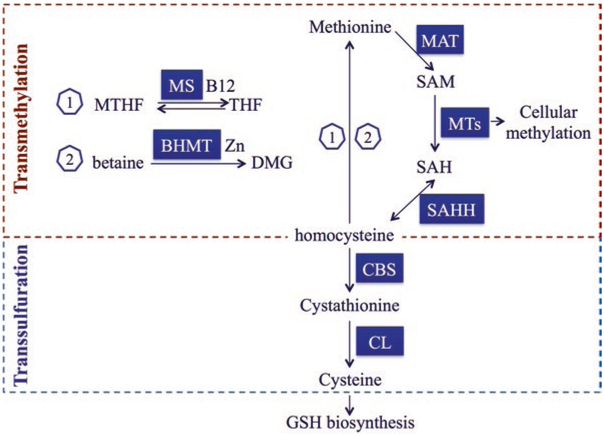
Transsulfuration is a biochemical pathway that connects glutathione biosynthesis to the metabolism of sulfur-containing amino acids, viz., methionine and cysteine (Fig. 3.2) [23]. In the methionine cycle, methionine forms S-adenosylmethionine (SAM) in a reaction catalyzed by methionine adenosyltransferase (MAT). SAM is converted to S-adenosylhomocysteine (SAH) by the actions of methyltransferases (MTs), which transfer the methyl group to accepting molecules. Homocysteine is then derived from SAH via a reversible reaction catalyzed by SAH hydrolase (SAHH). Methionine can be regenerated from homocysteine by one of two methylation pathways. In the first, methionine synthase (MS) catalyzes the transfer of a methyl group from N5-methyltetrahydrofolate (MTHF) to homocysteine creating methionine and tetrahydrofolate (THF); this reaction requires vitamin B12 as a cofactor. In the second pathway, betaine serves as the source of the methyl group transferred to homocysteine, a reaction catalyzed by the zinc-dependent enzyme betaine homocysteine methyltransferase (BHMT). The transsulfuration pathway involves homocysteine being irreversibly converted to cystathionine by the enzyme cystathioninβ-synthase (CBS). Cystathionine is converted to cysteine by cystathionine-γ-lyase (CL). The resulting cysteine can then be used for GSH biosynthesis. In the liver, approximately 50% of the cysteine used for GSH synthesis is derived from the transsulfuration pathway from methionine [24, 25].
Fig. 3.2. Major enzymes and intermediates in cellular transmethylation-transsulfuration pathways.
In the liver, the transsulfuration pathway connects transmethylation cycle (methionine cycle) to glutathione (GSH) biosynthesis. Methionine forms S-adenosylmethionine (SAM), the major biological methyl donor, by the action of methionine adenosyltransferase (MAT). SAM is then converted to S-adenosylhomocysteine (SAH) by the actions of various methyltransferases (MTs). These MTs transfer the methyl group to accepting molecules (e.g., DNA, RNA and proteins) undergoing methylation. Homocysteine is derived from the hydrolysis of SAH by the action of SAH hydrolase (SAHH). In the methionine cycle, methionine can be regenerated from homocysteine by one of two remethylation pathways. In one pathway (1), methionine synthase (MS) catalyzes the transfer of a methyl group from N5-methyltetrahydrofolate (MTHF) to homocysteine creating methionine and tetrahydrofolate (THF); this reaction requires vitamin B12 (B12) as a cofactor. In the other pathway (2), betaine is the source of the methyl group transferred to homocysteine, which is catalyzed by a zinc (Zn)-dependent enzyme, betaine homocysteine methyltransferase (BHMT). The transsulfuration pathway starts with homocysteine being irreversibly converted to cystathionine by the enzyme cystathionine-β-synthase (CBS). Cystathionine is further converted to cysteine by cystathionine-γ-lyase (CL). Cysteine can then feed GSH biosynthesis
The functional importance of these metabolic pathways is underscored by their essentiality for cellular methylation and for the maintenance of cellular redox homeostasis. The intermediate, SAM, serves as a primary methyl donor participating in epigenetic gene regulation, protein stability, and phospholipid and neurotransmitter production [26]. Through the transsulfuration pathway, SAM has been shown to increase GSH, inhibit lipid peroxidation, and protect against oxidative stress associated with ischemia-reperfusion injury in brain tissues [27]. Deficiencies in enzymes of the transsulfuration pathway may lead to ROS generation, homocysteine accumulation and macrophage synthesis of proinflammatory molecules, and thereby contribute to human pathologies like atherosclerosis and tumor development [23]. Homocysteine accumulation induces fibrin deposition, oxidant stress, cytokine release and inflammation, promoting atherosclerosis [28].
3.4. Glutathione and Transsulfuration in Alcohol-Related Non-Cancerous Diseases
Alcohol consumption can cause a variety of health issues. Heavy drinking is associated with numerous non-cancerous health conditions, including liver disease, cardiovascular disease, disorders of the digestive tract, pulmonary disease, and neurobehavioral disorders. Oxidative stress appears to be intimately involved in the initiation and progression of these diseases [4]. Alcohol consumption induces oxidative stress through a variety of cellular changes; an important one involves compromised cellular antioxidant defense mechanisms including alterations in GSH [4]. GSH levels and/or its redox status (e.g., GSH/GSSG ratio) in the plasma and tissues from ethanol-fed animals and chronic alcoholics have been investigated in numerous studies. In rodents, chronic ethanol consumption caused decreases in heart cytosolic and mitochondrial GSH levels and concomitant increases in cytosolic and mitochondrial levels of lipid peroxidation and protein carbonyls; such compromised oxidant buffering capacity has been proposed to contribute to the pathogenesis of alcoholic cardiomyopathy [29]. The impact of chronic alcoholism on systemic and pulmonary GSH redox status was investigated in a cohort comprising healthy alcohol-dependent subjects and control subjects [30]. Chronic alcoholics showed dramatic oxidant stress in the alveolar space manifesting as decreased GSH, increased GSSG, and a corresponding oxidative shift in the redox potential of GSH/GSSG. Systemic oxidative stress was observed in alcoholics who also smoked. Interestingly, alcohol-induced chronic oxidant stress in the alveolar space may sensitize alcohol abusers to acute respiratory distress syndrome [31].
The liver is a major organ subject to ethanol-induced toxicity. There is a wealth of data from studies in human and experimental animals documenting ethanol-induced changes in hepatic GSH homeostasis, including GSH/GSSG and GSH-related antioxidant enzymes [32–35]. Collectively, these studies suggest that depletion of hepatic GSH, particularly mitochondrial GSH, is one of the early changes associated with chronic ethanol consumption [36]. Importantly, plasma GSH concentrations are inversely correlated with the degree of liver damage and hepatic lipid peroxidation [32–34]. Prolonged ethanol consumption has been reported to inhibit multiple steps in methionine metabolism and transsulfuration pathways in the liver, resulting in increased homocysteine and SAH levels, and a lowered heptaic SAM/SAH ratio [37, 38]. Enzymes affected directly or indirectly by ethanol include MAT, BHMT and various methytransferases [37, 39]. The detrimental consequences of these changes include, but are not limited to, dysregulation of gene expression (due to altered DNA methylation), homocysteine-promoted inflammation, and inhibition of GSH biosynthesis [37, 39]. Importantly, serum levels of intermediates of the transsulfuration pathway (such as cystathionine) have been proposed as diagnostic markers for the severity of alcoholic liver disease (ALD) [40].
In a recent study, we utilized a transgenic mouse model to elucidate the role of GSH redox homeostasis in the hepatic response to chronic ethanol consumption [41]. Global disruption of the Gclm gene (GCLM knockout) results in mice that have greatly reduced (10–40% normal) tissue GSH and lower plasma GSH/GSSG [42]. In the liver, 85% depletion of GSH results in an oxidative shift of hepatic GSH redox potential by 65 mV, 60% decrease in mitochondrial GSH pool and yet mitochondrial functioning remains intact [43]. Thus, GCLM knockout (KO) mice represent a model of chronic hepatic and systemic oxidative stress. Following chronic ethanol consumption, these mice are unexpectedly protected from ethanol-induced steatosis and liver damage [41]. At the molecular level, this protective phenotype appears to involve following beneficial cellular adaptions: (i) suppression of lipogenic genes and induction of genes involved in fatty acid oxidation, (ii) induction of the nuclear-factor-erythroid 2–related-factor 2 (NRF2) antioxidant response, and (iii) activation of the AMP-activated protein kinase (AMPK) metabolic signaling pathway [41]. Our study showed unconventional beneficial cellular consequences associated with GSH deficiency, implying that hepatic GSH homeostasis may function to modulate metabolic and stress responses to ethanol consumption.
3.5. Alcohol-Mediated Carcinogenesis
Ethanol and its direct metabolite acetaldehyde have been identified as human carcinogens by the International Agency for Research on Cancer (IARC). Available epidemiological studies have established that alcohol consumption is strongly associated with an increased risk for cancers of stomach, oropharynx, larynx, oesophagus, head and neck, liver, pancreas, female breast, colorectum, and gallbladder [44–46]. In this context, alcohol is estimated to have contributed to 3.2–3.7% and 5.8% of cancer deaths worldwide and in the United States, respectively [47].
Drinking patterns play an important role in influencing the relationship between alcohol and cancer risk. An increased risk of breast cancer is associated with chronic alcohol consumption and it occurs in a dose-dependent manner [48, 49]. Consumption of 10 g alcohol each day raises the risk by 8% for post-menopausal breast cancer, 9% for pre-menopausal breast cancer, and 10% for overall breast cancer [49]; risk increases by ≈7% for every additional 10 g alcohol consumed each day [49]. A dose-dependent association also exists between lifetime alcohol intake and the risk of upper-aero digestive tract (UADT) cancer (e.g., of the oral cavity, pharynx, larynx or oesophagus) (multivariable-adjusted relative risk was 2.67 for an intake of ≥40 g/day, and 1.16 for a 10 g/day increment in intake) [50]. For the lower digestive tract, longer duration and higher amount of alcohol consumption were associated with increased colorectal cancer risk (relative risk was 2.24 for ≥30 g/day) [51–54]. While the main causal factor of hepatocellular carcinoma (HCC) is chronic infection with hepatitis B (HBV) and C (HCV) viruses, alcohol intake represents an independent risk factor for HCC [55, 56]. Chronic ethanol consumption can cause a spectrum of ALDs, which clinically can manifest as steatosis, steatohepatitis, fibrosis, and cirrhosis [57]. Only ≈1~2% of cirrhotic patients develop HCC [58]. Daily alcohol ingestion exceeding 20.44 g was associated with higher risks of both liver cancer occurring and liver disease mortality [59]. The dose-response relationship between alcohol consumption and liver cancer was apparent with relative risks of 1.54 for 50 g/day, 2.14 for 75 g/day, 3.21 for 100 g/day, and 5.20 for 125 g/day [60]. It should be noted that a J-shaped dose-response relationship between alcohol consumption and all-cause or all-cancer mortality was observed, implicating a possible beneficial effect of light drinking [61–63].
The exact molecular mechanisms causing alcohol-associated carcinogenesis are not well understood. Several have been proposed and are reviewed in depth elsewhere [58, 64, 65]. Alcohol is thought to exert carcinogenic effects at many levels, including acetaldehyde formation, induction of CYP2E1, oxidative stress, epigenetic alterations due to a reduced capacity for methyl moiety transfer, and modulation of cellular growth [58]. Alcohol is metabolized primarily via oxidation to acetaldehyde through the actions of alcohol dehydrogenases (ADHs) and, to a lesser extent, CYP2E1 and catalase. Acetaldehyde is then oxidatively detoxified to acetate by the aldehyde dehydrogenase enzymes (ALDHs) [66]. Acetaldehyde is a highly reactive molecule capable of adducting DNA and proteins [67, 68]. Mitochondrial ALDH2 is the primary ALDH enzyme responsible for the elimination of acetaldehyde [69]. Human subjects carrying a defective allele of the ALDH2 gene (ALDH2*2 allele) have a greatly reduced capacity (10–45% normal in heterozygotes and 1–5% normal in homozygotes) to metabolize acetaldehyde [70]. Epidemiological studies have revealed these individuals to be highly susceptible to the development of gastrointestinal cancers following excessive alcohol consumption [71]. Following chronic ethanol consumption, acetaldehyde-DNA adducts are elevated to a greater extent in the liver and stomach of Aldh2 KO mice than in wild-type mice [72, 73]. Studies in humans and experimental animals have established that acetaldehyde-DNA adduct formation is an initial step in ethanol-induced carcinogenesis [74]. Alcohol induction of CYP2E1 serves as an important molecular pathway by which ethanol can promote carcinogenicity [65]. Specifically, CYP2E1 activation may bioactivate other procarcinogens and is an important cellular source of ROS formation, including superoxide anion, hydrogen peroxide and the lipid peroxidation by-products malondialdehyde and 4-hydroxynonenal (4-HNE) [65]. 4-HNE can form highly mutagenic DNA-adducts; such adducts are more frequently observed in advanced stages of ALD [75, 76]. In addition to CYP2E1 activation, ethanol-induced oxidative stress can arise from dysfunctional mitochondrial respiration, iron overload, inflammation and/or compromised antioxidant defenses [77]. The epigenetic aspect of alcohol-induced carcinogenesis has been the subject of extensive studies in recent years and is covered in comprehensive reviews elsewhere [78, 79]. Accumulating lines of evidence suggest that ethanol consumption causes aberrant patterns of DNA methylation and thereby altered gene expression by inhibiting key enzymes involved in SAM bioavailability and DNA methyltransferases [79]. Finally, chronic ethanol consumption lowers hepatic concentrations of vitamin A and retinoic acid, which are critical modulators of cellular growth and differentiation. Importantly, an apparent inverse relationship appears to exist between serum concentrations of vitamin A and later development of HCC in humans [80, 81].
3.6. GSH and Transsulfuration in Cancer Biology and Alcohol-Related Cancers
GSH appears to play a paradoxical role in cancer biology. Firstly, oxidative stress due to production of ROS and/or electrophilic metabolites is an important mutagenic mechanism for numerous physical (e.g., ultraviolet light exposure) and chemical (e.g., alcohol) carcinogens [82–84]. GSH scavenges DNA-damaging free radicals directly or via enzymatic reactions (e.g., GPXs and GSTs), and in doing so, it may contribute to the prevention of tumor initiation [85, 86]. Secondly, some oncogenes (e.g., AP-1) and tumor suppressors (e.g., P53) are transcription factors that play key roles in controlling cell proliferation and death in response to genomic stress. The DNA-binding activity of these proteins requires the maintenance of some crucial cysteine residues in a reduced form [87, 88]. By acting as a major homeostatic redox buffer in subcellular compartments, GSH-GSSG couple may modulate the activities of tumor suppressors or oncoproteins, thereby contributing to tumor promotion [89]. Thirdly, many highly metastatic cancer cells attain high intracellular levels of GSH; such a situation is typically associated with higher expressions of γ-glutamyl cycle enzymes, such as GCL and GGT [90–92]. These biochemical features are believed to function at multiple levels to promote the growth advantage and metastasis of neoplastic cells, such as: (a) the γ-glutamyl cycle supplies the fast turnover of cysteine and other amino acids for protein synthesis, (b) high GSH helps to maintain mitochondrial functional integrity to meet the high metabolic demands of the neoplastic cells, and (c) GSH combats harmful ROS or reactive nitrogen species (RNS) released by vascular endothelial cells in response to cancer cell contact in the process of metastatic invasion. Lastly, resistance of cancer cells to radiation and chemotherapy appears to correlate directly to their GSH levels. This is often accompanied by over-expression of multidrug resistance-associated proteins (MRPs) and GST enzymes [93–96]. Several mechanisms have been proposed for the role of GSH in regulating drug resistance of cancer cells: (a) GSH may directly protect against oxidative cytotoxicities elicited by anti-cancer treatments, (b) MRPs are a family of ATP-binding cassette membrane transporters that mediate the efflux of GSH and GSH-conjugates; GSH may facilitate the export of anti-cancer drugs through the actions of MRP proteins, and (c) GSTs are phase II detoxification enzymes that catalyze GSH conjugation with different chemotherapeutic compounds for their safe elimination; GSH may promote GST-mediated metabolic elimination of anti-cancer drugs by serving as its cofactor. The latter two mechanisms may act independently or cooperatively to diminish the therapeutic effects of anticancer drugs in cancer cells expressing high levels of GSH. Taken together, GSH seems to have bidirectional functions such that it can protect against neoplastic transformation in non-tumor cells while also being able to promote metastasis and chemoresistance of neoplastic cells.
Deficiencies in the transsulfuration pathway have been documented to occur in cancer cells and cancerous tissues [23]. Genetic polymorphisms in the CBS gene (which encodes the enzyme converting homocysteine to cystathionine) have been associated with increased risks for breast, gastrointestinal and lung cancers [97–99]. The importance of the transsulfuration pathway in cancer biology attributes partially to its metabolic link to the metabolism of cysteine and GSH [23]. The trans-sulfuration pathway also connects to the methionine cycle through homocysteine. A blockade of this pathway results in homocysteine accumulation as well as altered cellular transmethylation [23], both of which have been implicated in tumor initiation and progression. Homocysteine is a pro-inflammatory intermediate that causes ROS production, cytokine release, and altered expression of adhesion molecules [100]. Elevated levels of homocysteine induce chronic inflammation and are an established risk factor for coronary heart disease [28]. Many tumor cells both require high methionine for growth and export large amounts of homocysteine [101]. The elevated production of homocysteine by methionine-dependent cancer cells is proposed to act as an adaptive mechanism that promotes a cancer microenvironment for cancer cell survival, colonization and vascular invasion [102, 103]. In the methionine cycle, accumulated homocysteine can be converted to SAH that, as a potent inhibitor of cellular methylation, can lead to SAM deficiency [104]. In agreement with this notion, aberrant DNA methylation is often observed in hyperhomocysteinemia-associated pathologies (including cancer) and is considered an important causal factor of the disease condition [104].
In the context of alcohol-related cancer, ethanol-induced depletion of the cellular GSH pool and inhibition of transsulfuration/transmethylation pathways are of particular importance for the development of alcoholic HCC. Clinically, low levels of hepatocellular GSH and SAM and a low SAM/SAH ratio are commonly observed in chronic alcoholics with advanced stage ALD and they correlate with the severity of liver damage [32, 37, 38]. The significance of reduced SAM production in the development of HCC is supported by several findings: SAM feeding blocked the transformation of pre-neoplastic lesions into HCCs [105], SAM administration inhibited the expressions of selected proto-oncogenes [106], SAM decreased the survival of liver tumor cells in vitro in a dose-dependent manner [107], and SAM treatment prevented liver tumor formation in a xenograft model [108]. The proposed mechanisms underlying a protective role of SAM against alcoholic HCC, including providing precursors for GSH biosynthesis and supplying methyl groups for balanced DNA methylation, are presented and discussed comprehensively in other articles [109, 110].
Along with abstinence from alcohol and anti-inflammatory treatment, nutrient (e.g., SAM) and antioxidants (e.g., GSH) supplementation represents an important element for preventive and therapeutic management of ALD including cancer [111, 112]. The use of GSH precursors (e.g., N-acetylcysteine) [113, 114], intermediates of transmethylation pathway (e.g., SAM, folate and betaine) [110] and compounds possessing antioxidant properties (e.g. vitamin E and plant extracts) [115, 116] have been investigated in experimental animal models and pilot human studies targeting at advanced ALD. These studies have provided inconsistent results in that human studies largely showed no beneficial effects in improving clinical markers of chronic liver damage or preventing degeneration into hepatocellular carcinoma [117, 118]. However, the lack of therapeutic efficacy of these compounds may be related to their complex pharmacokinetics in ALD patients. Nevertheless, it has been proposed that long-term use of antioxidants (including SAM) may assume a greater role for the treatment of ALD patients who are in the process of achieving sobriety and at risk for progression to cirrhosis and HCC.
3.7. Concluding Remarks
Individuals who abuse alcohol on a chronic basis are predisposed to the development of numerous diseases including cancer. GSH is a ubiquitous tripeptide that functions as a major cellular antioxidant and redox-buffering molecule. The transsulfuration pathway metabolically connects GSH biosynthesis with cellular transmethylation. Chronic alcohol consumption results in depletion of the cellular GSH pool and inhibition of cellular transsulfuration/transmethylation, which are key pathogenic events involved in alcohol-associated tissue injury and carcinogenesis. Molecular details of these processes are yet to be defined. Therapeutic strategies targeted at improving these metabolic changes are inconclusive and warrant further studies.
Acknowledgement
This work was supported in part by the USA National Institutes of Health grants K01AA025093, R24AA022057, U01AA021724, and the China Scholarship Council No.201508140059.
Contributor Information
Ying Chen, Department of Environmental Health Sciences, Yale School of Public Health, Yale University,New Haven, CT, USA.
Ming Han, Department of Environmental Health Sciences, Yale School of Public Health, Yale University, New Haven, CT, USA, College of Environment and Resource, Shanxi University, Taiyuan, Shanxi, China.
Akiko Matsumoto, Department of Social Medicine, Saga University School of Medicine, Saga, Japan.
Yewei Wang, Department of Environmental Health Sciences, Yale School of Public Health, Yale University, New Haven, CT, USA.
David C. Thompson, Department of Clinical Pharmacy, University of Colorado School of Pharmacy, Aurora, CO, USA
Vasilis Vasiliou, Department of Environmental Health Sciences, Yale School of Public Health, Yale University, New Haven, CT, USA.
References
- 1.Rana SV, Allen T, Singh R (2002) Inevitable glutathione, then and now. Indian J Exp Biol 40:706–716 [PubMed] [Google Scholar]
- 2.Jung YS (2015) Metabolism of sulfur-containing amino acids in the Liver: a link between hepatic injury and recovery. Biol Pharm Bull 38:971–974 [DOI] [PubMed] [Google Scholar]
- 3.Townsend DM, Tew KD, Tapiero H (2003) The importance of glutathione in human disease. Biomed Pharmacother 57:145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu D, Cederbaum AI (2003) Alcohol, oxidative stress, and free radical damage. Alcohol Res Health 27:277–284 [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Checa JC, Hirano T, Tsukamoto H, Kaplowitz N (1993) Mitochondrial glutathione depletion in alcoholic liver disease. Alcohol 10:469–475 [DOI] [PubMed] [Google Scholar]
- 6.Meister A (1988) Glutathione metabolism and its selective modification. J Biol Chem 263:17205–17208 [PubMed] [Google Scholar]
- 7.Meister A (1991) Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol Ther 51:155–194 [DOI] [PubMed] [Google Scholar]
- 8.Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG (2004) Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med 37:1511–1526 [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP (2005) Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J Biol Chem 280:33766–33774 [DOI] [PubMed] [Google Scholar]
- 10.Lu SC (2009) Regulation of glutathione synthesis. Mol Asp Med 30:42–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meister A (1982) Metabolism and function of glutathione: an overview. Biochem Soc Trans 10:78–79 [DOI] [PubMed] [Google Scholar]
- 12.Lu SC (1999) Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J 13:1169–1183 [PubMed] [Google Scholar]
- 13.Njalsson R, Norgren S (2005) Physiological and pathological aspects of GSH metabolism. Acta Paediatr 94:132–137 [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Forman HJ, Choi J (2005) Gamma-glutamyl transpeptidase in glutathione biosynthesis. Methods Enzymol 401:468–483 [DOI] [PubMed] [Google Scholar]
- 15.Kosower NS, Kosower EM (1978) The glutathione status of cells. Int Rev Cytol 54:109–160 [DOI] [PubMed] [Google Scholar]
- 16.Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760 [DOI] [PubMed] [Google Scholar]
- 17.Griffith OW (1999) Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 27:922–935 [DOI] [PubMed] [Google Scholar]
- 18.Arthur JR (2000) The glutathione peroxidases. Cell Mol Life Sci 57:1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rinaldi R, Eliasson E, Swedmark S, Morgenstern R (2002) Reactive intermediates and the dynamics of glutathione transferases. Drug Metab Dispos 30:1053–1058 [DOI] [PubMed] [Google Scholar]
- 20.Jones DP (2002) Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol 348:93–112 [DOI] [PubMed] [Google Scholar]
- 21.Green RM, Graham M, O’Donovan MR, Chipman JK, Hodges NJ (2006) Subcellular compartmentalization of glutathione: correlations with parameters of oxidative stress related to genotoxicity. Mutagenesis 21:383–390 [DOI] [PubMed] [Google Scholar]
- 22.Hwang C, Sinskey AJ, Lodish HF (1992) Oxidized redox state of glutathione in the endoplasmic reticulum. Science 257:1496–1502 [DOI] [PubMed] [Google Scholar]
- 23.Rosado JO, Salvador M, Bonatto D (2007) Importance of the trans-sulfuration pathway in cancer prevention and promotion. Mol Cell Biochem 301:1–12 [DOI] [PubMed] [Google Scholar]
- 24.Mosharov E, Cranford MR, Banerjee R (2000) The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 39:13005–13011 [DOI] [PubMed] [Google Scholar]
- 25.Vitvitsky V, Dayal S, Stabler S, Zhou Y, Wang H et al. (2004) Perturbations in homocysteine-linked redox homeostasis in a murine model for hyperhomocysteinemia. Am J Physiol Regul Integr Comp Physiol 287:R39–R46 [DOI] [PubMed] [Google Scholar]
- 26.Roje S (2006) S-Adenosyl-L-methionine: beyond the universal methyl group donor. Phytochemistry 67:1686–1698 [DOI] [PubMed] [Google Scholar]
- 27.Tchantchou F, Graves M, Falcone D, Shea TB (2008) S-adenosylmethionine mediates glutathione efficacy by increasing glutathione S-transferase activity: implications for S-adenosyl methionine as a neuroprotective dietary supplement. J Alzheimers Dis 14:323–328 [DOI] [PubMed] [Google Scholar]
- 28.Ganguly P, Alam SF (2015) Role of homocysteine in the development of cardiovascular disease. Nutr J 14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebbe S (1974) Thrombopoietin. Thrombopoietin Blood 44:605–608 [PubMed] [Google Scholar]
- 30.Yeh MY, Burnham EL, Moss M, Brown LA (2007) Chronic alcoholism alters systemic and pulmonary glutathione redox status. Am J Respir Crit Care Med 176:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaphalia L, Calhoun WJ (2013) Alcoholic lung injury: metabolic, biochemical and immunological aspects. Toxicol Lett 222:171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ucar G, Demir B, Ulug B (2005) Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type I and II alcoholics. Cell Biochem Funct 23:29–37 [DOI] [PubMed] [Google Scholar]
- 33.Singh M, Gupta S, Singhal U, Pandey R, Aggarwal SK (2013) Evaluation of the oxidative stress in chronic alcoholics. J Clin Diagn Res 7:1568–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta S, Pandey R, Katyal R, Aggarwal HK, Aggarwal RP et al. (2005) Lipid peroxide levels and antioxidant status in alcoholic liver disease. Indian J Clin Biochem 20:67–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Dong H, Thompson DC, Shertzer HG, Nebert DW et al. (2013) Glutathione defense mechanism in liver injury: insights from animal models. Food Chem Toxicol 60:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantena SK, King AL, Andringa KK, Landar A, Darley-Usmar V et al. (2007) Novel interactions of mitochondria and reactive oxygen/nitrogen species in alcohol mediated liver disease. World J Gastroenterol 13:4967–4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kharbanda KK (2013) Methionine metabolic pathway in alcoholic liver injury. Curr Opin Clin Nutr Metab Care 16:89–95 [DOI] [PubMed] [Google Scholar]
- 38.Lu SC, Tsukamoto H, Mato JM (2002) Role of abnormal methionine metabolism in alcoholic liver injury. Alcohol 27:155–162 [DOI] [PubMed] [Google Scholar]
- 39.Halsted CH, Medici V (2012) Aberrant hepatic methionine metabolism and gene methylation in the pathogenesis and treatment of alcoholic steatohepatitis. Int J Hepatol 2012:959746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medici V, Peerson JM, Stabler SP, French SW, Gregory JF 3rd, et al. (2010) Impaired homocysteine transsulfuration is an indicator of alcoholic liver disease. J Hepatol 53: 551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Singh S, Matsumoto A, Manna SK, Abdelmegeed MA, et al. (2016) Chronic glutathione depletion confers protection against alcohol-induced steatosis: implication for redox activation of AMP-activated protein kinase pathway. Sci Rep 6: 29743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW et al. (2002) Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(−/−) knockout mouse. Novel model system for a severely compromised oxidative stress response. J Biol Chem 277:49446–49452 [DOI] [PubMed] [Google Scholar]
- 43.Kendig EL, Chen Y, Krishan M, Johansson E, Schneider SN et al. (2011) Lipid metabolism and body composition in Gclm(−/−) mice. Toxicol Appl Pharmacol 257:338–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connor J (2017) Alcohol consumption as a cause of cancer. Addiction 112:222–228 [DOI] [PubMed] [Google Scholar]
- 45.Jayasekara H, MacInnis RJ, Hodge AM, Room R, Milne RL et al. (2016) Is breast cancer risk associated with alcohol intake before first full-term pregnancy? Cancer Causes Control 27:1167–1174 [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Cheng W, Li J, Zhu J (2016) A meta-analysis of alcohol consumption and thyroid cancer risk. Oncotarget 7:55912–55923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Praud D, Rota M, Rehm J, Shield K, Zatonski W et al. (2016) Cancer incidence and mortality attributable to alcohol consumption. Int J Cancer 138:1380–1387 [DOI] [PubMed] [Google Scholar]
- 48.Scoccianti C, Lauby-Secretan B, Bello PY, Chajes V, Romieu I (2014) Female breast cancer and alcohol consumption: a review of the literature. Am J Prev Med 46:S16–S25 [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Nguyen N, Colditz GA (2015) Links between alcohol consumption and breast cancer: a look at the evidence. Womens Health (Lond) 11:65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jayasekara H, MacInnis RJ, Hodge AM, Hopper JL, Giles GG et al. (2015) Lifetime alcohol consumption and upper aero-digestive tract cancer risk in the Melbourne collaborative cohort study. Cancer Causes Control 26:297–301 [DOI] [PubMed] [Google Scholar]
- 51.Laffoy M, McCarthy T, Mullen L, Byrne D, Martin J (2013) Cancer incidence and mortality due to alcohol: an analysis of 10-year data. Ir Med J 106:294–297 [PubMed] [Google Scholar]
- 52.Cho S, Shin A, Park SK, Shin HR, Chang SH et al. (2015) Alcohol drinking, cigarette smoking and risk of colorectal cancer in the Korean multi-center cancer cohort. J Cancer Prev 20:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawkins NA, Berkowitz Z, Rodriguez JL (2015) Awareness of dietary and alcohol guidelines among colorectal cancer survivors. Am J Prev Med 49:S509–S517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klarich DS, Brasser SM, Hong MY (2015) Moderate alcohol consumption and colorectal cancer risk. Alcohol Clin Exp Res 39:1280–1291 [DOI] [PubMed] [Google Scholar]
- 55.Chitapanarux T, Phornphutkul K (2015) Risk factors for the development of hepatocellular carcinoma in Thailand. J Clin Transl Hepatol 3:182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Testino G, Leone S, Borro P (2014) Alcohol and hepatocellular carcinoma: a review and a point of view. World J Gastroenterol 20:15943–15954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F et al. (1997) Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos study group. Gut 41:845–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seitz HK, Stickel F (2007) Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer 7:599–612 [DOI] [PubMed] [Google Scholar]
- 59.Schwartz LM, Persson EC, Weinstein SJ, Graubard BI, Freedman ND et al. (2013) Alcohol consumption, one-carbon metabolites, liver cancer and liver disease mortality. PLoS One 8:e78156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chuang SC, Lee YC, Wu GJ, Straif K, Hashibe M (2015) Alcohol consumption and liver cancer risk: a meta-analysis. Cancer Causes Control 26:1205–1231 [DOI] [PubMed] [Google Scholar]
- 61.Cai S, Li Y, Ding Y, Chen K, Jin M (2014) Alcohol drinking and the risk of colorectal cancer death: a meta-analysis. Eur J Cancer Prev 23:532–539 [DOI] [PubMed] [Google Scholar]
- 62.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L et al. (2006) Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 166:2437–2445 [DOI] [PubMed] [Google Scholar]
- 63.Jin M, Cai S, Guo J, Zhu Y, Li M et al. (2013) Alcohol drinking and all cancer mortality: a meta-analysis. Ann Oncol 24:807–816 [DOI] [PubMed] [Google Scholar]
- 64.Seitz HK, Mueller S (2015) Alcohol and cancer: an overview with special emphasis on the role of acetaldehyde and cytochrome P450 2E1. Adv Exp Med Biol 815:59–70 [DOI] [PubMed] [Google Scholar]
- 65.Seitz HK, Wang XD (2013) The role of cytochrome P450 2E1 in ethanol-mediated carcinogenesis. Subcell Biochem 67:131–143 [DOI] [PubMed] [Google Scholar]
- 66.Heit C, Dong H, Chen Y, Shah YM, Thompson DC et al. (2015) Transgenic mouse models for alcohol metabolism, toxicity, and cancer. Adv Exp Med Biol 815:375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seitz HK, Homann N (2007) The role of acetaldehyde in alcohol-associated cancer of the gastrointestinal tract. Novartis Found Symp 285:110–119 discussion 119–114, 198–119 [DOI] [PubMed] [Google Scholar]
- 68.Seitz HK, Stickel F (2010) Acetaldehyde as an underestimated risk factor for cancer development: role of genetics in ethanol metabolism. Genes Nutr 5:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinmetz CG, Xie P, Weiner H, Hurley TD (1997) Structure of mitochondrial aldehyde dehydrogenase: the genetic component of ethanol aversion. Structure 5:701–711 [DOI] [PubMed] [Google Scholar]
- 70.Yoshida A, Huang IY, Ikawa M (1984) Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in orientals. Proc Natl Acad Sci U S A 81:258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsumoto A, Thompson DC, Chen Y, Kitagawa K, Vasiliou V (2016) Roles of defective ALDH2 polymorphism on liver protection and cancer development. Environ Health Prev Med 21:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsuda T, Matsumoto A, Uchida M, Kanaly RA, Misaki K et al. (2007) Increased formation of hepatic N2-ethylidene-2′-deoxyguanosine DNA adducts in aldehyde dehydrogenase 2-knockout mice treated with ethanol. Carcinogenesis 28:2363–2366 [DOI] [PubMed] [Google Scholar]
- 73.Nagayoshi H, Matsumoto A, Nishi R, Kawamoto T, Ichiba M et al. (2009) Increased formation of gastric N(2)-ethylidene-2′-deoxyguanosine DNA adducts in aldehyde dehydrogenase-2 knockout mice treated with ethanol. Mutat Res 673:74–77 [DOI] [PubMed] [Google Scholar]
- 74.Yu HS, Oyama T, Isse T, Kitagawa K, Pham TT et al. (2010) Formation of acetaldehyde-derived DNA adducts due to alcohol exposure. Chem Biol Interact 188:367–375 [DOI] [PubMed] [Google Scholar]
- 75.Hu W, Feng Z, Eveleigh J, Iyer G, Pan J et al. (2002) The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis 23:1781–1789 [DOI] [PubMed] [Google Scholar]
- 76.Frank A, Seitz HK, Bartsch H, Frank N, Nair J (2004) Immunohistochemical detection of 1,N6-ethenodeoxyadenosine in nuclei of human liver affected by diseases predisposing to hepato-carcinogenesis. Carcinogenesis 25:1027–1031 [DOI] [PubMed] [Google Scholar]
- 77.Albano E (2008) Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Asp Med 29:9–16 [DOI] [PubMed] [Google Scholar]
- 78.French SW (2013) Epigenetic events in liver cancer resulting from alcoholic liver disease. Alcohol Res 35:57–67 [PMC free article] [PubMed] [Google Scholar]
- 79.Varela-Rey M, Woodhoo A, Martinez-Chantar ML, Mato JM, Lu SC (2013) Alcohol, DNA methylation, and cancer. Alcohol Res 35:25–35 [PMC free article] [PubMed] [Google Scholar]
- 80.Leo MA, Lieber CS (1982) Hepatic vitamin a depletion in alcoholic liver injury. N Engl J Med 307:597–601 [DOI] [PubMed] [Google Scholar]
- 81.Yu MW, Hsieh HH, Pan WH, Yang CS, CJ CH (1995) Vegetable consumption, serum retinol level, and risk of hepatocellular carcinoma. Cancer Res 55:1301–1305 [PubMed] [Google Scholar]
- 82.Ray G, Batra S, Shukla NK, Deo S, Raina V et al. (2000) Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res Treat 59:163–170 [DOI] [PubMed] [Google Scholar]
- 83.Ohshima H (2003) Genetic and epigenetic damage induced by reactive nitrogen species: implications in carcinogenesis. Toxicol Lett 140–141:99–104 [DOI] [PubMed] [Google Scholar]
- 84.Sander CS, Chang H, Hamm F, Elsner P, Thiele JJ (2004) Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int J Dermatol 43:326–335 [DOI] [PubMed] [Google Scholar]
- 85.Sies H (1999) Glutathione and its role in cellular functions. Free Radic Biol Med 27:916–921 [DOI] [PubMed] [Google Scholar]
- 86.Jakobisiak M, Lasek W, Golab J (2003) Natural mechanisms protecting against cancer. Immunol Lett 90:103–122 [DOI] [PubMed] [Google Scholar]
- 87.Nikitovic D, Holmgren A, Spyrou G (1998) Inhibition of AP-1 DNA binding by nitric oxide involving conserved cysteine residues in Jun and Fos. Biochem Biophys Res Commun 242:109–112 [DOI] [PubMed] [Google Scholar]
- 88.Wu HH, Momand J (1998) Pyrrolidine dithiocarbamate prevents p53 activation and promotes p53 cysteine residue oxidation. J Biol Chem 273:18898–18905 [DOI] [PubMed] [Google Scholar]
- 89.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P (2008) Redox regulation of cell survival. Antioxid Redox Signal 10:1343–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL et al. (2013) Role of glutathione in cancer progression and chemoresistance. Oxidative Med Cell Longev 2013: 972913, 1, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Corti A, Franzini M, Paolicchi A, Pompella A (2010) Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res 30:1169–1181 [PubMed] [Google Scholar]
- 92.Estrela JM, Ortega A, Obrador E (2006) Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci 43:143–181 [DOI] [PubMed] [Google Scholar]
- 93.Gatti L, Zunino F (2005) Overview of tumor cell chemoresistance mechanisms. Methods Mol Med 111:127–148 [DOI] [PubMed] [Google Scholar]
- 94.Townsend DM, Tew KD (2003) The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 22:7369–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oudard S, Levalois C, Andrieu JM, Bougaran J, Validire P et al. (2002) Expression of genes involved in chemoresistance, proliferation and apoptosis in clinical samples of renal cell carcinoma and correlation with clinical outcome. Anticancer Res 22:121–128 [PubMed] [Google Scholar]
- 96.Chaichenko GM, Tomilina LI (1990) Analysis of the process of learning in the formation of the conditioned reflex of avoidance in rats. Fiziol Zh 36:77–83 [PubMed] [Google Scholar]
- 97.Shames JM, Dhurandhar NR, Blackard WG (1968) Insulin-secreting bronchial carcinoid tumor with widespread metastases. Am J Med 44:632–637 [DOI] [PubMed] [Google Scholar]
- 98.Zhao H, Li Q, Wang J, Su X, Ng KM et al. (2012) Frequent epigenetic silencing of the folate-metabolising gene cystathionine-beta-synthase in gastrointestinal cancer. PLoS One 7:e49683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shen M, Rothman N, Berndt SI, He X, Yeager M et al. (2005) Polymorphisms in folate metabolic genes and lung cancer risk in Xuan Wei, China. Lung Cancer 49:299–309 [DOI] [PubMed] [Google Scholar]
- 100.Austin RC, Lentz SR, Werstuck GH (2004) Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ 11(Suppl 1):S56–S64 [DOI] [PubMed] [Google Scholar]
- 101.Cellarier E, Durando X, Vasson MP, Farges MC, Demiden A et al. (2003) Methionine dependency and cancer treatment. Cancer Treat Rev 29:489–499 [DOI] [PubMed] [Google Scholar]
- 102.Wu LL, Wu JT (2002) Hyperhomocysteinemia is a risk factor for cancer and a new potential tumor marker. Clin Chim Acta 322:21–28 [DOI] [PubMed] [Google Scholar]
- 103.Beylot C, Feuillatre F, Doutre MS (1987) Local corticotherapy in children. Rev Prat 37:2713–2718 [PubMed] [Google Scholar]
- 104.James SJ, Melnyk S, Pogribna M, Pogribny IP, Caudill MA (2002) Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J Nutr 132:2361s–2366s [DOI] [PubMed] [Google Scholar]
- 105.Pascale RM, Simile MM, Satta G, Seddaiu MA, Daino L et al. (1991) Comparative effects of L-methionine, S-adenosyl-L-methionine and 5′-methylthioadenosine on the growth of pre-neoplastic lesions and DNA methylation in rat liver during the early stages of hepatocarcinogenesis. Anticancer Res 11:1617–1624 [PubMed] [Google Scholar]
- 106.Dobritsa AP, Mikhailova TG, Dubovaya VI (1985) Physical and genetic structure of the IncN plasmid R15. Plasmid 14:99–105 [DOI] [PubMed] [Google Scholar]
- 107.Oliva J, Zhong J, Buslon VS, French SW (2012) The effect of SAMe and betaine on Hepa 1-6, C34 and E47 liver cell survival in vitro. Exp Mol Pathol 92:126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu SC, Ramani K, Ou X, Lin M, Yu V et al. (2009) S-adenosylmethionine in the chemoprevention and treatment of hepatocellular carcinoma in a rat model. Hepatology 50:462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu SC, Mato JM (2005) Role of methionine adenosyltransferase and S-adenosylmethionine in alcohol-associated liver cancer. Alcohol 35:227–234 [DOI] [PubMed] [Google Scholar]
- 110.Purohit V, Abdelmalek MF, Barve S, Benevenga NJ, Halsted CH et al. (2007) Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: summary of a symposium. Am J Clin Nutr 86:14–24 [DOI] [PubMed] [Google Scholar]
- 111.Lieber CS (2003) Relationships between nutrition, alcohol use, and liver disease. Alcohol Res Health 27:220–231 [PMC free article] [PubMed] [Google Scholar]
- 112.Kattie AL, Cottrel M, Le Cabellec MT, Kerebel LM (1989) The structure, ultrastructure and physicochemical analysis of the hard dental tissues of the Viperidae. Bull Group Int Rech Sci Stomatol Odontol 32:217–225 [PubMed] [Google Scholar]
- 113.Nguyen-Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O et al. (2011) Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 365:1781–1789 [DOI] [PubMed] [Google Scholar]
- 114.Ronis MJ, Hennings L, Stewart B, Basnakian AG, Apostolov EO et al. (2011) Effects of long-term ethanol administration in a rat total enteral nutrition model of alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 300:G109–G119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaur J, Shalini S, Bansal MP (2010) Influence of vitamin E on alcohol-induced changes in antioxidant defenses in mice liver. Toxicol Mech Methods 20:82–89 [DOI] [PubMed] [Google Scholar]
- 116.Adewusi EA, Afolayan AJ (2010) Effect of Pelargonium reniforme roots on alcohol-induced liver damage and oxidative stress. Pharm Biol 48:980–987 [DOI] [PubMed] [Google Scholar]
- 117.Rambaldi A, Gluud C (2006) S-adenosyl-L-methionine for alcoholic liver diseases. Cochrane Database Syst Rev:CD002235 [DOI] [PubMed] [Google Scholar]
- 118.Stewart S, Prince M, Bassendine M, Hudson M, James O et al. (2007) A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol 47:277–283 [DOI] [PubMed] [Google Scholar]