Abstract
Metabolic conditions during brain development may have long-term consequences on brain metabolism, thereby influencing the risk of neurodegenerative disease in later life. To ascertain the long-term consequences of ω3 fatty acid deficiency during brain development on oxidative fatty acid degradation in the brain and the development of Alzheimer-like pathology, wild-type (WT) female mice were fed diets that were either replete or deficient in ω3 fatty acids for five weeks. These females were then mated with hemizygous 5xFAD male transgenic (TG) mouse models of Alzheimer’s disease (AD), and the progeny were continued on diets that were either ω3-replete or ω3-deficient. When the progeny were six months of age, they received radiolabeled arachidonic acid (ARA) by intracerebroventricular injection. Five days after these injections, the brains were harvested and oxidative degradation of the radiolabeled ARA was characterized. Among the progeny of female mice on an ω3-replete diet, TG progeny had lower PSD-95 expression, and higher oxidative ARA degradation, than WT progeny. Progeny on an ω3-deficient diet, however, had no significant differences in PSD-95 expression between TG and WT mice, or in the extent of ARA degradation. In TG mice, an ω3-deficient diet reduced oxidative ARA degradation to a greater extent than in WT mice. The reductions in oxidative ARA degradation occurred even if the progeny of female mice on an ω3-deficient diet resumed an ω3-replete diet immediately upon weaning. These results demonstrate that dietary ω3 fatty acid deficiency during development can cause long-term changes in the expression of a synaptic marker, and long-term reductions in the rate of ARA degradation in the WT brain, that are not completely alleviated by an ω3-replete diet after weaning. The elimination of differences between TG and WT mice by an ω3-deficient diet suggest that mechanisms regulating PSD-95 expression and the oxidative degradation of ARA are related, and that the timing of dietary ω3 intake during development may influence AD-related pathological changes later in life.
Keywords: Alzheimer’s disease, polyunsaturated fatty acids, oxidative stress, neurodegeneration, docosahexaenoic acid, arachidonic acid
Introduction
Oxidative stress is frequently implicated in the pathogenesis of Alzheimer’s disease (AD) (Axelsen et al., 2011). Polyunsaturated fatty acids (PUFAs) are concentrated in the brain and among the most vulnerable compounds to direct chemical damage from oxidative stress. When damaged, they can degrade into highly reactive oxidative degradation products including hydroxyalkenals, oxoalkenals, malondialdehyde and acrolein, causing toxicity through the spontaneous covalent modification of proteins. Proteins modified by Michael addition to 4-hydroxy-2-nonenaI (HNE, an ω6 PUFA oxidation product), appear to concentrate around amyloid plaques in AD brains (Furman et al., 2016) and in mouse models of AD (Ellis et al., 2010; Furman et al., 2016), suggesting that amyloid plaques are active centers of oxidative PUFA degradation in AD.
It follows that altering the PUFA content of the brain may alter the pathogenesis of AD. This possibility is supported by observations that a dietary deficiency of docosahexaenoic acid (DHA) and its ω3 precursors such as α-linolenic acid reduces expression of the postsynaptic marker PSD-95 and lowers cognitive performance in mouse models of AD, whereas dietary ω3 supplementation improves cognitive test scores, decreases apoptosis, and lowers amyloid burden (Calon et al., 2004; Lim et al., 2005). However, dietary ω3 supplementation has not demonstrated any salutary effects in human cases of AD (Quinn et al., 2010; Freund-Levi et al., 2008; Plourde et al., 2007; Issa et al., 2006). One possible explanation for its failure is that ω3 fatty acids such as DHA may have their primary biological role at an early age. This idea is supported by the association observed between low ω3 consumption during pregnancy and suboptimal neurocognitive outcomes in childhood (Hibbeln et al., 2007). Conversely, dietary ω3 supplementation improved test scores and social behavior in children. A meta-analysis of 34 studies found that ω3 supplementation improved cognitive development in infants, while the effects on cognitive performance in adults are less clear (Heaton et al., 2013).
DHA is the most abundant polyunsaturated fatty acid in the human brain. Its concentration increases markedly during the third trimester spurt in brain growth, and this increase continues through 8 years of age (Martinez and Mougan, 1998). A corresponding increase of DHA through early childhood has been documented in primates (Miller et al., 2016) and during rodent development (Green and Yavin, 1996; Green et al., 1999). A failure to accumulate DHA during gestation has been shown to cause physical and behavioral abnormalities in rodents such as delayed cell migration, reduced proliferation in the dense cortical plates, CA1 region, and the dentate gyrus (Yavin et al., 2009a; Tang et al., 2016a), as well as an increase in GABA-antagonist-induced heart rate changes, and enhanced depressive-like behavior (Tang et al., 2016b; Chen and Su, 2013; Yavin et al., 2009b). An elevated ω6/ω3 PUFA ratio in the rodent diet impairs neocortical neurogenesis in the offspring and increases anxiety-related behavior in adulthood (Sakayori et al., 2016). Even if brain DHA concentrations are restored on a DHA-containing diet, concentrations in the brain are slower to normalize than in other organs, and abnormalities persist well after DHA concentrations return to normal (Lozada et al., 2017b).
DHA may be synthesized by the liver from an ω3-PUFA precursor, α-linolenic acid (Pauter et al., 2017). However, the capacity of endogenous synthetic mechanisms to produce this precursor is limited and therefore, the availability of DHA is critically dependent on dietary ω3-PUFA intake (Rapoport et al., 2010). Moreover, dietary ω3 deficiency promotes the formation of otherwise uncommon ω6 PUFAs (Axelsen et al., 2016), which alters the ratio of ω6 to ω3 PUFAs in the brain and may lead to neurological disorders (Simopoulos, 2016; Husted and Bouzinova, 2016).
Isotopically-labeled forms of ARA (U-14C-ARA and U-13C-ARA) (Lee et al., 2017) have been used in transgenic (TG) mouse models of AD and in human brain to show that oxidative chemical degradation of ARA is accelerated in the immediate vicinity of amyloid plaques (Furman et al., 2016), and that oxidative ARA degradation products accumulate in the cortical tissue of AD brain (Furman et al., 2018). In this work, U-14C-ARA was introduced by intracerebroventricular (ICV) injection, and the rate of oxidative ARA degradation in the brain was characterized by examining the distribution of 14C activity among oxidative degradation products. The effects of maternal dietary ω3 deficiency on the rate of oxidative ARA degradation in their progeny, was examined in WT mice, and in a mouse model of AD known to exhibit evidence of increased oxidative stress.
Results show that a dietary ω3 deficiency during development (i.e. gestation and weaning) has persistent effects in the brains of adult progeny, reducing the oxidative degradation of ARA and altering the expression of a synaptic marker. Restoring ω3 PUFAs in the diet after weaning partially restores brain DHA concentrations, but other effects of ω3 deficiency during development persist into adulthood.
Materials and Methods
Reagents. PSD-95 antibodies were purchased from Antibodies Incorporated (Davis, CA). Donkey anti-mouse antibodies were purchased from Santa Cruise Biotechnology (Dallas, Tx). Unlabeled ARA was purchased from Nu-Check Prep Inc. (Elysian, MN). Uniformly labeled [U-14C]-ARA was prepared as described (Lee et al., 2017). d5–DHA was purchased from Cayman Chemicals (Ann Arbor, MI). All other chemicals were purchased from Fisher Scientific (Pittsburgh, PA).
Animals and diets. Hemizygous transgenic 5xFAD (TG) and wild-type B6SJL/J (WT) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were fed ad libitum either an AIN-93G purified rodent diet or a custom formulated low ω3 diet, with compositions as listed in Table 1. All experimental procedures and animal care were in compliance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
Table 1.
Composition of the ω3-replete and ω3-deficient diets (all values are grams per kilogram).
| ω3-replete (#101093) |
ω3-deficient (#101094) |
|
|---|---|---|
| Casein | 200 | 200 |
| Sucrose | 99.98 | 99.98 |
| Cornstarch | 150 | 150 |
| Dextrose | 200 | 200 |
| Maltose Dextrin | 150 | 150 |
| Cellulose | 49.5 | 49.5 |
| TBHQ | 0.02 | 0.02 |
| Salt Mix #210025 | 35 | 35 |
| Vitamin Mix #310025 | 10 | 10 |
| L-Cystine | 3 | 3 |
| Choline Bitartrate | 2.5 | 2.5 |
| Fatty acids | ||
| Myristic | 11.6 | 12.8 |
| Palmitic | 8.2 | 8.4 |
| Stearic | 8.8 | 9.4 |
| Oleic | 5.3 | 4.1 |
| Linoleic | 26.4 | 26.4 |
| Linolenic | 4.4 | 0 |
| Octanoic | 3.7 | 4.1 |
| Decanoic | 3.2 | 3.6 |
| Lauric | 28.3 | 31.2 |
Female WT mice were maintained on ω3-replete or ω3-deficient diets for 5 weeks before being mated with hemizygous TG males. The progeny were genotyped upon weaning and divided into 3 groups according to the experimental scheme illustrated in figure 1: all of the progeny on an ω3-replete diet were continued on an ω3-replete diet (the “NN” group). Among the mice on an ω3-deficient diet, half of the progeny were continued on an ω3-deficient diet (“LL” group), while the other half were given an ω3-replete diet (“LN” group). All tested animals were first-litter pups, to avoid parity effects (Ozias et al., 2007).
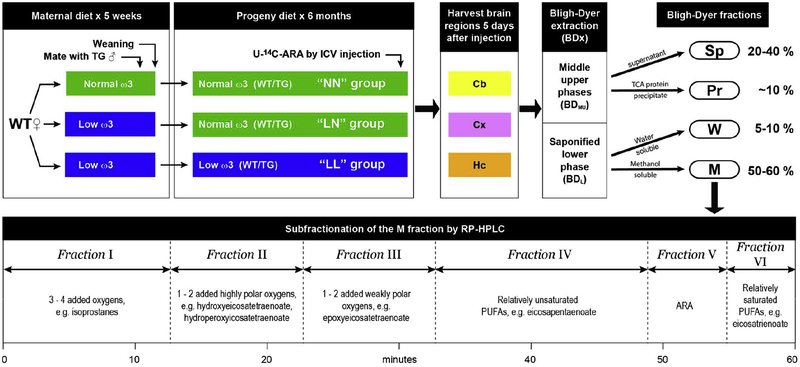
Figure 1. Experimental scheme.
There were 5 stages in the experimental scheme. Stage 1: Female WT mice, fed either an ω3-replete or an ω3-deficient diet for 5 weeks, were mated with hemizygous male TG mice. Stage 2: Upon weaning, the progeny of female mice on an ω3-deficient diet were either continued on an ω3-deficient diet or switched to a ω3-replete diet for 6 months, while the progeny of female mice on an ω3-replete diet were continued on an ω3-replete diet for 6 months. This protocol yielded WT and TG mice in 3 different “diet groups” designated NN, LN, and LL. Five days before brain tissue was harvested, U-14C-ARA was introduced by ICV injection. Stage 3: Three brain regions from each mouse were dissected, extracted, and fractionated as described in Experimental Methods. Cb – cerebellum; Cx – cortex; Hc–hippocampus; BDMU – middle/upper phase of the Bligh Dyer extract; BDL – lower phase of the Bligh Dyer extraction. Stage 4: The middle/upper and the lower phases of the Bligh Dyer extract were fractionated according to solubility. Sp – protein-depleted supernatant from the BDMU phase; Pr – Proteins precipitated from the BDMU by TCA; W – water-soluble fraction from the BDL phase; M – methanol-soluble fraction from the BDL phase. Stage 6: The M fraction was subfractionated by reversed-phase (RP) HPLC. Typical products identified by mass spectrometry in each subfraction are indicated.
Intracerebroventricular (ICV) injections. [U-14C]-ARA stock was stored in ethanol at −80 C. Immediately before use, the ethanol was evaporated and the residue was resuspended in 90% PBS / 10% DMSO by volumes. ICV injections of 0.3–0.5 μCi were performed as described previously (Furman et al., 2016). 5 days after injection, animals were sacrificed by CO2 asphyxiation and brains were removed and dissected on dry ice within 3 min post mortem. Tissue samples (~20 mg) from the hippocampus (Hc), parietal cortex (Cx), and cerebellum (Cb), were separated, weighted and transferred to glass vials (Microsolv Technology Corp., Eatontown, NJ).
Lipid extraction and protein precipitation. Bligh-Dyer Extractions (BDx) were performed as described (Furman et al., 2016). Tissues were placed in 760 3BCl of BDx monophase (400 μl methanol, 200 μl dichloromethane, and 160 μl of 5 mM ammonium acetate in water) and tip sonicated (F60 Sonic Dismembrator, Fisher Scientific, Pittsburgh, PA). The extract was transferred to a 15 ml glass tube (Fisher Scientific, Pittsburgh, PA) and an additional aliquot of 760 μl monophase was added for each 5 mg of tissue beyond 5 mg (i.e. two additional 760 μl aliquots of monophase would be added to a 17 mg sample). Then, for each 760 μl aliquot of monophase, 200 μl of dichloromethane and 160 μl of 5 mM ammonium acetate in water was added to break the monophase (i.e. a 17 mg sample of tissue would ultimately be mixed in 3 × 760 μl monophase plus 3 × 200 μl dichloromethane plus 3 × 160 μl of 5 mM ammonium acetate in water). The tube was vortexed for 1 minute and centrifuged at low speed for 2 minutes to separate the phases.
The lower phase of the BDx (BDL) was removed, evaporated under nitrogen, resuspended in 750 μl of 85% methanol and 250 μl of 2 M sodium hydroxide, and saponified for 1 h at 80°C. Following saponification samples were acidified with 150 μl of 5M HCl, dried under nitrogen, and dissolved in methanol (the M fraction). The remaining white residue was dissolved in 200 μl of water (the W fraction).
The middle and the upper phases of the BDx (BDMU) were vortexed and a sample was withdrawn for radioactivity measurement. The remainder was dried under nitrogen, resuspended in 300 μl of water, and transferred into a polypropylene bullet tube. The original tube was washed with 300 μl of acetone and added to the aqueous suspension (total 600 μl of acetone:water). Proteins were precipitated by adding 150 μl of 65% trichloroacetic acid (TCA) in acetone to yield a final TCA concentration of 13%. Following overnight incubation at −20°C, samples were centrifuged at 13,000 g for 12 minutes at 4°C. The supernatant (Sp) was transferred to a new bullet tube (~600 μl), while the protein pellet (Pr) was subjected to two wash cycles in 500 μl cold acetone. Following each wash, the supernatant was collected and added to the Sp. The final pellet was air dried and resuspended in 300 μl of cold Tris-buffered saline with Tween 20 (20 mM Tris, 150 mM NaCl and 0.1% Tween 20, pH 7.4) supplemented with protease inhibitors (PMSF 0.2 mM, leupeptine 1 μg/ml, and aprotinine 1 μg/ml). The suspension was agitated with a tip sonicator for 1 minute. Protein concentrations in the Pr and Sp were measured by BCA assay (ThermoFisher, Waltham, MA). The amount of protein in the Pr was >9 fold higher than the Sp.
Radioactivity measurements and data analysis. An aliquot of the BDMU and each of the four fractions (W, M, Sp, and Pr) was mixed with 4 ml Ecolite scintillation fluid (MP biomedicals, Santa Ana CA) in a 5 ml scintillation vial (Lake Charles, Lake Charles LA) and counted for 1 minute in LS 6500 Multipurpose Scintillation Counter (Beckman Coulter, Brea, CA) operating in auto-DPM mode. All results are reported in disintegrations-per-minute, DPM. A scale factor, fL, was calculated to correct for activity losses incurred during processing of the BDMU according to:
where A is the activity per unit volume, and V is the volume of the BDMU, Sp, and Pr fractions. Values of fL ranged from 1.0 – 1.6. Therefore, the total activity in the four fractions of each sample (AT) was given by:
Results for each fraction are reported as % of total activity in the Pr, Sp, M, and W fractions.
PSD 95 determinations.
Proteins were isolated from the BDMU using TCA precipitation. A portion of each Prfaction was loaded onto a Tris Tricine 16.5% PAGE gel (0.225 μg protein/well), electrophoresed, and transferred to a 0.2 μm nitrocellulose membrane using a Western transfer cassette (Mini Trans-Blot Cell, BIORAD, Ca, USA). The membrane was blocked overnight at 4°C with 5% fat free milk in PBS (137 mM NaCl, 12 mM Phosphate, 2.7 mM KCl, pH 7.4), washed with PBST (PBS+ 0.1% Triton X-100), and incubated overnight at 4°C with PSD-95 antibodies (1:1000) in 2% fat free milk in PBST. Blots were washed 3 times in PBST and then incubated with HRP donkey anti-mouse antibody (1:10000) in 2% fat free milk in PBST for 30 minutes at room temperature, followed by 30 minutes at 37°C. For detection, all nitrocellulose membranes were incubated with WEST Femto enhanced chemiluminescent HRP substrate (ThermoFisher, Waltham, MA) for 5 minutes, imaged with a Multi-application gel system (Syngene, PXi, Frederick, MD), and densitometrically quantified with image J (NIH, Washington DC).
Chromatography of radioactive samples.
Saponified M fractions from the BDL were injected onto a 4.6 × 150 mm XDB-C18 column (Agilent, Santa Clara, CA) with a mobile phase gradient running at 0.35 ml/min. Solvent A was 60% acetonitrile, 40% H2O, and 0.1% formic acid; solvent B was 100% acetonitrile and 0.1% formic acid. The mobile phase composition was changed linearly from 30% to 100% B over 55 min, and held at 100% B for 9 min. Column performance was characterized by the analysis of effluent from non-radioactive samples with an ABI 4000 mass spectrometer (Toronto, Canada) operating in negative ion multiple reaction monitoring (MRM) mode as described previously (Furman et al., 2016). Subfractions (6–12 ml) were collected over 60 min, and the radioactivity in each subfraction was divided by the total radioactivity that eluted from the column.
Quantitative PUFA analysis.
WT mice were euthanized by CO2 asphyxiation at 6 months of age. Samples (~20 mg) of Cx and Cb were excised, rapidly frozen on dry ice, transferred to autosampler vials, and weighed. To each sample 10 μl of 100 μM BHT, 10 μl of 10 mM d5-DHA in methanol and 10 μl of 10 mM d8-ARA in methanol were added as internal standards. The sample was tip-sonicated and BDx was performed as described above. BDL from each sample was saponified, dried and dissolved in methanol as described for radioactive samples above.
The saponified BDL from each sample was analyzed by injecting a 20 μl volume onto a 1 × 250 mm Zorbax 300SB 5 μm C18 column (Agilent, Santa Clara, CA). Solvent A was 8% acetonitrile, 92% water, and 0.1% formic acid. Solvent B was 100% acetonitrile and 0.1% formic acid. The mobile phase was pumped at 100 μl/min as the composition was changed linearly from 8% to 80% solvent B over 25 min, and then held at 80% B. The eluent was alkalinized post-column with 0.15 M ammonium hydroxide in methanol flowing at 50 μl/min before being introduced via electrospray ionization into an ABI 4000 mass spectrometer (Sciex, Toronto, Canada) operating in negative ion mode. The declustering potential was −70V, the ionization energy was −4500V, and the drying gas temperature was 200°C. The collision gas was nitrogen at 7 psi and the collection time for each transition in MRM-LC/MSMS methods was 100 msec. Results are reported as the ratio of integrated MRM signals from analyte to internal standard; i.e. DHA / d5-DHA and ARA/d8-ARA per gram wet tissue. There was no significant difference in PUFA concentrations in the Cx and Cb, so the results from both regions were combined.
Statistical analysis. Statistical analysis.
Statistical analysis was performed within Sigma Plot (Systat Software Inc.) using one-way ANOVA for single variants, two-way ANOVA for multiple variants, and the Holm-Sidak method for post hoc testing. The latter has the potential to indicate a lower p-value than would obtained when applying a more stringent test such as Bonferroni to comparisons that are independent. Data is presented as means of at least 4 biological repeats, and all error bars represent standard errors of the means.
Results
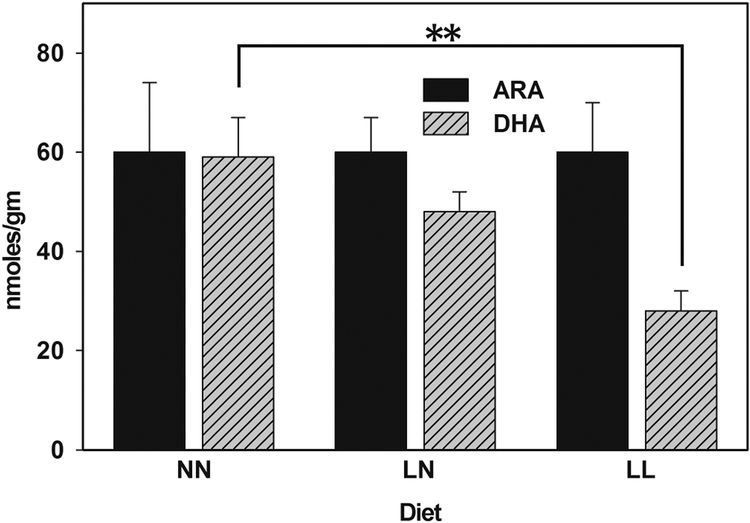
DHA and ARA concentrations were measured at 6 months of age in the cortical brain tissue of WT progeny of mice fed on NN, LN and LL diets. Significant amounts of both PUFAs were only recovered from the BDL (data not shown). WT animals in the NN group had 60 mmoles/gm of DHA in their cortical brain tissue, consistent with prior results from GC-MS (figure 2 and table S1) (Axelsen and Murphy, 2010). DHA concentrations declined by 53% (to 28 mmoles/gm) in the LL group compared to the NN group. The DHA concentration in the LN group (48 nmoles/gm) was not significantly different from the concentrations in the LL or NN groups, suggesting that DHA concentrations were not fully replenished in the brain after 6 months on an ω3-replete diet. ARA concentrations were similar in all of the diet groups (~ 60 mmoles/gm).
Figure 2. An ω3-deficient diet reduces DHA concentrations in offspring.
The data represents the mean and standard error of 8 samples from 4 animals. Numerical results are provided in table S1. “**” indicates a difference with P < 0.01 by T-test. NN diet – replete with ω3 fatty acids throughout development, and thereafter for 6 months; LN – an ω3-deficient diet during development, then switched to an ω3-replete diet; LL – mice fed on an ω3-deficient diet throughout.
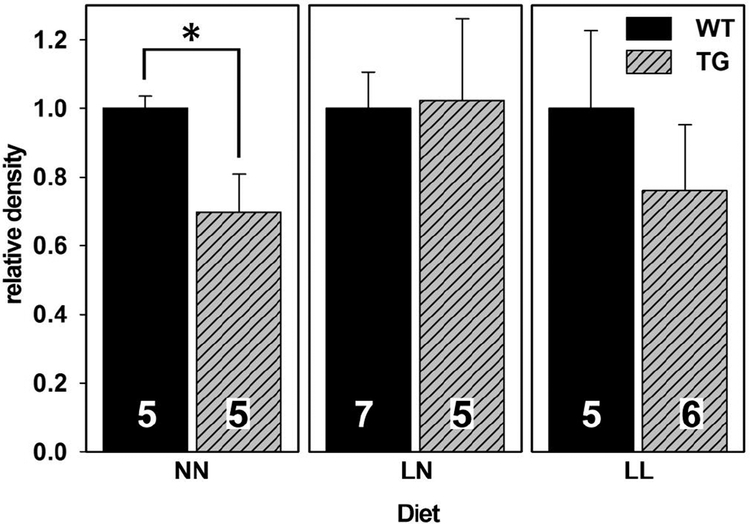
The effects of dietary ω3 deficiency on the expression of PSD-95 (a synaptic marker) was assayed in the cortical brain tissue of WT and TG mice. This marker is known to be lower in mouse models of AD (Oakley et al., 2006) and indeed, it was found to be 30% lower among TG mice in the NN group (the TG/WT ratio was 0.7, figure 3 and table S2). In contrast, the TG/WT ratio was indistinguishable from 1.0 in the LN and LL diet groups.
Figure 3. An ω3-deficient diet reduces differences between WT and TG mice in hippocampal PSD95 expression.
Each panel represents a different diet group, and results are normalized to 1.0 for results from WT brains. The number of animals represented by each vertical bar is indicated. “*” indicates a difference with P < 0.05 by T-test. Because Western blots for each diet group were done on different nitrocellulose membranes, valid comparisons may only be made within each diet group.
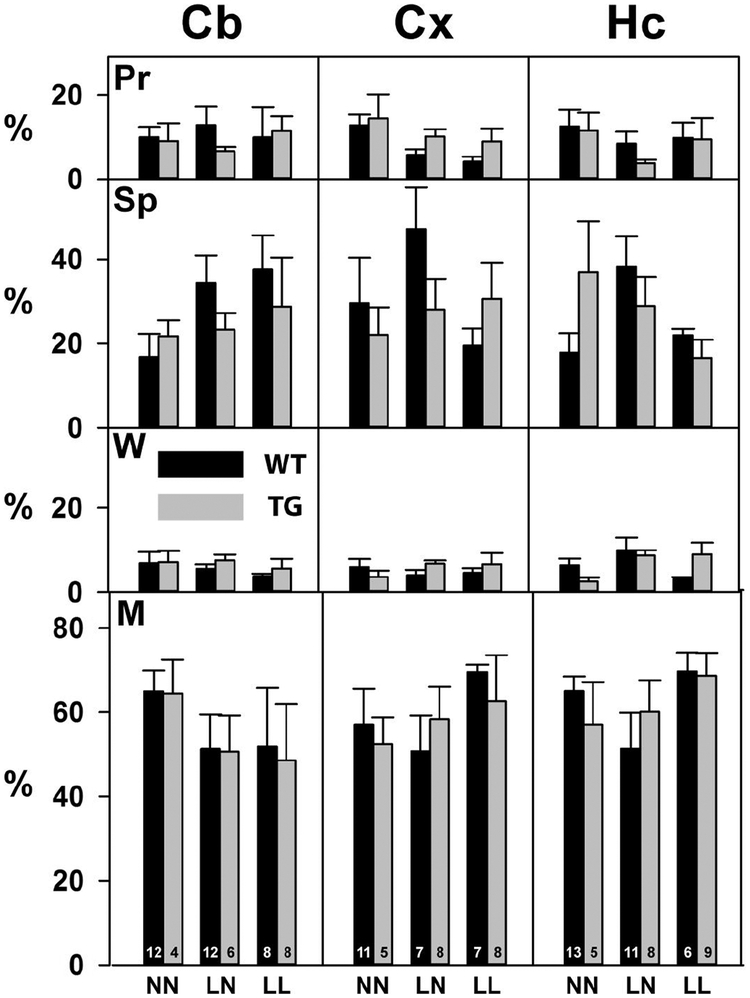
The consequences of dietary ω3 deficiency on oxidative PUFA degradation were explored by examining the fate of ICV-injected [U-14C]-ARA. Because the radiolabel was uniformly distributed along the hydrocarbon chain of ARA, measurements of radioactivity were quantitative with respect to the fate of ARA-derived carbon atoms. Each mouse received 0.3–0.5 μCi of [U-14C]-ARA via ICV. Cx, Cb and Hc regions of the brains were harvested after 5 days, and samples from each region were processed as outlined in figure 1. The 14C activity recovered from the Pr and Sp fractions of the BDMU, and from the W and M fractions of the BDL, are shown in figure 4 and table S3.
Figure 4. Changes in the ω3 content of diets do not significantly change the distribution of 14C-ARA.
Extracts from the Cb, Cx and Hc of WT and TG mice in the three diet groups were separated into four fractions as described in Figure 1. The radioactivity in each fraction is expressed as a percentage of the total in all fractions for each sample. (Pr) The protein-rich pellet from the BDMU. (Sp) The protein depleted BDMU supernatant. (W) Water-soluble methanol-insoluble materials in the BDL. (M) Methanol-soluble materials in the BDL. There were no statistical differences between groups. The data represent the mean and standard error of results from the number of animals indicated at the base of the bars in the bottom panel.
Most (50–70%) of the 14C activity was recovered in the M fraction of the saponified BDL. This fraction includes esterified ARA species such as phospholipids and triglycerides (Furman et al., 2016). Between 18–45% of the activity was recovered in the Sp fraction of the BDMU. This fraction includes unesterified oxidized ARA species such as isoprostanes and most likely any intermediates of beta oxidation (Furman et al., 2018). Between 5–15% was recovered from the Pr fraction. Because this fraction mainly includes proteins, whereas the 14C activity was originally only in ARA, we infer that this activity was most likely due to proteins that have been covalently modified by chemically reactive PUFA oxidation products such as HNE, MDA, and acrolein. There were no statistically significant differences in the distribution of 14C activity between diet groups, brain regions, or genotypes, suggesting that the distribution of 14C activity into these fractions was not significantly changed by ω3 deficiency in the maternal diet.
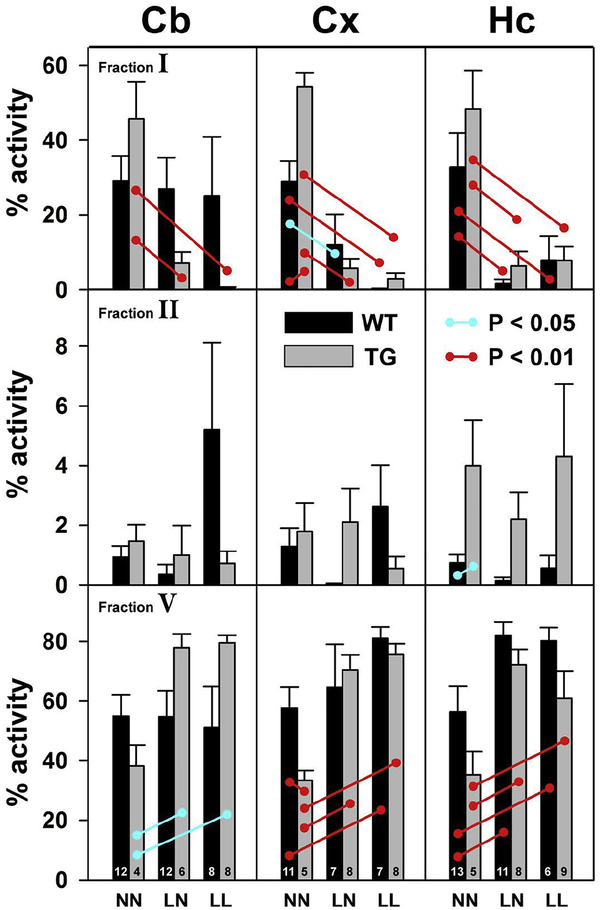
The distribution of 14C activity within the M fraction of the BDL was separated by HPLC into six subfractions according to retention time (figures 1 and S1). These subfractions have been previously characterized by mass spectrometry with respect to the classes of compounds they contain (Furman et al., 2016). Subfraction I contains early-eluting extensively oxidized polar degradation products such as isoprostanes, subfraction II contains oxidation products of intermediate polarity such as HETEs, and subfraction V contains intact ARA.
In the NN diet group, the amount of 14C activity recovered as intact ARA (subfraction V) was similar in the Cb, Cx, and Hc of WT animals (~55%), suggesting that extent of ARA degradation does not differ greatly across brain regions in WT animals on an ω3-replete diet (figure 5, lower panels, figure S1, and table S4). However, subfraction V activity was significantly lower in the Cx of TG animals in the NN diet group, consistent with previously published results showing that the oxidative degradation of ARA is increased in TG animals (Anderson et al., 1987). Subfraction V activity also trended lower in the Cb and Hc of TG animals, but results did not meet criteria for statistical significance.
Figure 5. A low ω3 diet reduces ARA degradation.
The methanol-soluble, M fractions were separated by HPLC into the 6 subfractions illustrated in figure 1 (see also figure S1B). The activity in each subfraction is expressed as a percentage of the total in all 6 subfractions. Most of the activity was found in fractions I (earliest eluting, most polar), II (monooxygenated species), and V (unmodified ARA). Blue barbells indicate results for which the difference is significant with P < 0.05. Red barbells indicate results for which the difference is significant with P < 0.01.
There were numerous statistically significant differences between diet groups in subfractions I and V of fraction M (figure 5). In general, subfraction V activity was frequently greater in the LN and LL diet groups compared to NN, while the activity in subfraction I was frequently lower. These results – an increasing in subfraction I compensated for by a reduction in subfraction V – indicate that dietary ω3 deficiency tends to reduce oxidative ARA degradation. This finding in the LN diet group has special significance in that ω3 deficiency only during development was sufficient to reduce ARA degradation in mature animals, even despite months of an ω3 replete diet. The reduction of ARA degradation was evident in both WT and TG mice, and both Cx and Hc brain regions. It was also evident in the Cb of TG mice, but not in WT mice. There were relatively small amounts of activity recovered in in subfractions III, IV, and VI, and no significant differences in recovered activity between diet groups.
Discussion
These results demonstrate that a dietary ω3 PUFA deficiency during development has long-term effects on brain metabolism and synaptic proteins that persisted into adulthood, even if an ω3-replete diet is provided after weaning. Unexpectedly, the effects of ω3 deficiency included a reduction in the oxidative degradation of ARA in both WT and TG progeny, and normalization of the relatively low expression of PSD-95 in adult TG mice.
These results were unexpected because dietary ω3 supplementation – not deficiency – in adult TG mice has been found to preserve synaptic markers and reduce AD-like pathology (Calon et al., 2004; Calon and Cole, 2007; Lim et al., 2005). Maternal dietary ω3 PUFA deficiency has been shown in other systems to cause delayed cell migration (Yavin et al., 2009a), impaired neurogenesis (Tang et al., 2016a), reduced expression of synaptic proteins (Lozada et al., 2017b), and increased appetite (Mathai et al., 2004). Restoration of ω3 PUFA levels in rodent and monkey brain after a period of dietary deficiency requires weeks-months (Youyou et al., 1986; Connor et al., 1990; Moriguchi et al., 2001; Kodas et al., 2002; Xiao et al., 2005). Therefore, it was expected that dietary ω3 deficiency during gestation would have effects that persisted into adulthood, but the nature of the effects observed in these experiments – a reduction of oxidative PUFA degradation – was not expected.
To date, only a very limited number of studies have addressed the link between maternal diet and neurodegenerative disorders in adults. Maekawa et al., revealed that maternal DHA depletion elicited schizophrenia-like phenotypes in mature mice through hyper methylation of the RXR and PPAR promoters (Maekawa et al., 2017), Wesier et al., demonstrated that DHA supplementation during gestation, alleviated autism-like behavior in the progeny (Weiser et al., 2016b), and Lozada et al., showed that maternal ω3 deficiency elicited long term effects on cognition that could not be reversed by repletion at an older age (Lozada et al., 2017a). The biochemical role of ω3 PUFAs in the brain is not known, but it is widely recognized that they are essential for brain development (Weiser et al., 2016a; Lauritzen et al., 2016; Morse, 2012), and that their concentrations increase markedly in human (Ballabriga and Martinez, 1978; Martinez and Mougan, 1998) and rat brain (Sinclair and Crawford, 1972) late in gestation. This concentration increase coincides with a correspondingly marked increase in brain mass and the development of synaptic connections (Stiles and Jernigan, 2010; Dobbing and Sands, 1979). An ω3 deficiency in adult TG mice has been shown by others to aggravate an AD-related change in postsynaptic markers, including PSD-95, and these effects could be reversed by ω3 supplementation (Calon et al., 2004). Indeed, dietary ω3 PUFA supplementation increases oxidative lipid degradation in rat synaptosomal membranes (Salvati et al., 1993a).
It has been reported that ω3 deficiency during adulthood reduces PSD-95 concentrations in the brain, and that this effect could be ameliorated by ω3 supplementation (Calon et al., 2004; Lozada et al., 2017a). However, the experimental results reported herein suggest that ω3 deficiency during development may not be ameliorated by ω3 supplementation. This difference may have its basis in a need for ω3 PUFAs during neuronal development, but not for neuronal ‘maintenance’ during adulthood. In other words, the effect of ω3 deficiency during neuronal development may be irreversible, while its effect during adulthood may be reversible. This explanation is consistent with the findings of Lozada et al., who showed that maternal ω3 depletion alters hippocampal synaptic protein expression, spatial learning, and memory; effects that can be reversed by providing ω3 PUFAs at 3 weeks of age, but not at 2 and 4 months (Lozada et al., 2017a), and that may be mediated by the pharmacological effects of oxidative ARA degradation products. Although we observed no significant changes in the Sp fraction, where we would expect to find pharmacologically active eicosanoids, the amount of activity in this fraction was small, and the compounds can be potent, so that pharmacologically significant changes may have escaped detection. For example, an F2 isoprostane caused memory deficits, an increase in tau phosphorylation, an activation of the cyclin kinase 5 pathway, and neuroinflammation in mice (Lauretti and Pratico, 2017).
Oxidative ARA degradation may occur via enzyme-mediated, or so-called “chemically” mediated nonenzymatic mechanisms, and both mechanisms of ARA degradation have been documented in AD patients. For example, an increase in 5, 12, and 15 lipoxygenase activity has been associated with tau pathology, synaptic integrity, β secretase expression, and mitochondrial damage during oxidative stress in TG mice, as well as in people with mild cognitive impairment and AD. (Chu et al., 2012; Giannopoulos et al., 2013; Pallast et al., 2009) In contrast, compounds such as 4-hydroxynonenal are well documented in the brain, and are believed to originate solely from the chemical degradation of ω6 PUFAs (Lee and Blair, 2000; Zhang et al., 2003; Lin et al., 2005). The analyses performed herein do not distinguish between these mechanisms of ARA degradation. However, the products of enzyme-mediated ARA degradation are likely to be non-esterified and consequently, more likely to partition into the BDMU (Furman et al., 2018). Analysis of BDMU fractions revealed no clear differences attributable to diet (figure 4). The statistically significant results of this study were all found in subfractions of methanol-soluble material in the BDL, suggesting that diet exerted its principal effects on chemically-mediated ARA degradation.
A possible explanation for decreased ARA degradation in the setting of ω3 PUFA deficiency is that an ω3 deficiency is likely to decrease DHA degradation which, in turn, reduces the production of toxic or inflammatory DHA-derived oxidation products that activate antioxidant and radical scavenging mechanisms. Since Aβ-mediated oxidative stress appears to precede neuronal loss in APPswe/hPS1ΔE9 TG mice (Xie et al., 2013), the ability of an ω3-enriched diet to reduce neuronal damage may be mediated by ω3-derived activators of the cellular antioxidant response (Salvati et al., 1993b; Leonardi et al., 2005; Schebb et al., 2014). This explanation is supported by data showing that regulators of oxidative stress such as Nrf2 and Gpx4 are specifically activated by oxidative ω3 degradation products such as J3 isoprostanes, HHE and hydroxyl-DHA (Cao et al., 2004; Groeger et al., 2010; Zhang et al., 2014; Gruber et al., 2015). Nrf2 is located primarily in the cytoplasm where it cannot induce the expression of antioxidant proteins (Ramsey et al., 2007). However, oxidative DHA degradation products cause the translocation of Nrf2 into the nucleus where it can induce an antioxidant response and mitigate oxidative degradation (Cao et al., 2004). Consequently, a reduction in oxidative DHA degradation products could render the mechanisms that protect ARA relatively unresponsive to oxidative stress, and thereby result in increased oxidative ARA damage.
PSD-95 is immunoreactive material associated with the post-synaptic density of neuronal glutamatergic receptors; it is frequently used as a surrogate measure of synaptic health or abundance (Savioz et al., 2014; Won et al., 2017; Hunt et al., 1996). PSD-95 expression was significantly affected by both diet and genotype in these experiments. TG animals in the NN diet group had significantly lower PSD-95 expression compared to WT animals on the same diet, a finding that reproduces results previously reported by others for this mouse model of AD (Oakley et al., 2006). PSD-95 expression in TG animals normalized in the LN and LL diet groups, compared to WT animals in the same diet groups. This finding echoes the normalization of PSD-95 expression in the same mouse model with bexarotene treatment (Mariani et al., 2017), and normalization following the implantation of mesenchymal human stem cells into the animal brain (Kim et al., 2018). Because PSD-95 determinations are relative, not absolute, it remains unclear whether the normalization in PSD-95 expression in among TG mice in the LN and LL diet groups is due to greater expression of PSD-95 under these conditions, or to reduced expression of PSD-95 among WT mice in the LN and LL diet groups.
The mechanism by which diet, genotype, and PSD-95 expression are linked is not known. One or more protein components of the post-synaptic density binds Aβ peptides, and it has been suggested that this interaction may mediate some of the neurotoxic effects attributed to some forms of these peptides (Savioz et al., 2014). WT mice on a diet enriched in an ω6 PUFA (ARA) for 12 weeks exhibited a marked reduction in PSD-95 expression – an effect that was partially reproduced by the ICV injection of Aβ42 (Thomas et al., 2017). Within the 5xFAD model of AD, PSD-95 expression declines with age, and to a greater extent in TG than in WT animals (Shao et al., 2011; Neuman et al., 2015).
Conclusion
This work demonstrates that maternal ω3 deficiency has an effect on the rate of oxidative ARA degradation in their progeny that persists into adulthood, and that this effect is in an unexpected direction: ω3 deficiency reduces oxidative ARA degradation. These effects are similar in WT and TG mice, and quantitatively greater in TG mice such that they eliminate differences between adult WT and TG mice. There are implications in these results for understanding the pathogenesis of AD because they suggest that nutrient factors available in the diet during development may profoundly affect the development of disease much later in life.
Supplementary Material
Highlights:
Mice were subject to a dietary omega-3 fatty acid deficiency during development
This deficiency reduced oxidative omega-6 fatty acid degradation during maturity
These effects were more pronounced in a mouse model of Alzheimer’s disease
Verification.
We have no conflicts of interest to disclose
Sources of support, namely NIH and private foundation support, are stated in the manuscript.
The text, data, and figures have not been submitted or published elsewhere.
Animal use followed protocols approved by our university IACUC.
Both authors approve of this submission
Funding:
This work was supported by grants from the NIH, the Alzheimer’s Association, and the American Health Assistance Foundation (to P.H.A.).
Abbreviations:
- ARA
arachidonic acid
- DHA
docosahexaenoic acid
- PBS
phosphate-buffered saline
- BDx
Bligh-Dyer extraction
- BDMU
middle and upper phases of the BDx
- BDL
the lower phase of the BDx
- ICV
intracerebroventricular
- MRM-LC/MSMS
multiple reaction monitoring liquid chromatography tandem mass spectrometry
- TCA
trichloroacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
Reference List
- Anderson AB, Darst SA, and Robertson CR Protein adsorption at polymer surfaces: a study using total internal reflection fluorescence In Proteins at Interfaces, unknown, ed. American Chemical Society; 1987, pp. 306–323. [Google Scholar]
- Axelsen PH, Komatsu H, Murray IVJ, 2011. Oxidative Stress and Cell Membranes in the Pathogenesis of Alzheimer’s Disease. Physiol., 26, 54–69. [DOI] [PubMed] [Google Scholar]
- Axelsen PH, Murphy RC, 2010. Quantitative Analysis of Phospholipids Containing Arachidonate and Docosahexaenoate Chains in Microdissected Regions of Mouse Brain. J. Lipid Res, 51, 660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsen PH, Murphy RC, Igarashi M, Rapoport SI, 2016. Increased Omega6-Containing Phospholipids and Primary Omega6 Oxidation Products in the Brain Tissue of Rats on an Omage3-Deficient Diet. PLoS ONE, 11, e0164326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabriga A, Martinez M, 1978. Chemical Study on Development of Human Forebrain and Cerebellum During Brain Growth Spurt Period.2. Phosphoglyceride Fatty-Acids. Brain Res, 159, 363–370. [DOI] [PubMed] [Google Scholar]
- Calon F, Cole G, 2007. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: Evidence from animal studies. Prost. Leuk. Ess. Fat. Acid, 77, 287–293. [DOI] [PubMed] [Google Scholar]
- Calon F, Lim GP, Yang FS, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Ashe KH, Frautschy SA, Cole GM, 2004. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron, 43, 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Tao JQ, Bates SR, Beers MF, Haczku A, 2004. IL-4 induces production of the lung collectin surfactant protein-D. J. All. Clin. Imm, 113, 439–444. [DOI] [PubMed] [Google Scholar]
- Chen HF, Su HM, 2013. Exposure to a maternal n-3 fatty acid-deficient diet during brain development provokes excessive hypothalamic-pituitary-adrenal axis responses to stress and behavioral indices of depression and anxiety in male rat offspring later in life. J. Nutr. Biochem, 24, 70–80. [DOI] [PubMed] [Google Scholar]
- Chu J, Zhuo JM, Pratico D, 2012. Transcriptional regulation of beta secretase-1 by 12/15-lipoxygenase results in enhanced amyloidogenesis and cognitive impairments. Ann. Neurol, 71, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Connor WE, Neuringer M, Lin DS, 1990. Dietary-Effects on Brain Fatty-Acid Composition - the Reversibility of N-3 Fatty-Acid Deficiency and Turnover of Docosahexaenoic Acid in the Brain, Erythrocytes, and Plasma of Rhesus-Monkeys. J. Lipid Res, 31, 237–247. [PubMed] [Google Scholar]
- Dobbing J, Sands J, 1979. Comparative Aspects of the Brain Growth Spurt. Early Hum. Dev, 3, 79–83. [DOI] [PubMed] [Google Scholar]
- Ellis G, Fang E, Maheshwari M, Roltsch E, Holcomb L, Zimmer D, Martinez D, Murray IVJ, 2010. Lipid Oxidation and Modification of Amyloid-beta (A beta) in vitro and in vivo. J. Alz. Dis, 22, 593–607. [DOI] [PubMed] [Google Scholar]
- Esposito G, Giovacchini G, Liow JS, Bhattacharjee AK, Greenstein D, Schapiro M, Hallett M, Herscovitch P, Eckelman WC, Carson RE, Rapoport SI, 2008. Imaging neuroinflammation in Alzheimer’s disease with radiolabeled arachidonic acid and PET. J. Nucl. Med, 49, 1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Levi Y, Basun H, Cederholm T, Faxen-Irving G, Garlind A, Grut M, Vedin I, Palmblad J, Wahlund LO, Eriksdotter-Jonhagen M, 2008. Omega-3 supplementation in mild to moderate Alzheimer’s disease: effects on neuropsychiatric symptoms. Int. J. Geriatr. Psychiatry, 23, 161–169. [DOI] [PubMed] [Google Scholar]
- Furman R, Lee JV, Axelsen PH, 2018. Analysis of eicosanoid oxidation products in Alzheimer brain by LCMS with uniformly 13C-labeled internal standards. Free Rad. Biol. Med, 118, 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman R, Murray IVJ, Schall HE, Liu Q, Ghiwot Y, Axelsen PH, 2016. Amyloid Plaque-Associated Oxidative Degradation of Uniformly Radiolabeled Arachidonic Acid. ACS Chem. Neurosci, 7, 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannopoulos PF, Joshi YB, Chu J, Pratico D, 2013. The 12–15-lipoxygenase is a modulator of Alzheimer’s-related tau pathology in vivo. Aging Cell, 12, 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P, Glozman S, Kamensky B, Yavin E, 1999. Developmental changes in rat brain membrane lipids and fatty acids: the preferential prenatal accumulation of docosahexaenoic acid. J. Lipid Res, 40, 960–966. [PubMed] [Google Scholar]
- Green P, Yavin E, 1996. Fatty acid composition of late embryonic and early postnatal rat brain. Lipids, 31, 859–865. [DOI] [PubMed] [Google Scholar]
- Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ, 2010. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat. Chem. Biol, 6, 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber F, Ornelas CM, Karner S, Narzt MS, Nagelreiter IM, Gschwandtner M, Bochkov V, Tschachler E, 2015. Nrf2 deficiency causes lipid oxidation, inflammation, and matrix-protease expression in DHA-supplemented and UVA-irradiated skin fibroblasts. Free Rad. Biol. Med, 88, 439–451. [DOI] [PubMed] [Google Scholar]
- Heaton AE, Meldrum SJ, Foster JK, Prescott SL, Simmer K, 2013. Does docosahexaenoic acid supplementation in term infants enhance neurocognitive functioning in infancy? Front. Hum. Neurosci, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J, 2007. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet, 369, 578–585. [DOI] [PubMed] [Google Scholar]
- Hunt CA, Schenker LJ, Kennedy MB, 1996. PSD-95 is associated with the postsynaptic density and not with the presynaptic membrane at forebrain synapses. J. Neurosci, 16, 1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted KS, Bouzinova EV, 2016. The importance of n-6/n-3 fatty acids ratio in the major depressive disorder. Medicina-Lithuania, 52, 139–147. [DOI] [PubMed] [Google Scholar]
- Issa AM, Mojica WA, Morton SC, Traina S, Newberry SJ, Hilton LG, Garland RH, MacLean CH, 2006. The efficacy of omega-3 fatty acids on cognitive function in aging and dementia: A systematic review. Dement. Geriat. Cogn. Dis, 21, 88–96. [DOI] [PubMed] [Google Scholar]
- Kim DH, Lim H, Lee D, Choi SJ, Oh W, Yang YS, Oh JS, Hwang HH, Jeon HB, 2018. Thrombospondin-1 secreted by human umbilical cord blood-derived mesenchymal stem cells rescues neurons from synaptic dysfunction in Alzheimer’s disease model. Sci. Rep, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodas E, Vancassel S, Lejeune B, Guilloteau D, Chalon S, 2002. Reversibility of n-3 fatty acid deficiency-induced changes in dopaminergic neurotransmission in rats: critical role of developmental stage. J. Lipid Res, 43, 1209–1219. [PubMed] [Google Scholar]
- Lauretti E, Pratico D, 2017. Effect of canola oil consumption on memory, synapse and neuropathology in the triple transgenic-ámouse model of Alzheimer’s disease. Sci. Rep, 7, 17134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen L, Brambilla P, Mazzocchi A, Harslof LBS, Ciappolino V, Agostoni C, 2016. DHA Effects in Brain Development and Function. Nutrients, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JV, Furman R, Axelsen PH, 2017. Biosynthesis of uniformly labeled C-13- and C-14-arachidonic acid in Mortierella alpina. Biores. Technol, 227, 142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Blair IA, 2000. Characterization of 4-oxo-2-nonenal as a novel product of lipid peroxidation. Chem. Res. Tox, 13, 698–702. [DOI] [PubMed] [Google Scholar]
- Leonardi F, Attorri L, Di Benedetto R, Di Biase A, Sanchez M, Nardini M, Salvati S, 2005. Effect of arachidonic, eicosapentaenoic and docosahexaenoic acids on the oxidative status of C6 glioma cells. Free Rad. Res, 39, 865–874. [DOI] [PubMed] [Google Scholar]
- Lim GP, Calon F, Morihara T, Yang FS, Teter B, Ubeda O, Salem N, Frautschy SA, Cole GM, 2005. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci, 25, 3032–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Lee HG, Liu Q, Perry G, Smith MA, Sayre LM, 2005. 4-oxo-2-nonenal is both more neurotoxic and more protein reactive than 4-hydroxy-2-nonenal. Chem. Res. Tox, 18, 1219–1231. [DOI] [PubMed] [Google Scholar]
- Lozada LE, Desai A, Kevala K, Lee JW, Kim HY, 2017a. Perinatal Brain Docosahexaenoic Acid Concentration Has a Lasting Impact on Cognition in Mice. J. Nutr, 147, 1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozada LE, Desai A, Kevala K, Lee JW, Kim HY, 2017b. Perinatal Brain Docosahexaenoic Acid Concentration Has a Lasting Impact on Cognition in Mice. J. Nutr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Watanabe A, Iwayama Y, Kimura T, Hamazaki K, Balan S, Ohba H, Hisano Y, Nozaki Y, Ohnishi T, Toyoshima M, Shimamoto C, Iwamoto K, Bundo M, Osumi N, Takahashi E, Takashima A, Yoshikawa T, 2017. Polyunsaturated fatty acid deficiency during neurodevelopment in mice models the prodromal state of schizophrenia through epigenetic changes in nuclear receptor genes. Transl. Psych, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani MM, Malm T, Lamb R, Jay TR, Neilson L, Casali B, Medarametla L, Landreth GE, 2017. Neuronally-directed effects of RXR activation in a mouse model of Alzheimer’s disease. Sci. Rep, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Mougan I, 1998. Fatty acid composition of human brain phospholipids during normal development. J. Neurochem, 71, 2528–2533. [DOI] [PubMed] [Google Scholar]
- Mathai ML, Soueid M, Chen N, Jayasooriya AP, Sinclair AJ, Wlodek ME, Weisinger HS, Weisinger RS, 2004. Does perinatal omega-3 polyunsaturated fatty acid deficiency increase appetite signaling? Obesity Research, 12, 1886–1894. [DOI] [PubMed] [Google Scholar]
- Miller LR, Jorgensen MJ, Kaplan JR, Seeds MC, Rahbar E, Morgan TM, Welborn A, Chilton SM, Gillis J, Hester A, Rukstalis M, Sergeant S, Chilton FH, 2016. Alterations in levels and ratios of n-3 and n-6 polyunsaturated fatty acids in the temporal cortex and liver of vervet monkeys from birth to early adulthood. Physiology & Behavior, 156, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Loewke J, Garrison M, Catalan JN, Salem N, 2001. Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J. Lipid Res, 42, 419–427. [PubMed] [Google Scholar]
- Morse NL, 2012. Benefits of Docosahexaenoic Acid, Folic Acid, Vitamin D and Iodine on Foetal and Infant Brain Development and Function Following Maternal Supplementation during Pregnancy and Lactation. Nutrients, 4, 799–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman KM, Molina-Campos E, Musial TF, Price AL, Oh KJ, Wolke ML, Buss EW, Scheff SW, Mufson EJ, Nicholson DA, 2015. Evidence for Alzheimer’s disease-linked synapse loss and compensation in mouse and human hippocampal CA1 pyramidal neurons. Brain Struct. Funct, 220, 3143–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik LJ, Berry R, Vassar R, 2006. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci, 26, 10129–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozias MK, Carlson SE, Levant B, 2007. Maternal parity and diet (n-3) polyunsaturated fatty acid concentration influence accretion of brain phospholipid docosahexaenoic acid in developing rats. J. Nutr, 137, 125–129. [DOI] [PubMed] [Google Scholar]
- Pallast S, Arai K, Wang XY, Lo EH, van Leyen K, 2009. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. J. Neurochem, 111, 882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauter AM, Trattner S, Gonzalez-Bengtsson A, Talamonti E, Asadi A, Dethlefsen O, Jacobsson A, 2017. Both maternal and offspring Elovl2 genotypes determine systemic DHA levels in perinatal mice. J. Lipid Res, 58, 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plourde M, Fortier M, Vandal M, Tremblay-Mercier J, Freemantle E, Begin M, Pifferi F, Cunnane SC, 2007. Unresolved issues in the link between docosahexaenoic acid and Alzheimer’s disease. Prost. Leuk. Ess. Fat. Acid, 77, 301–308. [DOI] [PubMed] [Google Scholar]
- Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR, Weiner M, Shinto L, Aisen PS, 2010. Docosahexaenoic Acid Supplementation and Cognitive Decline in Alzheimer Disease A Randomized Trial. J. Am. Med. Assoc, 304, 1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, Chia LA, Hamilton RL, Chu CT, Jordan-Sciutto KL, 2007. Expression of Nrf2 in neurodegenerative diseases. J. Neuropath. Exp. Neurol, 66, 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport SI, Igarashi M, Gao F, 2010. Quantitative contributions of diet and liver synthesis to docosahexaenoic acid homeostasis. Prost. Leuk. Ess. Fat. Acid, 82, 273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakayori N, Kikkawa T, Tokuda H, Kiryu E, Yoshizaki K, Kawashima H, Yamada T, Arai H, Kang JX, Katagiri H, Shibata H, Innis SM, Arita M, Osumi N, 2016. Maternal Dietary Imbalance between Omega-6 and Omega-3 Polyunsaturated Fatty Acids Impairs Neocortical Development via Epoxy Metabolites. Stem Cells, 34, 470–482. [DOI] [PubMed] [Google Scholar]
- Salvati S, Campeggi LM, Benedetti PC, Difelice M, Gentile V, Nardini M, Tomassi G, 1993a. Effects of Dietary Oils on Fatty-Acid Composition and Lipid-Peroxidation of Brain Membranes (Myelin and Synaptosomes) in Rats. J. Nutr. Biochem, 4, 346–350. [Google Scholar]
- Salvati S, Campeggi LM, Benedetti PC, Difelice M, Gentile V, Nardini M, Tomassi G, 1993b. Effects of Dietary Oils on Fatty-Acid Composition and Lipid-Peroxidation of Brain Membranes (Myelin and Synaptosomes) in Rats. J. Nutr. Biochem, 4, 346–350. [Google Scholar]
- Savioz A, Leuba G, Vallet PG, 2014. A framework to understand the variations of PSD-95 expression in brain aging and in Alzheimer’s disease. Age. Res. Rev, 18, 86–94. [DOI] [PubMed] [Google Scholar]
- Schebb NH, Ostermann AI, Yang J, Hammock BD, Hahn A, Schuchardt JP, 2014. Comparison of the effects of long-chain omega-3 fatty acid supplementation on plasma levels of free and esterified oxylipins. Prost. Other Lip. Med, 113, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao CY, Mirra SS, Sait HBR, Sacktor TC, Sigurdsson EM, 2011. Postsynaptic degeneration as revealed by PSD-95 reduction occurs after advanced A beta and tau pathology in transgenic mouse models of Alzheimer’s disease. Acta Neuropath, 122, 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP, 2016. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AJ, Crawford MA, 1972. Accumulation of Arachidonate and Docosahexaenoate in Developing Rat-Brain. J. Neurochem, 19, 1753–&. [DOI] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL, 2010. The Basics of Brain Development. Neuropsychol. Rev, 20, 327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MM, Zhang M, Cai HL, Li HD, Jiang P, Dang RL, Liu YP, He X, Xue Y, Cao LJ, Wu YQ, 2016a. Maternal diet of polyunsaturated fatty acid altered the cell proliferation in the dentate gyrus of hippocampus and influenced glutamatergic and serotoninergic systems of neonatal female rats. Lipids Health Dis, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MM, Zhang M, Cai HL, Li HD, Jiang P, Dang RL, Liu YP, He X, Xue Y, Cao LJ, Wu YQ, 2016b. Maternal diet of polyunsaturated fatty acid altered the cell proliferation in the dentate gyrus of hippocampus and influenced glutamatergic and serotoninergic systems of neonatal female rats. Lipids Health Dis, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MH, Paris C, Magnien M, Colin J, Pelleieux S, Coste F, Escanye MC, Pillot T, Olivier JL, 2017. Dietary arachidonic acid increases deleterious effects of amyloid-beta oligomers on learning abilities and expression of AMPA receptors: putative role of the ACSL4-cPLA(2) balance. Alz. Res. Ther, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Butt CM, Mohajeri MH, 2016a. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Mucha B, Denheyer H, Atkinson D, Schanz N, Vassiliou E, Benno RH, 2016b. Dietary docosahexaenoic acid alleviates autistic-like behaviors resulting from maternal immune activation in mice. Prost. Leuk. Ess. Fat. Acid, 106, 27–37. [DOI] [PubMed] [Google Scholar]
- Won S, Levy JM, Nicoll RA, Roche KW, 2017. MAGUKs: multifaceted synaptic organizers. Curr. Opin. Neurobiol, 43, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Huang Y, Chen ZY, 2005. Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Brit. J. Nutr, 94, 544–550. [DOI] [PubMed] [Google Scholar]
- Xie H, Hou S, Jiang J, Sekutowicz M, Kelly J, Bacskai BJ, 2013. Rapid cell death is preceded by amyloid plaque-mediated oxidative stress. Proceedings of the National Academy of Sciences of the United States of America, 110, 7904–7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavin E, Himovichi E, Eilam R, 2009a. Delayed Cell Migration in the Developing Rat Brain Following Maternal Omega 3 Alpha Linolenic Acid Dietary Deficiency. Neuroscience, 162, 1011–1022. [DOI] [PubMed] [Google Scholar]
- Yavin E, Himovichi E, Eilam R, 2009b. Delayed Cell Migration in the Developing Rat Brain Following Maternal Omega 3 Alpha Linolenic Acid Dietary Deficiency. Neuroscience, 162, 1011–1022. [DOI] [PubMed] [Google Scholar]
- Youyou A, Durand G, Pascal G, Piciotti M, Dumont O, Bourre JM, 1986. Recovery of Altered Fatty-Acid Composition Induced by A Diet Devoid of N-3 Fatty-Acids in Myelin, Synaptosomes, Mitochondria, and Microsomes of Developing Rat-Brain. J. Neurochem, 46, 224–228. [DOI] [PubMed] [Google Scholar]
- Zhang MJ, Wang SP, Mao LL, Leak RK, Shi YJ, Zhang WT, Hu XM, Sun BL, Cao GD, Gao YQ, Xu Y, Chen J, Zhang F, 2014. Omega-3 Fatty Acids Protect the Brain against Ischemic Injury by Activating Nrf2 and Upregulating Heme Oxygenase 1. J. Neurosci, 34, 1903–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WH, Liu JY, Xu GZ, Yuan Q, Sayre LM, 2003. Model studies on protein side chain modification by 4-oxo-2-nonenal. Chem. Res. Tox, 16, 512–523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.