Abstract
Background: Recent studies in nondisabled individuals have demonstrated that low-volume high-intensity interval training (HIIT) can improve cardiometabolic health similar to moderate-intensity training (MIT) despite requiring 20% of the overall time commitment. To date, there have been no studies assessing the effects of HIIT for improving cardiometabolic health in individuals with SCI. Objectives: The primary purpose of this pilot study was to compare the effects of 6 weeks of low-volume HIIT vs MIT using arm crank ergometer exercise to improve body composition, cardiovascular fitness, glucose tolerance, blood lipids, and blood pressure in a cohort of individuals with longstanding SCI. Methods: Participants were randomized to 6 weeks of HIIT or MIT arm crank exercise training. Aerobic capacity, muscular strength, blood lipids, glucose tolerance, blood pressure, and body composition were assessed at baseline and 6 weeks post training. Results: Seven individuals (6 male, 1 female; n = 3 in MIT and n = 4 in HIIT; mean age 51.3 ± 10.5 years) with longstanding SCI completed the study. The preliminary findings from this pilot study demonstrated that individuals with SCI randomized to either 6 weeks of HIIT or MIT displayed improvements in (a) insulin sensitivity, (b) cardiovascular fitness, and (c) muscular strength (p < .05). However, MIT led to greater improvements in arm fat percent and chest press strength compared to HIIT (p < .05). Conclusion: No differences between MIT and HIIT were observed. Both conditions led to improvements in insulin sensitivity, aerobic capacity, muscle strength, and blood lipids in individuals with SCI. Future larger cohort studies are needed to determine if the shorter amount of time required from HIIT is preferable to current MIT exercise recommendations.
Keywords: cardiometabolic health, high-intensity interval training, moderate-intensity exercise training, spinal cord injury
Spinal cord injury (SCI) is currently estimated to be prevalent in over two million individuals worldwide.1 Individuals with chronic SCI often experience severe muscle atrophy and increased adiposity and have increased risk of developing cardiometabolic diseases, such as obesity, dyslipidemia, insulin resistance, and type 2 diabetes, compared to age- and weight-matched nondisabled individuals.2–5 The benefits of exercise and physical activity for delaying and in many instances reversing health conditions associated with cardiometabolic diseases are well known in nondisabled6 and disabled persons,7 however, two-thirds of the SCI population remain physically inactive.8 Thus, it is important to identify novel exercise strategies that can not only improve health outcomes but also lead to greater exercise adherence in individuals with SCI.
One mode of training that has gained traction in the literature for improving cardiometabolic health in nondisabled individuals is low-volume high-intensity interval training (HIIT).9–12 A growing body of evidence from our group9 and others11,13 has demonstrated the potential for HIIT to provide comparable or superior improvements in a number of cardiometabolic health outcomes compared to continuous moderate-intensity training (MIT) that requires 60% to 80% greater overall time commitment. For example, we recently found similar improvements in % fat, blood lipids, and insulin sensitivity in obese males following 6 weeks of training despite HIIT only requiring 20% of the total time commitment as MIT.9 Importantly, this mode of exercise has been shown to be well tolerated in many different clinical populations, including type 2 diabetes,13,14 overweight/obesity,9,15,16 cardiovascular disease,17 and metabolic syndrome.18 A recent review by Nightingale et al19 suggests that HIIT may be a time-efficient solution for improving cardiometabolic health in SCI. However, the majority of data presented were based on evidence from nondisabled individuals.
To our knowledge, there have been only two published studies that have assessed the effects of exercise intensity in the SCI population.20,21 De Groot et al20 found greater improvements in VO2peak and the total cholesterol/high-density lipoprotein (HDL) ratio in the high-intensity group (70%–80% heart rate reserve [HRR]) compared to the low-intensity group (40%–50% HRR). In contrast, there were nonsignificant decreases in insulin sensitivity in the high-intensity group while the low-intensity group experienced a nonsignificant increase in insulin sensitivity. Hooker and Wells21 found no significant changes in VO2max in either group but did see significant improvements in blood lipids in the moderate-intensity group with no changes in the low-intensity group. While these studies lend valuable insight into the effects of exercise intensity for improving health outcomes in SCI, the protocols used required a significant time commitment (1 hour) and were similar between the two intensity groups. Thus, there is a need for comparative efficacy studies between HIIT and MIT to determine if low-volume HIIT is a viable alternative mode of exercise for improving cardiometabolic health in the SCI population.
An additional important gap in the SCI literature regarding HIIT is identifying the optimal dose of exercise (ie, interval duration and intensity) that achieves the greatest health benefits in the shortest time duration. Thus far, there have been no studies that have modeled low-volume HIIT in an SCI population. Recent HIIT studies in nondisabled individuals have used the Wingate protocol to determine exercise intensity, and the training protocol consisted of extremely short duration intervals at supramaximal intensities.9,22–24 This form of HIIT is uniquely different from traditional HIIT studies that have utilized greater volumes of interval training at an intensity based on percentage of VO2max.25 There are no training studies that have determined if individuals with SCI can even tolerate exercise training using a Wingate-based high-intensity and low-volume approach. Therefore, the primary purpose of this pilot study was to compare the effects of 6 weeks of low-volume HIIT versus MIT using arm crank ergometer exercise to improve body composition, cardiovascular fitness, glucose tolerance, blood lipids, and blood pressure in a cohort of individuals with longstanding SCI.
Methods
Participants
Seven individuals (6 male, 1 female; n = 3 in MIT and n = 4 in HIIT; mean age 51.3 ± 10.5 years) with longstanding SCI completed the study. Individuals were considered eligible if they met the following criteria: (a) diagnosed with a traumatic SCI at the lower cervical, thoracic, and upper lumbar level (C5-L2); (b) classified as A, B, C, D (motor and sensory complete or incomplete) on the American Spinal Injury Association (ASIA) Impairment Scale (AIS); and (c) >3 years post injury. Individuals with cardiovascular disease, renal disease, or orthopedic problems were considered ineligible. Potential participants were identified by a computer-generated list of patients who are enrolled in the SCI Model System and Lakeshore Foundation Member Database and currently reside in a large metropolitan city. The study was approved by the Institutional Review Board at a large university medical center.
Study design
This pilot study was a 6-week, randomized controlled trial that compared the effects of HIIT versus MIT on cardiometabolic health outcomes in SCI. Subjects were randomly assigned to either the HIIT (C6 ASIA B, L1 ASIA B, T8 ASIA A, C8 ASIA B) group or the MIT (C7 ASIA B, T6 ASIA A, T12-L1 ASIA D) group. To ensure randomization, 20 assignments were placed in 20 nontransparent envelopes and individually distributed to participants after they completed baseline testing. Nine participants enrolled in the study (n = 5 for HIIT and n = 4 for MIT). In total, two participants dropped out of the study, one from the MIT group due to an injury unrelated to this intervention and one from the HIIT group due to inability to adhere to the exercise training time commitment. Participants underwent assessment at baseline, performed 6 weeks of HIIT or MIT exercise training, and were evaluated again after exercise training.
Pretraining testing protocol
Eligible participants attended three baseline visits: Day 1, following an overnight fast, resting metabolic rate, body composition, and blood pressure were assessed; Day 2, an oral glucose tolerance test (OGTT) was performed and baseline blood samples were stored at −80°C and analyzed for HDL, low-density lipoprotein (LDL), total cholesterol, and triglyceride levels; and Day 3, VO2peak was assessed using indirect calorimetry during a graded arm cycle ergometer test, and peak power was determined by the standard 30-second Wingate test on a Lode (The Netherlands) arm ergometer. Additionally, four one-repetition maximum (1RM) strength assessments were performed using the upper body.
Exercise training
HIIT was performed on an electronically braked Lode arm ergometer. Participants performed a total of 20 minutes of exercise consisting of 4 minutes of arm crank exercise at 25% of HRR determined from the VO2peak test, followed by 30 seconds at 50% of peak power obtained from the Wingate Test. This cycle was repeated four times ending with 2 minutes of recovery at 25% of HRR. Participants in the HIIT group exercised twice a week with at least 24 hours of rest between each training session. MIT was performed on a SCIFIT Arm Ergometer (SCIFIT; Tulsa, OK). MIT consisted of 30 minutes of continuous arm crank exercise at 55% of VO2peak as determined by the baseline VO2peak assessment. MIT participants exercised three times each week. Heart rate was recorded for each HIIT and MIT session. Heart rate was logged every 5 minutes during MIT and immediately after each 30-second work interval and 4-minute recovery period for HIIT. Exercise training data and weekly time commitment by exercise group are shown in Table 1.
Table 1.
Exercise training data and weekly time commitment
| Variable | HIIT group (n = 4) | MIT group (n = 3) |
|---|---|---|
| Protocol | 30s × 4 repeats; 4 min rest | 30 min arm cycling |
| 2 sessions per week | 3 sessions per week | |
| Workload (watts) | 50% peak power: 145 ± 62 W | 55–65% VO2peak: 30 ± 11.3 W |
| 25% HRR: 15± 1.2 W | ||
| HR (bpm) | Interval: 133 ± 43 bpm | 103 ± 11 bpm |
| Recovery: 109 ± 23 bpm | ||
| Weekly training time | Interval: 4 min | 90 min |
| Recovery: 36 min | ||
| Total: 40 min |
Note: Values are mean ± SD. HIIT = high-intensity interval training; HR = heart rate; HRR = heart rate reserve; MIT = moderate-intensity training.
Clinical measures
Body composition
Total body composition, including fat mass, lean mass, percent body fat, percent arm fat, and percent leg fat, were measured by dual-energy X-ray absorptiometry (DXA). Participants were lying supine with arms at their sides on a padded table. Scans were analyzed using ADULT software version 1.33 (Lunar Radiation).
Resting blood pressure
Resting blood pressure (systolic and diastolic) was taken by automatic auscultation (Omron Blood Pressure Monitor, model HEM-780; Omron Healthcare, Inc., Bannockburn, IL) while participants were seated in a wheelchair. Readings were taken after 12 hours of fasting between 7:00 and 9:00 a.m.
Resting energy expenditure
Resting energy expenditure (REE) was measured between 7:00 and 9:00 a.m. following a 12-hour overnight fast. Participants remained supine following the DXA measurement. Subjects remained awake in a quiet, dimly lit room while oxygen uptake and carbon dioxide production were measured continuously using a ventilated hood system. Oxygen uptake was measured using a computerized, open-circuit indirect calorimetry system (ParvoMedics). After a 10-minute equilibration period, the data from the remaining 20-minute steady state period was used for analysis. REE was generated from the ParvoMedics system from Weir equation 12.26
Oral glucose tolerance test and insulin sensitivity
An OGTT was performed on an in-patient basis at the Clinical Research Unit (CRU). Participants fasted overnight and arrived at the CRU between 7:00 and 9:00 a.m. The post training OGTT was performed at least 24 hours after the last exercise session. Prior to testing, a flexible intravenous catheter was inserted into the antecubital space of one arm. Within the first 20 minutes, two baseline blood samples were taken to establish basal glucose and insulin. At time 0, the patient was instructed to drink a 75-g oral glucose load within 5 minutes. After consumption, blood samples were taken at 10, 20, 30, 60, 90, and 120 minutes to assess plasma glucose and plasma insulin. Following completion of the test, blood samples were immediately centrifuged, separated for serum, and frozen at −80°C until analysis. Assays were performed in the Center for Clinical and Translational Sciences Metabolism Core. Serum glucose assays were performed on an automated glucose analyzer (Sirrus analyzer; Stanbio Laboratory, Boerne, TX), and serum insulin was measured using an immunofluorescent method with an AIA-600 II analyzer (TOSOH Bioscience, South San Francisco, CA) per manufacturers' instructions. Insulin sensitivity was calculated using Quantitative Insulin Sensitivity Check Index (QUICKI).27 QUICKI was calculated as 1/[log glucose (mg/dL) + log insulin (μU/mL)]. Insulin resistance (IR) was assessed using the Homeostasis Model of Assessment of Insulin Resistance (HOMA-IR). HOMA-IR was calculated as [fasting insulin (μU/mL) × fasting glucose (mmol/L)]/22.5.
Blood lipids
Laboratory analyses took place in the Core Laboratory of CRU, Nutrition Obesity Research Center, and Diabetes Research Center. Total cholesterol, HDL, and triglycerides were assessed in serum using an automated glucose analyzer (Sirrus analyzer; Stanbio Laboratory, Boerne, TX). The Friedwald method was used to calculate LDL values.28
Strength assessment
Muscle strength was determined using a standard 1RM protocol. The maximum load that could be lifted one time was measured for chest press, overhead press, lateral pull down, and tricep extension (Cybex VR3 for chest press, overhead press, and lateral pulldown; and Free Motion EXT Dual Cable for tricep extension). 1RM was determined after subjects lifted progressively heavier loads through full range of motion until failure occurred.
Peak oxygen uptake (VO2peak)
Peak aerobic assessment, which determined aerobic capacity, was defined by a VO2peak test on a Lode arm cycle ergometer. Subjects were instructed to cycle on an arm crank ergometer at 10W for 2 minutes. Every 2 minutes thereafter, power output was increased by 10W until voluntary fatigue. Due to the variability of heart rates after SCI, VO2peak was determined by (a) volitional exhaustion, (b) failure to maintain 60–65 revolutions per minute (RPM), (c) RER ≥ 1.10, and (d) rate of perceived exertion (RPE) >18 using the 6–20 Borg scale.29 Minute ventilation, oxygen uptake, and carbon dioxide production were continuously analyzed and recorded via open circuit spirometry (ParvoMedics).
Peak anaerobic power assessment
Peak power was determined by the standard 30-second Wingate protocol on a Lode arm cycle ergometer. Participants were seated in front of the Lode ergometer and remained seated throughout the entire test. Prior to each test, participants completed a 5-minute warm up at 25 W, which included three short sprint efforts followed by a 5-minute recovery period. Following the warm up, participants were instructed to hand cycle as fast as possible. Verbal encouragement was given to all participants to maintain their highest possible cadence throughout the test. The resistance was determined by the body weight (0.075 kg/kg body weight), with data collected and analyzed using the Monark Anaerobic Test software. Peak power, mean anaerobic power, fatigue rate, and relative peak power were recorded following the test.
Statistical analysis
Statistical analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC). Descriptive statistics were computed for each group at baseline and post exercise training. A mixed-model, repeated measures analysis of variance (ANOVA) was used to assess the effects of time (pre and post intervention), group, and Group × Time interactions for each variable of interest. A compound symmetry covariance matrix was assumed for these analyses. Due to the pilot nature of this study and the small sample size, post hoc comparisons were performed without adjustments for multiple pairwise comparisons. Statistical tests were two-sided, and p < .05 was deemed statistically significant.
Results
Exercise training data and weekly time commitment between exercise groups are shown in Table 1. Average peak power for HIIT was 145 ± 62 W during the intervals and 15 ± 1.2 W during the recovery period. Average peak power for the MIT group was 30 ± 11.3 W. These workloads corresponded to 276% and 28.5% of VO2peak during the HIIT sessions and 55% of VO2peak during the MIT sessions. Average heart rate during the HIIT was 133 ± 43 bpm for intervals and 109 ± 23 bmp during recovery and 103 ± 11 bpm during MIT.
Baseline characteristics are shown in Table 2. There were no significant differences between groups on any baseline measures. Table 3 highlights the body composition and cardiometabolic data. With the exception of a significant time effect for QUICKI (p = .042), there were no other significant differences between groups on body composition and cardiometabolic markers. However, there was a significant Group × Time interaction for arm fat percent (p = .043) such that MIT led to greater improvements in arm fat percent compared to HIIT and a trend for an overall time effect for HOMA-IR (p = .072) and arm fat percent (p = .061). Table 4 presents the data on aerobic capacity, anaerobic power, and muscular strength. There was a significant time effect for chest press (p = .035) and lateral pulldown (p = .021). Additionally, there was a significant Group × Time interaction (p = .039) for chest press, such that MIT led to greater improvements in chest press strength.
Table 2.
Baseline descriptive data between groups (N = 8)
| Descriptive data | HIIT | MIT | p |
|---|---|---|---|
| Age (years) | 49.4 ± 13 | 51.3 ± 1.2 | .823 |
| Height (cm) | 177.1 ± 14.9 | 177.2 ± 11.7 | .986 |
| Body weight (kg) | 81.4 ± 24.9 | 92.7 ± 23.4 | .565 |
| BMI (kg/m2) | 26 ± 4.1 | 31.1 ± 12.8 | .420 |
| Lean mass (kg) | 47.3 ± 14.4 | 48.1 ± 4.4 | .936 |
| Fasting glucose (mg/dL) | 105.4 ± 18.9 | 154 ± 102.7 | .319 |
| Fasting insulin (mg/dL) | 18.1 ± 11.8 | 21.6 ± 25.5 | .801 |
| HOMA-IR | 4.9 ± 3.3 | 12.5 ± 18.8 | .387 |
| Cholesterol (mg/dL) | 158 ± 37.8 | 177 ± 38.5 | .506 |
| Triglycerides (mg/dL) | 83.4 ± 23.4 | 97.3 ± 61.7 | .654 |
| HDL (mg/L) | 52 ± 9.4 | 57 ± 9.8 | .501 |
| LDL (mg/dL) | 89.3 ± 33.7 | 101.2 ± 38.6 | 0.662 |
| SBP (mm Hg) | 120 ± 14 | 125 ± 26.9 | 0.735 |
| DBP (mm Hg) | 67.7 ± 8.2 | 73.3 ± 15.5 | 0.516 |
| VO2peak (mL/kg/min) | 13.5 ± 5.5 | 11.5 ± 2.6 | 0.588 |
Note: Values are mean ± SD. BMI = body mass index; DBP = diastolic blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SBP = systolic blood pressure.
Table 3.
Changes in body composition and cardiometabolic health markers by intervention group
| HIIT (n = 4) | MIT (n = 3) | G | T | GxT | |||
|---|---|---|---|---|---|---|---|
| Baseline | 6 weeks post | Baseline | 6 weeks post | p | p | p | |
| Body weight (kg) | 87.8 ± 24.3 | 86.8 ± 23.4 | 92.7 ± 23.4 | 92.7 ± 21.6 | .778 | .408 | .436 |
| Fat (%) | 26.8 ± 4.3 | 26.4 ± 4.8 | 31.1 ± 12.8 | 31 ± 12.8 | .536 | .365 | .589 |
| Fat mass (kg) | 33.7 ±10 | 32.7 ± 9.3 | 41.8 ± 24.6 | 41.8 ± 24.7 | .544 | .356 | .356 |
| Lean mass (kg) | 50.9 ± 13.7 | 50.9 ± 13.6 | 48.1 ± 4.4 | 46.5 ± 4.9 | .680 | .333 | .389 |
| Arm fat (%) | 25.9 ± 3.3 | 25.9 ± 3.4 | 37.9 ± 14.1 | 36.1 ± 13.9 | .175 | .061 | .043 |
| Leg fat (%) | 39.6 ± 8.3 | 39.7 ±8.7 | 45.4 ± 18.5 | 44.8 ± 19.8 | .631 | .657 | .538 |
| QUICKI | .3004 ± 0.02 | .3223 ± 0.01 | .3208 ±0.07 | .3555 ± 0.04 | .374 | .042 | .560 |
| HOMA-IR | 5.9 ± 2.7 | 3.2 ± 0.63 | 12.5 ± 18.9 | 1.9 ±1.0 | .525 | .072 | .572 |
| Glucose (mL/dL) | 109.9 ±18.5 | 108.4 ±17.5 | 154 ±102.8 | 135.7 ±80.4 | .468 | .131 | .186 |
| Insulin (mL/dL) | 21.8 ±9.9 | 12.1 ±2.9 | 21.6 ±25.5 | 5.9 ±2.3 | .674 | .113 | .671 |
| Cholesterol (mg/dL) | 161 ±43 | 146.5 ±46.2 | 177.7 ±38.5 | 173.3 ±29.8 | .482 | .478 | .696 |
| HDL (mg/dL) | 48.5 ±6.0 | 46.8 ±3.4 | 57 ±9.8 | 57 ±10.8 | .133 | .737 | .737 |
| LDL (mg/dL) | 94.7 ±36.4 | 81 ±42.3 | 101.2 ±38.6 | 96.4 ±21.2 | .686 | .427 | .696 |
| TRG (mg/dL) | 89.3 ±22.4 | 93.5 ±20.8 | 97.3 ±61.7 | 99.7 ±45.6 | .812 | .649 | .893 |
| SBP (mm Hg) | 118.5 ±15.7 | 114 ±23.1 | 125 ±26.9 | 119.7 ±25.7 | .736 | .120 | .880 |
| DBP (mm Hg) | 66.1 ±8.6 | 66.3 ±15.4 | 73.3 ±15.5 | 71.2 ±15.4 | .572 | .773 | .723 |
| REE (kcal/day) | 1951 ±612 | 1951 ±879 | 1840 ±388 | 1718 ±462 | .723 | .749 | .751 |
Note: Values are mean ± SD. Bold values indicate statistical significance (p < .05). DBP = diastolic blood pressure; G = intervention group; GxT = Group × Time interaction; HDL = high density lipoprotein, LDL = low density lipoprotein REE = resting energy expenditure; SBP = systolic blood pressure; T = time; TRG = triglycerides.
Table 4.
Changes in aerobic capacity, anaerobic power, and muscular strength by intervention group
| HIIT (n = 4) | MIT (n = 3) | G | T | GxT | |||
|---|---|---|---|---|---|---|---|
| Baseline | 6 weeks post | Baseline | 6 weeks post | p | p | p | |
| VO2peak (mL/kg/min) | 14.2 ± 6 | 15.3 ± 7.3 | 11.5 ± 2.6 | 13.9 ± 1.3 | .631 | .048 | .347 |
| Peak power (watts) | 347 ± 131 | 377 ± 72 | 278 ± 71 | 308 ± 77 | .313 | .458 | .997 |
| Relative PP (watts) | 4.0 ±1.6 | 4.4 ± 0.87 | 2.9 ± 0.35 | 3.3 ± 0.78 | .190 | .409 | .919 |
| Overhead press (kg) | 45.3 ± 18.9 | 48.5 ± 22.2 | 37.9 ± 9.2 | 39.4 ± 4.7 | .541 | .266 | .678 |
| Tricep extension (kg) | 23.3 ± 7.9 | 31.8 ± 5.7 | 34.1 ± 16.1 | 38.6 ± 9.6 | .299 | .168 | .450 |
| Chest press (kg) | 75.2 ± 27.9 | 75.3 ± 29.7 | 56.1 ± 21.8 | 63.6 ± 20.2 | .473 | .035 | .039 |
| Lat pulldown (kg) | 45.9 ± 12.6 | 53..2 ± 19 | 36.4 ±8.2 | 47.7 ± 13.8 | .513 | .021 | .509 |
Note: Bold values indicate statistical significance (p < .05). G = intervention group; T = time; GxT = Group × Time interaction.
Individual blood lipid results are shown in Figure 1. As illustrated in Figure 1, there was an overall reduction in total cholesterol and LDL-C in all participants except for participant 8 who had type 2 diabetes. Changes in serum triglycerides and HDL were variable between participants. Figure 2 illustrates the glucose tolerance findings. All participants improved insulin sensitivity determined by QUICKI and HOMI-IR. However, participant 8 had an unusual insulin response to the OGTT, such that there was no change in her insulin response during her 6-week post OGTT. We are uncertain if this response was due to medication or something associated with her diabetes. The extreme variability between her test results and the small sample size affected the primary blood lipid and glucose tolerance outcomes. When she was excluded from the data set, there was a significant reduction in total cholesterol and LDL in addition to the reduction in QUICKI (p < .05) and a trend for a decrease in fasting glucose (p = .07) in the remaining six participants.
Figure 1.
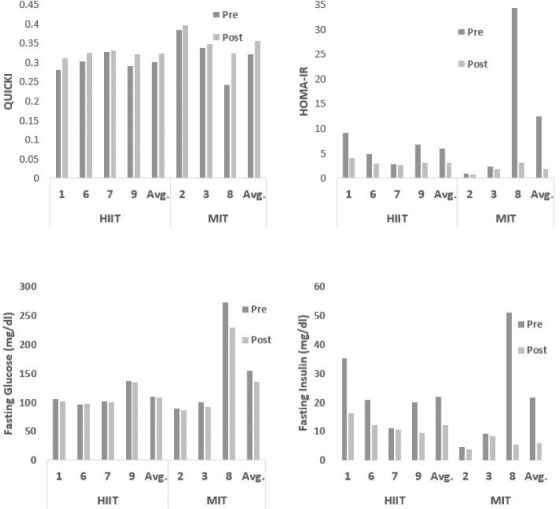
Individual blood lipid responses following high-intensity interval training (HIIT) and moderate-intensity training (MIT).
Figure 2.
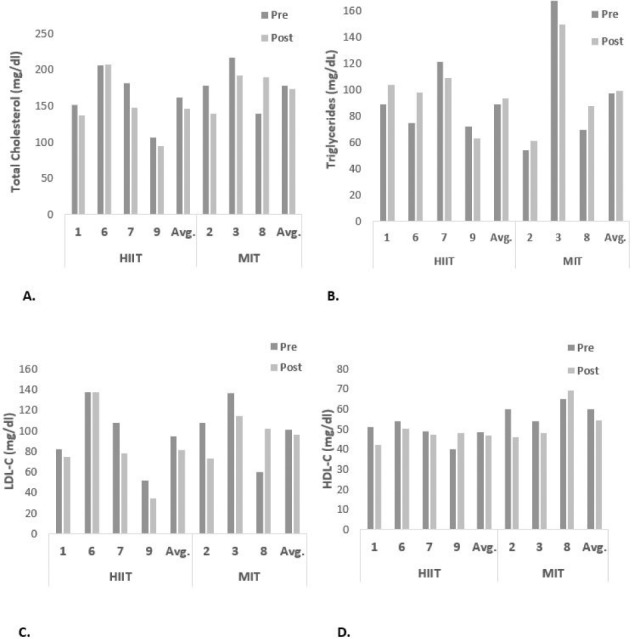
Individual glucose and insulin responses following high-intensity interval training (HIIT) and moderate-intensity training (MIT).
Discussion
The high rates of physical inactivity among people with SCI is a medical and public health concern that demands further study on identifying promising, dose-specific exercise strategies that can be readily achieved by this underactive/inactive population. The preliminary findings from this pilot study demonstrated that individuals with long-standing SCI randomized to either 6 weeks of HIIT or MIT arm crank ergometer training displayed improvements in (a) insulin sensitivity, (b) cardiovascular fitness, and (c) muscular strength. However, MIT led to greater improvements in arm fat percent and chest press strength compared to HIIT. To our knowledge, this is the first study in SCI that has compared a low-volume HIIT protocol at an exercise intensity well above VO2peak to a MIT exercise group.
Overall, this pilot study demonstrated three important findings. First, both HIIT and MIT improved a few cardiometabolic risk factors in individuals with long-standing SCI. This is the first study to demonstrate that supramaximal HIIT can be tolerated by participants with SCI. HIIT has gained popularity in the general population and has also been shown to be effective for improving many aspects of cardiometabolic health in individuals with obesity, type 2 diabetes, and older age.9,12,13 In general, participants tolerated HIIT well in this study. Several participants enjoyed the overall lower weekly time commitment required from the HIIT, and they liked the brevity of the exercise. Future studies are needed to optimize the duration, intensity, and volume of HIIT in individuals with SCI.
Finally, although this is a pilot study utilizing a relatively small sample size, the results are similar to what has been reported in other populations. Previous studies have reported that as few as 2 days of HIIT could improve cardiometabolic health markers to a similar degree as individuals performing MIT exercise for double the weekly time commitment.9,13 One study involving nondisabled individuals demonstrated similar improvements in glucose tolerance between traditional moderate-intensity exercise and low-volume HIIT training.30 In addition, HIIT has been shown to significantly improve insulin sensitivity in as little as 2 weeks in healthy adults31 and potentially has similar long-term (>24 hours) benefits as MIT.32
In research on HIIT in SCI populations, Hasnan et al showed no improvements in oral glucose tolerance after 6 weeks of hybrid arm and leg exercise suggesting that 6 weeks of training may not be a sufficient dose (ie, duration) to improve glucose tolerance in individuals with SCI.33 However, Jeon et al showed favorable results for both glucose tolerance and insulin sensitivity using electrical stimulated-assisted cycling in individuals with SCI. Glucose tolerance improved for all seven subjects while insulin sensitivity improved in two out of three subjects tested on this measure.34 A recent 6-week training study that looked at moderate-intensity exercise in individuals with chronic paraplegia showed an improvement in hepatic insulin sensitivity.19 Our study is in agreement with Nightingale et al who also reported a significant time effect for QUICKI (p < .05), as well as a trend toward significance in HOMA. In the present study, six out of seven participants reported decreases in HOMA-IR from baseline to post exercise training. However, there were no significant changes in fasting glucose or fasting insulin for either the HIIT or MIT group, although there was a clinically meaningful effect in insulin sensitivity for HIIT and MIT from baseline to post exercise training in all participants (7.3% and 10.8%, respectively). Therefore, it appears that as little as 6 weeks of HIIT or MIT exercise training can improve insulin sensitivity in individuals with long-standing SCI for as long as 24 hours following the last exercise bout.
In the present study, we did not find significant differences in total cholesterol, LDL, and HDL between HIIT and MIT groups. However, if we omit our one participant with type 2 diabetes who had abnormal blood glucose and blood lipid responses, we did see a mean decrease 11.3% and 17.9% decrease in total cholesterol and LDL levels across groups. Thus, these data suggest that 6 weeks of either HIIT or MIT training can improve the blood lipid profile in previously sedentary individuals with longstanding SCI. This is in contrast to several HIIT studies in nondisabled individuals where no changes in total cholesterol were observed after prolonged HIIT training.30,35
Although the literature on HIIT in the SCI population is scarce, two studies assessed blood lipids following HIIT training. One study utilized a hybrid HIIT protocol combining arm and lower functional electrical stimulation using a leg tricycle and found no significant changes in total cholesterol or LDL.33 In another study using arm cycling only, there were no significant changes in total cholesterol or LDL.20 Furthermore, a previous investigation by our group found no improvement in blood lipids following 8 weeks of combined aerobic and resistance exercise (unpublished observations). Therefore, the improvements in blood lipids in this study are the first to demonstrate that both MIT and HIIT exercise training can lead to an improved blood lipid profile and reduced cardiovascular disease risk.
VO2peak is a strong predictor of mortality and morbidity,36 thus it is important for health professionals to implement treatments that can increase aerobic capacity. This study found an overall 7.2% and 17.2% improvement in aerobic capacity in the HIIT and MIT groups. In general, prior research studies have shown improvements in VO2peak following a variety of exercise interventions in individuals with SCI. For example, Brurok et al compared changes in VO2peak following 8 weeks of high-intensity hybrid training that consisted of arm crank exercise with functional electrical stimulation cycling, an arm crank exercise only group, and a functional electrical stimulation cycling group. They found significant improvements in VO2peak (23.5%–25.9%) in all three training groups with no differences between groups.37 Additionally, Horiuchi and Okita found an 11.6% improvement in VO2peak following 10 weeks of moderate-intensity arm crank exercise performed 4 days per week.38 In addition to aerobic training protocols, Jacobs et al also showed that 12 weeks of upper body resistance training improved VO2peak by 15% in individuals with paraplegia.39 Overall, our study consisted of a much shorter training duration and significantly lower weekly exercise volume compared to previous investigations. While these data are preliminary in nature, this is the first study to demonstrate an improvement in aerobic capacity following only 2 days a week of low-volume HIIT performed at an exercise intensity greater than VO2peak.
SCI generally leads to significant skeletal muscle atrophy below the level of injury. Skeletal muscle atrophy of 27% to 56% has been observed in SCI participants from 6 to 24 weeks post injury with a cross-sectional area of only one-third that of able-bodied controls.40 Electrical stimulation has been shown to improve muscular strength. Both resistance and endurance training with the assistance of NMES or FES in individuals with chronic SCI have demonstrated significant increases in muscle hypertrophy in the lower body41 and overall muscular strength in the upper body, specifically the shoulder flexors, extensors, abductors, and adductors.42 Additionally, high-intensity aerobic arm cycling of 90 minutes per week is enough to improve upper extremity muscular strength in individuals with tetraplegia.43 Although our study did not look at cross-sectional area, we did see significant improvements in chest press and lateral pulldown in both groups. Thus, we showed that as little as 40 minutes per week of HIIT and 90 minutes of MIT is sufficient to improve upper body strength. This is important as muscle strength and function is critical for enabling individuals with SCI to perform activities of daily living.
Regular moderate-to-vigorous physical activity is associated with less visceral adipose tissue in persons with SCI.44 Additionally, endurance training has been shown to improve body composition in this population.45,46 While many studies performed in nondisabled individuals have shown improved body composition following HIIT in both men and women,47–49 we are aware of only one study that assessed body composition following HIIT in the SCI population. This case report found increases in lean mass, total body fat mass, and body fat percentage following 10 weeks of resistance-guided FES cycling.50 While the present study observed no significant changes in fat mass, fat-free mass, or percent body fat, we did obtain a greater reduction of arm fat % following MIT compared to HIIT. Overall, 6 weeks is a relatively short training duration to see significant changes in body composition. Future studies should incorporate a longer training period and a larger sample size to assess the effects of HIIT for improving body composition.
The present pilot study demonstrated that both HIIT and MIT are effective for improving a number of cardiometabolic health markers in individuals with SCI. With only 45% of the time commitment as MIT, HIIT was just as effective for improving insulin sensitivity, aerobic capacity, muscle strength, and blood lipid profiles in individuals with SCI. Overall strengths of this study include (a) inclusion of a MIT training group to compare to HIIT; (b) the longitudinal design; (c) the use of state-of-the-art methods to assess body composition, blood biomarkers, and cardiovascular fitness; and (d) 100% adherence to the exercise protocol in both HIIT and MIT groups. The primary limitation in this study was the small sample size, which limits our ability to confer inference for the entire SCI population. Additionally, we did not control for potential dietary differences between participants. Future research is needed to conclusively recommend HIIT as an alternative mode of exercise for individuals with SCI.
Footnotes
Conflicts of Interest
G. Fisher reports grants from UAB/Lakeshore Research Collaborative during the conduct of the study.
REFERENCES
- 1.Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: Update 2011, global incidence rate. Spinal Cord. 2014;52(2):110–116. doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 2.Liusuwan RA, Widman LM, Abresch RT, Styne DM, McDonald CM. Body composition and resting energy expenditure in patients aged 11 to 21 years with spinal cord dysfunction compared to controls: Comparisons and relationships among the groups. J Spinal Cord Med. 2007;30(Suppl 1):S105–111. doi: 10.1080/10790268.2007.11754613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widman LM, Abresch RT, Styne DM, McDonald CM. Aerobic fitness and upper extremity strength in patients aged 11 to 21 years with spinal cord dysfunction as compared to ideal weight and overweight controls. J Spinal Cord Med. 2007;30(Suppl 1):S88–96. doi: 10.1080/10790268.2007.11754611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yarar-Fisher C, Bickel CS, Windham ST, McLain AB, Bamman MM. Skeletal muscle signaling associated with impaired glucose tolerance in spinal cord-injured men and the effects of contractile activity. J Appl Physiol (1985) 2013;115(5):756–764. doi: 10.1152/japplphysiol.00122.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adriaansen JJ, van Asbeck FW, Lindeman E, van der Woude LH, de Groot S, Post MW. Secondary health conditions in persons with a spinal cord injury for at least 10 years: Design of a comprehensive long-term cross-sectional study. Disabil Rehabil. 2013;35(13):1104–1110. doi: 10.3109/09638288.2012.712196. [DOI] [PubMed] [Google Scholar]
- 6.Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: Primary prevention through exercise biology. J Appl Physiol (1985) 2000;88(2):774–787. doi: 10.1152/jappl.2000.88.2.774. [DOI] [PubMed] [Google Scholar]
- 7.Phillips WT, Kiratli BJ, Sarkarati M et al. Effect of spinal cord injury on the heart and cardiovascular fitness. Curr Probl Cardiol. 1998;23(11):641–716. doi: 10.1016/s0146-2806(98)80003-0. [DOI] [PubMed] [Google Scholar]
- 8.Miller LE, Herbert WG. Health and economic benefits of physical activity for patients with spinal cord injury. Clinicoecon Outcomes Res. 2016;8:551–558. doi: 10.2147/CEOR.S115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher G, Brown AW, Bohan Brown MM et al. High intensity interval- vs moderate intensity-training for improving cardiometabolic health in overweight or obese males: A randomized controlled trial. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0138853. e0138853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgomaster KA, Howarth KR, Phillips SM et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586(1):151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillen JB, Martin BJ, MacInnis MJ, Skelly LE, Tarnopolsky MA, Gibala MJ. Twelve weeks of sprint interval training improves indices of cardiometabolic health similar to traditional endurance training despite a five-fold lower exercise volume and time commitment. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0154075. e0154075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590(Pt 5):1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Little JP, Gillen JB, Percival ME et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol (1985) 2011;111(6):1554–1560. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 14.Boudou P, Sobngwi E, Mauvais-Jarvis F, Vexiau P, Gautier JF. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. Eur J Endocrinol. 2003;149(5):421–424. doi: 10.1530/eje.0.1490421. [DOI] [PubMed] [Google Scholar]
- 15.Gillen JB, Percival ME, Ludzki A, Tarnopolsky MA, Gibala MJ. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity (Silver Spring) 2013;21(11):2249–2255. doi: 10.1002/oby.20379. [DOI] [PubMed] [Google Scholar]
- 16.Whyte LJ, Gill JM, Cathcart AJ. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism. 2010;59(10):1421–1428. doi: 10.1016/j.metabol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Warburton DE, McKenzie DC, Haykowsky MJ et al. Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease. Am J Cardiol. 2005;95(9):1080–1084. doi: 10.1016/j.amjcard.2004.12.063. [DOI] [PubMed] [Google Scholar]
- 18.Tjønna AE, Lee SJ, Rognmo Ø et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: A pilot study. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nightingale TE, Walhin JP, Thompson D, Bilzon JLJ. Impact of exercise on cardiometabolic component risks in spinal cord-injured humans. Med Sci Sports Exerc. 2017;49(12):2469–2477. doi: 10.1249/MSS.0000000000001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Groot PC, Hjeltnes N, Heijboer AC, Stal W, Birkeland K. Effect of training intensity on physical capacity, lipid profile and insulin sensitivity in early rehabilitation of spinal cord injured individuals. Spinal Cord. 2003;41(12):673–679. doi: 10.1038/sj.sc.3101534. [DOI] [PubMed] [Google Scholar]
- 21.Hooker SP, Wells CL. Effects of low- and moderate-intensity training in spinal cord-injured persons. Med Sci Sports Exerc. 1989;21(1):18–22. doi: 10.1249/00005768-198902000-00004. [DOI] [PubMed] [Google Scholar]
- 22.MacInnis MJ, Zacharewicz E, Martin BJ et al. Superior mitochondrial adaptations in human skeletal muscle after interval compared to continuous single-leg cycling matched for total work. J Physiol. 2017;595(9):2955–2968. doi: 10.1113/JP272570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J Physiol. 2010;588(Pt 6):1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibala MJ, Little JP, van Essen M et al. Short-term sprint interval versus traditional endurance training: Similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575(Pt 3):901–911. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peake JM, Tan SJ, Markworth JF, Broadbent JA, Skinner TL, Cameron-Smith D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am J Physiol Endocrinol Metab. 2014;307(7):E539–552. doi: 10.1152/ajpendo.00276.2014. [DOI] [PubMed] [Google Scholar]
- 26.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition. 1990;6(3):213–221. [PubMed] [Google Scholar]
- 27.Katz A, Nambi SS, Mather K et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 28.Friedwald WT LR, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of preparative ultracentrifuge. Clim Chem. 1972:499–502. [PubMed] [Google Scholar]
- 29.Borg G. Borgs Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 30.Nybo L, Sundstrup E, Jakobsen MD et al. High-intensity training versus traditional exercise interventions for promoting health. Med Sci Sports Exerc. 2010;42(10):1951–1958. doi: 10.1249/MSS.0b013e3181d99203. [DOI] [PubMed] [Google Scholar]
- 31.Babraj JA, Vollaard NB, Keast C, Guppy FM, Cottrell G, Timmons JA. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr Disord. 2009;9:3. doi: 10.1186/1472-6823-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bajpeyi S, Tanner CJ, Slentz CA et al. Effect of exercise intensity and volume on persistence of insulin sensitivity during training cessation. J Appl Physiol (1985) 2009;106(4):1079–1085. doi: 10.1152/japplphysiol.91262.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasnan N, Engkasan JP, Husain R, Davis GM. High-intensity virtual-reality arm plus FES-leg interval training in individuals with spinal cord injury. Biomed Tech (Berl) 2013 doi: 10.1515/bmt-2013-4028. doi: 10.1515/bmt-2013-4028. [DOI] [PubMed] [Google Scholar]
- 34.Jeon JY, Weiss CB, Steadward RD et al. Improved glucose tolerance and insulin sensitivity after electrical stimulation-assisted cycling in people with spinal cord injury. Spinal Cord. 2002;40(3):110–117. doi: 10.1038/sj.sc.3101260. [DOI] [PubMed] [Google Scholar]
- 35.Musa DI, Adeniran SA, Dikko AU, Sayers SP. The effect of a high-intensity interval training program on high-density lipoprotein cholesterol in young men. J Strength Cond Res. 2009;23(2):587–592. doi: 10.1519/JSC.0b013e318198fd28. [DOI] [PubMed] [Google Scholar]
- 36.Liu R, Sui X, Laditka JN et al. Cardiorespiratory fitness as a predictor of dementia mortality in men and women. Med Sci Sports Exerc. 2012;44(2):253–259. doi: 10.1249/MSS.0b013e31822cf717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brurok B, Helgerud J, Karlsen T, Leivseth G, Hoff J. Effect of aerobic high-intensity hybrid training on stroke volume and peak oxygen consumption in men with spinal cord injury. Am J Phys Med Rehabil. 2011;90(5):407–414. doi: 10.1097/PHM.0b013e31820f960f. [DOI] [PubMed] [Google Scholar]
- 38.Horiuchi M, Okita K. Arm-cranking exercise training reduces plasminogen activator inhibitor 1 in people with spinal cord injury. Arch Phys Med Rehabil. 2017;98(11):2174–2780. doi: 10.1016/j.apmr.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs PL. Effects of resistance and endurance training in persons with paraplegia. Med Sci Sports Exerc. 2009;41(5):992–997. doi: 10.1249/MSS.0b013e318191757f. [DOI] [PubMed] [Google Scholar]
- 40.Castro MJ, Apple DF, Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol (1985) 1999;86(1):350–358. doi: 10.1152/jappl.1999.86.1.350. [DOI] [PubMed] [Google Scholar]
- 41.Gorgey AS, Lester RM, Wade RC et al. A feasibility pilot using telehealth videoconference monitoring of home-based NMES resistance training in persons with spinal cord injury. Spinal Cord Ser Cases. 2017;3 doi: 10.1038/scsandc.2017.39. 17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim DI, Park DS, Lee BS, Jeon JY. A six-week motor-driven functional electronic stimulation rowing program improves muscle strength and body composition in people with spinal cord injury: A pilot study. Spinal Cord. 2014;52(8):621–624. doi: 10.1038/sc.2014.76. [DOI] [PubMed] [Google Scholar]
- 43.Hjeltnes N, Wallberg-Henriksson H. Improved work capacity but unchanged peak oxygen uptake during primary rehabilitation in tetraplegic patients. Spinal Cord. 1998;36(10):691–698. doi: 10.1038/sj.sc.3100687. [DOI] [PubMed] [Google Scholar]
- 44.Pelletier CA, Omidvar M, Miyatani M, Giangregorio L, Craven BC. Participation in moderate-to-vigorous leisure time physical activity is related to decreased visceral adipose tissue in adults with spinal cord injury. Appl Physiol Nutr Metab. 2018;43(2):139–144. doi: 10.1139/apnm-2017-0304. [DOI] [PubMed] [Google Scholar]
- 45.Karelis AD, Carvalho LP, Castillo MJ, Gagnon DH, Aubertin-Leheudre M. Effect on body composition and bone mineral density of walking with a robotic exoskeleton in adults with chronic spinal cord injury. J Rehabil Med. 2017;49(1):84–87. doi: 10.2340/16501977-2173. [DOI] [PubMed] [Google Scholar]
- 46.Hjeltnes N, Aksnes AK, Birkeland KI, Johansen J, Lannem A, Wallberg-Henriksson H. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol. 1997;273(3 Pt 2):R1072–1079. doi: 10.1152/ajpregu.1997.273.3.R1072. [DOI] [PubMed] [Google Scholar]
- 47.Grossman JA, Arigo D, Bachman JL. Meaningful weight loss in obese postmenopausal women: A pilot study of high-intensity interval training and wearable technology. Menopause. 2018;25(4):465–470. doi: 10.1097/GME.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 48.Hazell TJ, Hamilton CD, Olver TD, Lemon PW. Running sprint interval training induces fat loss in women. Appl Physiol Nutr Metab. 2014;39(8):944–950. doi: 10.1139/apnm-2013-0503. [DOI] [PubMed] [Google Scholar]
- 49.Macpherson RE, Hazell TJ, Olver TD, Paterson DH, Lemon PW. Run sprint interval training improves aerobic performance but not maximal cardiac output. Med Sci Sports Exerc. 2011;43(1):115–122. doi: 10.1249/MSS.0b013e3181e5eacd. [DOI] [PubMed] [Google Scholar]
- 50.Dolbow DR, Credeur DP. Effects of resistance-guided high intensity interval functional electrical stimulation cycling on an individual with paraplegia: A case report. J Spinal Cord Med. 2017:1–5. doi: 10.1080/10790268.2017.1367358. [DOI] [PMC free article] [PubMed] [Google Scholar]


