Abstract
Background
Neisseria gonorrhoeae and Neisseria meningitidis are closely-related bacteria that cause a significant global burden of disease. Control of gonorrhoea is becoming increasingly difficult, due to widespread antibiotic resistance. While vaccines are routinely used for N. meningitidis, no vaccine is available for N. gonorrhoeae. Recently, the outer membrane vesicle (OMV) meningococcal B vaccine, MeNZB, was reported to be associated with reduced rates of gonorrhoea following a mass vaccination campaign in New Zealand. To probe the basis for this protection, we assessed the cross-reactivity to N. gonorrhoeae of serum raised to the meningococcal vaccine Bexsero, which contains the MeNZB OMV component plus 3 recombinant antigens (Neisseria adhesin A, factor H binding protein [fHbp]-GNA2091, and Neisserial heparin binding antigen [NHBA]-GNA1030).
Methods
A bioinformatic analysis was performed to assess the similarity of MeNZB OMV and Bexsero antigens to gonococcal proteins. Rabbits were immunized with the OMV component or the 3 recombinant antigens of Bexsero, and Western blots and enzyme-linked immunosorbent assays were used to assess the generation of antibodies recognizing N. gonorrhoeae. Serum from humans immunized with Bexsero was investigated to assess the nature of the anti-gonococcal response.
Results
There is a high level of sequence identity between MeNZB OMV and Bexsero OMV antigens, and between the antigens and gonococcal proteins. NHBA is the only Bexsero recombinant antigen that is conserved and surfaced exposed in N. gonorrhoeae. Bexsero induces antibodies in humans that recognize gonococcal proteins.
Conclusions
The anti-gonococcal antibodies induced by MeNZB-like OMV proteins could explain the previously-seen decrease in gonorrhoea following MeNZB vaccination. The high level of human anti-gonococcal NHBA antibodies generated by Bexsero vaccination may provide additional cross-protection against gonorrhoea.
Keywords: STI, gonorrhea, Neisseria gonorrhoeae, immune response, meningococcal vaccine
This study demonstrates that Neisseria gonorrhoeae shares a high level of sequence identity with outer membrane vesicle antigens in 2 serogroup B meningococcal vaccines: MeNZB and Bexsero. The Bexsero neisserial heparin binding antigen (NHBA) recombinant antigen is also conserved in N. gonorrhoeae. Furthermore, serum from humans vaccinated with Bexsero is able to recognize several gonococcal proteins, including the gonococcal NHBA homologue.
The sexually transmitted infection gonorrhoea is a global public health concern [1]. It is estimated that there are approximately 100 million cases of gonorrhoea worldwide each year [2], with the number of cases rising in recent years [3, 4]. Symptomatic gonococcal infections most commonly present as urethritis in males and cervicitis in females, although mucosal infections of the rectum, pharynx, and eye frequently occur. Furthermore, asymptomatic infections are common and, if undiagnosed or untreated, gonorrhoea can lead to severe sequelae, including pelvic inflammatory disease, adverse pregnancy outcomes, neonatal complications, and infertility. Gonococcal infection also increases the risk of human immunodeficiency virus [1, 5]. The effectiveness of antibiotics has been significantly compromised, and strains with high-level resistances to the last line of antibiotics, the expanded-spectrum cephalosporins, have been isolated from around the world [6]. As such, N. gonorrhoeae has been prioritized as an urgent public health threat for which immediate action is needed [7, 8], including the development of a gonococcal vaccine [9].
Vaccine development has been challenging for N. gonorrhoeae, and none of the vaccine candidates tested in clinical trials have afforded protection against gonorrhoea. This is largely due to its various mechanisms of immune evasion, the lack of an animal model that mimics natural disease, and our limited understanding of what is required to induce a protective immune response [1, 10]. However, several different approaches have identified promising gonococcal vaccine candidates [11–17]. In addition, recently, a vaccine to a closely-related pathogen, the Neisseria meningitidis serogroup B outer membrane vesicle (OMV) vaccine MeNZB, was associated with decreased rates of gonorrhoea [18, 19]. MeNZB was developed in response to a meningococcal epidemic in New Zealand, and over 1 million people were vaccinated between 2004 and 2008 [20]. A retrospective case-control study showed that individuals vaccinated with MeNZB were significantly less likely to contract gonorrhoea compared with unvaccinated controls, with a predicted vaccine efficacy of 31% [18].
OMVs are spherical, bi‐layered membrane structures that are naturally released from the outer membrane of Gram-negative bacteria and contain phospholipids, lipopolysaccharides (LPS), and a mix of outer membrane proteins [21, 22]. The most abundant proteins in meningococcal OMVs include PorA, PorB, and OpcA, with the antigenically-diverse PorA being immunodominant and the main target of serum bactericidal antibodies [23]. However, functional antibodies are raised against other OMV components, and some cross-protection against heterologous strains with mismatched PorA types has been reported [24]. Despite causing distinct diseases, N. meningitidis and N. gonorrhoeae are genetically and antigenically very similar, with 80–90% nucleotide identity across the genome and many proteins sharing high levels of identity (eg, PorB shares 60–70% amino acid homology) [14, 25]. As such, meningococcal OMV vaccines may induce functional antibodies against gonococcal strains. Several other observational studies have also reported reduced rates of gonorrhoea following the use of OMV-based meningococcal vaccines [26–30].
MeNZB is no longer available. However, the broad-spectrum serogroup B vaccine Bexsero contains the MeNZB OMV antigen plus 3 recombinant antigens (Neisseria adhesin A [NadA], factor H binding protein [fHbp]-GNA2091, and Neisserial heparin binding antigen [NHBA]-GNA1030) [31]. NadA, fHbp, and NHBA induce serum bactericidal antibodies against diverse strains [32, 33]. The accessory proteins GNA2091 [34] and GNA1030 [35] are fused with fHbp and NHBA, respectively, and increase their immunogenicity and serum bactericidal titers [33]. In N. gonorrhoeae, the gene encoding NadA is absent [36, 37]; the gene encoding fHbp is present, but not surface exposed [38]; and genes encoding NHBA, GNA2091, and GNA1030 are present [36, 37], but have not been characterized in detail. NHBA was found to be present in 17/17 N. gonorrhoeae strains studied, with an average identity of 81.2% to NHBA-2 peptide in Bexsero [37], and in 97/111 strains, N. gonorrhoeae had a 65.6% identity to the non-vaccine NHBA-3 peptide from N. meningitidis strain MC58 [36]. Here, we investigated the similarity of antigens present in MeNZB and Bexsero to gonococcal proteins, the capacity of MeNZB-like OMVs and Bexsero recombinant antigens to induce anti-gonococcal antibodies, and the specificity of antibodies induced by Bexsero-vaccinated humans to recognize gonococcal surface antigens.
METHODS
Bacterial Strains
N. gonorrhoeae strains 1291, FA1090, and WHO K were grown at 37°C with 5% CO2 on GC agar or broth (Oxoid) supplemented with IsoVitalex (Becton Dickinson).
Sequence Analysis
Allele and protein sequences of vaccine antigens are shown in Table 1. Sequences were aligned with CLUSTAL in MEGA7, and the percentage of amino acid identity and similarity were calculated (BLOSUM90, threshold 0). The protein identity between gonococcal strains was determined using the Basic Local Alignment Search Tool program (BLASTp) with sequences from N. gonorrhoeae 1291 against 438 gonococcal genomes in GenBank.
Table 1.
Bexsero Vaccine Components and Their Homology to Gonococcal Proteins
| OMV Protein Antigens | ||||
|---|---|---|---|---|
| NMB Locusa | Protein | Abundance in OMVsb | %ID to Ng FA1090c | %ID Between Ng Strainsd |
| NMB2039e | PorB (porin, major OMP PIB) | 42.54 | 67.3 | 88.6–100 |
| NMB1429e | PorA (porin, serosubtype P1.4) | 28.63 | n/a | n/a |
| NMB1497 | TonB-dependent receptor | 4.60 | 96.1 | 98–100 |
| NMB0382e | RmpM (OMP class 4) | 3.08 | 93.4 | 99.6–100 |
| NMB0964 | TonB-dependent receptor | 2.87 | 96.9 | 96.2–100 |
| NMB1812 | PilQ (Tfp assembly protein) | 1.44 | 91.4 | 79.1–100 |
| NMB0634e | FbpA (iron ABC transporter substrate-binding protein) | 1.29 | 99.1 | 99.1–100 |
| NMB1126/NMB1164 | Putative lipoprotein NMB1126/1164 | 1.06 | 94.2 | 99.1–100 |
| NMB1988e | FrpB (FetA, iron-regulated OMP) | 0.96 | 94.3 | 94.6–100 |
| NMB0461 | Tbp1 (transferrin binding protein 1) | 0.92 | 93.7 | 38.3–100 |
| NMB0182e | OMP85 | 0.87 | 95 | 99.2–100 |
| NMB1053e | OpcA (class 5 OMP) | 0.75 | 43.8 | 98.9–100 |
| NMB0088 | OMP P1 | 0.54 | 94 | 98.9–100 |
| NMB1540 | LbpA (lactoferrin binding protein A) | 0.46 | n/af | 41.0–100 |
| NMB0280 | LptD (LPS assembly protein/organic solvent tolerance protein [OstA]) | 0.44 | 89.8 | 99.4–100 |
| NMB1714 | MtrE (outer membrane efflux protein) | 0.29 | 96.4 | 95.3–100 |
| NMB0109 | LysM peptidoglycan-binding domain containing protein | 0.26 | 88.7 | 97.3–100 |
| NMB1333 | hypothetical protein | 0.24 | 96.3 | 97.3–100 |
| NMB1567 | FkpA (macrophage infectivity protein) | 0.23 | 97.8 | 98.9–100 |
| NMB0946 | antioxidation AhpC TSA family glutaredoxin | 0.20 | 98.5 | 99.6–100 |
| NMB0375 | MafA adhesin (mafA-1) | 0.18 | 98.8 | 59.2–100 |
| NMB0633e | NspA (OMP) | n/a | 93.7 | 25.4–100 |
| Recombinant Protein Antigens | ||||
|---|---|---|---|---|
| NMB Locusa | Protein | Variant (strain)g | %ID to Ngc | %ID Between Ng Strainsd |
| NMB2132 | Neisseria heparin binding antigen (NHBA) | peptide 2 (NZ98/254) | 68.8 | 93.7–100 |
| NMB1870 | Factor H binding protein (fHbp) | peptide 8, variant 1.1 (MC58) | 62.6h | 98.9–100 |
| NMB1994 | Neisseria Adhesin A (NadA) | peptide 8, variant 2/3 (2996) | n/a | n/a |
| NMB1030 | GNA1030 (NUbp) | n/a (2996) | 92.6 | 98.8–100 |
| NMB2091 | GNA2091 | n/a (2996) | 95.6 | 99.5–100 |
Abbreviations: %ID, percent identity; ABC, ATP-binding cassette; AhpC, alkyl hydroperoxide reductase C; FbpA, ferric binding protein A; fHbp, factor H binding protein; FkpA, FKBP-type peptidyl-prolyl cis-trans isomerase; FrpB, Fe-regulated protein B; LbpA, lactoferrin binding protein A; LPS, lipopolysaccharides; LptD, LPS-assembly protein; LysM, Lysin Motif; MafA, multiple adhesin family A; MeNZB, OMV meningococcal B vaccine; MtrE, outer membrane efflux protein; n/a, not available; NadA, Neisseria adhesin A; Ng, Neisseria gonorrhoeae; NHBA, Neisserial heparin binding antigen; NMB, Neisseria meningitidis strain MC58; NspA, Neisseria gonorrhoeae surface protein A; OMP, outer membrane protein; OMV, outer membrane vesicle; OpcA, opacity protein A; PilQ, pili associated protein Q; PorA, porin, serosubtype P1.4; PorB, porin, major OMP PIB; RmpM, reduction modifiable protein M; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; Tbp1, transferrin binding protein 1; Tfp, type IV pili; TSA, thiol specific antioxidant.
For the distribution and homology analysis of OMV proteins, allele and protein sequences were obtained from strains Neisseria meningitidis NZ98/254 (isolate id 34532) from the PubMLST database (https://pubmlst.org/neisseria) [39]. When the sequence for NZ98/254 was not available, the sequence from the N. meningitidis NZ05/33 (isolate id 19263) was used. NMB locus tags corresponding to N. meningitidis strain MC58 (accession NC_003112) are used as this genome is fully annotated.
Average abundance, calculated from average of 6 lots of Bexsero from Table 2 in Tani et al [44]. NspA protein is detected poorly by the proteomic approached used, compared with its abundance on SDS-PAGE [44].
Sequence from Neisseria meningitidis strain NZ05/33 (NZ98/254 genome is not available) was compared with Ng strain FA1090.
Conservation of antigen in the 438 Ng genomes in GenBank.
An antibody response is induced to this protein post–MeNZB vaccination [45].
The gene encoding LbpA is a pseudogene in FA1090 but is expressed by the majority of gonococcal strains.
Previously established nomenclature for Bexsero NHBA, fHbp, and NadA was used, where every unique peptide sequence is assigned a unique identification number (eg, NHBA peptide 2 [NHBA-2] is in Bexsero). Gray shading indicates the level of identity: dark >90%; medium >80%; light >60%.
The gonococcal fHbp is not expressed on the surface of the gonococcus due to the absence of a signal sequence for export [38]. It has previously been shown that the gonococcal fHbp signal sequence differs from that of Neisseria meningitidis and is identical in 111 gonococcal isolates examined [36]. We confirm that the N-terminal 33 amino acids are identical in all annotated fHbp sequences in the gonococcal genome strains available in GenBank.
NHBA distribution was investigated in >3000 N. gonorrhoeae genomes (PubMLST [39]) using BLASTx with NHBA from N. gonorrhoeae 1291 (GenBank Accession EEH61857.1). Amino acid sequence alignments, phylogeny tree construction, and annotation were performed using Clustal Omega at EMBL-EBI, MEGA (v7.0.26), and iTOL (v3.5.4), respectively.
Outer Membrane Vesicle Preparation
Naturally-secreted gonococcal OMVs were isolated as described previously [40]. Briefly, OMVs were harvested from a 6-hour culture (OD600 ~0.8) by brief centrifugation (5000 x g), the supernatant was filtered (0.22µm filter), the filtrate was centrifuged (100 000 x g, 1 hour, 4°C), the pellet was washed with phosphate buffered saline (PBS), then OMVs were solubilized in PBS-0.2% sodium dodecyl sulfate (SDS).
Expression of Recombinant Neisserial Heparin Binding Antigen
Escherichia coli BL21(DE3) was transformed with pET19b carrying the mature NHBA (no signal sequence) from N. gonorrhoeae 1291 (amplified using 5’-ATTActcgagTCGCCCGATGTCAAGTC-3’ and 5’-TGAAggatccCGGCATCAACATCAATC-3’ primers containing XhoI and BamHI sites shown in lower case, in the respective primers). The expression of recombinant NHBA (rNHBA) was induced (100 mM isopropyl β-d-1-thiogalactopyranoside [IPTC], 16 hours, 25°C) and protein-purified using TALON affinity resin (Clontech), as described previously [40].
Polyclonal Rabbit Serum
The rabbit sera to Bexsero vaccine antigens were generated as per Giuliani et al [33] and were provided by Novartis Vaccines. On days 0, 21, and 35, New Zealand White rabbits were immunized with 10 µg NZ98/254 OMV (α-OMV) or a combination of 25 µg each of the recombinant antigens NadA, fHbp-GNA2091, and NHBA-GNA1030 (α-rMenB) with aluminum hydroxide. Blood was taken on day 49.
Human Serum
Pre- and post-vaccination human serum was obtained from a previous phase II trial, in which adult laboratory staff were vaccinated with 3 doses of Bexsero at 0, 3, and 6 months [41]. Pre-vaccination (month 0) and 1 month post–dose 3 of Bexsero (month 7) serum samples from 10 individuals were tested. In addition, samples from healthy adults with no history of meningococcal disease who were vaccinated with 2 doses of Bexsero at 0 and 2 months (as per Australian recommendations [42]) were collected in accordance with the guidelines of the Griffith University Human Ethics Committee (HREC 2012/798).
Western Blot
Western blot analysis [40] was performed with whole-cell lysates, OMV, or rNHBA, that were separated using polyacrylamide gel electrophoresis (PAGE) with 12% Bis-Tris NuPAGE gels. A rabbit (1:2000) or human (1:4000) primary antibody and a horseradish peroxidase (HRP)–conjugated anti-immunoglobulin secondary antibody (Sigma-Aldrich) were used for protein detection. Duplicate gels were Coomassie stained to confirm equal sample loading.
Enzyme-linked Immunosorbent Assays
Enzyme-linked immunosorbent assays (ELISAs) [40] were performed with 96-well MaxiSorp (NUNC) plates coated with N. gonorrhoeae or N. meningitidis (50 µL/well of OD600 0.2), OMVs (2 µg/mL), rNHBA (1 µg/mL), or LPS (1 µg/mL [43]). Binding by rabbit or human antibodies was detected using Goat Anti-Rabbit HRP (1:2000; Dako) or Goat Anti-Human immunoglobin G Fc HRP (1:20 000; ThermoFisher), respectively. The ELISA titer is the highest serum dilution with absorbance at 450 nm > mean negative (all reagents excluding primary sera) + 3 standard deviations.
RESULTS
Gonococcal Proteins Share a High Level of Identity With Serogroup B Meningococcal Vaccine Antigens
To investigate the sequence conservation between serogroup B meningococcal vaccine antigens and N. gonorrhoeae, the major OMV protein antigens present in MeNZB and Bexsero and the recombinant protein antigens present in Bexsero were compared to gonococcal proteins from available N. gonorrhoeae genomes. OMVs contain a heterogeneous mix of numerous proteins; however, proteomic analysis of OMVs from Bexsero vaccine preparations identified a core set of 22 proteins that comprise >90% of OMV content [44]. Several of these major OMV proteins induce an antibody response to meningococcal strains post–MeNZB vaccination [45]. Our bioinformatic analysis identified homologues of 20 of the 22 core OMV proteins in N. gonorrhoeae strain FA1090 (Table 1). Of these 20 homologues, 16 proteins have >90% identity, 2 proteins have >80%, and 2 proteins are poorly conserved in FA1090 (PorB [24] and OpcA [46]). For the 2 proteins absent in FA1090, porA is a pseudogene in N. gonorrhoeae [47] and lbpA is a pseudogene in N. gonorrhoeae strain FA1090, but is expressed by the majority of gonococcal strains. Of the major OMV proteins that have a homologue in N. gonorrhoeae, 14 of these also have a high level of sequence identity (94–100%) in the 438 gonococcal genome strains available in GenBank (Table 1).
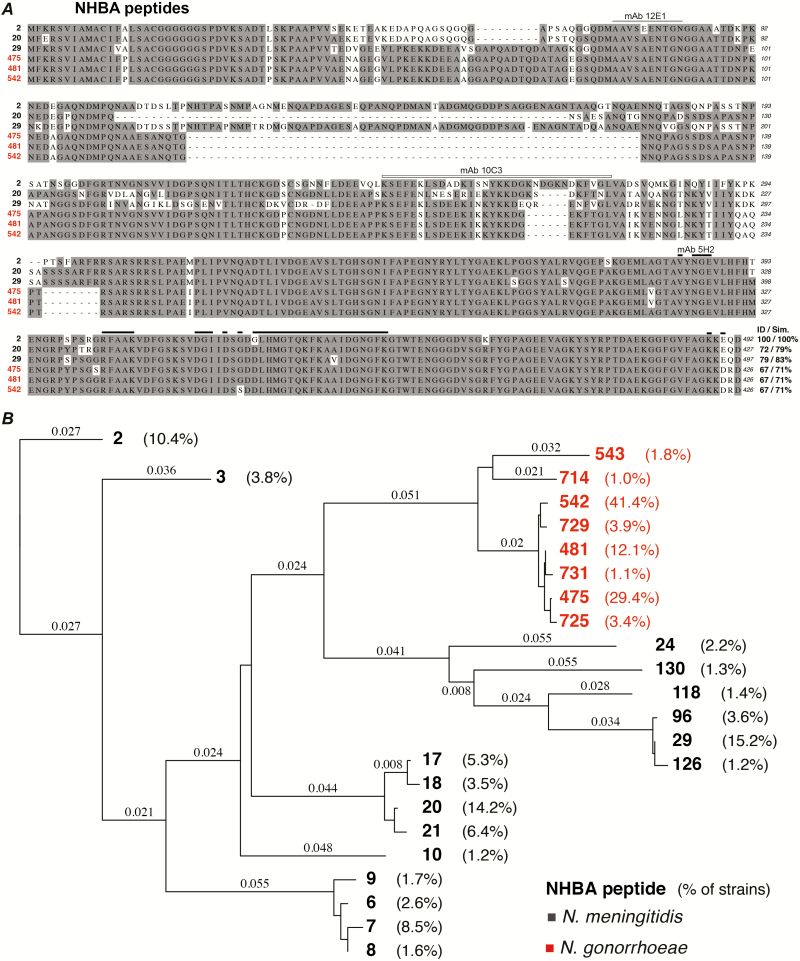
An analysis of the recombinant Bexsero antigens confirmed previous findings from smaller strain panels [36, 37] that the gene encoding NadA is absent in N. gonorrhoeae, while homologues of fHbp, NHBA, GNA2091, and GNA1030 are conserved N. gonorrhoeae strains (Table 1). Since fHbp [38], GNA2091 [34], and GNA1030 [35] are not believed to be surface-exposed in N. gonorrhoeae, further investigation focused on NHBA. Bexsero contains NHBA-2, which shares 68.8% identity to the NHBA variant from strain FA0190 (NHBA-527; Table 1). An investigation of NHBA in N. gonorrhoeae genome strains in GenBank revealed that nhba is present in 100% of strains. An expanded search in the PubMLST database indicated that 72% of gonococcal strains (3068/4953) have an annotated nhba gene (NEIS2109). This is likely an underestimate of the presence of nhba, due to duplicate and incompletely-annotated genomes. There are 41 unique NHBA variants reported for N. gonorrhoeae and 393 for N. meningitidis in PubMLST. There are 3 NHBA variants, represented by N. gonorrhoeae strains WHO K (NHBA-475), PID332 (NHBA-481), and 1291 (NHBA-542), that account for 82% of gonococcal strains, and these variants each share 67% identity to the NHBA-2 in Bexsero (Figure 1A). The phylogenetic relatedness of the most common NHBA variants (ie, variants present in ≥1% of N. meningitidis or N. gonorrhoeae strains in the database) is shown in Figure 1B. This tree highlights the relatively high conservation of NHBA in N. gonorrhoeae, with 93.7–100% amino acid identities between strains (Figure 1; Table 1). Epitope mapping indicates that the human monoclonal antibodies 12E1 and 10C3 bind to the N-terminal region [48], while 5H2 interacts with a large, cross-reactive, conformational epitope in the C-terminal of NHBA-2 [49]. The regions bound by 12E1, 10C3, and 5H2 are conserved in the main gonococcal NHBA variants (Figure 1).
Figure 1.
Conservation of Neisserial heparin binding antigen (NHBA) in Neisseria meningitidis and Neisseria gonorrhoeae. A, Alignment of the amino acid sequence of the 3 main NHBA peptide variants in N. meningitidis (black) and N. gonorrhoeae (red). Bexsero contains NHBA peptide 2 from N. meningitidis strain NZ98/254. The percent identity and similarity of each NHBA peptide to the Bexsero NHBA peptide 2 is shown on the left. Amino acids identified in epitopes bound by the human monoclonal antibodies 12E1 (gray bar; 9/10 amino acids identical to gonococcal NHBA) and 10C3 (open bar; 24/32 amino acids identical to gonococcal NHBA) in the N-terminal region [48], and 5H2 (black bar, 32/35 amino acids identical to gonococcal NHBA) interacts with in the C-terminal of NHBA-2 [49] are indicated above the sequence. B, Phylogenetic tree of the most common NHBA peptides present in N. meningitidis (black) and N. gonorrhoeae (red); the percent of strains that express this peptide are shown (all peptides present in ≥1% of strains are shown). The Bexsero NHBA peptide 2 is boxed, and the peptides included in the alignment in panel A are indicated with an asterisk. Abbreviation: NHBA, Neisserial heparin binding antigen.
Bexsero Antigens Elicit Antibodies in Rabbits
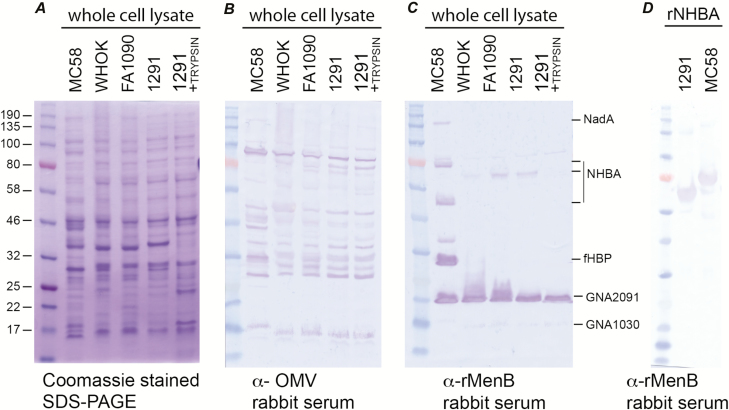
To investigate the ability of antibodies raised to serogroup B meningococcal vaccine antigens to recognize gonococcal proteins, Western blot and ELISA analyses were performed using serum from rabbits immunized with either the OMV present in MeNZB and Bexsero (anti-OMV) or a combination of the recombinant antigens of Bexsero (anti-rMenB). Several bands in whole-cell lysates of N. gonorrhoeae strains WHO K, FA1090, and 1291 are recognized by the anti-OMV sera, and these proteins are consistent between the 3 gonococcal strains and are similar, but not identical, to proteins recognized in N. meningitidis (Figure 2A and 2B; Supplementary Figure S1). The anti-OMV sera had an ELISA titer of 128 000 to OMVs from N. gonorrhoeae 1291 (Supplementary Figure S1).
Figure 2.
Reactivity of rabbit serum raised against Bexsero antigens to Neisseria gonorrhoeae antigens. A, Coomassie stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE); B, western blot with rabbit serum immunized with the NZ98/254 outer membrane vesicle component of Bexsero (α-OMV); and C, western blot with rabbit serum immunized to the recombinant protein component of Bexsero (α-rMenB). Samples shown are whole-cell lysates (equivalent to a final optical density at 600 nm of 5) from Neisseria meningitidis (strain MC58) and N. gonorrhoeae (strains WHO K, FA1090, 1291), and N. gonorrhoeae strain 1291, treated with trypsin for 60 min to remove surface proteins (1291+TRYPSIN). The protein ladder is shown on the left of each panel, with the protein sizes (kDa) on the far left. On the right of panel C, the recombinant proteins are indicated. For MC58 NHBA, the upper band is the full length NHBA protein, and the lower band is the fragment generated by NalP cleavage. For GNA1030, the protein is weakly expressed, and a digitally overexposed blot is shown in Supplementary Figure S1A, where GNA1030 is more evident. D, Western blot with α-rMenB rabbit serum against recombinant NHBA from N. meningitidis strain MC58 and N. gonorrhoeae strain 1291. Abbreviations: fHBP, factor H binding protein; NadA, Neisseria adhesin A; NalP, Neisseria autotransporter lipoprotein; NHBA, Neisserial heparin binding antigen; OMV, outer membrane vesicles; rNHBA, recombinant NHBA; SDS-PAGE, sodium dodecyl sulfate poly-acrylamide gel electrophoresis.
The anti-rMenB sera recognized all Bexsero protein antigens in N. meningitidis strain MC58 (NadA, fHbp, NHBA, GNA2091, and GNA1030), while only NHBA, GNA2091, and GNA1030 were recognized in N. gonorrhoeae (Figure 2A and 2C). NHBA was not recognized in trypsin-treated N. gonorrhoeae, confirming that NHBA is surface-exposed (Figure 2A and 2C). The detection of GNA2091 and GNA1030 were unchanged by trypsin treatment of N. gonorrhoeae (Figure 2A and 2C), indicating that they are located inside the cell, as previously described for N. meningitidis [34, 35]. The anti-rMenB sera recognized rNHBA from N. meningitidis MC58 and N. gonorrhoeae 1291 equally (Figure 2D), with ELISA titers of 2 048 000 to both proteins (Supplementary Figure S1).
Antibody Generation From Bexsero Vaccination in Humans
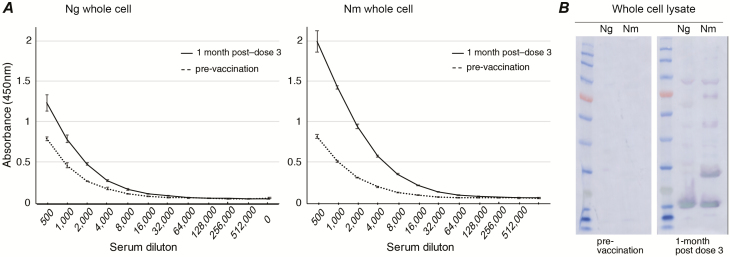
To investigate the ability of human, Bexsero-induced antibodies to recognize gonococcal proteins, Western blot and ELISA analyses were performed using serum from humans vaccinated with either 3 (0, 1, and 3 months) or 2 (0 and 1 months) doses of Bexsero. For the 10 individuals given 3 doses of Bexsero, there was no significant increase in the geometric mean ELISA titer (GMT) of the samples from pre-vaccination to 1 month post–dose 3 for gonococcal OMVs, which is likely due to the high pre-vaccine titers of some individuals. However, titers were significantly increased from pre- to post-vaccination for whole-cell N. gonorrhoeae (1.8-fold increased GMT, compared to 5.7-fold increase against whole-cell N. meningitidis) and gonococcal NHBA (34-fold increase; Table 2; Figure 3; Supplementary Figures S2–S5). A Western blot analysis of whole-cell lysates supports the ELISA data and shows reactivity to several gonococcal and meningococcal antigens with vaccinated, but not pre-immune, serum (Figure 3). There is a minimal amount of LPS present in detergent-extracted OMVs, which can induce a weak increase in antibodies to meningococcal LPS [45]. We see a minor increase in antibodies to meningococcal LPS, but no response to gonococcal LPS in sera from Bexsero-vaccinated individuals (Supplementary Figure S6).
Table 2.
Enzyme-linked Immunosorbent Assay Geometric Mean Titers Against Bexsero Vaccine Components in Serum From Bexsero-vaccinated Humans
| Pre-vaccination | 1 Month Post–dose 3b | ||
|---|---|---|---|
| Antigena | GMT (95% CI) | GMT (95% CI) | P Value |
| Ng OMV | 34 297 | 42 224 | .596 |
| (20 946–56 156) | (29 853–59 722) | ||
| Ng whole cell | 48 503 | 78 793 | .035 |
| (25 906–90 811) | (49 228–126 115) | ||
| Nm whole cell | 97 006 | 388 023 | .0091 |
| (42 879–219 456) | (183 938–818 550) | ||
| Ng rNHBA | 34 297 | 1 176 267 | .0051 |
| (20 946–56 156) | (669 930–2 065 300) |
GMT is the arithmetic mean of the logarithms of individuals’ serum titers. P values were calculated using the Wilcoxon signed-rank test.
Abbreviations: CI, confidence interval; GMT, geometric mean titers; Ng, Neisseria gonorrhoeae; Nm, Neisseria meningitidis; OMV, outer membrane vesicles; rNHBA, recombinant Neisseria heparin binding antigen.
OMV, intact, whole cells from Ng, whole cells from Nm, or rNHBA from Ng strain 1291.
From a 3-dose vaccine schedule (0, 1, and 3 months) and 10 donors.
Figure 3.
Reactivity of Bexsero-vaccinated human serum to whole-cell Neisseria gonorrhoeae (Ng) and Neisseria meningitidis (Nm). Reactivity of pooled Bexsero-vaccinated human serum from 10 donors vaccinated with 3 doses of Bexsero, at 0, 3, and 6 months. A, Enzyme-linked immunosorbent assay titration curves pre-vaccination (dashed line) and 1 month post–dose 3 (black line) against intact, whole-cell Ng 1291 and Nm MC58 are shown as the average absorbance (+/– standard deviation) at 450 nm versus reciprocal serum dilutions. B, Western blot analysis of whole-cell lysates shows recognition of several gonococcal and meningococcal antigens from post-vaccination, but not pre-vaccination, serum. Proteins recognized include those running at a molecular weight consistent with recombinant Bexsero antigens (Neisserial heparin binding antigen [NHBA], GNA2091, and GNA1030 in Ng and Neisseria adhesin A, NHBA, factor H binding protein, GNA2091, and GNA1030 in Nm). Abbreviations: Ng, Neisseria gonorrhoeae; Nm, Neisseria meningitidis.
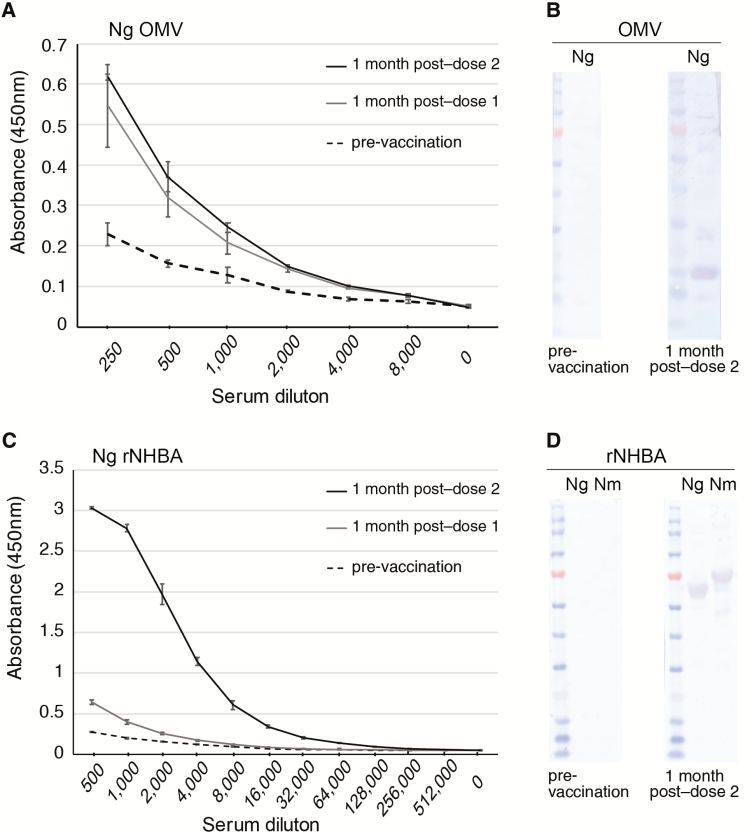
An analysis of serum from individuals who received 2 doses of Bexsero was also performed, as this is the current recommended adolescent schedule in Australia, the United Kingdom, Canada, and the United States. Antibodies recognizing gonococcal OMV proteins were induced above the pre-vaccination baseline to similar levels at 1 month post–dose 1 and 1 month post–dose 2 (ELISA titer of 8000; Figure 4A). A Western blot analysis indicated that the pre-vaccination serum did not cross-react with gonococcal OMV proteins, while post–dose 2 sera reacted with several proteins (Figure 4B). Antibodies recognizing the gonococcal NHBA were induced after dose 1 (titer of 64 000) and to a very high-level at 1 month post–dose 2 (titer of 512 000; Figure 4C). A Western blot analysis indicated that pre-vaccination serum did not cross-react with gonococcal or meningococcal rNHBA (Figure 4C), while post–dose 2 sera reacted equally well with these rNHBA proteins (Figure 4D).
Figure 4.
Reactivity of Bexsero-vaccinated human serum to Neisseria gonorrhoeae (Ng) outer membrane vesicles (OMVs) antigens and Neisserial heparin binding antigen (NHBA). Reactivity of Bexsero-vaccinated human serum from 1 donor vaccinated with 2 doses of Bexsero at 0 and 2 months to (A and B) Ng strain 1291 OMVs and (C and D) recombitant NHBA (rNHBA) from Ng strain 1291 or N. meningitidis (Nm) strain MC58. (A and C) ELISA titration curves of pre-vaccination (month 0, dashed line), 1 month post–dose 1 (month 1, grey line), and 1 month post–dose 2 (month 3, black line) are shown as the average absorbance (+/- standard deviation) at 450 nm versus reciprocal serum dilutions. Western blot analysis shows recognition of (B) several gonococcal OMV proteins and (D) rNHBA in post-vaccination, but not pre-vaccination serum. Abbreviations: Ng, Neisseria gonorrhoeae strain 1291; Nm, Neisseria meningitidis strain MC58; OMV, outer membrane vesicles; rNHBA, recombitant Neisserial heparin binding antigen.
DISCUSSION
This study provides both bioinformatic and serological data on the potential of meningococcal vaccine antigens to generate an immune response that recognizes gonococcal proteins. These data provide experimental evidence for the concept that cross-reactive antibodies may be the mechanism that underlies the recent observation that the meningococcal serogroup B OMV vaccine MeNZB was associated with reduced rates of gonorrhoea [18]. The broad-spectrum serogroup B vaccine Bexsero, which contains the MeNZB OMV antigen plus 3 recombinant antigens (NadA, fHbp-GNA2091, and NHBA-GNA1030), is now licensed worldwide. In this study, we determined that there is a high level of amino acid identity between most of the major MeNZB/Bexsero OMV proteins and N. gonorrhoeae homologues, and that OMV-induced antibodies recognize gonococcal proteins. Furthermore, we have shown that NHBA is the only Bexsero recombinant protein antigen with a homologue in N. gonorrhoeae that is exposed on the surface of the bacteria and, therefore, accessible to vaccine-induced antibodies. We have also identified a high level of homology and cross-reactivity between the meningococcal and gonococcal NHBA proteins, which suggests that Bexsero may result in additional cross-protection against gonorrhoea, above that predicted for MeNZB.
The highly-variable PorA protein is the main antigen in meningococcal OMV vaccines that induces bactericidal antibodies, and it has long been considered likely that OMV vaccines do not protect against N. meningitidis strains expressing heterologous PorA types [23]. However, there is increasing evidence that some level of protection is provided against heterologous meningococcal strains, potentially due to minor OMV antigens, synergy between antigens, and/or a general immunomodulatory effect of OMVs induced by bacterial components such as LPS [24, 45, 50–52]. It is important to note that, although serum bactericidal activity is the established correlate of immune protection for N. meningitidis, the mechanisms of immune protection against N. gonorrhoeae are unknown and may involve cell-mediated killing or bactericidal, opsonophagocytic, and/or functional blocking activity of antibodies. Since N. gonorrhoeae rarely causes the invasive, life-threatening sepsis that is typical of meningococcal infection, then the sterilizing immunity conferred by meningococcal vaccines may not be required or appropriate to prevent gonococcal transmission and disease. Rather, a vaccine that is able to reduce mucosal colonization and transmission (ie, if antibodies that target a gonococcal adhesin are able to block bacterial adherence) or reduce pathology (ie, if antibodies that target a gonococcal virulence factor are able to reduce bacterial ascension to the upper genital tract) may be sufficient to reduce prevalence and disease burden [53]. Other studies have shown various levels of cross-reactivity or functional activity to N. gonorrhoeae in antibodies raised to meningococcal vaccines. For example, mouse sera raised to an intranasal serogroup B Proteoliposome vaccine recognize N. gonorrhoeae by ELISA [54] and mouse sera raised to the meningococcal NHBA or NHBA-GNA1030 fusion protein can cross-react with N. gonorrhoeae F62 and induce complement deposition, as detected by flow cytometry [55]. Mouse sera raised to Bexsero or OMVs were able to reduce gonococcal adherence to epithelial cells. Mouse sera raised to either Bexsero, the Bexsero OMV or recombinant protein component, or NHBA, GNA1030, or GNA2091 alone showed serum bactericidal activity against N. gonorrhoeae FA1090. However, similar serum bactericidal activity (SBA) titers were seen for all these antigens despite whether they are surface localized on N. gonorrhoeae or not [55]. Mice immunized with Bexsero have been reported to have a significant reduction in the percentage of mice colonized and the bacterial burden through 7 days post-infection [56]. Mouse sera raised to native meningococcal OMVs have also been shown to induce SBA activity against N. gonorrhoeae FA1090, but no SBA activity was seen with serum from humans vaccinated with Bexsero in this study [57].
Irrespective of the functional immune response required for protection against gonorrhoea, there was a high level of immune reactivity of human, Bexsero-induced antibodies to N. gonorrhoeae. There was a significant increase in ELISA GMTs between pre– and post–Bexsero vaccine sera against gonococcal whole cells and, although the GMT to OMVs was not significantly increased, >50% of the samples did have an increased response to OMVs post-vaccination. Several individuals had high pre-immune titers against whole cells and OMVs, which was consistent with findings from the original study, where high baseline immunity against serogroup B meningococcal strains were seen (71% of individuals had pre-vaccination bactericidal antibody titers above the cut-off for PorA) [41], potentially due to prior meningococcal carriage or exposure in this at-risk laboratory cohort. The use of sera from laboratory workers is a potential limitation, and a larger study in the general population is needed. However, it is important to note that carriage rates of up to 35% are seen in young adults [58]. Of the recombinant antigens present in Bexsero that are not components of MeNZB, NHBA is potentially the only protein that may be able to provide an additive, protective effect towards N. gonorrhoeae. The Bexsero NHBA-2 shares 69% identity to the NHBA-527 variant from N. gonorrhoeae strain FA1090 and, as previously reported, the main difference between NHBA-2 and gonococcal NHBA peptides is due to a 189 nucleotide deletion in the N-terminal half of the gonococcal nhba gene [36]. Despite the presence of different NHBA variants, Western blot and ELISA data presented here show strong immune reactivity of anti-NHBA antibodies to N. gonorrhoeae, with a 34-fold increase in the ELISA GMT between pre– and post–Bexsero vaccine sera against the gonococcal NHBA antigen. This cross-reactivity between NHBA variants is supported by a recent analysis of meningococcal strains circulating in the United States, which demonstrated the immune reactivity of anti-NHBA antibodies with 99.5% of isolates, irrespective of the NHBA genotype [59]. This meningococcal antigen typing system (MATS) analysis predicted that NHBA provided high (84–100%) coverage of strains that expressed 1 of the 8 major NHBA variants present in the US isolates [59], including NHBA-10, 20, 21, and 29 (shown in Figure 1), which share a similar level of identity to the NHBA-2 and to the 3 major gonococcal NHBA variants (72–79% identity for meningococcal vs 67% identity for gonococcal variants). NHBA induces antibodies that have serum bactericidal activity against a diverse collection of meningococcal strains [32, 33], are opsonophagocytic [60, 61], and are able to block the adherence of N. meningitidis to epithelial cells [62]. NHBA antibodies may have similar functional activities against N. gonorrhoeae.
Mathematical modelling of hypothetical gonococcal vaccines indicated that a vaccine efficacy of 31%, as predicted for MeNZB [18], could decrease gonorrhoea prevalence by >30% in the 20 years after vaccine implementation, if vaccine-induced protection could be maintained for longer than 10 years [53]. A higher level of vaccine efficacy would be optimal and may potentially be afforded by Bexsero, due to the additional NHBA component. Given that antibiotic-resistant gonococcal infections are a rapidly-emerging health problem worldwide, vaccine development is an increasing priority [9] and, if untreatable gonorrhoea [63] becomes widespread, then a modestly-effective vaccine would be better than no vaccine. Ideally, a gonococcal-specific vaccine consisting of a combination of promising candidate gonococcal antigens (as recently reviewed [1, 10]) should enter human clinical trials as soon as possible to determine whether a higher vaccine efficacy can be achieved. However, until now there have been limited tools to enable the discovery of what is required to induce a protective immune response, and there has been little to no clinical progress towards a gonococcal vaccine in the last 3 decades [1, 10]. The landmark finding that individuals vaccinated with MeNZB were significantly less likely to contract gonorrhoea compared with unvaccinated controls [18] represents the first time that any vaccine has been associated with protection against gonorrhoea in humans. This observation, and the data on cross-reactivity outlined here, provides a new opportunity to progress gonococcal vaccine development, guided by the human immune response to the vaccine-mediated presentation of antigens that are common between N. gonorrhoeae and the closely-related N. meningitidis. Further work is needed to identify the full set of gonococcal targets recognized by Bexsero-induced antibodies and the mechanism(s) of protection against gonorrhoea that are mediated by these antibodies or by other components of the immune system. However, this study provides both a new framework to advance gonococcal vaccine development and firm evidence to justify new human trials to investigate the potential level of Bexsero-induced protection against gonorrhoea.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Michael P. Jennings for valuable discussions and critical review of the manuscript, and Novartis Vaccines, Siena, Italy, for the provision of rabbit sera. This publication made use of the Neisseria Multi Locus Sequence Typing website (https://pubmlst.org/neisseria/), developed by Keith Jolley and hosted at the University of Oxford; the development of this site has been funded by the Wellcome Trust and European Union.
Financial support. This work was supported by the Australian National Health and Medical Research Council (Career Development Fellowship and Project Grants 1028326 and 1099278 to K. L. S.).
Potential conflicts of interest. R. B. performs contract research on behalf of Public Health England for GlaxoSmithKline, Pfizer, and Sanofi Pasteur. K. L. S. worked for Novartis Vaccines on the development of Bexsero, from 2006 to 2012. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Edwards JL, Jennings MP, Apicella MA, Seib KL. Is gonococcal disease preventable? The importance of understanding immunity and pathogenesis in vaccine development. Crit Rev Microbiol 2016; 42:928–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization; Global incidence and prevalence of selected curable sexually transmitted infections – 2008. Geneva, Switzerland: World Health Organization, 2012. [Google Scholar]
- 3. Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2017. University of New South Wales: Sydney: Kirby Institute, 2017. [Google Scholar]
- 4. Centers for Disease Control and Prevention; Sexually transmitted disease surveillance 2016. Atlanta, Georgia: Centers for Disease Control and Prevention, US Department of Health and Human Services, 2017. [Google Scholar]
- 5. Hook EW, Handsfield HH. Gonococcal infection in the adult. In: Holmes KK. Sexually transmitted diseases. New York, New York: McGraw-Hill, 2008:627–45. [Google Scholar]
- 6. Unemo M, Jensen JS. Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium. Nat Rev Urol 2017; 14:139–52. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention; Antibiotic resistance threats in the United States, 2013 Available at: https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf Accessed 8 April 2018. [Google Scholar]
- 8. World Health Organization; Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics Available at: http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ Accessed 8 April 2018. [Google Scholar]
- 9. Unemo M, Bradshaw CS, Hocking JS, et al. . Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17:e235–79. [DOI] [PubMed] [Google Scholar]
- 10. Rice PA, Shafer WM, Ram S, Jerse AE. Neisseria gonorrhoeae: drug resistance, mouse models, and vaccine development. Annu Rev Microbiol 2017; 71:665–86. [DOI] [PubMed] [Google Scholar]
- 11. Zielke RA, Wierzbicki IH, Baarda BI, et al. . Proteomics-driven antigen discovery for development of vaccines against gonorrhea. Mol Cell Proteomics 2016; 15:2338–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shewell LK, Jen FE, Jennings MP. Refinement of immunizing antigens to produce functional blocking antibodies against the AniA nitrite reductase of Neisseria gonorrhoeae. PLOS One 2017; 12:e0182555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Semchenko EA, Day CJ, Seib KL. MetQ of Neisseria gonorrhoeae is a surface-expressed antigen that elicits bactericidal and functional blocking antibodies. Infect Immun 2017; 85:e00898–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Massari P, Ram S, Macleod H, Wetzler LM. The role of porins in neisserial pathogenesis and immunity. Trends Microbiol 2003; 11:87–93. [DOI] [PubMed] [Google Scholar]
- 15. Edwards JL, Apicella MA. Neisseria gonorrhoeae PLD directly interacts with Akt kinase upon infection of primary, human, cervical epithelial cells. Cell Microbiol 2006; 8:1253–71. [DOI] [PubMed] [Google Scholar]
- 16. Price GA, Russell MW, Cornelissen CN. Intranasal administration of recombinant Neisseria gonorrhoeae transferrin binding proteins A and B conjugated to the cholera toxin B subunit induces systemic and vaginal antibodies in mice. Infect Immun 2005; 73:3945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gulati S, Zheng B, Reed GW, et al. . Immunization against a saccharide epitope accelerates clearance of experimental gonococcal infection. PLOS Pathog 2013; 9:e1003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petousis-Harris H, Paynter J, Morgan J, et al. . Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 2017; 390:1603–10. [DOI] [PubMed] [Google Scholar]
- 19. Seib KL. Gonorrhoea vaccines: a step in the right direction. Lancet 2017; 390:1567–9. [DOI] [PubMed] [Google Scholar]
- 20. Arnold R, Galloway Y, McNicholas A, O’Hallahan J. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine 2011; 29:7100–6. [DOI] [PubMed] [Google Scholar]
- 21. Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol 2010; 64:163–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Pol L, Stork M, van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol J 2015; 10:1689–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin DR, Ruijne N, McCallum L, O’Hallahan J, Oster P. The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin Vaccine Immunol 2006; 13:486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holst J, Oster P, Arnold R, et al. . Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum Vaccin Immunother 2013; 9:1241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tinsley CR, Nassif X. Analysis of the genetic differences between Neisseria meningitidis and Neisseria gonorrhoeae: two closely related bacteria expressing two different pathogenicities. Proc Natl Acad Sci USA 1996; 93:11109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perez O, Campo JD, Cuello M, et al. . Mucosal approaches in Neisseria vaccinology. Vacci Monitor 2009; 18:53–5. [Google Scholar]
- 27. Ochoa-Azze RF. Cross-protection induced by VA-MENGOC-BC® vaccine. Hum Vaccin Immunother 2018; 14:1064–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petousis-Harris H. Impact of meningococcal group B OMV vaccines, beyond their brief. Hum Vaccin Immunother 2018; 14:1058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Longtin J, Dion R, Simard M, et al. . Possible impact of wide-scale vaccination against serogroup B neisseria meningitidis on gonorrhea incidence rates in one region of Quebec, Canada. Open Forum Infect Dis 2017; 4:S734–5. [Google Scholar]
- 30. Whelan J, Kløvstad H, Haugen IL, Holle MR, Storsaeter J. Ecologic study of meningococcal B vaccine and Neisseria gonorrhoeae infection, Norway. Emerg Infect Dis 2016; 22:1137–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toneatto D, Pizza M, Masignani V, Rappuoli R. Emerging experience with meningococcal serogroup B protein vaccines. Expert Rev Vaccines 2017; 16:433–51. [DOI] [PubMed] [Google Scholar]
- 32. Pizza M, Scarlato V, Masignani V, et al. . Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 2000; 287:1816–20. [DOI] [PubMed] [Google Scholar]
- 33. Giuliani MM, Adu-Bobie J, Comanducci M, et al. . A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci USA 2006; 103:10834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bos MP, Grijpstra J, Tommassen-van Boxtel R, Tommassen J. Involvement of Neisseria meningitidis lipoprotein GNA2091 in the assembly of a subset of outer membrane proteins. J Biol Chem 2014; 289:15602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donnarumma D, Golfieri G, Brier S, et al. . Neisseria meningitis GNA1030 is a ubiquinone-8 binding protein. FASEB J 2015; 29:2260–7. [DOI] [PubMed] [Google Scholar]
- 36. Hadad R, Jacobsson S, Pizza M, et al. . Novel meningococcal 4CMenB vaccine antigens - prevalence and polymorphisms of the encoding genes in Neisseria gonorrhoeae. APMIS 2012; 120:750–60. [DOI] [PubMed] [Google Scholar]
- 37. Muzzi A, Mora M, Pizza M, Rappuoli R, Donati C. Conservation of meningococcal antigens in the genus Neisseria. MBio 2013; 4:e00163–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jongerius I, Lavender H, Tan L, et al. . Distinct binding and immunogenic properties of the gonococcal homologue of meningococcal factor H binding protein. PLOS Pathog 2013; 9:e1003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jolley KA, Maiden MC. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 2010; 11:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Semchenko EA, Day CJ, Seib KL. MetQ of Neisseria gonorrhoeae is a surface-expressed antigen that elicits bactericidal and functional blocking antibodies. Infect Immun 2017; 85:e00898-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Findlow J, Bai X, Findlow H, et al. . Safety and immunogenicity of a four-component meningococcal group B vaccine (4CMenB) and a quadrivalent meningococcal group ACWY conjugate vaccine administered concomitantly in healthy laboratory workers. Vaccine 2015; 33:3322–30. [DOI] [PubMed] [Google Scholar]
- 42. Australian Technical Advisory Group on Immunisation (ATAGI). The Australian immunisation handbook, 10th ed (2017 update). Canberra, Australia, 2017. [Google Scholar]
- 43. Mubaiwa TD, Hartley-Tassell LE, Semchenko EA, et al. . The glycointeractome of serogroup B Neisseria meningitidis strain MC58. Sci Rep 2017; 7:5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tani C, Stella M, Donnarumma D, et al. . Quantification by LC-MS(E) of outer membrane vesicle proteins of the Bexsero® vaccine. Vaccine 2014; 32:1273–9. [DOI] [PubMed] [Google Scholar]
- 45. Wedege E, Bolstad K, Aase A, et al. . Functional and specific antibody responses in adult volunteers in New Zealand who were given one of two different meningococcal serogroup B outer membrane vesicle vaccines. Clin Vaccine Immunol 2007; 14:830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhu P, Morelli G, Achtman M. The opcA and (psi)opcB regions in Neisseria: genes, pseudogenes, deletions, insertion elements and DNA islands. Mol Microbiol 1999; 33:635–50. [DOI] [PubMed] [Google Scholar]
- 47. Unemo M, Norlén O, Fredlund H. The porA pseudogene of Neisseria gonorrhoeae–low level of genetic polymorphism and a few, mainly identical, inactivating mutations. APMIS 2005; 113:410–9. [DOI] [PubMed] [Google Scholar]
- 48. Maritan M, Cozzi R, Lo Surdo P, Veggi D, Bottomley MJ, Malito E. Crystal structures of human Fabs targeting the Bexsero meningococcal vaccine antigen NHBA. Acta Crystallogr F Struct Biol Commun 2017; 73:305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maritan M, Veggi D, Cozzi R, et al. . Structures of NHBA elucidate a broadly conserved epitope identified by a vaccine induced antibody. PLOS One 2018; 13:e0201922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Findlow J, Borrow R, Snape MD, et al. . Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant Meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis 2010; 51:1127–37. [DOI] [PubMed] [Google Scholar]
- 51. Ochoa-Azze RF. Cross-protection induced by VA-MENGOC-BC(R) vaccine. Hum Vaccin Immunother 2018; 14:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Petousis-Harris H. Commentary: Impact of meningococcal group B OMV vaccines, beyond their brief. Hum Vaccin Immunother 2017; 14:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Craig AP, Gray RT, Edwards JL, et al. . The potential impact of vaccination on the prevalence of gonorrhea. Vaccine 2015; 33:4520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cuello M, Cabrera O, Acevedo R, et al. . Nasal immunization with AFCo1 induces immune response to N. gonorrhoea in mice. VacciMonitor 2014; 18:76–8. [Google Scholar]
- 55. Pizza M, inventor; Glaxosmithkline Biologicals SA, assignee Vaccines for Neisseria gonorrhoeae. United States patent application US20180064801A1, 8 March 2018.
- 56. Connolly K, Leduc I, Rahman N, Sempowski G, Jerse A. The group B meningococcal vaccine Bexsero induces antibodies that recognize several candidate gonorrhea vaccine targets and shows protective efficacy against experimental Neisseria gonorrhoeae genital tract infection in mice [abstract O10]. In: Program and abstracts of the 21st International Pathogenic Neisseria Conference. Asilomar, CA, USA, 2018. [Google Scholar]
- 57. Beernink PT, Ispasanie E, Lewis LA, Ram S, Moe GR, Granoff DM. A meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and overexpressed Factor H binding protein elicits gonococcal bactericidal antibodies. J Infect Dis 2018; doi: 10.1093/infdis/jiy609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Caugant DA, Tzanakaki G, Kriz P. Lessons from meningococcal carriage studies. FEMS Microbiol Rev 2007; 31:52–63. [DOI] [PubMed] [Google Scholar]
- 59. Rajam G, Stella M, Kim E, et al. . Meningococcal antigen typing system (MATS)-based Neisseria meningitidis serogroup B coverage prediction for the MenB-4C vaccine in the United States. mSphere 2017; 2:e00261–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Welsch JA, Moe GR, Rossi R, Adu-Bobie J, Rappuoli R, Granoff DM. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J Infect Dis 2003; 188:1730–40. [DOI] [PubMed] [Google Scholar]
- 61. Plested JS, Granoff DM. Vaccine-induced opsonophagocytic immunity to Neisseria meningitidis group B. Clin Vaccine Immunol 2008;15:799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vacca I, Del Tordello E, Gasperini G, et al. . Neisserial heparin binding antigen (NHBA) contributes to the adhesion of Neisseria meningitidis to human epithelial cells. PLOS One 2016; 11:e0162878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Public Health England. UK case of Neisseria gonorrhoeae with high-level resistance to azithromycin and resistance to ceftriaxone acquired abroad. Health Protection Report 12(11). Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/694655/hpr1118_MDRGC.pdf Accessed 8 April 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.