ABSTRACT
Epilepsy is a chronic neurological disorder that affects many people worldwide. Temporal lobe epilepsy is the most common and most studied type of epilepsy, but the pathological mechanisms underlying this condition are poorly understood. More than 20 antiepileptic drugs (AEDs) have been developed and used for the treatment of epilepsy; however, 30% of patients still experience uncontrolled epilepsy and associated comorbidities, which impair their quality of life. In addition, various side effects have been reported for AEDs, such as drowsiness, unsteadiness, dizziness, blurred or double vision, tremor (shakiness), greater risk of infections, bruising, and bleeding. Thus, critical medical needs remain unmet for patients with uncontrolled epilepsy. Flavonoids belong to a subclass of polyphenols that are widely present in fruits, vegetables, and certain beverages. Recently, many studies have reported that some flavonoids elicit various beneficial effects in patients with epilepsy without causing the side effects associated with conventional medical therapies. Moreover, flavonoids may have a property of regulating microRNA expression associated with inflammation and cell survival. These findings suggest that flavonoids, which are more effective but impose fewer adverse effects than conventional AEDs, could be used in the treatment of epilepsy.
Keywords: epilepsy, granule cell dispersion, flavonoids, antiepilepsy, antiepileptic drugs
Introduction
Epilepsy is a chronic neurological disorder that affects >70 million people worldwide. Globally, an estimated 2.4 million people are diagnosed with epilepsy per year (1, 2), and the adult epilepsy patient population in the United States has increased ≥30%, from ∼2.3 million in 2010 to 3 million in 2015. During the same period, pediatric patient diagnoses have increased from 450,000 to 470,000 (3). In addition to epilepsy and the associated comorbidities (e.g., depression and anxiety), many epilepsy patients also experience social discrimination and alienation. Currently, epilepsy is classified into 4 types depending on the onset of seizures: focal, generalized, combined generalized and focal, and unknown (4). Focal seizures arise in 1 brain hemisphere only, whereas generalized seizures arise in both brain hemispheres. Temporal lobe epilepsy (TLE) was defined in 1985 by the International League Against Epilepsy. It is the most common type of epilepsy, with recurrent, unprovoked focal seizures originating from the medial or lateral temporal lobe. Several studies have demonstrated that recurrent seizures affect cognitive function, including memory, attention, language, praxis, executive function, judgment, insight, and problem-solving (5, 6). These cognitive impairments have been suggested to result from changes in neural circuitry by seizure-induced structural and functional changes in the brain (7). More than 20 antiepileptic drugs (AEDs) have been developed, including valproic acid, lamotrigine, phenobarbital, gabapentin, felbamate, and topiramate (8). Despite the fact that >10 different epilepsy treatments are available, ∼30% of patients respond poorly to treatment (9). In contrast, 70% of patients can achieve long-term remission under AED treatment. However, many AEDs are associated with adverse side effects that are experienced by a considerable number of patients. These include somnolence, dizziness, gastrointestinal events, psychotic episodes, behavioral problems, depression, impaired cognition, osteoporosis, and leukopenia (10). Furthermore, previous clinical trials reported that treatment with AEDs could lead to seizure worsening, and AED therapeutic effects were not significantly different from placebo treatments (11–14). Thus, significant unmet medical needs still must be overcome for the effective and safe treatment of epilepsy.
Recently, there has been increasing evidence for the pharmacological effects of plant-derived flavonoids on epilepsy. Flavonoids are most widely found in plant-based products such as fruits, vegetables, grains, nuts, seeds, tea, and traditional medicinal herbs. More than 8000 different flavonoids have been isolated from natural sources. In general, flavonoids are compounds of low molecular weight containing 2 benzene rings linked by a heterocyclic pyran or pyrone ring (15). Flavonoids have been considered as novel AED candidates because they have many relevant biological and medicinal properties, including antioxidative, anti-inflammatory, and neuroprotective properties. We previously reported that the flavonoids naringin (16, 17), naringenin (18), eugenol (19), silibinin (20), morin (21), and hesperetin (22) attenuated epileptic symptoms. These effects seemed to be taking place via 2 main mechanisms: 1) by the alleviation of hippocampal structural changes, including through granule cell dispersion (GCD) in the dentate gyrus (DG), and 2) by the reduction of inflammatory responses, which is well represented in TLE, by inhibiting the expression of pro-inflammatory cytokines in kainic acid (KA)-induced seizure model mice. These findings suggest that flavonoids can be used as an alternative medicine for the treatment of epilepsy. In this review, we discuss the beneficial effects of flavonoids as potential antiepileptic agents with less adverse effects than conventional AEDs.
Basic Mechanisms Underlying TLE
TLE is frequently associated with Ammon's horn sclerosis. The latter is characterized by loss of principal neurons in the CA1 and CA3 regions of the hippocampus and in the hilus of the DG, where widening of the granule cell layer is typical (23–26) and was named GCD by Houser (23). GCD is associated with hippocampal sclerosis in ∼40% of epilepsy patients, but its underlying pathological mechanisms and clinical significance are poorly understood. Nevertheless, it has been implicated in the decreased expresstion of the extracellular matrix protein Reelin in the hippocampus of TLE patients (27) and seizure model mice (28–31). Hyperactivation of the mammalian target of rapamycin (mTOR) signaling pathway in the hippocampi of human patients and animal models has also been associated with GCD (18, 20, 32, 33); in some cases, treatment with rapamycin, which inhibits the activity of mTOR, suppressed GCD in vivo (33, 34).
Currently Available Epilepsy Treatments
Many studies have suggested that imbalances between excitatory and inhibitory signals may cause epilepsy (35–37). AEDs currently used to stop epileptic seizures act mostly by blocking ion channels and inhibiting neuronal excitability (Table 1). The targets of AEDs include voltage-gated sodium channels [for carbamazepine (38–41), felbamate (42), lacosamide (42), lamotrigine (43), oxcarbazepine (42), phenytoin (44–47), rufinamide (46, 48), topiramate (49), valproic acid (50–52), and zonisamide (42, 53)] and T-type calcium channels [for valproic acid (42)]. Other AEDs reduce abnormal excitatory action potentials via the inhibition of synaptic neurotransmitter release. These targets and drugs include the synaptic vesicle ligands, glycoprotein 2A [for levetiracetam (42)], the α2δ subunit of the voltage-gated calcium channel [for gabapentin (54) and pregabalin (55)], and glutamate receptors [e.g., N-methyl-d-aspartate (NMDA) receptor for felbamate (42)]. Approximately 70% of epilepsy patients become seizure-free after receiving medication, but 30% of patients are often resistant to AED treatment. When seizures are not successfully controlled by single-drug treatments, multiple-drug treatments are typically attempted. However, a study demonstrated that 1 such combination therapy elicited a positive therapeutic effect in only 3% of patients (9). In addition, current treatments with AEDs exert only transient effects on recurrent seizures and insufficient suppression of GCD in TLE model animals (56, 57). Rapamycin, which was approved by the FDA as an anticancer drug, has been demonstrated as another potential antiepileptic agent with broader clinical relevance (58, 59). Unfortunately, rapamycin can inhibit cell proliferation and motility; thus, the safety of long-term rapamycin treatments must be assessed in advance. Nevertheless, the role of the mTOR inhibition strategy for the treatment of epilepsy remains viable.
TABLE 1.
Current AEDs used in epilepsy1
| Agent | Mechanism of action | Advantage | Adverse effects | References |
|---|---|---|---|---|
| Carbamazepine | Sodium channel blockerAction on monoamine, acetylcholine, and NMDA receptors | Highly effectiveSuitable in adults and children for many types of epilepsy | Diplopia, dizziness, ataxia, hyponatremia, dermatological, hepatic, hematological toxicity | (38–41) |
| Ethotoin | Calcium channel blocker | Highly effective | Dizziness, fatigue, headache, insomnia, numbness, rash, diarrhea, chest pain, diplopia, nystagmus, lymphadenopathy, ataxia, vomiting or nausea | (77) |
| Felbamate | NMDA antagonistSodium channel conductance | Powerful broad-spectrum action | Occasional case of severe hepatic and aplastic anemiaUsed only by specialists as last-resort therapy | (42) |
| Gabapentin | Unknown; possibly GAD modulation | Lack of side effects at low doses | Seizure exacerbation at high doses | (54) |
| Lacosamide | Sodium channel blocker | Highly effective | Dizziness, diplopia, tremors, sleepiness, headache, loss of coordination, nausea | (42) |
| Lamotrigine | Sodium channel blocker | Moderate efficacy | High instance of rash (occasionally severe) | (43) |
| Dizziness, diplopia, tremors, sleepiness, headache, loss of coordination, nausea | ||||
| Levetiracetam | Action via binding to the SV2A synaptic vesicle proteinAction via binding to the SV2A synaptic vesicle protein | Highly effective and generally well tolerated; mode of action not shared by other drugs | Mood and behavioral changes | (42) |
| Oxcarbazepine | Sodium channel, potassium conductance blockerNMDA antagonist | Powerful antiepileptic actionAn alternative to carbamazepine | Adverse event profile is different and involves fewer drug interactions than does carbamazepine. | (42) |
| Higher incidence of hyponatremia than that with carbamazepine | ||||
| Phenobarbital | Enhances activity of GABAA receptor | Highly effective and low-cost AEDHighly effective well-tested AED | SedationRash | (42) |
| Depresses glutamate excitability and affects sodium, potassium, and calcium conductance | ||||
| Phenytoin | Sodium channel blocker | Highly effectiveLow cost | Teratogenic and carcinogenicSedation, dizziness, ataxia, gingival hyperplasia | (44–47) |
| Pregabalin | Calcium channel modulationReduces release of glutamate | Effective and well tolerated | Dizziness, vertigo, incoordination, balance disorder, ataxia, diplopia, blurred vision, amblyopia, tremor, somnolence, confusional state, disturbance in attention, thinking abnormal, euphoria, asthenia, fatigue, edema, peripheral edema, dry mouth, constipation | (55) |
| Rufinamide | Sodium channel modulation | Highly effective | Dizziness, headache, nausea, somnolence, double vision, fatigue, ataxia, vomiting, abnormal vision | (46, 48) |
| Tiagabine | Inhibits GABA reuptake | Highly effective | Dizziness, asthenia, somnolence, nausea, irritability, tremor, abdominal pain, difficulty with concentration | (78, 79) |
| Topiramate | AMPA/kainic acid antagonistInhibition of voltage-gated sodium channels | Powerful antiepileptic actionRare serious adverse effects | Weight loss, anorexia, somnolence and fatigue, sedation, cognitive complaints, paresthesia | (49) |
| Potentiation inhibitor of benzodiazepine GABAA receptor | ||||
| Inhibition of high-voltage calcium channels | ||||
| Valproate | GAD modulation | A wide spectrum of activity | Weight gain, nausea, tremor, hair loss | (50–52) |
| Effects on GABA | Hepatic disturbance in children, teratogenicity | |||
| Vigabatrin | GAD modulationInhibition of GABA transaminase activity | Highly effective antiepileptic drugExcellent effect in West syndrome | Adverse effect on visual fields and potential for cognitive effects | (42) |
| Inhibition of GABA transaminase activity | Excellent effect in West syndrome | Because of visual field effects, prescriptions are currently restricted to last-resort use in partial epilepsy. | ||
| Zonisamide | Sodium channel blocker | Highly effective | Drowsiness, dizziness | (42, 53) |
| Inhibition benzodiazepine GABAA receptor | Problems with memory or concentration | |||
| Loss of coordination, trouble walking | ||||
| Renal stones, oligohydrosis, hypersensitivity, teratogenicity |
1AED, antiepileptic drug; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; GABA, γ-aminobutyric acid; GAD, glutamic acid decarboxylase; NMDA, N-methyl-d-aspartate.
In addition to poor efficiency, AEDs can induce a variety of dose-related side effects, such as drowsiness, unsteadiness, dizziness, blurred or double vision, tremor (shakiness), greater risk of infections, bruising, and bleeding (60). Thus, there is a critical need for effective and safe alternative drugs to use in the treatment of epilepsy.
Therapeutic Advantages of Flavonoids in Neurological Diseases
With respect to neurological disorders, many flavonoids are known to provide neuroprotective effects, including in Alzheimer's disease (61, 62), Parkinson's disease (63–66), and ischemic stroke (67, 68). Emerging evidence suggests that the beneficial effects of flavonoids on these neurological diseases may be associated with modulation of γ-aminobutyric acid (GABA) receptors (69–71); mitochondrial dysfunction (72, 73); and regulation of antioxidative and anti-inflammatory mediators such as glutathione (GSH), superoxide dismutase, and cytokines (74–76). Furthermore, some flavonoids prevented mossy fiber sprouting, GCD formation, and mTOR activation in the hippocampi of KA-induced TLE model mice (16–22). Antiepileptic effects of flavonoids have been verified in some preclinical studies, but the effects of flavonoids as antiepileptic agents for the treatment of epilepsy have not been reported in clinical trials (80).
Blood–brain barrier (BBB) penetration is the largest obstacle for drugs targeting the central nervous system. Several studies have demonstrated that flavonoids can penetrate the BBB and exert effects in the brain (81–86). Both naringenin and naringin were detected in the cortex after intravenous administration of naringenin (20 mg/kg) (81). In addition, the concentration of morin in the brain was measured as 10 µg/mL at 3 h post intranasal administration with 160 mg/40 μL morin, indicating that morin may have the ability to cross the BBB (86). Another study reported that quercetin was significantly elevated in the brain tissue of quercetin-fed rats (after 1 mo compared with 1 wk of quercetin feeding); the actual amounts of brain quercetin were ∼8% (83). Despite flavonoids being safer than conventional drugs, there is little evidence regarding the specific mechanisms whereby they enter the brain. It has been suggested that flavonoids may penetrate the BBB via mechanisms such as transcellular diffusion, carrier-mediated transcellular transport (87, 88), or paracellular diffusion through tight junctions between the endothelial cells of the BBB (82). Yang et al. (89) used an in vitro BBB model to investigate the rates of transportation of some lipophilic or lipophobic flavonoids, including rutin, hesperidin, quercetin, genistein, and apigenin. Their results showed that the transportation rates of flavonoids increase linearly with their concentration. However, saturation was not observed, indicating that the permeation process may be mainly driven by the concentration gradient for these flavonoids.
Using Flavonoids to Treat Epilepsy and Seizures
Apigenin
Apigenin (5,7,4′-trihydroxyflavone) is a dietary flavonoid present in large amounts in many fruits and vegetables. Han et al. (90) reported that pretreatment with apigenin (25 and 50 mg/kg, intraperitoneally) for 5 d reduced the seizure scores and delayed the convulsion onset time in KA-injected mice; it also blocked the KA-induced electroencephalography changes in the cortex. In addition, increases in GSH concentrations in hippocampal neurons after KA-induced seizures were significantly prevented by apigenin pretreatment; this resulted in a significant reduction in the amount of mitochondrial reactive oxygen species and degree of apoptotic neuronal cell death in vitro and in vivo. In picrotoxin-induced seizure model rats, apigenin (25 and 50 mg/kg, intraperitoneally) significantly reduced the latency of seizure onset (91).
Luteolin
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a flavonoid commonly present in many fruits, vegetables, and medicinal herbs. According to a previous report (92), pretreatment with luteolin (50 and 100 mg/kg/d by oral administration for 36 d) reduced seizure onset, frequency, and severity after pentylenetetrazol (PTZ) injection; in addition, it significantly reduced the amount of mitochondrial reactive oxygen species, resulting in protection of hippocampal neurons against PTZ toxicity. Furthermore, luteolin led to the expression induction of brain-derived neurotrophic factor and activation of protein kinase A and cAMP response element binding protein in the hippocampus of PTZ-injected rats. Tambe et al. (93) investigated the effects of pretreatment with luteolin (5, 10, and 20 mg/kg, intraperitoneally) in PTZ-induced, acute and chronic epilepsy model mice. They found that luteolin attenuated the malondialdehyde (MDA) concentrations, restored concentrations of reduced GSH, and inhibited kindling behavior induced by PTZ injection.
Genistein
Genistein (4',5,7-trihydroxyisoflavone) is an isoflavone found in soybeans and known to exhibit, among others, anti-inflammatory, antioxidative, and antitumorigenic biological properties. The effects of genistein on KA-induced behavioral and neuronal dysfunctions in ovariectomized rats have been reported (94). Pretreatment with genistein (0.5 mg/kg, intraperitoneally once a day for 4 consecutive days) significantly improved seizure-induced spatial learning and memory impairments, early long-term potentiation deficits, and damage to hippocampal neurons 7 d post-seizure onset. In another study, pretreated genistein (10 mg/kg, intraperitoneally 30 min before PTZ injection) exerted anticonvulsant effects against PTZ-induced seizure in ovariectomized mice, which might have been mediated via the estrogenic/serotonergic systems (95). Elsayed et al. (96) also tested the anticonvulsant effects of genistein on PTZ-induced epilepsy in ovariectomized rats. Pretreatment with genistein (10 and 20 mg/kg, intraperitoneally 30 min before PTZ injection) delayed seizure onset, reduced seizure duration, reduced the concentrations of oxidative stress indicators such as MDA and GSH, decreased estrogen receptor expression, reduced apoptosis, and improved histopathological patterns.
Baicalin
Baicalin (7-glucuronic acid, 5,6-dihydroxyflavone) is a major flavonoid component of the herbal medicine prepared from Scutellaria baicalensis. A previous study (97) found that administration of baicalin (100 mg/kg, intraperitoneally injected twice at 1 and 8 h after seizure onset) significantly reduced the expression of cleaved caspase-3 and induced the expression of B cell lymphoma 2, resulting in reduction of neuronal cell death in the hippocampus of KA-treated mice. Furthermore, treatment of KA upregulated the expression of microRNA-497 (miR-497) in the hippocampus, but baicalin significantly attenuated this effect. In another study, Liu et al. (98) showed anticonvulsant effects of baicalin in pilocarpine-induced epilepsy model rats. Baicalin (100 mg/kg, administered intraperitoneally 30 min before pilocarpine) significantly delayed the onset of pilocarpine-induced seizures. In addition, baicalin significantly reduced nitrite/nitrate and MDA concentrations while upregulating the GSH concentration in the hippocampus of pilocarpine-injected rats, which is indicative of its antioxidant properties.
Silibinin
Silibinin [2,3-dihydro-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-6-(3, 5, 7-trihydroxy-4-oxobenzopyran-2-yl)-benzodioxin] is a flavonoid extracted from milk thistle seeds. A recent report (99) demonstrated that silibinin has beneficial effects in lithium–pilocarpine-induced seizure model rats. The authors administered silibinin intragastrically 30 min before (at 100 mg/kg) pilocarpine injection and daily for 13 d thereafter (at 100 mg/kg for days 1–3 and at 50 mg/kg for days 4–13). Under these conditions, silibinin significantly downregulated the mRNA expression of hypoxia-inducible factor-1α, TNF-α, IL-1β, and IL-6. Furthermore, these effects resulted in the reduction of neuronal loss in the hippocampus of rats after seizure induction. In a separate study, we reported positive therapeutic effects of silibinin in KA-injection-induced epilepsy model mice (20). Treatment with 200 mg/kg of silibinin (intraperitoneally injected 1 d and 1 h before KA injection and daily for 35 d thereafter) significantly attenuated seizure susceptibility, spontaneous recurrent seizure frequency, and GCD, a morphological alteration characteristic of the DG. Moreover, silibinin significantly reduced the expression of apoptotic, autophagic, and pro-inflammatory molecules that are normally produced after KA injection, resulting in neuroprotection of hippocampal neurons in the KA-injected mice.
Naringin and its metabolite, naringenin
Naringin (4′,5,7-trihydroxyflavanone-7-rhamnoglucoside) and its metabolite, naringenin (4′,5,7-trihydroxyflavanone), are flavanone glycosides found in grapes and citrus fruits that have strong antioxidative and anti-inflammatory properties. Golechha et al. (100, 101) reported on the effects of naringin in both KA- and PTZ-induced epileptic rat models. Pretreatment with naringin (20, 40, and 80 mg/kg, intraperitoneally) for 7 d significantly delayed the latency of seizure and reduced the expression of TNF-α induced by KA injection in a dose-dependent manner. Naringin pretreatment (40 and 80 mg/kg) also increased GSH concentrations and prevented lipid oxidation in the hippocampus of KA-treated rats, supporting the antioxidant effects of naringin (100). Similar to the observations in the KA-induced model rats, pretreatment with naringin (80 mg/kg, intraperitoneally) for 7 d significantly attenuated oxidative damage, inflammation, and cognitive impairment in PTZ-induced seizure model rats. In addition, rats pretreated with the GABA receptor antagonist flumazenil showed a significant decrease in the latency of myoclonic jerks compared with naringin-treated rats, indicating that naringin may have GABA receptor modulation properties (101). Moreover, 80 mg/kg of naringin (administered intraperitoneally 1 d before KA injection and daily for 6 d thereafter) could delay the onset of KA-induced seizures and attenuate autophagic stress and GCD in KA-treated mice via the regulation of mTOR complex 1 (mTORC1) activity (16, 17). The beneficial effects of naringenin have also been reported in various other epileptic models. Khodayar et al. (102) investigated the anticonvulsant effect of naringenin on both maximal electroshock and PTZ-induced seizures in mice. Naringenin (200 mg/kg, intraperitoneally 30 min before seizure onset) reduced the duration of hindlimb extension in the maximal electroshock-induced seizure model and decreased the duration of myoclonic jerks in the PTZ-injected mice. In another study (103), naringenin (20 and 40 mg/kg) was administered orally for 15 d before pilocarpine-induced seizure onset in mice. Under these conditions, naringenin restored the antioxidant status and reduced lipid peroxidation in the hippocampus. Our previous report (18) showed that pretreatment with naringenin for 8 d (100 mg/kg/day, intraperitoneally) reduced the extent of morphological alterations of the DG and attenuated the expression of neurotoxic inflammatory cytokines such as TNF-α, IL-1β, and inducible nitric oxide synthase in the hippocampus of KA-treated mice. Furthermore, a mild binding affinity of naringenin for the GABAA receptor benzodiazepine site (104, 105) indicates that naringenin may act as an agonist of GABA receptors.
Morin
Morin [2-(2, 4-dihydroxyphenyl)-3, 5, 7-trihydroxy-4H-1-benzopyran-4-one], is a flavonoid isolated from Maclura pomifera (Osage orange), Maclura tinctoria (old fustic), and the leaves of Psidium guajava (common guava). Kandhare et al. (106) showed that morin has antiepileptic effects in PTZ-induced seizure model mice. Morin (20 and 40 mg/kg, intraperitoneally 45 min before PTZ injection) significantly reduced seizure behavior and improved the locomotor impairment caused by PTZ-induced seizures. In addition, morin significantly limited the seizure-induced reductions in GABA, dopamine, and Na+/K+-ATPase concentrations and the seizure-induced increases in xanthine oxidase activity and oxidonitrosative stress. Therefore, the authors suggested that the anticonvulsive effects of morin were elicited via modulation of the concentrations of GABA, Na+/K+-ATPase, and antioxidant status. Moreover, we recently reported (21) that the activation mTORC1 due to KA-induced seizure in mice was inhibited by treatment of morin (80 mg/kg; orally 1 d and 1 h before KA injection and daily for 2 d thereafter). Decreases in inflammation, mossy fiber sprouting, and GCD formation were also observed in the hippocampus of the KA-treated mice after administration of morin for 7 d.
Rutin
Rutin (3, 3′, 4′, 5, 7-pentahydroxyflavone-3-rhamnogluco-side) is a flavonoid of the flavonol type that is an important dietary component of foods and plant-based beverages. Nassiri-Asl et al. (107) showed the antiseizure effects of rutin on PTZ-induced model rats. Pretreatment with rutin (at 50 and 100 mg/kg, intraperitoneally for 14 d and up to 30 min before PTZ injection on day 14) led to a reduction in seizure severity and significantly increased the step-through latency in the passive avoidance paradigm. In another study with KA-induced seizure model mice (108), rutin treatment (at 100 and 200 mg/kg, intraperitoneally for 7 d and up to 30 min prior to KA injection) had anticonvulsant effects and attenuated oxidative stress indicators such as MDA concentrations.
Quercetin
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is one of the most widely occurring flavonoids and is often present in vegetables and fruits. Nassiri-Asl et al. (109) investigated the anticonvulsant and antioxidant effects of quercetin in PTZ-induced seizure model rats. Quercetin (administered at 25, 50, or 100 mg/kg/day, intraperitoneally for 15 d and up to 30 min before PTZ treatment) resulted in anticonvulsant effects; although protection against memory impairment was observed at a dose of 50 mg/kg, no antioxidant effects were observed. On the other hand, it was reported that quercetin treatment (at 10 mg/kg, intraperitoneally 30 min before PTZ injection in rats) significantly prolonged the onset and reduced the severity of the seizure (110). In that study, 20 mg/kg of quercetin administered 30 min before picrotoxin injection had anticonvulsant effects. Moreover, in another study with KA-induced model mice, it was shown that quercetin administration (50 and 100 mg/kg/d, intraperitoneally for 7 d) decreased seizure severity in a dose-dependent manner; in addition, it reduced the expression levels of the GABAA α5 mRNA (111). Other studies have also reported an increase in GABAA α5 expression in KA and pilocarpine models (112–114), suggesting that compensatory mechanisms are involved in disease pathogenesis (114).
Hesperidin and its aglycone, hesperetin
Hesperidin is a natural flavone that is predominantly and abundantly found in citrus fruits. Hesperidin and its aglycone, hesperetin (3′,5,7-trihydroxy-4′-methoxyflavanone), have been shown to exert beneficial effects in the treatment of epilepsy. Kumar et al. (115) reported that 7 d of pretreatment with hesperidin (100 and 200 mg/kg, orally) prolonged the latency of onset of clonic and tonic phases of convulsion and increased the concentrations of antioxidant enzymes such as glutathione, superoxide dismutase, and catalase in PTZ-induced model mice. Another study (116) suggested that hesperidin pretreatment (10 and 50 mg/kg, intraperitoneally) attenuated neuronal loss in the hippocampus of KA-treated rats via reduction of glutamate release. We previously (22) showed that hesperetin (20 mg/kg/d, administered orally 1 d before KA injection and 7 d after seizure onset) prevented structural and functional abnormalities such as GCD and mTORC1 activation in the hippocampus of KA-injected mice.
Vitexin
Vitexin (5, 7, 4-trihydroxyflavone-8-glucoside) is a C-glycosylated flavone, which has been found in various plants such as Passiflora sp., bamboo leaves, pigeon pea leaves, and mung bean. Abbasi et al. (117) investigated the neuroprotective effects of vitexin on PTZ-induced model rats. In that study, vitexin (100 and 200 µM, administered intracerebroventricularly 30 min before PTZ injection) exerted anticonvulsant effects by significantly reducing minimal clonic seizure and generalized tonic–clonic seizure. In addition, the authors showed that such antiepileptic effects of vitexin were abolished by pretreatment with flumazenil, indicating that vitexin may act as a GABAA receptor modulator.
Modulation of microRNAs as a Potential Target of Flavonoids in Epilepsy
MicroRNAs (miRNAs) are small endogenous noncoding RNAs critical for the post-transcriptional regulation of most mRNAs. miRNA biogenesis is a conserved process, which generates 21–25-nucleotide-long products that regulate gene expression by complementary binding to the target mRNA. Some studies have profiled alteration of miRNAs in the hippocampus of animal models and patients with TLE (118–130). Peng et al. (123) demonstrated that the expression of miR-132, which belongs to the miR-212/132 family, is increased in the hippocampus of human TLE patients and experimental models. miR-132 could modulate neurite outgrowth and dendritic morphology via regulation of the protein kinase A-mediated cAMP response element binding protein signaling pathway. Han et al. (118) measured the miRNA profile in the hippocampus of rats after lithium–pilocarpine-induced epilepsy using microarrays and qPCR. This approach revealed the presence of 4 upregulated miRNAs (miR-146a, -210, -34a, and -27a) and 2 downregulated miRNAs (miR-135b and -33) in the hippocampus of TLE model rats. This work highlighted a role for miR-34a in TLE-associated neuronal cell death. In addition, it showed that miR-34a antagomir treatment significantly inhibited the activity of caspase-3 and led to an increase in neuronal survival 7 d post-epilepsy in the rat hippocampus (122). Liu et al. (126) found that miR-344a expression was downregulated in the hippocampus of PTZ-induced epilepsy model rats. Overexpression of miR-344a by intracerebroventricular injection of miR-344a agomir significantly reduced neuronal cell death and the seizure behaviors associated with TLE in the PTZ-injected rats. Alsharafi et al. (124) investigated the miRNA expression patterns in the hippocampus of patients with TLE and pilocarpine-induced model rats. The results showed that the expression of miR-139–5p was significantly downregulated in both TLE patients and pilocarpine-induced model rats. These findings suggested that alternation of miRNA expression may play an important role in epileptogenesis.
Several studies found that miR-155 expression was increased in the hippocampus of epilepsy model rats and TLE patients (128, 131–133). In addition, the TNF-α and phosphoinositide 3-kinase/Akt/mTOR epileptogenic pathways were regulated by miR-155 in epilepsy model rats and TLE patients (128, 131, 132). Another study showed that silencing miR-155 reduced apoptotic cell death associated with pilocarpine-induced seizure in the CA3 hippocampal area of rats (133). The effects of silibinin on miR-155 expression were evaluated in cell cultures of MCF-7, which is a breast cancer cell line. Treatment with 100 µg/mL of silibinin significantly downregulated expression of miR-155, resulting in inhibition of cell proliferation and migration (134). Arango et al. (135) reported that apigenin (50 mg/kg, intraperitoneally) prevented LPS-induced inflammatory activity by reducing miR-155 expression in murine macrophage culture and lung tissue. Quercetin (10 μmol/L) significantly decreased the mRNA and protein expression of TNF-α through the downregulation of miR-155 in LPS-stimulated murine macrophages (136). In another in vivo study, elevation of miR-497 expression was observed in the hippocampus of KA-injected mice at 12 h post-seizure onset (97). However, double treatment with baicalin (100 mg/kg) by intraperitoneal injection at 1 and 8 h after seizure onset significantly inhibited the expression of miR-497 in the mouse hippocampus.
There are few studies on miRNAs as molecular targets of flavonoids in TLE model animals. However, there are several studies on the miRNA-related mechanisms of action of plant-derived compounds, including flavonoids, in human diseases such as hypertension, diabetes, atherosclerosis, metabolic disorders, and cancer. Therefore, further studies are required to examine the extent of correlation between flavonoids and miRNA in the treatment and prevention of TLE.
Conclusions
Although there are some medications to control epilepsy that successfully regulate seizures, 30–40% of epilepsy patients do not respond to typical treatments with AEDs. In addition, current AEDs have a narrow therapeutic window due to several adverse effects, such as dose-related neurotoxicity and impaired systemic processes. Therefore, alternative effective and safe medical treatments of epilepsy are highly desirable. In recent years, studies have reported beneficial effects of flavonoids, particularly TLE, in epilepsy (Table 2), including reductions in neuronal cell death, neurotoxic inflammation, mossy fiber sprouting, and GCD formation, as well as modulation of miRNA expression in the hippocampus (Figure 1). Current understanding of the specific molecular mechanisms preventing structural changes and mossy fiber sprouting in the hippocampus is limited. In addition, there are insufficient data from clinical trials on the therapeutic and adverse effects of flavonoids. Nevertheless, the beneficial effects of many flavonoids described recently suggest that various flavonoids have the potential to become effective and safe alternative medicines. Thus, flavonoids could be useful for developing novel therapeutic strategies for the treatment of epilepsy.
TABLE 2.
Preclinical studies of the effects of flavonoids on epileptic models1
| Flavonoids | Study model | Dosage | Main targets | References |
|---|---|---|---|---|
| Apigenin | KA-induced mouse model | 25, 50 mg/kg, i.p. | Antioxidant | (90) |
| Picrotoxin-induced rat model | Pretreatment, 25, 50 mg/kg, i.p. | GABA receptor antagonism | (91) | |
| Luteolin | PTZ-induced rat model | 50 or 100 mg/kg/d, p.o. | Antioxidant, induction of trophic factor | (92) |
| PTZ-induced mouse model | 5, 10, 20 mg/kg, i.p. | Antioxidant effects, inhibition of kindling behavior | (93) | |
| Genistein | KA-induced rat model (ovariectomized) | Pretreatment, 0.5, 5 mg/kg/d, i.p. for 4 consecutive days | Seizure-induced spatial learning and memory impairment, early long-term potentiation deficit, damage to hippocampal neurons | (94) |
| PTZ-induced mouse model (ovariectomized) | Pretreatment, 10 mg/kg, i.p. 30 min before PTZ injection | Inhibition of estrogen and serotonin system | (95) | |
| PTZ-induced rat model (ovariectomized) | Pretreatment, 10, 20 mg/kg, i.p. 30 min before PTZ injection | Antioxidative stress (MDA and GSH), inhibition of estrogen receptor expression | (96) | |
| Baicalin | KA-induced mouse model | 100 mg/kg, i.p. twice at 1 and 8 h after KA treatment | Antiapoptotic effects via inhibition of miR-497 | (97) |
| Pilocarpine-induced rat model | Pretreatment, 100 mg/kg, i.p. 30 min before pilocarpine injection | Antioxidant effects | (98) | |
| Silibinin | Lithium–pilocarpine induced rat model | Pretreatment, 100 mg/kg, p.o. 30 min before pilocarpine injection and 100 mg/kg at 1–3 d and 50 mg/kg at 4–13 d post onset | Anti-inflammatory effects | (99) |
| KA-induced mouse model | 200 mg/kg, i.p. 1 d and 1 h before KA injection and daily treatment for 35 d | Antiapoptotic, autophagic, inflammatory effect and anti-GCD effect | (20) | |
| Naringin | KA-induced rat model | Pretreatment, 20, 40, 80 mg/kg/d for 7 d, i.p. | Antioxidant and anti-inflammatory effects | (100, 101) |
| PTZ-induced rat model | Modulation of GABA receptor and ameliorate cognitive impairment (80 mg/kg) | (16, 17) | ||
| KA-induced mouse model | 80 mg/kg, i.p. 1 d before KA injection and daily treatment for 7 d | Anti-autophagic stress and GCDModulation of mTORC1 activity | ||
| Naringenin | Maximal electroshock and PTZ-induced mouse model | 200 mg/kg, i.p. 30 min before seizure onset | Anticonvulsant effects | (102) |
| Pilocarpine-induced mouse model | 20, 40 mg/kg, p.o. for 15 d before seizure onset | Antioxidant effects | (103) | |
| KA-induced mouse model | Pretreatment, 100 mg/kg/d i.p. for 8 days | Anti-inflammatory effects and anti-GCD | (18) | |
| Morin | PTZ-induced mouse model | 20, 40 mg/kg, i.p. 45 min before seizure onset | Preservation of GABA, dopamine, and Na+/K+ ATPase concentrations and antioxidant effects | (106) |
| KA-induced mouse model | 80 mg/kg, p.o. 1 d and 1 h before KA injection and daily treatment for 2–7 d | Inhibition of GCD formation via mTORC1 inhibition, anti-inflammatory and anti-apoptotic effects | (21) | |
| Rutin | PTZ-induced rat model | 50, 100 mg/kg/d, i.p. for 14 d 30 min before PTZ injection | Prevention of seizure behaviors | (107) |
| KA-induced mouse model | 100, 200 mg/kg/d, i.p. for 7 d | Prevention of seizure behaviors and antioxidant effects | (108) | |
| Quercetin | PTZ-induced rat model | 50 mg/kg/day, i.p. 30 min before PTZ injection for 15 days | Anticonvulsant effects and protection against memory impairment | (109) |
| PTZ- or picrotoxin-induced rat model | 10, 20 mg/kg, i.p. 30 min before seizure onset | Anticonvulsant effects | (110) | |
| KA-induced mouse model | 50, 100 mg/kg/d, i.p. for 7 d | Anticonvulsant effects and reduction in the expression of the GABAA α5 mRNA | (111) | |
| Hesperidin | PTZ-induced mouse model | Pretreatment, 100, 200 mg/kg, p.o. for 7 d before seizure onset | Anticonvulsant effects and modulation of antioxidant enzymes concentrations | (115) |
| KA-induced rat model | Pretreatment, 10, 50 mg/kg, i.p. | Neuroprotection, reduction of glutamate release | (116) | |
| Hesperetin | KA-induced mouse model | 20 mg/kg/day, p.o. 1 d before KA injection and 7 d after seizure onset | Prevention of GCD and inhibition of mTORC1 activity | (22) |
| Vitexin | PTZ-induced rat model | 100, 200 µM, i.c.v. 30 min before PTZ injection | Anticonvulsant effects and GABA receptor modulation | (117) |
1GABA, γ-aminobutyric acid; GCD, granule cell dispersion; GSH, glutathione; i.c.v., intracerebroventricular; KA, kainic acid; MDA, malondialdehyde; p.o., oral administration; PTZ, pentylenetetrazol.
FIGURE 1.
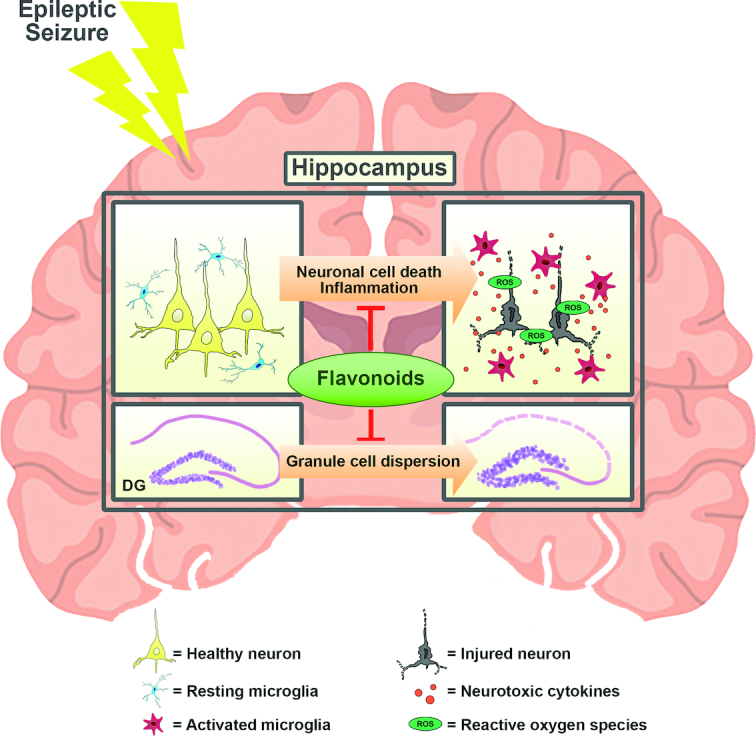
Schematic of the beneficial effects of flavonoids in the hippocampus with epilepsy in vivo. The treatment with various flavonoids, such as apigenin, silibinin, and naringin, may provide antioxidative and anti-inflammatory effects and reduce granule cell dispersion in the hippocampus, resulting in attenuation of status epilepticus. DG, dentate gyrus; ROS, reactive oxygen species.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—JYK, M-TJ, UJJ, GJM, and SRK: conceived and designed the study; JYK, M-TJ, GJM, and SRK: generated the figure; UJJ, DWK, GJM, and SRK: provided the references for the tables; GJM and SRK: wrote and edited the manuscript; and all authors: contributed to preparation of the manuscript and read and approved the final manuscript.
Notes
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
This study was supported by a grant from the National Research Foundation of Korea (NRF-2017R1A2B4002675) by the Korean government.
Author disclosures: JYK, M-TJ, UJJ, DWK, GJM, and SRK, no conflicts of interest.
JYK, M-TJ, and UJJ contributed equally to this work.
Abbreviations used: AED, antiepileptic drug; BBB, blood–brain barrier; DG, dentate gyrus; GABA, γ-aminobutyric acid; GCD, granule cell dispersion; GSH, glutathione; KA, kainic acid; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; MDA, malondialdehyde; miRNA, microRNA; PTZ, pentylenetetrazol; TLE, temporal lobe epilepsy.
References
- 1. WHO. Atlas: Epilepsy Care in the World. Geneva (Switzerland):WHO; 2005. [Google Scholar]
- 2. Singh A, Trevick S.. The epidemiology of global epilepsy. Neurol Clin. 2016;34(4):837–47. [DOI] [PubMed] [Google Scholar]
- 3. Zack MM, Kobau R.. National and state estimates of the numbers of adults and children with active epilepsy—United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(31):821–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, Hirsch E, Jain S, Mathern GW, Moshé SL et al.. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, Bell B. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60(1):80–7. [DOI] [PubMed] [Google Scholar]
- 6. Keary TA, Frazier TW, Busch RM, Kubu CS, Iampietro M. Multivariate neuropsychological prediction of seizure lateralization in temporal epilepsy surgical cases. Epilepsia. 2007;48(8):1438–46. [DOI] [PubMed] [Google Scholar]
- 7. Holmes GL. Cognitive impairment in epilepsy: the role of network abnormalities. Epileptic Disord. 2015;17(2):101–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. French JA, Gazzola DM. New generation antiepileptic drugs: what do they offer in terms of improved tolerability and safety?. Ther Adv Drug Saf. 2011;2(4):141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kwan P, Brodie MJ.. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–9. [DOI] [PubMed] [Google Scholar]
- 10. Sorensen AT, Kokaia M. Novel approaches to epilepsy treatment. Epilepsia. 2013;54(1):1–10. [DOI] [PubMed] [Google Scholar]
- 11. Matsuo F, Bergen D, Faught E, Messenheimer JA, Dren AT, Rudd GD, Lineberry CG. Placebo-controlled study of the efficacy and safety of lamotrigine in patients with partial seizures: U.S. Lamotrigine Protocol 0.5 Clinical Trial Group. Neurology. 1993;43(11):2284–91. [DOI] [PubMed] [Google Scholar]
- 12. Messenheimer J, Ramsay RE, Willmore LJ, Leroy RF, Zielinski JJ, Mattson R, Pellock JM, Valakas AM, Womble G, Risner M. Lamotrigine therapy for partial seizures: a multicenter, placebo-controlled, double-blind, cross-over trial. Epilepsia. 1994;35(1):113–21. [DOI] [PubMed] [Google Scholar]
- 13. French J, Edrich P, Cramer JA. A systematic review of the safety profile of levetiracetam: a new antiepileptic drug. Epilepsy Res. 2001;47(1–2):77–90. [DOI] [PubMed] [Google Scholar]
- 14. Bauer J. Seizure-inducing effects of antiepileptic drugs: a review. Acta Neurol Scand. 1996;94(6):367–77. [DOI] [PubMed] [Google Scholar]
- 15. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52(4):673–751. [PubMed] [Google Scholar]
- 16. Jeong KH, Jung UJ, Kim SR. Naringin attenuates autophagic stress and neuroinflammation in kainic acid-treated hippocampus in vivo. Evid Based Complement Alternat Med. 2015;2015:354326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jang H, Jeong KH, Kim SR. Naringin attenuates granule cell dispersion in the dentate gyrus in a mouse model of temporal lobe epilepsy. Epilepsy Res. 2016;123:6–10. [DOI] [PubMed] [Google Scholar]
- 18. Park J, Jeong KH, Shin WH, Bae YS, Jung UJ, Kim SR. Naringenin ameliorates kainic acid-induced morphological alterations in the dentate gyrus in a mouse model of temporal lobe epilepsy. Neuroreport. 2016;27(15):1182–9. [DOI] [PubMed] [Google Scholar]
- 19. Jeong KH, Lee DS, Kim SR. Effects of eugenol on granule cell dispersion in a mouse model of temporal lobe epilepsy. Epilepsy Res. 2015;115:73–6. [DOI] [PubMed] [Google Scholar]
- 20. Kim S, Jung UJ, Oh YS, Jeon MT, Kim HJ, Shin WH, Hong J, Kim SR. Beneficial effects of silibinin against kainic acid-induced neurotoxicity in the hippocampus in vivo. Exp Neurobiol. 2017;26(5):266–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee JM, Hong J, Moon GJ, Jung UJ, Won SY, Kim SR. Morin prevents granule cell dispersion and neurotoxicity via suppression of mTORC1 in a kainic acid-induced seizure model. Exp Neurobiol. 2018;27(3):226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwon JY, Jung UJ, Kim DW, Kim S, Moon GJ, Hong J, Jeon MT, Shin M, Chang JH, Kim SR. Beneficial effects of hesperetin in a mouse model of temporal lobe epilepsy. J Med Food. 2018; [Epub ahead of print]. doi:10.1089/jmf.2018.4183. [DOI] [PubMed] [Google Scholar]
- 23. Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res. 1990;535(2):195–204. [DOI] [PubMed] [Google Scholar]
- 24. Armstrong DD. The neuropathology of temporal lobe epilepsy. J Neuropathol Exp Neurol. 1993;52(5):433–43. [DOI] [PubMed] [Google Scholar]
- 25. Lurton D, Sundstrom L, Brana C, Bloch B, Rougier A. Possible mechanisms inducing granule cell dispersion in humans with temporal lobe epilepsy. Epilepsy Res. 1997;26(2):351–61. [DOI] [PubMed] [Google Scholar]
- 26. Harding B, Thom M. Bilateral hippocampal granule cell dispersion: autopsy study of 3 infants. Neuropathol Appl Neurobiol. 2001;27(3):245–51. [DOI] [PubMed] [Google Scholar]
- 27. Haas CA, Dudeck O, Kirsch M, Huszka C, Kann G, Pollak S, Zentner J, Frotscher M. Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy. J Neurosci. 2002;22(14):5797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heinrich C, Nitta N, Flubacher A, Muller M, Fahrner A, Kirsch M, Freiman T, Suzuki F, Depaulis A, Frotscher M et al.. Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. J Neurosci. 2006;26(17):4701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tinnes S, Schafer MK, Flubacher A, Munzner G, Frotscher M, Haas CA. Epileptiform activity interferes with proteolytic processing of Reelin required for dentate granule cell positioning. FASEB J. 2011;25(3):1002–13. [DOI] [PubMed] [Google Scholar]
- 30. Orcinha C, Munzner G, Gerlach J, Kilias A, Follo M, Egert U, Haas CA. Seizure-induced motility of differentiated dentate granule cells is prevented by the central Reelin fragment. Front Cell Neurosci. 2016;10:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frotscher M, Haas CA.. Epilepsy-associated Reelin Dysfunction Induces Granule Cell Dispersion in the Dentate Gyrus. In: Reference module in neuroscience and biobehavioral psychology. New York:Elsevier;2017. [Google Scholar]
- 32. Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29(21):6964–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shima A, Nitta N, Suzuki F, Laharie AM, Nozaki K, Depaulis A. Activation of mTOR signaling pathway is secondary to neuronal excitability in a mouse model of mesio-temporal lobe epilepsy. Eur J Neurosci. 2015;41(7):976–88. [DOI] [PubMed] [Google Scholar]
- 34. Kim SR. Control of granule cell dispersion by natural materials such as eugenol and naringin: a potential therapeutic strategy against temporal lobe epilepsy. J Med Food. 2016;19(8):730–6. [DOI] [PubMed] [Google Scholar]
- 35. McNamara JO. Emerging insights into the genesis of epilepsy. Nature. 1999;399(6738 Suppl):A15–22. [DOI] [PubMed] [Google Scholar]
- 36. Bialer M, White HS.. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9(1):68–82. [DOI] [PubMed] [Google Scholar]
- 37. White HS, Smith MD, Wilcox KS. Mechanisms of action of antiepileptic drugs. Int Rev Neurobiol. 2007;81:85–110. [DOI] [PubMed] [Google Scholar]
- 38. Liu L, Zheng T, Morris MJ, Wallengren C, Clarke AL, Reid CA, Petrou S, O'Brien TJ. The mechanism of carbamazepine aggravation of absence seizures. J Pharmacol Exp Ther. 2006;319(2):790–8. [DOI] [PubMed] [Google Scholar]
- 39. Tateno A, Sawada K, Takahashi I, Hujiwara Y. Carbamazepine-induced transient auditory pitch-perception deficit. Pediatr Neurol. 2006;35(2):131–4. [DOI] [PubMed] [Google Scholar]
- 40. Shorvon SD. Drug treatment of epilepsy in the century of the ILAE: the second 50 years, 1959–2009. Epilepsia. 2009;50(Suppl 3):93–130. [DOI] [PubMed] [Google Scholar]
- 41. Jentink J, Dolk, Loane H, Morris MA, Wellesley JK, Garne D, de Jong-van den Berg E, Group EASW L. Intrauterine exposure to carbamazepine and specific congenital malformations: systematic review and case–control study. BMJ. 2010;341:c6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perucca P, Gilliam FG.. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):792–802. [DOI] [PubMed] [Google Scholar]
- 43. Nevitt SJ, Tudur Smith C, Weston J, Marson AG. Lamotrigine versus carbamazepine monotherapy for epilepsy: an individual participant data review. Cochrane Database Syst Rev. 2018;2018(6):CD001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharafuddin MJ, Spanheimer RG, McClune GL. Phenytoin-induced agranulocytosis: a nonimmunologic idiosyncratic reaction?. Acta Haematol. 1991;86(4):212–3. [DOI] [PubMed] [Google Scholar]
- 45. Scheinfeld N. Phenytoin in cutaneous medicine: its uses, mechanisms and side effects. Dermatol Online J. 2003;9(3):6. [PubMed] [Google Scholar]
- 46. Rogawski MA, Loscher W.. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5(7):553–64. [DOI] [PubMed] [Google Scholar]
- 47. Handoko KB, Souverein PC, van Staa TP, Meyboom RH, Leufkens HG, Egberts TC, van den Bemt PM. Risk of aplastic anemia in patients using antiepileptic drugs. Epilepsia. 2006;47(7):1232–6. [DOI] [PubMed] [Google Scholar]
- 48. Rogawski MA. Diverse mechanisms of antiepileptic drugs in the development pipeline. Epilepsy Res. 2006;69(3):273–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Faught E. Topiramate in the treatment of partial and generalized epilepsy. Neuropsychiatr Dis Treat. 2007;3(6):811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. St Louis EK. Minimizing AED adverse effects: improving quality of life in the interictal state in epilepsy care. Curr Neuropharmacol. 2009;7(2):106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eom TH, Lee HS, Jang PS, Kim YH. Valproate-induced panhypo-gammaglobulinemia. Neurol Sci. 2013;34(6):1003–4. [DOI] [PubMed] [Google Scholar]
- 52. Asif M. A review on antiepileptic drug and their uses, mechanism of actions, adverse effects and drug interaction. Curr Sci Perspect. 2016;2(2):19–38. [Google Scholar]
- 53. Stephen LJ, Kelly K, Wilson EA, Parker P, Brodie MJ. A prospective audit of adjunctive zonisamide in an everyday clinical setting. Epilepsy Behav. 2010;17(4):455–60. [DOI] [PubMed] [Google Scholar]
- 54. Rowan AJ, Ramsay RE, Collins JF, Pryor F, Boardman KD, Uthman BM, Spitz M, Frederick T, Towne A, Carter GS et al.. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology. 2005;64(11):1868–73. [DOI] [PubMed] [Google Scholar]
- 55. Zaccara G, Gangemi P, Perucca P, Specchio L. The adverse event profile of pregabalin: a systematic review and meta-analysis of randomized controlled trials. Epilepsia. 2011;52(4):826–36. [DOI] [PubMed] [Google Scholar]
- 56. Riban V, Bouilleret V, Pham-Le BT, Fritschy JM, Marescaux C, Depaulis A. Evolution of hippocampal epileptic activity during the development of hippocampal sclerosis in a mouse model of temporal lobe epilepsy. Neuroscience. 2002;112(1):101–11. [DOI] [PubMed] [Google Scholar]
- 57. Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW. Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol. 2007;35(7):984–99. [DOI] [PubMed] [Google Scholar]
- 58. Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40(1):193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wong M. Rapamycin for treatment of epilepsy: antiseizure, antiepileptogenic, both, or neither?. Epilepsy Curr. 2011;11(2):66–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cramer JA, Mintzer S, Wheless J, Mattson RH. Adverse effects of antiepileptic drugs: a brief overview of important issues. Expert Rev Neurother. 2010;10(6):885–91. [DOI] [PubMed] [Google Scholar]
- 61. Jimenez-Aliaga K, Bermejo-Bescos P, Benedi J, Martin-Aragon S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011;89(25–26):939–45. [DOI] [PubMed] [Google Scholar]
- 62. Lu JH, Ardah MT, Durairajan SS, Liu LF, Xie LX, Fong WF, Hasan MY, Huang JD, El-Agnaf OM, Li M. Baicalein inhibits formation of α-synuclein oligomers within living cells and prevents Aβ peptide fibrillation and oligomerisation. Chembiochem. 2011;12(4):615–24. [DOI] [PubMed] [Google Scholar]
- 63. Lee MH, Lin RD, Shen LY, Yang LL, Yen KY, Hou WC. Monoamine oxidase B and free radical scavenging activities of natural flavonoids in Melastoma candidum D. Don. J Agric Food Chem. 2001;49(11):5551–5. [DOI] [PubMed] [Google Scholar]
- 64. Cheng Y, He G, Mu X, Zhang T, Li X, Hu J, Xu B, Du G. Neuroprotective effect of baicalein against MPTP neurotoxicity: behavioral, biochemical and immunohistochemical profile. Neurosci Lett. 2008;441(1):16–20. [DOI] [PubMed] [Google Scholar]
- 65. Jiang M, Porat-Shliom Y, Pei Z, Cheng Y, Xiang L, Sommers K, Li Q, Gillardon F, Hengerer B, Berlinicke C et al.. Baicalein reduces E46K α-synuclein aggregation in vitro and protects cells against E46K α-synuclein toxicity in cell models of familiar Parkinsonism. J Neurochem. 2010;114(2):419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hung KC, Huang HJ, Wang YT, Lin AM. Baicalein attenuates α-synuclein aggregation, inflammasome activation and autophagy in the MPP(+)-treated nigrostriatal dopaminergic system in vivo. J Ethnopharmacol. 2016;194:522–9. [DOI] [PubMed] [Google Scholar]
- 67. Jiang J, Dai J, Cui H. Vitexin reverses the autophagy dysfunction to attenuate MCAO-induced cerebral ischemic stroke via mTOR/Ulk1 pathway. Biomed Pharmacother. 2018;99:583–90. [DOI] [PubMed] [Google Scholar]
- 68. Park DJ, Shah FA, Koh PO. Quercetin attenuates neuronal cells damage in a middle cerebral artery occlusion animal model. J Vet Med Sci. 2018;80(4):676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hanrahan JR, Chebib M, Johnston GA. Flavonoid modulation of GABA(A) receptors. Br J Pharmacol. 2011;163(2):234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wasowski C, Marder M.. Flavonoids as GABAA receptor ligands: the whole story?. J Exp Pharmacol. 2012;4:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hanrahan JR, Chebib M, Johnston GA. Interactions of flavonoids with ionotropic GABA receptors. Adv Pharmacol. 2015;72:189–200. [DOI] [PubMed] [Google Scholar]
- 72. Sandhir R, Mehrotra A.. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: implications in Huntington's disease. Biochim Biophys Acta. 2013;1832(3):421–30. [DOI] [PubMed] [Google Scholar]
- 73. Jiang K, Wang W, Jin X, Wang Z, Ji Z, Meng G. Silibinin, a natural flavonoid, induces autophagy via ROS-dependent mitochondrial dysfunction and loss of ATP involving BNIP3 in human MCF7 breast cancer cells. Oncol Rep. 2015;33(6):2711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA, Kandaswami C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor α) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-κβ system. Clin Vaccine Immunol. 2006;13(3):319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Impellizzeri D, Cordaro M, Campolo M, Gugliandolo E, Esposito E, Benedetto F, Cuzzocrea S, Navarra M. Anti-inflammatory and antioxidant effects of flavonoid-rich fraction of bergamot juice (BJe) in a mouse model of intestinal ischemia/reperfusion injury. Front Pharmacol. 2016;7:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Leyva-Lopez N, Gutierrez-Grijalva EP, Ambriz-Perez DL, Heredia JB. Flavonoids as cytokine modulators: a possible therapy for inflammation-related diseases. Int J Mol Sci. 2016;17(6):E921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schmidt D. Drug treatment of epilepsy: options and limitations. Epilepsy Behav. 2009;15(1):56–65. [DOI] [PubMed] [Google Scholar]
- 78. Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Perucca E, Tomson T. Progress report on new antiepileptic drugs: a summary of the Eighth Eilat Conference (EILAT VIII). Epilepsy Res. 2007;73(1):1–52. [DOI] [PubMed] [Google Scholar]
- 79. Bauer J, Cooper-Mahkorn D.. Tiagabine: efficacy and safety in partial seizures—current status. Neuropsychiatr Dis Treat. 2008;4(4):731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lasoń W, Leśkiewicz M.. Effect of plant polyphenols on seizures—animal studies. J Epileptol. 2013;21(2):79–87. [Google Scholar]
- 81. Peng HW, Cheng FC, Huang YT, Chen CF, Tsai TH. Determination of naringenin and its glucuronide conjugate in rat plasma and brain tissue by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1998;714(2):369–74. [DOI] [PubMed] [Google Scholar]
- 82. Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C. Interaction between flavonoids and the blood–brain barrier: in vitro studies. J Neurochem. 2003;85(1):180–92. [DOI] [PubMed] [Google Scholar]
- 83. Ishisaka A, Ichikawa S, Sakakibara H, Piskula MK, Nakamura T, Kato Y, Ito M, Miyamoto K, Tsuji A, Kawai Y et al.. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic Biol Med. 2011;51(7):1329–36. [DOI] [PubMed] [Google Scholar]
- 84. Faria A, Mateus N, Calhau C. Flavonoid transport across blood–brain barrier: implication for their direct neuroprotective actions. Nutr Aging. 2012;1(2):89–97. [Google Scholar]
- 85. Faria A, Meireles M, Fernandes I, Santos-Buelga C, Gonzalez-Manzano S, Duenas M, de Freitas V, Mateus N, Calhau C. Flavonoid metabolites transport across a human BBB model. Food Chem. 2014;149:190–6. [DOI] [PubMed] [Google Scholar]
- 86. Sharma D, Singh M, Kumar P, Vikram V, Mishra N. Development and characterization of morin hydrate loaded microemulsion for the management of Alzheimer's disease. Artif Cells Nanomed Biotechnol. 2017;45(8):1620–30. [DOI] [PubMed] [Google Scholar]
- 87. Youdim KA, Shukitt-Hale B, Joseph JA. Flavonoids and the brain: interactions at the blood–brain barrier and their physiological effects on the central nervous system. Free Radic Biol Med. 2004;37(11):1683–93. [DOI] [PubMed] [Google Scholar]
- 88. Chen Z, Ma T, Huang C, Zhang L, Zhong J, Han J, Hu T, Li J. Efficiency of transcellular transport and efflux of flavonoids with different glycosidic units from flavonoids of Litsea coreana L. in a MDCK epithelial cell monolayer model. Eur J Pharm Sci. 2014;53:69–76. [DOI] [PubMed] [Google Scholar]
- 89. Yang Y, Bai L, Li X, Xiong J, Xu P, Guo C, Xue M. Transport of active flavonoids, based on cytotoxicity and lipophilicity: an evaluation using the blood–brain barrier cell and Caco-2 cell models. Toxicol in Vitro. 2014;28(3):388–96. [DOI] [PubMed] [Google Scholar]
- 90. Han JY, Ahn SY, Kim CS, Yoo SK, Kim SK, Kim HC, Hong JT, Oh KW. Protection of apigenin against kainate-induced excitotoxicity by anti-oxidative effects. Biol Pharm Bull. 2012;35(9):1440–6. [DOI] [PubMed] [Google Scholar]
- 91. Avallone R, Zanoli P, Puia G, Kleinschnitz M, Schreier P, Baraldi M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem Pharmacol. 2000;59(11):1387–94. [DOI] [PubMed] [Google Scholar]
- 92. Zhen JL, Chang YN, Qu ZZ, Fu T, Liu JQ, Wang WP. Luteolin rescues pentylenetetrazole-induced cognitive impairment in epileptic rats by reducing oxidative stress and activating PKA/CREB/BDNF signaling. Epilepsy Behav. 2016;57(Pt A):177–84. [DOI] [PubMed] [Google Scholar]
- 93. Tambe R, Patil A, Jain P, Sancheti J, Somani G, Sathaye S. Assessment of luteolin isolated from Eclipta alba leaves in animal models of epilepsy. Pharm Biol. 2017;55(1):264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Khodamoradi M, Asadi-Shekaari M, Esmaeili-Mahani S, Esmaeilpour K, Sheibani V. Effects of genistein on cognitive dysfunction and hippocampal synaptic plasticity impairment in an ovariectomized rat kainic acid model of seizure. Eur J Pharmacol. 2016;786:1–9. [DOI] [PubMed] [Google Scholar]
- 95. Amiri Gheshlaghi S, Mohammad Jafari R, Algazo M, Rahimi N, Alshaib H, Dehpour AR. Genistein modulation of seizure: involvement of estrogen and serotonin receptors. J Nat Med. 2017;71(3):537–44. [DOI] [PubMed] [Google Scholar]
- 96. Elsayed AA, Menze ET, Tadros MG, Ibrahim BMM, Sabri NA, Khalifa AE. Effects of genistein on pentylenetetrazole-induced behavioral and neurochemical deficits in ovariectomized rats. Naunyn Schmiedebergs Arch Pharmacol. 2018;391(1):27–36. [DOI] [PubMed] [Google Scholar]
- 97. Liao ZJ, Liang RS, Shi SS, Wang CH, Yang WZ. Effect of baicalin on hippocampal damage in kainic acid-induced epileptic mice. Exp Ther Med. 2016;12(3):1405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Liu YF, Gao F, Li XW, Jia RH, Meng XD, Zhao R, Jing YY, Wang Y, Jiang W. The anticonvulsant and neuroprotective effects of baicalin on pilocarpine-induced epileptic model in rats. Neurochem Res. 2012;37(8):1670–80. [DOI] [PubMed] [Google Scholar]
- 99. Wu L, Li YS, Yang F, Wu B, Yu MH, Tu MQ, Xu HB. Silibinin inhibits inflammation and apoptosis in a rat model of temporal lobe epilepsy. Int J Clin Exp Med. 2018;11(3):1891–9. [Google Scholar]
- 100. Golechha M, Chaudhry U, Bhatia J, Saluja D, Arya DS. Naringin protects against kainic acid-induced status epilepticus in rats: evidence for an antioxidant, anti-inflammatory and neuroprotective intervention. Biol Pharm Bull. 2011;34(3):360–5. [DOI] [PubMed] [Google Scholar]
- 101. Golechha M, Sarangal V, Bhatia J, Chaudhry U, Saluja D, Arya DS. Naringin ameliorates pentylenetetrazol-induced seizures and associated oxidative stress, inflammation, and cognitive impairment in rats: possible mechanisms of neuroprotection. Epilepsy Behav. 2014;41:98–102. [DOI] [PubMed] [Google Scholar]
- 102. Khodayar MJ, Salehi S, Rezaei M, Siahpoosh A, Khazaei A, Houshmand G. Evaluation of the effect of naringenin on pentylenetetrazole and maximal electroshock-induced convulsions in mice. Jundishapur J Natural Pharmaceutical Products. 2016;12(1):e31384. [Google Scholar]
- 103. Shakeel S, Rehman MU, Tabassum N, Amin U, Mir MUR. Effect of naringenin (a naturally occurring flavanone) against pilocarpine-induced status epilepticus and oxidative stress in mice. Pharmacogn Mag. 2017;13(Suppl 1):S154–S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jäger AK, Almqvist JP, Vangsøe SAK, Stafford GI, Adsersen A, Van Staden J. Compounds from Mentha aquatica with affinity to the GABA-benzodiazepine receptor. South Afr J Bot. 2007;73(4):518–21. [Google Scholar]
- 105. Salah SM, Jager AK.. Screening of traditionally used Lebanese herbs for neurological activities. J Ethnopharmacol. 2005;97(1):145–9. [DOI] [PubMed] [Google Scholar]
- 106. Kandhare AD, Mukherjee AA, Bodhankar SL. Anti-epileptic effect of morin against experimental pentylenetetrazol-induced seizures via modulating brain monoamines and oxidative stress. Asian Pacific J Trop Biomed. 2018;8(7):352–9. [Google Scholar]
- 107. Nassiri-Asl M, Mortazavi SR, Samiee-Rad F, Zangivand AA, Safdari F, Saroukhani S, Abbasi E. The effects of rutin on the development of pentylenetetrazole kindling and memory retrieval in rats. Epilepsy Behav. 2010;18(1–2):50–3. [DOI] [PubMed] [Google Scholar]
- 108. Nassiri-Asl M, Naserpour Farivar T, Abbasi E, Sadeghnia HR, Sheikhi M, Lotfizadeh M, Bazahang P. Effects of rutin on oxidative stress in mice with kainic acid-induced seizure. J Integr Med. 2013;11(5):337–42. [DOI] [PubMed] [Google Scholar]
- 109. Nassiri-Asl M, Moghbelinejad S, Abbasi E, Yonesi F, Haghighi MR, Lotfizadeh M, Bazahang P. Effects of quercetin on oxidative stress and memory retrieval in kindled rats. Epilepsy Behav. 2013;28(2):151–5. [DOI] [PubMed] [Google Scholar]
- 110. Sefil F, Kahraman I, Dokuyucu R, Gokce H, Ozturk A, Tutuk O, Aydin M, Ozkan U, Pinar N. Ameliorating effect of quercetin on acute pentylenetetrazole induced seizures in rats. Int J Clin Exp Med. 2014;7(9):2471–7. [PMC free article] [PubMed] [Google Scholar]
- 111. Moghbelinejad S, Alizadeh S, Mohammadi G, Khodabandehloo F, Rashvand Z, Najafipour R, Nassiri-Asl M. The effects of quercetin on the gene expression of the GABAA receptor α5 subunit gene in a mouse model of kainic acid-induced seizure. J Physiol Sci. 2017;67(2):339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bouilleret V, Loup F, Kiener T, Marescaux C, Fritschy J-M. Early loss of interneurons and delayed subunit-specific changes in GABAA-receptor expression in a mouse model of mesial temporal lobe epilepsy. Hippocampus. 2000;10(3):305–24. [DOI] [PubMed] [Google Scholar]
- 113. Schwarzer C, Tsunashima K, Wanzenbock C, Fuchs K, Sieghart W, Sperk G. GABA(A) receptor subunits in the rat hippocampus II: altered distribution in kainic acid-induced temporal lobe epilepsy. Neuroscience. 1997;80(4):1001–17. [DOI] [PubMed] [Google Scholar]
- 114. Pavlov I, Walker MC. Tonic GABA(A) receptor-mediated signalling in temporal lobe epilepsy. Neuropharmacology. 2013;69:55–61. [DOI] [PubMed] [Google Scholar]
- 115. Kumar A, Lalitha S, Mishra J. Hesperidin potentiates the neuroprotective effects of diazepam and gabapentin against pentylenetetrazole-induced convulsions in mice: possible behavioral, biochemical and mitochondrial alterations. Indian J Pharmacol. 2014;46(3):309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chang CY, Lin TY, Lu CW, Huang SK, Wang YC, Chou SS, Wang SJ. Hesperidin inhibits glutamate release and exerts neuroprotection against excitotoxicity induced by kainic acid in the hippocampus of rats. Neurotoxicology. 2015;50:157–69. [DOI] [PubMed] [Google Scholar]
- 117. Abbasi E, Nassiri-Asl M, Shafeei M, Sheikhi M. Neuroprotective effects of vitexin, a flavonoid, on pentylenetetrazole-induced seizure in rats. Chem Biol Drug Des. 2012;80(2):274–8. [DOI] [PubMed] [Google Scholar]
- 118. Hu K, Zhang C, Long L, Long X, Feng L, Li Y, Xiao B. Expression profile of microRNAs in rat hippocampus following lithium-pilocarpine-induced status epilepticus. Neurosci Lett. 2011;488(3):252–7. [DOI] [PubMed] [Google Scholar]
- 119. Kan AA, van Erp S, Derijck AA, de Wit M, Hessel EV, O'Duibhir E, de Jager W, Van Rijen PC, Gosselaar PH, de Graan PN et al.. Genome-wide microRNA profiling of human temporal lobe epilepsy identifies modulators of the immune response. Cell Mol Life Sci. 2012;69(18):3127–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Jimenez-Mateos EM, Bray I, Sanz-Rodriguez A, Engel T, McKiernan RC, Mouri G, Tanaka K, Sano T, Saugstad JA, Simon RP et al.. miRNA expression profile after status epilepticus and hippocampal neuroprotection by targeting miR-132. Am J Pathol. 2011;179(5):2519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Song YJ, Tian XB, Zhang S, Zhang YX, Li X, Li D, Cheng Y, Zhang JN, Kang CS, Zhao W. Temporal lobe epilepsy induces differential expression of hippocampal miRNAs including let-7e and miR-23a/b. Brain Res. 2011;1387:134–40. [DOI] [PubMed] [Google Scholar]
- 122. Hu K, Xie YY, Zhang C, Ouyang DS, Long HY, Sun DN, Long LL, Feng L, Li Y, Xiao B. MicroRNA expression profile of the hippocampus in a rat model of temporal lobe epilepsy and miR-34a-targeted neuroprotection against hippocampal neurone cell apoptosis post-status epilepticus. BMC Neurosci. 2012;13:115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123. Peng J, Omran A, Ashhab MU, Kong H, Gan N, He F, Yin F. Expression patterns of miR-124, miR-134, miR-132, and miR-21 in an immature rat model and children with mesial temporal lobe epilepsy. J Mol Neurosci. 2013;50(2):291–7. [DOI] [PubMed] [Google Scholar]
- 124. Alsharafi WA, Xiao B, Li J. MicroRNA-139-5p negatively regulates NR2A-containing NMDA receptor in the rat pilocarpine model and patients with temporal lobe epilepsy. Epilepsia. 2016;57(11):1931–40. [DOI] [PubMed] [Google Scholar]
- 125. Wang D, Li Z, Zhang Y, Wang G, Wei M, Hu Y, Ma S, Jiang Y, Che N, Wang X et al.. Targeting of microRNA-199a-5p protects against pilocarpine-induced status epilepticus and seizure damage via SIRT1-p53 cascade. Epilepsia. 2016;57(5):706–16. [DOI] [PubMed] [Google Scholar]
- 126. Liu X, Liao Y, Wang X, Zou D, Luo C, Jian C, Wu Y. MicroRNA expression profiles in chronic epilepsy rats and neuroprotection from seizures by targeting miR-344a. Neuropsychiatr Dis Treat. 2017;13:2037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Reschke CR, Silva LF, Norwood BA, Senthilkumar K, Morris G, Sanz-Rodriguez A, Conroy RM, Costard L, Neubert V, Bauer S et al.. Potent anti-seizure effects of locked nucleic acid antagomirs targeting miR-134 in multiple mouse and rat models of epilepsy. Mol Ther Nucleic Acids. 2017;6:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Li TR, Jia YJ, Wang Q, Shao XQ, Zhang P, Lv RJ. Correlation between tumor necrosis factor alpha mRNA and microRNA-155 expression in rat models and patients with temporal lobe epilepsy. Brain Res. 2018;1700:56–65. [DOI] [PubMed] [Google Scholar]
- 129. Tang C, Gu Y, Wang H, Wu H, Wang Y, Meng Y, Han Z, Gu Y, Ma W, Jiang Z et al.. Targeting of microRNA-21-5p protects against seizure damage in a kainic acid-induced status epilepticus model via PTEN-mTOR. Epilepsy Res. 2018;144:34–42. [DOI] [PubMed] [Google Scholar]
- 130. Wang W, Guo Y, He L, Chen C, Luo J, Ma Y, Li J, Yang Y, Yang Q, Du C et al.. Overexpression of miRNA-137 in the brain suppresses seizure activity and neuronal excitability: a new potential therapeutic strategy for epilepsy. Neuropharmacology. 2018;138:170–81. [DOI] [PubMed] [Google Scholar]
- 131. Ashhab MU, Omran A, Kong H, Gan N, He F, Peng J, Yin F. Expressions of tumor necrosis factor α and microRNA-155 in immature rat model of status epilepticus and children with mesial temporal lobe epilepsy. J Mol Neurosci. 2013;51(3):950–8. [DOI] [PubMed] [Google Scholar]
- 132. Duan W, Chen Y, Wang XR. MicroRNA155 contributes to the occurrence of epilepsy through the PI3K/Akt/mTOR signaling pathway. Int J Mol Med. 2018;42(3):1577–84. [DOI] [PubMed] [Google Scholar]
- 133. Huang LG, Zou J, Lu QC. Silencing rno-miR-155-5p in rat temporal lobe epilepsy model reduces pathophysiological features and cell apoptosis by activating Sestrin-3. Brain Res. 2018;1689:109–22. [DOI] [PubMed] [Google Scholar]
- 134. Zadeh MM, Motamed N, Ranji N, Majidi M, Falahi F. Silibinin-induced apoptosis and downregulation of microRNA-21 and microRNA-155 in MCF-7 human breast cancer cells. J Breast Cancer. 2016;19(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Arango D, Diosa-Toro M, Rojas-Hernandez LS, Cooperstone JL, Schwartz SJ, Mo X, Jiang J, Schmittgen TD, Doseff AI. Dietary apigenin reduces LPS-induced expression of miR-155 restoring immune balance during inflammation. Mol Nutr Food Res. 2015;59(4):763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Boesch-Saadatmandi C, Loboda A, Wagner AE, Stachurska A, Jozkowicz A, Dulak J, Doring F, Wolffram S, Rimbach G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: role of miR-155. J Nutr Biochem. 2011;22(3):293–9. [DOI] [PubMed] [Google Scholar]


