ABSTRACT
Emerging evidence suggests that gastrointestinal (GI) microbiota dysbiosis is associated with chronic kidney disease (CKD) and metabolite concentrations. The purpose of this systematic review was to evaluate and contextualize the research characterizing GI microbiota in patients with CKD. We searched for full-text, peer-reviewed, English studies in PubMed, Cumulative Index to Nursing and Allied Health Literature, Web of Science, and Google Scholar using a combination of MeSH terms and keywords. Eleven of the 20 studies examined GI microbiota in patients with CKD, and 9 studies focused on the effect of interventions on GI microbiota or metabolites. Available data characterizing GI microbiota in patients with CKD suggest a decline in saccharolytic bacteria and an increase in fermenters of nitrogen-containing compounds, serving as a source for circulating uremic toxins. However, studies examined limited sets of predetermined microbes, which do not reflect the entire GI microbial community and its influence on host physiology. We recommend further studies examining the entire microbial community and the potential role in regulating host physiology in CKD.
Keywords: chronic kidney disease, gastrointestinal microbiota, microbiome, metabolite, nephropathy, systematic review
Introduction
Chronic kidney disease (CKD) is characterized by a progressive decline in kidney function over time, affecting an estimated 15% of the US population (1). Patients with CKD experience symptoms that significantly impact quality of life (QOL), including fatigue, pain, pruritus, anorexia, and irritability (2, 3). CKD is staged progressively, with stage 1 being the mildest level of impaired function, progressing to stage 5, end-stage renal disease (ESRD) or kidney failure, which requires some form of renal replacement therapy for survival, including hemodialysis (HD), peritoneal dialysis (PD), or kidney transplant. Regardless of the type of dialysis treatment, the more advanced disease puts patients at greater risk for adverse symptoms, extensive complications, and elevated comorbidity risk (3–5).
The relation between CKD and the gastrointestinal (GI) microbiota is an emerging area of investigation. The GI microbiota refers to the population of micro-organisms residing in the GI tract and is composed of trillions of bacteria, viruses, archea, protozoa, and fungi, representing an estimated 1000 species (6). The importance of the microbiota in supporting immune function, GI and systemic health, and regulating physiologic homeostasis that may influence symptoms, complications, and disease risk is well established (7–9). Indeed, a myriad list of diseases are associated with a dysbiosis, or deviation from normal microbial populations (10). Despite the rapid increase in knowledge regarding the composition and functionality of the GI microbiota, much remains to be elucidated, particularly the relation between the microbiota and CKD. This is especially true for the associations among changes in the microbial populations, shifts in the predominate circulating metabolites, the GI originating metabolites, CKD progression, and its relation with comorbidity risk and QOL.
Although the precise mechanism of its contribution is unclear, studies reveal profound GI microbiota alterations in ESRD patients (11, 12). Intestinal dysbiosis is proposed to be associated with decreased consumption of dietary fiber (13–15), frequent use of antibiotics (16), slow colonic transit (17), alterations of intestinal barrier function (18), uremic toxin production (19), and metabolic acidosis (20)—conditions that are often seen in patients with CKD. Among these, elevated circulating uremic toxins and low-fiber diets are two factors commonly considered to underlie microbial dysbiosis in patients with CKD (21).
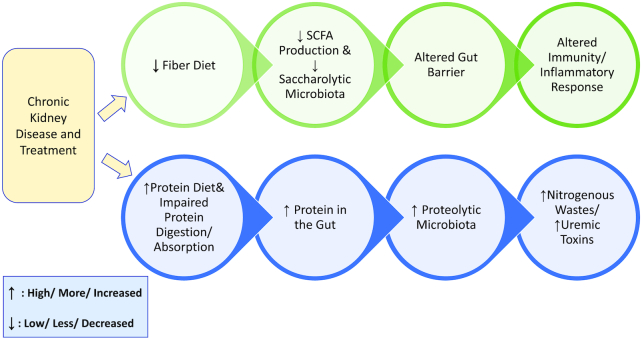
As kidney function declines, nitrogenous metabolic waste products increase in circulation. Uremic toxins, namely p-cresyl sulfate and indoxyl sulfate, are synthesized from amino acids tyrosine, phenylalanine, and tryptophan (22) by the microbiota and are associated with cardiovascular disease, which accounts for ∼41% of deaths among dialysis patients (1). Under normal conditions, these toxins are filtered by the kidneys and excreted in urine, but the diseased kidney is unable to perform this task, resulting in elevated circulating concentrations of toxins (12). This is further exacerbated by the increased nitrogenous substrate availability secondary to impaired protein digestion and absorption in CKD, with subsequently greater concentrations reaching the large intestine (23, 24), as well as increased circulating urea and creatinine diffusing into the GI lumen. In fact, research suggests that even in healthy individuals, the microbiota populations are influenced by the delivery of nitrogen to the large intestine, with increased amounts of nitrogen associated with unhealthy microbiota populations (25). Recent work suggests that small intestine absorption is impaired in individuals with CKD, resulting in greater than average amounts of protein reaching the large intestine (26). This is hypothesized to be mediated, at least in part, by profound inflammation associated with ESRD (27). Moreover, individuals with CKD, especially ESRD, are advised to follow a restrictive diet designed to minimize circulating electrolytes and fluid retention. In the early stages of CKD, protein intake is decreased in order to preserve remaining kidney function and limit the amount of nitrogenous waste in the blood. Upon entrance into CKD stage 5, complete failure of the kidney, and initiation of dialysis, patients are advised to increase protein consumption beyond recommendations for healthy individuals. This recommendation is based on the need to preserve lean body mass, which decreases upon dialysis initiation (28, 29). Similar to impaired digestion and absorption, lean body mass loss is associated with chronic inflammation and decreased muscle protein synthesis (30, 31). The dialysis procedure removes urea from the blood, making the high-protein diet's corresponding increase in nitrogenous waste production less important. Overall, this dietary pattern during ESRD results in low consumption of fruits and vegetables, beans and legumes, and whole grains and high consumption of protein from animal sources—essentially a low-fiber, atherogenic profile. The altered metabolism in conjunction with the low-fiber diet may predispose CKD patients for dysbiosis (Figure 1).
FIGURE 1.
Proposed mechanism for dysbiosis in chronic kidney disease.
Prebiotic, probiotic, and/or synbiotic supplementation is a possibility for maintaining microbial balance. Prebiotics are digestion-resistant food ingredients fermented by the host microbiota resulting in selective growth and/or activity of beneficial bacteria (32). Probiotics are live micro-organisms that, when consumed in adequate amounts, confer a health benefit to the host (33). Synbiotics are a combination of both pre- and probiotics. The provision of prebiotics and/or probiotics is hypothesized to restore microbial populations with a preference toward carbohydrate fermentation rather than proteolytic fermentation, thereby decreasing the GI synthesis of uremic toxins (34). Furthermore, normalization of the microbiota would facilitate optimal immune function and metabolic regulation.
The GI microbiota and the dysbiosis associated with CKD are a growing area of investigation with the potential to greatly impact patients suffering from this disease. To better understand how the microbiome affects symptoms, metabolite concentrations, risk of comorbidities, and strategies to optimize interventions, the characteristic microbial profile of patients with CKD must be understood. Therefore, the objective of this systematic review was to evaluate and contextualize the available data categorizing the GI microbiota in those with CKD.
Methods
Literature search and screening
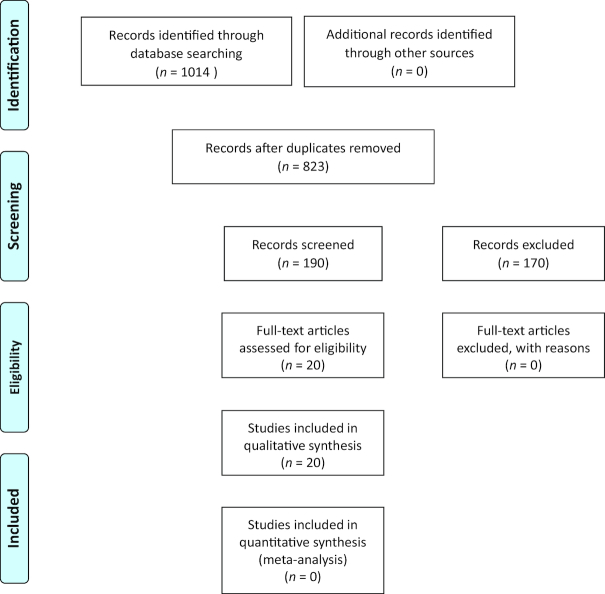
This systematic review was conducted in May–July 2017, guided by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (35) using Covidence, a platform selected by Cochrane to standardize reviews, manage literature, and track screening results. We identified studies by searching electronic databases with a librarian consultant. We searched 4 databases: PubMed, Cumulative Index to Nursing and Allied Health Literature, Web of Science, and Google Scholar. The search terms used to capture studies on kidney disease were MeSH terms “kidney disease,” “kidney failure, chronic,” and “dialysis patients” and keywords “renal” and “kidney” in the abstract. Search terms used to capture studies on GI microbiota were “gut microbiota,” “probiotics,” “prebiotics,” and “synbiotics.” The searches located studies examining GI microbiota in patients with kidney disease, attempting to answer the following question: What are the composition, relative abundance, and diversity of GI microbiota in patients with kidney disease? Inclusion criteria included full-text papers available in English with any study design or publication date and examining any stage of CKD and GI microbiota. Intervention studies including pre-, pro-, syn-, and antibiotics and/or nutrients that can alter the GI microbiota or metabolites were also included. Exclusion criteria included studies investigating kidney transplant patients; studies done in animal models or in vitro; studies examining microbes other than GI, including oral; and those examining metabolites only, unless there was an intervention altering the GI microbiota or metabolites. Nonretrievable articles or abstract-only papers were also excluded. The literature search identified 1014 records, of which 823 remained after removing duplicates (Figure 2). Two reviewers (SYC and JLB) independently reviewed all 823 titles and abstracts. Full-text articles selected by either reviewer were retrieved and reviewed independently, with agreement between reviewers achieved at each step. Twenty studies were identified for qualitative review.
FIGURE 2.
PRISMA flow diagram: review of records for gut microbiota in patients with kidney disease. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The quality of each study was rated by two reviewers (SYC and JLB) using the appropriate study design tool from the National Heart, Lung, and Blood Institute (36). These tools assess risk bias by evaluating components related but not limited to study population, exposures, outcome measures, blinding, and statistical analysis, yielding an overall rating of study quality as poor, fair, or good. Chung et al. (37) provide a detailed review protocol.
Results
Search results and study characteristics
The majority of the 20 studies included in the final analysis (Table 1) were nonexperimental (n = 9; 1 longitudinal, 8 cross-sectional), followed by quasi-experimental (n = 4), randomized controlled trials (n = 4), secondary data analysis (n = 2), and case report (n = 1). The quality of studies was fair (n = 9), good (n = 5), or poor (n = 6). The studies involved a total of 5137 patients with kidney disease from 13 countries. Most studies included adults with CKD, and only 2 studies examined pediatric patients (38, 39). In general, the studies can be differentiated into those examining the GI microbiota (n = 11) and those introducing interventions to alter the GI microbiota or metabolites (n = 9). Measurement of the GI microbiota was done mostly using stool specimens (n = 16). Many studies (n = 14) used qPCR or 16S rRNA gene sequencing to analyze biospecimens.
TABLE 1.
Studies examining gastrointestinal microbiota in patients with chronic kidney disease (n = 20)1
| Reference | Year | Study design | Subject sample (n) | Age, y2 | Gender (n/%) | Disease (stages of CKD) | Treatment | Specimens | Quality of study |
|---|---|---|---|---|---|---|---|---|---|
| GI microbiota | |||||||||
| Strid et al. (40) | 2003 | NE, cross-sectional (3-group comparison) | Sweden (total n = 56) HC (n = 34) Patients with CRF (n = 22) CRF GI symptoms (n = 12) CRF no GI symptoms (n = 10) | — CRF with GI symptoms: 62 CRF without GI symptoms: 57 | — CRF with GI symptoms: 10 men, 2 women CRF without GI symptoms: 5 men, 5 women | CRF | PD, HD, and predialysis | Obtained from small intestine through manometry catheter | Poor |
| Barros et al. (45) | 2015 | NE, cross-sectional (2-group comparison) | Brazil (total n = 39) CKD (n = 20) HC (n = 19) | CKD: 64.4 ± 9.1 HC: 51.6 ± 6.6 | CKD: 6 men, 14 women HC: 11 men, 8 women | CKD 3 and 4 (55% diabetic nephrosclerosis, 40% hypertensive nephrosclerosis, 5% polycystic kidney disease) | Conservative treatment, nondialysis | Stool, blood | Fair |
| De Angelis et al. (46) | 2014 | NE, longitudinal observation (3-group comparison) | Italy (total n = 48) IgAN-NP (n = 16) IgAN-P (n = 16) HC (n = 16) | NP: 41 ± 10 P: 45 ± 6 Control: 43 ± 8 | NP: 69% men P: 63% men Control: 60% men | No CKD stage specified; IgAN may cause CKD | None | Stool, blood, urine | Fair |
| Barrios et al. (44) | 2015 | Secondary data analysis (from TwinsUK cohort), cross-Sectional (1-group observation) | Large population-based cohort (TwinsUK, n = 4439) For microbiota n = 855 For metabolites n = 4439 | 58.39 ± 10.88 | For microbiota analysis: 98.2% women For metabolites analysis: 93.7% women | Healthy to early CKD mean eGFR = 83–85 mL/min/1.73 m2 Microbiota subsample: 7.2% had eGFR ≤60 Metabolite subsample: 7.4% had eGFR ≤60 | N/A | Stool, blood (secondary data) | Fair |
| I. K. Wang et al. (50) | 2012 | NE, cross-sectional (2-group comparison) | Taiwan (total n = 70) PD patients (n = 29) HC (n = 41) | PD Patients: 53.7 ± 11.7 Control: 58.2 ± 12.8 | PD: 15 men, 26 women Control: 10 men, 19 women | ESRD/5 | PD | Stool | Fair |
| F. Wang et al. (47) | 2012 | NE, cross-sectional (2-group comparison) | China (total n = 40) Nondialyzed ESRD patients (n = 30) HC (n = 10) | ESRD: 57 (range:37–71) Control: 55 (range, 41–67) | ESRD group: 16 men, 14 women Control: 5 men, 5 women | ESRD | Nondialyzed | Stool, blood | Fair |
| Vaziri et al. (11) | 2013 | NE, cross-sectional (2-group comparison) | United States (total n = 36) ESRD (n = 24) HC (n = 12) | ESRD group: 57 ± 14 Control: 51 ± 12 | ESRD: 6 men, 18 women Control: 4 men, 8 women | ESRD/5 | HD | Stool, blood | Fair |
| Crespo-Salgado et al. (39) | 2016 | NE, cross-sectional (4-group comparison) | United States (total n = 39) Pediatric PD (n = 8) HD (n = 8) Post-kidney transplant (n = 10) HC (n = 13) | HD: 13.6 (range, 8–178) PD: 11.9 (range, 3–17) Transplant: 13.2 (range, 2–18) Control: 9.5 (range, 3–16) | HD: 5 men, 3 women PD: 4 men, 4 women Transplant: 10 men, 0 women Control: 6 men, 7 women | ESRD/5, posttransplant | PD, HD, Post-kidney transplant, HC | Stool, blood | Good |
| Gut microbes possessing specific functions | |||||||||
| Wong et al. (48) | 2014 | Secondary data analysis [from Vaziri et al. (11)], cross-sectional comparison study | United States (total n = 36) Stable ESRD HD patients (n = 24) Control (n = 12) | HD: 57 ± 14 Control: 51 ± 12 | HD: 6 men, 18 women Control: 4 men, 8 women | ESRD/5 | HD | Stool | Fair |
| Jiang et al. (43) | 2016 | NE, cross-sectional (2-group comparison) | China (total n = 85) CKD (n = 65) HC (n = 20) | CKD: 43.45 ± 16.9 Control: 43.05 ± 9.88 | CKD: 30 men, 35 women Control: 6 men, 14 women | CKD 1–5 | No dialysis | Stool, blood | Fair |
| Gulhan et al. (49) | 2015 | NE, cross-sectional (1-group observation) | Turkey (total n = 50) HD (n = 50) | 62 ± 14.9 | 54% men, 46% women | ESRD/5 | HD | Stool, blood | Good |
| Probiotics and prebiotics | |||||||||
| Hida et al. (12) | 1996 | QE, 1-group pretest/posttest with control group | Japan (total n = 32) HD (n = 20) Control (n = 12) | HD: 56.4 ± 12.8 Control: 49.2 ± 6.8 | HD: 8 men, 12 women Control: 6 men, 6 women | ESRD/5 | HD | Stool, blood | Poor |
| Natarajan et al. (51) | 2014 | RCT (double-blind, placebo-controlled crossover study) | United States (total n = 22 completed out of 28) | 55 (range, 29–79) | 16 women, 6 men | ESRD/5 | HD | Blood (did not report microbiota data) | Good |
| Hyun et al. (38) | 2013 | QE (1-group pretest/posttest with multiple posttest) | South Korea Pediatric (total n = 32 completed out of 36) On dialysis (total n = 20; n = 16 completed study) HD (n = 5) PD (n = 11) Control (asymptomatic microscopic hematuria, n = 16) | HD: 8 (range, 3–16) PD: 15 (range, 7–21) Control: 12 (range, 7–16) | HD: 4 men, 1 woman | ESRD/5 | PD, HD | Blood (did not report microbiota data) | Good |
| Tayebi-Khosroshahi et al. (52) | 2016 | RCT | Iran (total n = 32) Intervention (n = 16) Control (n = 16) Placebo (n = 16) | 58.09 ± 12.75 | 14 men, 18 women | CKD 3 and 4 | Predialysis/none | Stool, blood | Poor |
| Synbiotics | |||||||||
| M. Rossi et al. (34) | 2016 | RCT | Australia (total n = 37) Treatment 1 (n = 17) Treatment 2 (n = 20) Complete fecal sample (n = 20) | 69 ± 10 | 21 men, 35 women | ESRD 4 and 5 | Predialysis/none | Stool, blood, urine | Good |
| Cruz-Mora et al. (54) | 2014 | RCT | Mexico (total n = 18) HD (n = 18) Intervention (n = 8) Control (n = 10) | Intervention: 34 ± 10 Control: 30.6 ± 9.5 | Intervention: 75% men, 25% women Control: 90% men, 10% women | ESRD/5 | HD | Stool | Fair |
| Borges et al. (55) | 2016 | Case report | Brazil (total n = 1) | 66 | man (1 case report) | ESRD/5 | HD | Unclear, presumably stool and blood | Poor |
| Antibiotics and nutrients | |||||||||
| Nazzal et al. (56) | 2017 | QE (1-group pretest/posttest with multiple posttest) | United States (total n = 10) | 48.7 (range, 27–67) | 9 men, 1 woman | ESRD/5 | HD | Stool, blood | Poor |
| Irie et al. (57) | 2017 | QE (1-group pretest/posttest) | Japan (total n = 15) | 72 ± 10 | 6 men, 9 women | ESRD/5 | HD | Stool, blood | Poor |
CKD, chronic kidney disease; CRF, chronic renal failure; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease (CKD stage 5); GI, gastrointestinal; HC, healthy controls; HD, hemodialysis; IgAN, immunoglobulin A nephropathy (P, progressing; NP, nonprogressing); N/A, not applicable; NE, nonexperimental; PD, peritoneal dialysis; QE, quasi-experimental; RCT, randomized controlled trial.
Values are means ± SDs (ranges).
GI microbiota in individuals with CKD
More than half of the studies (n = 11) focused on examining the GI microbiota in patients (Table 2). Strid et al. (40) found 36% of individuals with CKD had bacterial overgrowth in the small intestine. The most common bacterial strains seen in patients with small intestine bacterial overgrowth were Escherichia coli and Enterobacter (Gammaproteobacteria class), both more commonly classified as pathogenic, suggesting their contribution to the GI symptoms experienced in this group of patients (41, 42). Jiang and colleagues (43) investigated butyrate-producing bacteria Roseburia spp. and Faecalibacterium prausnitzii, both of which are negatively associated with C-reactive protein and positively associated with renal function. Researchers found significantly reduced amounts of butyrate-producing bacteria in patients with ESRD compared to healthy controls (HC) and even those with early stages of CKD, proposing an association with CKD progression and concomitant inflammation.
TABLE 2.
Common gastrointestinal microbiota in patients with chronic kidney disease compared to healthy controls1
| Stage of kidney disease and GI microbiota | Reference |
|---|---|
| CKD (predialysis and dialysis) | |
| CKD patients, predialytic or receiving HD and PD, compared to HC | Strid et al. (40) |
| 36% had SIBO (25% of patients with GI symptoms and 50% without) | |
| Most common bacterial strains seen in patients with SIBO: Escherichia coli and Enterobacter (Gammaproteobacteria class) | |
| Comparison group (without kidney disease) | |
| 18% had SIBO | |
| Patients with all stages of CKD, compared to HC | Jiang et al. (43) |
| Studied butyrate-producing microbiota | |
| ↓ Roseburia spp. in all stages of CKD compared to HC, with marked ↓ in ESRD | |
| Markedly ↓ in Faecalibacterium prausnitzii in ESRD compared to HC, even compared to early stage CKD (stages 1 and 2) | |
| Mild CKD (predialysis) | |
| Patients with minimal decline in renal function | Barrios et al. (44) |
| Identified three anaerobic gram-positive families in Clostridiales order: Christensenellaceae, Ruminococcaceae, and Lachnospiraceae | |
| Predialysis patients with stage 3 or 4 CKD, compared to HC | Barros et al. (45) |
| Identified Listeria monocytogenes in Firmicutes phylum and Flavobacteriaceae bacterium in Bacteroidetes phylum | |
| Comparison group: HC | |
| Identified uncultured Lachnospiraceae bacterium and Butyrivibrio crossotus in Firmicutes phylum | |
| Patients with IgAN, progressing and nonprogressing, compared to HC | De Angelis et al. (46) |
| ↑ number of metabolically active Firmicutes phylum | |
| ↓ number of Bacteroidetes phylum and Bifidobacterium spp. (Actinobacteria phylum) | |
| NP | |
| ↑ number of Firmicutes phylum | |
| ↑ number of species from Proteobacteria phylum: Sutterellaceae and Enterobacteriaceae | |
| Lowest Prevotellaceae family (Bacteroidetes phylum) | |
| Markedly ↓ Bifidobacteriaceae (Actinobacteria phylum) compared to HC | |
| P | |
| ↑ number of genera/species in Firmicutes phylum: Ruminococcaceae, Lachnospiraceae, Eubacteriaceae, and Streptococcaeae | |
| ↑ number of species from Proteobacteria phylum: Sutterellaceae and Enterobacteriaceae | |
| Lowest microbial diversity | |
| Comparison group: HC | |
| Highest metabolically active Actinobacteria | |
| Lowest Proteobacteria | |
| Advanced CKD (dialysis) | |
| Nondialyzed patients with ESRD, compared to HC | F. Wang et al. (47) |
| Five species were overgrown: Klebsiella spp., Proteus spp., Escherichia spp., Enterobacter spp., and Pseudomonas spp.; they were also detected in the blood of 20% of the patients | |
| Patients with ESRD, compared to HC | Vaziri et al. (11) |
| ↑ β diversity (between subjects) | |
| ↑ OTUs from Brachybacterium, Catenibacterium, Enterobacteriaceae, Halomonadaceae, Moraxellaceae, Nesterenkonia, Polyangiaceae, Pseudomonadaceae, and Thiothrix families | |
| Largest ↑ from the Actinobacteria, Firmicutes (especially subphylum Clostridia), and Proteobacteria (primarily Gammaproteobacteria) phyla | |
| Patients with ESRD compared to HC | Wong et al. (48) |
| Studied microbiota related to uremic toxins, short-chain fatty acids | |
| Dominant: 19 microbial families, 12 of which possessed urease-producing enzymes, 5 possessed uricase, and 4 possessed indole and p-cresol-producing enzymes | |
| Diminished: 4 microbial families, 2 of which possessed butyrate-producing enzymes | |
| Patients receiving HD | Gulhan et al. (49) |
| Studied microbiota related to calcium oxalate stones | |
| Identified Oxalobacter formigenes in 2 of 50 patients | |
| Patients on PD, compared to HC | I. K. Wang et al. (50) |
| Less frequently detected: Bifidobacterium catenulatum, B. longum, B. bifidum, Lactobacillus plantarum, L. paracasei, and Klebsiella pneumoniae | |
| ↓ mean colony count in samples for all bacteria | |
| Pediatric patients receiving PD, compared to HC | Crespo-Salgado et al. (39) |
| ↓ Firmicutes and Actinobacteria phyla, and beneficial Bifidobacteria family | |
| ↑ Enterobacteriaceae, taxa | |
| Pediatric patients receiving HD, compared to HC | |
| ↑ Bacteriodetes | |
| Pediatric patients receiving PD, compared to HD | |
| ↑ Proteobacteria | |
| Less diverse | |
CKD, chronic kidney disease; ESRD, end-stage renal disease (CKD stage 5); GI, gastrointestinal; HC, healthy controls; HD, hemodialysis; IgAN, immunoglobulin A nephropathy (P, progressing; NP, nonprogressing); OTUs, operational taxonomic units; PD, peritoneal dialysis; SIBO, small intestine bacterial overgrowth; ↑, increased; ↓, decreased.
GI microbiota in patients with mild CKD
Three studies were specific to patients with mild CKD. In adults with minimal decline in renal function (44), researchers identified Ruminococcus (Firmicutes phylum, Clostridia class) that was associated negatively with renal function and positively with an indoxyl sulfate metabolite as well as 2 genera in the Lachnospiraceae family that were associated positively with renal function and negatively with phenylacetylglutamine metabolite. Although specific functional roles are unclear, Barros and colleagues (45) detected Listeria monocytogenes (Firmicutes phylum) and Flavobacteriaceae bacterium (Bacteroidetes phylum) in patients with stage 3 or 4 CKD. De Angelis et al. (46) found increased amounts of metabolically active Firmicutes and decreased amounts of Bacteroidetes and Bifidobacterium species (Actinobacteria phylum) in patients with IgA nephropathy. This suggests a relation between early stages of declining kidney function and increasing numbers of uremic toxin-producing bacteria.
GI microbiota in patients with advanced CKD
Six studies were specific to patients with advanced CKD. A group of researchers (47) detected 5 species overgrown in the GI tracts of patients with ESRD. In addition, Klebsiella spp., Proteus spp., Escherichia spp., Enterobacter spp., and Pseudomonas spp. were also detected in the blood of 20% of the patients with ESRD, suggesting translocation of the GI microbiota into the bloodstream. Although the functional roles are unclear, Vaziri and colleagues (11) found a marked increase in the operational taxonomic units from 9 families of microbiota, with the largest increase from the Actinobacteria, Firmicutes (especially subphylum Clostridia), and Proteobacteria (primarily Gammaproteobacteria) phyla in patients with ESRD compared to HC. Wong et al. (48) examined the GI microbes possessing specific enzymes that contribute to producing uremic toxins, such as urease and uricase, and enzymes that produce indole and p-cresol, as well as SCFAs, including butyrate, which have beneficial effects in regulating inflammatory responses. In patients with ESRD, 19 microbial families were dominant, 12 of which possessed urease-producing enzymes, 5 possessed uricase, and 4 possessed indole- and p-cresol-producing enzymes, suggesting a contribution to uremic toxins. Two microbial families that possessed butyrate-producing enzymes were diminished, suggesting a decreased ability to synthesize SCFAs and a potentially impaired inflammatory response in patients with ESRD.
In adult patients receiving HD, Gulhan et al. (49) explored fecal Oxalobacter formigenes, which is crucial in mitigating oxalate deposition in the kidney, and detected it in only 4% of the patients, concluding its absence in this patient population. In adult patients on PD, Wang and colleagues (50) detected Bifidobacteria, B. catenulatum, B. longum, B. bifidum, Lactobacillus plantarum, L. paracasei, and Klebsiella pneumonia in a lower percentage of samples from this group, and in lower mean colony counts for all identified bacteria, compared to those from healthy controls. In a study conducted in pediatric patients with ESRD (39), researchers reported decreased relative abundances of Firmicutes phylum, Actinobacteria phylum, and beneficial Bifidobacteria as well as significantly increased Enterobacteriaceae, with the latter a taxa that produces indole and p-cresol, specifically in those receiving PD compared to HC. In those receiving HD, Bacteriodetes was increased compared to HC. Similar to mild CKD, research in advanced CKD and ESRD suggests that microbial populations continue to further deviate from HC in favor of pathogenic strains at the expense of SCFA-producing bacteria. Additional studies conducted in patients on HD or PD and in pediatric patients are needed to support these findings.
Studies introducing interventions to alter GI microbiota or metabolites
Nine studies focused on determining the effect of interventions related to altering the microbiota or their byproducts, such as uremic toxins or butyrate. Interventions included probiotics (n = 3), prebiotics (n = 1), synbiotics (n = 3), antibiotics (n = 1), and a nutrient (n = 1). These investigations are diverse in participant characteristics, length of intervention, supplement provided, and methodology for evaluating the microbial populations (Table 3).
TABLE 3.
Findings of intervention studies in patients with chronic kidney disease1
| Sample | Intervention | Post intervention results | Other findings | Reference |
|---|---|---|---|---|
| Probiotics (n = 3) | ||||
| Patients on HD (n = 20), compared to HC (n = 12) | One capsule, twice a day, for 4 wk Lebenin, containing lactic acid bacteria Bifidobacteria infantis, Lactobacillus acidophilus, and Enterococcus faecalis | Measured prior to and 2 and 4 wk post intervention; gut microbiota in patients on HD at baseline, compared to HC No significant difference in total number of bacteria Significantly ↑ aerobic bacteria, including Escherichia coli, Klebsiella, and Enterococci, in HD patients compared to controls Significantly ↑ number of anaerobic Clostridia perfringens, significantly decreased number of Bifidobacteria in HD patients compared to controls Gut microbiota in patients on HD, post intervention Significant ↓ in number of aerobic Enterobacteria, 2 wk post treatment Significant ↓ in frequency of detection of Klebsiella and Clostridia perfringens, 2 wk post treatment | Plasma and fecal uremic metabolites, at baseline Significantly ↑ concentrations of plasma phenol, p-cresol, and indican in HD patients (prior to HD) compared to controls at baseline, and remained elevated post-HD Significantly ↑ concentrations of fecal p-cresol in HD patients compared to controls Plasma and fecal uremic metabolites, post treatment Significant ↓ in fecal p-cresol and indole at 2 wk Significant ↓ in plasma indicant at 2 wk, which was maintained at 4 wk | Hida et al. (12) |
| Patients with ESRD, on HD, 2 mo of randomized placebo-controlled probiotic treatment, 6-mo cross-over study (n = 22/28) | 2 capsules 3 times a day with meals, for 2 mo Renadyl that contains Streptococcus thermophiles KB19, Lactobacillus acidophilus KB27, and Bifidobacterium longum KB31 | Measured prior to and 2, 4, and 6 mo post intervention Did not report microbiota data | No significant changes in QOL, selected biochemical parameters, markers of inflammation or oxidative stress, or uremic toxins including indoxyl sulfate or p-cresyl sulfate | Natarajan et al. (51) |
| Pediatric patients with ESRD, on PD (n = 16) or HD (n = 20) | Once a day, for 12 wk, dose by age and weight VSL#3 that contains Lactobacillus casei, L. plantarum, L. acidophilus, and L. delbrueckii subsp. bulgaricus; Bifidobacterium longum, B. breve, and B. infantis; as well as Streptococcus salivarius subsp. thermophiles | Measured prior to and 4, 8, and 12 wk post intervention Did not report microbiota data | Significantly higher baseline serum concentrations of p-cresyl sulfate in PD compared to HD No significant changes in serum concentrations of p-cresyl sulfate and indoxyl sulfate after probiotic treatment | Hyun et al. (38) |
| Prebiotics (n = 1) | ||||
| Patients with CKD stages 3 or 4, randomized placebo-controlled (n = 16) or prebiotic treatment (n = 16) | Three times a day, for 8 wk 30 mm lactulose syrup | Prebiotic group, compared to placebo Significantly ↑ quantities of Bifidobacteria and Lactobacilli | Significantly ↓ creatinine in intervention group; increased in placebo group | Tayebi-Khosroshahi et al. (52) |
| Synbiotics (n = 3) | ||||
| Patients with stages 4 and 5 CKD, not on dialysis, randomized placebo-controlled synbiotic treatment, 18-wk cross-over study (n = 31/37) | Dose escalation, 1–2 times a day, for 6 wk Prebiotic inulin, fruto-oligosaccharides, galacto-oligosaccharides Probiotic Lactobacillus, Bifidobacteria, and Streptococcus strains | Synbiotic group, compared to placebo Significantly ↑ Bifidobacterium spp. and Lachnospiraceae (Bifidobacteria not sustained upon cessation of supplement) | Significant ↓ in serum p-cresyl sulfate and a numerical reduction in indoxyl sulfate ↓ in the measured uremic solutes | M. Rossi et al. (34) |
| Patients on HD randomized to synbiotic (n = 10) or placebo treatment (n = 8) | For 2 mo, frequency unclear Prebiotic inulin Probiotic Lactobacillus acidophilus and Bifidobacterium bifidum 1.5 g of omega-3 fatty acids, B vitamins, vitamin C, and vitamin E | Synbiotic group, compared to placebo Significant ↑ in Bifidobacterium after synbiotic | ↓ gastrointestinal symptoms scores in the synbiotic group compared to the placebo group | Cruz-Mora et al. (54) |
| A patient on HD suffering from refractory diarrhea (n = 1) | Once a day, for 3 mo Prebiotic fructo-oligosaccharides Probiotic Lactobacillus paracasei, L. rhamnosus, L. acidophilus, Bifidobacterium lactis | Did not report microbiota data | Relief from refractory diarrhea within 15 d of synbiotic initiation ↑ hemoglobin, hematocrit, urea, albumin Weight gain as early as 3 mo and continued at 6 mo of supplementation | Borges et al. (55) |
| Antibiotics | ||||
| Patients with ESRD, on HD (n = 10) | Vancomycin 250-mg capsule, 1 time | Measured twice prior to (days –2 and 0) and on days 7 and 28 post intervention ↓ richness and evenness ↓ abundance in Enterobacteriaceae, Lachnospiraceae, Blautia, Clostridia, Enterococcaceae, and Bateroidales ↑ Veillonellaceae Remained significantly ↓ on day 28: Lachnospiraceae and Clostridiales | ↓ concentrations of indoxyl sulfate and p-cresyl sulfate on day 2 or day 5 that returned to baseline by day 28 | Nazzal et al. (56) |
| Nutrients | ||||
| Patients on HD (n = 15) | l-Carnitine tablets 900 mg, for 3 mo | Measured prior to and 3 mo post intervention | ↑ frequency of defecation from 4.2 to 4.8 times per week | Irie et al. (57) |
| Frequency unclear | ↓ Clostridium subcluster 4 genus, including Clostridium leptum | ↓ scores for myasthenia | ||
CKD, chronic kidney disease; ESRD, end-stage renal disease (CKD stage 5); HC, healthy controls; HD, hemodialysis; PD, peritoneal dialysis; QOL, quality of life; ↑, increased; ↓, decreased.
Probiotic and prebiotic supplementation
Of the 3 probiotic intervention studies, 1 included data on how probiotic supplementation influences the GI microbiota. Hida et al. (12) noted that 2 weeks of probiotic supplementation in the HD patients resulted in a significant decrease in fecal p-cresol and indole as well as plasma indican. In addition, decreases in Enterobacteria and an increase in Clostridia perfringens were noted. Among 2 studies that investigated a probiotic supplement but failed to report data on its impact on resident microbiota, 1 study (51) found no changes in uremic toxins, including indoxyl sulfate or p-cresyl sulfate, in adults on HD. The other (38) reported a nonsignificant decrease in blood uremic solutes, p-cresyl sulfate, and indoxyl sulfate in pediatric patients with ESRD following probiotic supplementation. In a single prebiotic intervention study, researchers (52) reported significantly increased Bifidobacteria and Lactobacilli quantities in patients with CKD stages 3 and 4 following lactulose supplement, which has been shown to increase Bifidobacteria (53). The limited number of studies and lack of continuity in reported outcomes make generalization difficult.
Synbiotic supplementation
Among 3 studies investigating how synbiotic supplementation influences the GI microbiota, Rossi and colleagues (34) reported significantly decreased serum p-cresyl sulfate and indoxyl sulfate following 6 wk of synbiotic treatment. Moreover, Bifidobacteria and Lachnospiraceae significantly increased following synbiotic therapy, and the increase in Bifidobacteria was correlated with a corresponding decrease in the measured uremic solutes noted previously. Similarly, Cruz-Mora and colleagues (54) found a significant increase in Bifidobacterium after 2 mo of synbiotic treatment in patients on HD. Finally, a case study reported considerable improvement in clinical markers, including urea, following supplementation (55). These reported increases in Bifidobacteria are promising for the efficacy of synbiotic administration in increased saccharolytic bacteria.
Antibiotic administration and nutrient supplementation
Nazzal and colleagues (56) examined the effects of oral consumption of vancomycin, an antibiotic commonly used clinically to treat gram-positive bacterial infections, on the GI microbiota and their uremic byproducts. Following antibiotic administration, Enterobacteriaceae, Lachnospiraceae, Blautia, Clostridia, Enterococcaceae, and Bateroidales decreased in abundance, whereas Veillonellaceae increased. Lachnospiraceae and Clostridiales, both involved in degrading tryptophan and tyrosine to yield uremic solutes such as indoxyl sulfate and p-cresyl sulfate, remained significantly decreased on day 28. Although the authors suggest the use of antibiotics to reduce the amount of uremic solutes in patients with ESRD, more studies are warranted to support this proposal. Irie and colleagues (57) studied the effects of supplementation of l-carnitine, a compound stored mainly in the muscles and that supports oxidation of fatty acids, on the microbiota; they hypothesized that carnitine deficiency is associated with GI discomfort and alterations in the GI microbiota. Although the composition of the GI microbiota was not altered significantly following supplementation, the relative abundance of Clostridium subcluster 4 genus was significantly deceased and was accompanied by an increased frequency of defecation and decreased scores for myasthenia. More studies employing larger experimental and control groups are needed to support this outcome.
Discussion
Overall, patients with any stage of CKD, from early decline to receiving HD or PD, exhibited evidence of a dysbiosis associated with declining kidney function. Specifically, a reduction in Roseburia spp. in patients compared with HC is reported (43). This bacteria produces the beneficial SCFA butyrate, a product of fiber fermentation that contributes to the integrity of the intestinal barrier and regulation of the inflammatory response (58). This may be attributed, at least in part, to the recommendation for patients with CKD to modify their diet in such a way that results in decreased fiber consumption. In addition, patients with ESRD had markedly decreased F. prausnitzii (43), a microbe correlated with anti-inflammatory properties (59). Bacteria commonly considered as pathogens, such as E. coli and Enterobacter (both of which belong to the Enterobacteriaceae family), were most commonly overgrown in the small intestine in CKD patients compared with HC (36% compared with 18%, respectively) (40). Together, this information suggests the influence of perturbed GI microbial composition on the compromised epithelial barrier and increased susceptibility to infection and the inflammatory state in this patient population.
GI microbial composition in patients with advanced CKD receiving renal replacement therapy further supported a possible role of dysbiosis. Compared with HC, the GI microbiota in patients with advanced CKD were markedly increased in microbial families associated with production of uremic toxins and markedly decreased in those associated with production of SCFAs (48). Moreover, the beneficial microbes from Bifidobacteria and Lactobacilliwere less frequently detected while the microbes from pathogenic Enterobacteriaceae were increased in patients receiving PD compared with HC (39, 50). Similarly, patients in early stages of CKD also had L. monocytogenes, a commonly known foodborne pathogen (60), and F. bacterium (44), which is less commonly found in humans (45). However, these patients also had somewhat beneficial microbes such as Christensenellaceae, which is associated with reduced weight gain (61), and Ruminococcaceae and Lachnospiraceae, both of which are enriched in the general population and are capable of degrading polysaccharides (62) or mucin provided by the mucus layer in the GI tract when there is a lack of dietary carbohydrates (63). This may suggest a “healthier” composition in the earlier stages of CKD.
Clinical implications
Healthy microbial populations possess robust saccharolytic or carbohydrate fermentative properties. The ability to effectively ferment dietary fiber results in the production of SCFAs. Among other properties, SCFAs, particularly butyrate, serve as an energy source for colonocytes, support intestinal integrity, stimulate water and sodium absorption, and are associated with a decreased risk of cancer (64). Furthermore, SCFAs are associated with modulation in inflammation (65), which is a significant contributor to symptoms and comorbidities, particularly as CKD progresses into later stages (66). In order to support a healthy microbiota profile and production of SCFAs, adequate dietary fiber must be consumed. This is problematic in CKD patients, especially due to the restrictive nature of the dietary recommendations (67, 68) that significantly limit dietary fiber intake, which is already lower than the recommended amount in most people (69). This dietary restriction could limit the fermentable substrate available for saccharolytic bacteria, thereby restricting their prominence in microbial populations.
The modification in fermentation end products may be further exacerbated by impaired protein digestion and amino acid absorption in patients with CKD, with greater nitrogenous substrate reaching the large intestine and a corresponding upregulation in proteolytic bacteria (21, 70, 71). Patients with ESRD are additionally counseled to increase their protein intake in an effort to preserve lean body mass and counteract the catabolism associated with dialysis (72), thereby providing greater protein-based substrate for fermentation, whereas those in earlier stages of CKD are advised to limit protein. Proliferation of proteolytic bacteria and the corresponding protein-fermentation products is associated with upregulation of disease-propagating and pro-inflammatory pathways, even in the metabolically healthy (73). These properties are achieved through products of proteolytic fermentation entering circulation. Patients with ESRD who have increased concentrations of circulating metabolites and waste products between dialysis treatments are at greater risk. Specifically, production of uremic metabolites, including but not limited to phenols and indoles, further exacerbates the uremic load, cardiovascular disease, or mortality in patients with CKD (74, 75).
Supplementation with prebiotics and/or probiotics is hypothesized to manipulate GI micro-organism populations to support a more beneficial fermentation profile and corresponding metabolite production. Indeed, several interventions reviewed in this article report some success in restoring desirable microbial species, including Bifidobacteria species (12, 34, 52, 54). These studies utilized a variety of prebiotic and/or probiotic cocktails, making a specific recommendation difficult, but they suggest supplementation must be sustained for continued benefit. Corresponding decreases in circulating uremic toxins were reported in some but not all interventions (12, 34); it remains unknown if long-term intervention would ultimately result in decreased incidence of comorbidities and improved QOL. Nevertheless, dietary supplementations are relatively inexpensive interventions, with early investigations warranting further study.
Implications for future research
There was a paucity of studies examining the GI microbiota in patients with kidney disease, limiting our understanding of the GI microbiota in this population. Of those available, most examined a limited set of predetermined microbes, and none of the studies considered the whole community and network-like correlations among the microbes. It is important to examine the entire community and to consider the correlations among them because a limited number of species do not mirror the natural existence and complexity of the microbial community, thereby restricting our understanding of the implications they have on human physiology. The GI microbiota have been studied in clusters called enterotypes, or clusters of communities that possibly represent functions of the GI microbiota (76–80). Furthermore, with the advent of high-throughput sequencing techniques, entire GI microbial communities as well as their functions and interaction with the host can now be studied in a more efficient and cost-effective manner using a multi-omics approach. Additional studies examining the entire microbial community and clusters they may form, in relation to their functional role, are needed (81, 82). A more complete understanding of the GI microbiota in CKD and the interaction with the host will allow improved interventions to optimize the microbial composition and the functional role.
To date, the interventions that have been studied have mostly focused on dietary supplements that may modulate the composition and abundance of the GI microbiota and/or their metabolites, such as SCFAs or uremic toxins. Apart from prebiotic, probiotic, and/or synbiotic supplements, lifestyle factors such as diet and physical activity are well studied and have been found to influence the microbial structure in other populations (83, 84). Future intervention studies examining the effects of modifiable lifestyle factors on the microbiota and their functional role in patients with CKD are warranted.
The number of studies examining the GI microbiota in patients was small for each of the 5 stages of CKD or the stages were not clearly reported, limiting the capacity to understand the role of the GI microbiota in disease progression. More studies on the GI microbiota for patients in each of the 5 CKD stages and longitudinal studies examining changes of the microbiota may be helpful to understand the influence of the GI microbiota on the progression of CKD and the development of side effects or adverse symptoms, such as fatigue, pruritus, cramps, and GI symptoms.
Conclusion
The evolution of the GI microbiota with CKD disease progression is a relatively novel area of study with many opportunities for investigation and subsequent interventions targeted to improve symptoms and QOL associated with this debilitating disease. Currently available data suggest that the microbiota shift from normal populations to an environment that is depleted in SCFA-producing bacteria with a concomitant increase in proteolytic bacteria. Furthermore, an overall decrease in diversity is reported. Taken together, this can impair localized GI function but also elicit negative ramifications systemically, not limited to increased inflammation in the face of impaired immune function. Dietary supplementation remains a promising avenue for manipulating the resident populations in a beneficial way. Further investigation is needed to characterize the GI microbiota in patients in various CKD stages, determine how they evolve as the disease progresses, and identify efficacious interventions with an emphasis on dietary approaches.
ACKNOWLEDGEMENTS
We thank Sue Franzen, an assistant professor and librarian at Illinois State University, for her support with identifying the databases and search terms ideal for this review; and Alyssa Welte, a graduate student at Illinois State University Department of Family and Consumer Sciences, for her support with creating tables to organize the articles resulting from this systematic review. We also appreciate assistance from In-Seo La, a graduate student at University of Maryland School of Nursing, with the final edits and formatting of the manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: SYC, JLB, and KSA, no conflicts of interest.
SYC and JLB contributed equally to this work.
Abbreviations used: CKD, chronic kidney disease; ESRD, end-stage renal disease; GI, gastrointestinal; HC, healthy control; HD, hemodialysis; PD, peritoneal dialysis; QOL, quality of life.
References
- 1. United States Renal Data System. 2015 USRDS annual data report: epidemiology of kidney disease in the United States [Internet]. Bethesda (MD): National Institutes of Health;2018. Available from: https://www.usrds.org/2015/view/Default.aspx. [Accessed 2019 Mar 31]. [Google Scholar]
- 2. Almutary H, Bonner A, Douglas C. Symptom burden in chronic kidney disease: a review of recent literature. J Ren Care. 2013;39(3):140–50. [DOI] [PubMed] [Google Scholar]
- 3. Kwok AO, Yuen S-k, Yong DS, Tse DM. The symptoms prevalence, medical interventions, and health care service needs for patients with end-stage renal disease in a renal palliative care program. Am J Hosp Palliat Med. 2016;33(10):952–8. [DOI] [PubMed] [Google Scholar]
- 4. Dai L, Golembiewska E, Lindholm B, Stenvinkel P. End-stage renal disease, inflammation and cardiovascular outcomes. Contrib Nephrol. 2017;191:32–43. [DOI] [PubMed] [Google Scholar]
- 5. Iwagami M, Yasunaga H, Matsui H, Horiguchi H, Fushimi K, Noiri E, Nangaku M, Doi K. Impact of end-stage renal disease on hospital outcomes among patients admitted to intensive care units: a retrospective matched-pair cohort study. Nephrology. 2017;22:617–23. [DOI] [PubMed] [Google Scholar]
- 6. Lagier J-C, Khelaifia S, Alou MT, Ndongo S, Dione N, Hugon P, Caputo A, Cadoret F, Traore SI, Seck EH et al.. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol. 2016;1:16203. [DOI] [PubMed] [Google Scholar]
- 7. Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–9. [DOI] [PubMed] [Google Scholar]
- 8. Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227–38. [DOI] [PubMed] [Google Scholar]
- 9. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Selber-Hnativ S, Rukundo B, Ahmadi M, Akoubi H, Al-Bizri H, Aliu A, Ambeaghen T, Avetisyan L, Bahar I, Baird A et al.. Human gut microbiota: toward an ecology of disease. Front Microbiol. 2017;8:1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83(2):308–15. [DOI] [PubMed] [Google Scholar]
- 12. Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron. 1996;74(2):349–55. [DOI] [PubMed] [Google Scholar]
- 13. Sirich TL. Dietary protein and fiber in end stage renal disease. Semin Dial. 2014;28(1):75–80. [DOI] [PubMed] [Google Scholar]
- 14. Kalantar-Zadeh K, Brown A, Chen JLT, Kamgar M, Lau W, Moradi H, Rhee CM, Streja E, Kovesdy CP. Dietary restrictions in dialysis patients: is there anything left to eat?. Semin Dial. 2015;28(2):159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002;12(1):17–31. [DOI] [PubMed] [Google Scholar]
- 16. Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108(Supp1):4554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu M, Chang C, Cheng C, Chen CH, Lee WC, Hsu YH, Shu KH, Tang MJ. Colonic transit time in long-term dialysis patients. Am J Kidney Dis. 2004;44(2):322–7. [DOI] [PubMed] [Google Scholar]
- 18. Sabatino A, Regolisti G, Brusasco I, Cabassi A, Morabito S, Fiaccadori E, Clinica M, Gramsci V. Alterations of intestinal barrier and microbiota in chronic kidney disease. 2015;30:924–33. [DOI] [PubMed] [Google Scholar]
- 19. Kikuchi M, Ueno M, Itoh Y, Suda W, Hattori M. Uremic toxin-producing gut microbiota in rats with chronic kidney disease. Nephron. 2017;135(1):51–60. [DOI] [PubMed] [Google Scholar]
- 20. Kraut JA, Madias NE. Narrative review: metabolic acidosis of CKD: an update. Am J Kidney Dis. 2016;67(2):307. [DOI] [PubMed] [Google Scholar]
- 21. Rossi M, Johnson DW, Xu H, Carrero JJ, Pascoe E, French C, Campbell KL. Dietary protein–fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutr Metab Cardiovasc Dis. 2015;25(9):860–5. [DOI] [PubMed] [Google Scholar]
- 22. Aronov PA, Luo FJ-G, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW. Colonic contribution to uremic solutes. J Am Soc Nephrol. 2011;22(9):1769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sorensen LB. Role of the intestinal tract in the elimination of uric acid. Arthritis Rheum. 1965;8(5):694–706. [DOI] [PubMed] [Google Scholar]
- 24. Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis. 2016;67(3):483–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holmes AJ, Chew YV, Colakoglu F, Raubenheimer D, Le CDG, Simpson SJ. Diet–microbiome interactions in health are controlled by intestinal nitrogen source constraints. Cell Metab. 2017;25(1):140–51. [DOI] [PubMed] [Google Scholar]
- 26. Grant CJ, Harrison LE, Hoad CL, Marciani L, Gowland PA, McIntyre CW. Patients with chronic kidney disease have abnormal upper gastro-intestinal tract digestive function: a study of uremic enteropathy. J Gastroenterol Hepatol. 2017;32:372–7. [DOI] [PubMed] [Google Scholar]
- 27. Jankowska M, Cobo G, Lindholm B, Stenvinkel P. Inflammation and protein-energy wasting in the uremic milieu. Contrib Nephrol. 2017;191:58–71. [DOI] [PubMed] [Google Scholar]
- 28. Pupim LB, Heimburger O, Qureshi AR, Ikizler TALP, Stenvinkel P. Accelerated lean body mass loss in incident chronic dialysis patients with diabetes mellitus. Kidney Int. 2005;68(5):2368–74. [DOI] [PubMed] [Google Scholar]
- 29. Johansen KL, Shubert T, Doyle J, Soher B, Sakkas GK, Kent-Braun JA. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003;63:291–7. [DOI] [PubMed] [Google Scholar]
- 30. Wang XH, Mitch WE. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol. 2014;10(9):504–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Vliet S, Skinner SK, Beals JW, Pagni BA, Fang H, Ulanov AV, Li Z, Paluska SA, Mazzulla M, West DWD et al.. Dysregulated handling of dietary protein. Kidney Int Rep. 2018;3(6):1403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17(2):259. [DOI] [PubMed] [Google Scholar]
- 33. Food and Agriculture Organization of the United Nations/World Health Organization, Probiotics in food (vol 85). Rome (Italy): Food and Agriculture Organization of the United Nations;2006. [Google Scholar]
- 34. Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, Szeto CC, McWhinney BC, Ungerer JPJ, Campbell KL. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol. 2016;11(2):223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. National Heart, Lung, and Blood Institute.Study quality assessment tools. [Internet]. 2017. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. [Accessed 2017 Aug 2]. [Google Scholar]
- 37. Chung S, Barnes J, Astroth K. Gastrointestinal microbiota in patients with chronic kidney disease: a systematic review. [Internet]. PROSPERO. 2017. Available from:; http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017080100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hyun HS, Paik KH, Cho HY. p-Cresyl sulfate and indoxyl sulfate in pediatric patients on chronic dialysis. Korean J Pediatr. 2013;56(4):159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crespo-Salgado J, Vehaskari VM, Stewart T, Ferris M, Zhang Q, Wang G, Blanchard EE, Taylor CM, Kallash M, Greenbaum LA et al.. Intestinal microbiota in pediatric patients with end stage renal disease: a Midwest pediatric nephrology consortium study. Microbiome. 2016;4(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strid H, Simren M, Stotzer P-O, Ringstrom G, Abrahamsson H, Bjornsson ES. Patients with chronic renal failure have abnormal small intestinal motility and a high prevalence of small intestinal bacterial overgrowth. Digestion. 2003;67(3):129–37. [DOI] [PubMed] [Google Scholar]
- 41. Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis. 2014;20(7):1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Forsythe SJ, Abbott SL, Pitout J. Klebsiella, Enterobacter, Citrobacter, Cronobacter, Serratia, Plesiomonas, and ther Enterobacteriaceae. In: Manual of clinical microbiology (11th ed. .) American Society of Microbiology; 2015:714–37. [Google Scholar]
- 43. Jiang S, Xie S, Lv D, Zhang Y, Deng J, Zeng L, Chen Y. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Int J Gen Mol Microbiol. 2016;109(10):1389–96. [DOI] [PubMed] [Google Scholar]
- 44. Barrios C, Beaumont M, Pallister T, Villar J, Goodrich JK, Clark A, Pascual J, Ley RE, Spector TD, Bell JT et al.. Gut–microbiota–metabolite axis in early renal function decline. PLoS One. 2015;10(8):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barros AF, Borges NA, Ferreira DC, Carmo FL, Rosado AS, Fouque D, Mafra D. Is there interaction between gut microbial profile and cardiovascular risk in chronic kidney disease patients?. Future Microbiol. 2015;10(4):517–26. [DOI] [PubMed] [Google Scholar]
- 46. De Angelis M, Montemurno E, Piccolo M, Vannini L, Lauriero G, Maranzano V, Gozzi G, Serrazanetti D, Dalfino G, Gobbetti M et al.. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS One. 2014;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang F, Jiang H, Shi K, Ren Y, Zhang P, Cheng S. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology. 2012;17(8):733–8. [DOI] [PubMed] [Google Scholar]
- 48. Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39(3):230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gulhan B, Turkmen K, Aydin M, Gunay M, Cıkman A, Kara M. The relationship between serum oxalic acid, central hemodynamic parameters and colonization by oxalobacter formigenes in hemodialysis patients. Cardiorenal Med. 2015;5(3):164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang IK, Lai HC, Yu CJ, Liang CC, Chang CT, Kuo HL, Yang YF, Lin CC, Lin HH, Liu YL et al.. Real-time PCR analysis of the intestinal microbiotas in peritoneal dialysis patients. Appl Environ Microbiol. 2012;78(4):1107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Natarajan R, Pechenyak B, Vyas U, Ranganathan P, Weinberg A, Liang P, Mallappallil MC, Norin AJ, Friedman EA, Saggi SJ. Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed Res Int. 2014;2014:568571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tayebi-Khosroshahi H, Habibzadeh A, Niknafs B, Ghotaslou R, Yeganeh Sefidan F, Ghojazadeh M, Moghaddaszadeh M, Parkhide S. The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial. J Ren Inj Prev. 2016;5(3):162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tuohy KM, Ziemer CJ, Klinder A, Knöbel Y, Beatrice L, Gibson GR. A human volunteer study to determine the prebiotic effects of lactulose powder on human colonic microbiota. Microb Ecol Health Dis. 2002;14(3):165–73. [Google Scholar]
- 54. Cruz-Mora J, Martínez-Hernández NE, Martín del Campo-López F, Viramontes-Hörner D, Vizmanos-Lamotte B, Muñoz-Valle JF, García-García G, Parra-Rojas I, Castro-Alarcón N. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J Ren Nutr. 2014;24(5):330–5. [DOI] [PubMed] [Google Scholar]
- 55. Borges NA, Farage NE, Barros AF, Ferreira DC, Fouque D, Mafra D. Synbiotic supplementation promotes improvement of chronic diarrhea of unknown etiology in patient with chronic kidney disease and provides better outcomes in dialysis. Nutr Hosp. 2016;33(1):182–4. [DOI] [PubMed] [Google Scholar]
- 56. Nazzal L, Roberts J, Singh P, Jhawar S, Matalon A, Gao Z, Holzman R, Liebes L, Blaser MJ, Lowenstein J. Microbiome perturbation by oral vancomycin reduces plasma concentration of two gut-derived uremic solutes, indoxyl sulfate and p-cresyl sulfate, in end-stage renal disease. Nephrol Dial Transplant. 2017; 32(11):1809–11. [DOI] [PubMed] [Google Scholar]
- 57. Irie J, Kanno Y, Kikuchi R, Yoshida T, Murai S, Watanabe M, Itoh H, Hayashi M. l-Carnitine improves gastrointestinal disorders and altered the intestinal microbiota in hemodialysis patients. Biosci Microbiota Food Health. 2017;36(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zheng L, Kelly CJ, Battista KD, Schaefer R, Lanis JM, Alexeev EE, Wang RX, Onyiah JC, Kominsky DJ, Colgan SP. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of claudin-2. J Immunol. 2017;199(8):2976–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rossi O, van Berkel LA, Chain F, Tanweer KM, Taverne N, Sokol H, Duncan SH, Flint HJ, Harmsen HJM, Langella P et al.. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci Rep. 2016;6(1):18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maury MM, Tsai Y, Charlier C, Touchon M. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat Genet. 2016;48(3):308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT et al.. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Biddle A, Stewart L, Blanchard J, Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5(3):627–40. [Google Scholar]
- 63. Tailford LE, Crost EH, Kavanaugh D, Juge N. Mucin glycan foraging in the human gut microbiome. Front Genet. 2015;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. De Haan JB, Gevers W, Parker MI. Effects of sodium butyrate on the synthesis and methylation of DNA in normal cells and their transformed counterparts. Cancer Res. 1986;46(2):713–6. [PubMed] [Google Scholar]
- 65. Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gungor O, Unal HU, Guclu A, Gezer M, Eyileten T, Guzel FB, Altunoren O, Erken E, Oguz Y, Kocyigit I et al.. IL-33 and ST2 levels in chronic kidney disease: associations with inflammation, vascular abnormalities, cardiovascular events, and survival. PLoS One. 2017;12(6):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(Suppl 2):S1–180. [DOI] [PubMed] [Google Scholar]
- 68. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850–86. [DOI] [PubMed] [Google Scholar]
- 69. US Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 dietary guidelines for Americans (8th ed.). Washington (DC): US Department of Health and Human Services; 2015;18. [Google Scholar]
- 70. Briskey D, Tucker P, Johnson DW, Coombes JS. The role of the gastrointestinal tract and microbiota on uremic toxins and chronic kidney disease development. Clin Exp Nephrol. 2017;21(1):7–15. [DOI] [PubMed] [Google Scholar]
- 71. Zha Y, Qian Q. Protein nutrition and malnutrition in CKD and ESRD. Nutrients. 2017;9(3):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Keane D, Gardiner C, Lindley E, Lines S, Woodrow G, Wright M. Changes in body composition in the two years after initiation of haemodialysis: a retrospective cohort study. Nutrients. 2016;8(11):702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yao CK, Muir JG, Gibson PR. Review article: insights into colonic protein fermentation, its modulation and potential health implications. Aliment Pharmacol Ther. 2016;43(2):181–96. [DOI] [PubMed] [Google Scholar]
- 74. Lin CJ, Wu V, Wu PC, Wu CJ. Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One. 2015;10(7):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Poesen R, Evenepoel P, de Loor H, Kuypers D, Augustijns P, Meijers B. Metabolism, protein binding, and renal clearance of microbiota-derived p-cresol in patients with CKD. Clin J Am Soc Nephrol. 2016;11(7):1136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Koren O, Knights D, Gonzalez A, Waldron L, Segata N, Knight R, Huttenhower C, Ley RE. A guide to enterotypes across the human body: meta-analysis of microbial community structures in human microbiome datasets. PLoS Comput Biol. 2013;9(1):e1002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Arumugam M, Raes J, Pelletier E, Paslier DL, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto J et al.. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R et al.. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen T, Long W, Zhang C, Liu S, Zhao L, Hamaker B. Fiber utilizing capacity varies with Prevotella versus Bacteroides enterotypes. FASEB J. 2016;30(1 Suppl):683.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Costea PI, Hildebrand F, Arumugam M, Bäckhed F, Blaser MJ, Bushman FD, De Vos WM, Erlich SD, Fraser CM, Hattori M et al.. Enterotypes in the landscape of gut microbial community composition. Nat Microbiol. 2018;3(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Goodrich JK, Di Rienzi SC, Poole AC, Koren O, Walters WA, Caporaso JG, Knight R, Ley RE. Conducting a microbiome study. Cell. 2014;158(2):250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Knight R, Navas J, Quinn RA, Sanders JG, Zhu Q. Best practices for analysing microbiomes. Nat Rev Microbiol. 2018;16:410–22. [DOI] [PubMed] [Google Scholar]
- 83. Mach N, Fuster-Botella D. Endurance exercise and gut microbiota : a review. J Sport Heal Sci. 2017;6(2):179–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Flint HJ, Duncan SH, Scott KP, Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc. 2015;74(1):13–22. [DOI] [PubMed] [Google Scholar]