Abstract
Background
The United Kingdom documented a decline of >30% in imported cases of malaria annually between 1996 and 2003; however, there are still approximately 1700 cases and 5–10 deaths each year. Prophylaxis health messages focus on families returning to their country of origin.
Methods
We reviewed 225 records of patients seen in Cambridge University Hospital Foundation Trust [CUHFT], a tertiary referral center in Cambridge, England. All records of patients seen in CUHFT between 2002–2016 were analyzed in the context of national figures from Public Health England.
Results
Between 2004–2016, there was no decrease in imported cases of malaria locally or nationally. Plasmodium falciparum remains responsible for most imported infections (66.7%); Plasmodium vivax contributed 15.1%, Plasmodium malariae 4%, and Plasmodium ovale 6.7%; 7.5% (17/225) of patients had an incomplete record. Most cases were reported in people coming from West Africa. Sierra Leone and the Ivory Coast had the highest proportions of travelers being infected at 8 and 7 per 1000, respectively. Visiting family in the country of origin (27.8%) was the commonest reason for travel. However, this was exceeded by the combined numbers traveling for business and holidays (22.5% and 20.1%, respectively). Sixty percent of patients took no prophylaxis. Of those who did, none of the patients finished their chemoprophylaxis regimen.
Conclusions
Significant numbers of travelers to malarious countries still take no chemoprophylaxis. Health advice about prophylaxis before travel should be targeted not only at those visiting family in their country of origin but also to those traveling for holiday and work.
Keywords: malaria, imported, risk factors
After a decline in the early 2000s, imported malaria has reached a plateau. Focus on people returning to countries of origin as the at-risk population oversimplifies intervention planning. Holiday and business reasons together exceed this as a risk factor.
(See the Editorial Commentary by Schlagenhauf and Patel on pages 1163–4.)
Malaria is a disease caused by the protozoan parasites of the genus Plasmodium transmitted by the Anopheles mosquito. The human types of malaria are Plasmodium falciparum, which is the most serious and can be fatal; Plasmodium vivax; Plasmodium malariae; Plasmodium ovale; and, rarely, Plasmodium knowlesi, which can each cause acute, severe disease but with a low mortality [1]. Worldwide in 2015 there were an estimated 214 million new cases of malaria with 429 000 deaths (World Health Organization [WHO] malaria factsheet, 2015 [2]). The WHO African region accounts for 90% of these cases, Southeast Asia 7%, and the Eastern Mediterranean region 2%.
Malaria in the United Kingdom (UK) is always imported. At 1500 cases per year, it is the most common imported tropical disease [2, 3]. Much of this morbidity has been attributed to people returning to the country of origin of their family; this group was previously shown to report the least use of chemoprophylaxis [4]. Regional experience of imported malaria in the UK has not been reported since 2015 [5]. We were curious to know whether the epidemiology had changed and whether health advice messages were still being appropriately targeted. We undertook a review and analysis of the epidemiological data of malaria patients seen in Addenbrooke’s Hospital (Cambridge University Hospital Foundation Trust [CUHFT]) from 2002 to 2016 and compared this with national data.
METHODS
This was a retrospective review of patients with confirmed malaria seen in CUHFT from 2002 to 2016. Malaria infection was confirmed using a combination of antigen testing, clinical data, and blood films. While most of the patient records were paper-based, the patient record software Epic was accessed for more detailed demographic data. National figures on imported malaria were kindly provided by the Public Health England Malaria Reference Laboratory.
RESULTS
General Trend
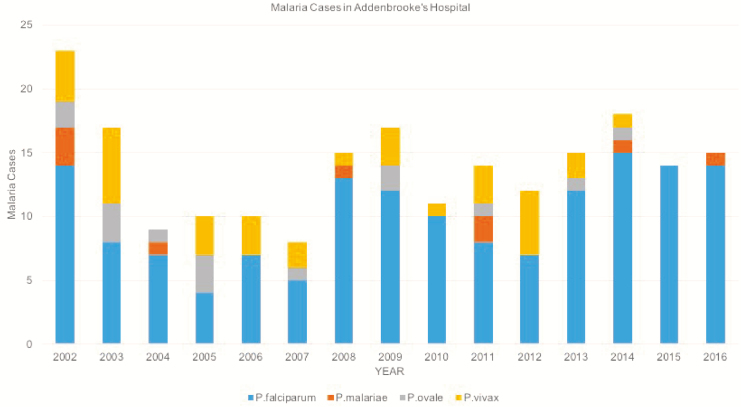
Two hundred twenty-five patients had confirmed malaria between 2002 and 2016, with a mean of 15 cases per year. There was little evidence of any change in trend from 2004 onward. Most of these infections were due to P. falciparum, which accounted for 66.7% (150/225) of cases in this 15-year period (Figure 1).
Figure 1.
Malaria cases in Addenbrooke’s Hospital, Cambridge University Hospital Foundation Trust, by year, over a 15-year period: Plasmodium falciparum, 66.7% (150/225); Plasmodium vivax, 15.1% (34/225); Plasmodium malariae, 4% (9/225); and Plasmodium ovale, 6.7% (15/225). For 17 patients, the causative organism was not recorded.
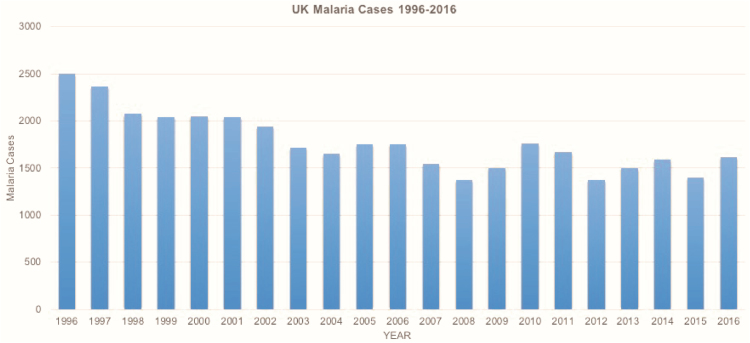
Malaria cases in the UK show a decline of 31.2% between 1996 and 2003; however, this is not maintained as the numbers of malaria cases per year in the UK plateaued thereafter (Figure 2).
Figure 2.
United Kingdom malaria cases by year, 1996–2016. Data source: Public Health England (PHE) Malaria Reference Laboratory, London School of Hygiene and Tropical Medicine, supplied by the Travel and Migrant Health Section, PHE National Infections Service, Colindale, London.
Age and Sex
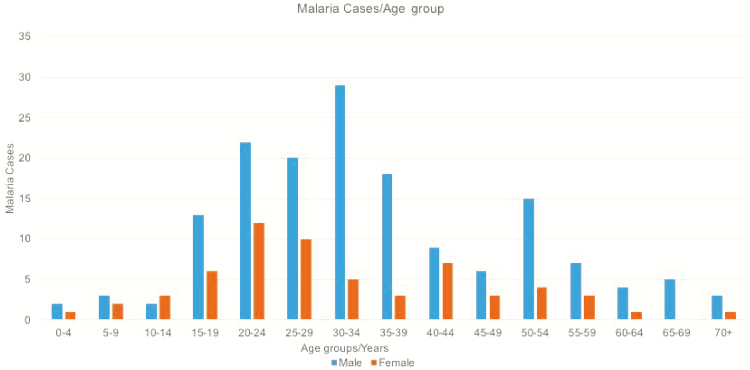
Data on age and sex were available for 219 patients. There were more male cases of malaria at all ages except 10–14 years. The highest number of malaria cases for men was in those 30–34 years of age, peaking at 29 cases; for females, it was in those 20–24 years of age, peaking at 12 cases (Figure 3).
Figure 3.
Malaria cases in Cambridge University Hospital Foundation Trust by age group and sex; there were 158 male patients and 61 female patients over the 15-year period.
Travel History
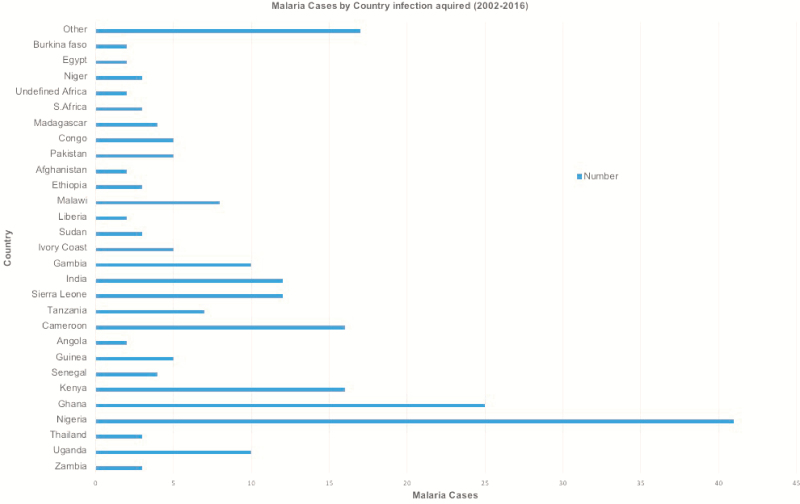
Most of the malaria cases seen in CUHFT between 2002 and 2016 were imported from Africa. West Africa (in particular Ghana and Nigeria) contributed the most cases (66 cases). Some patients visited >1 malarious country, and it was unclear where their malaria was acquired (Figure 4).
Figure 4.
Malaria cases in Cambridge University Hospital Foundation Trust by country in which infection was acquired, 2002–2016. “Other” represents 17 countries that each contributed 1 case (Mauritius, Nepal, Laos, Vietnam, Cambodia, Namibia, Botswana, South America [country unspecified], Mali, Benin, Iraq, Gabon, Malaysia, Somalia, Algeria, Mozambique, and Zimbabwe).
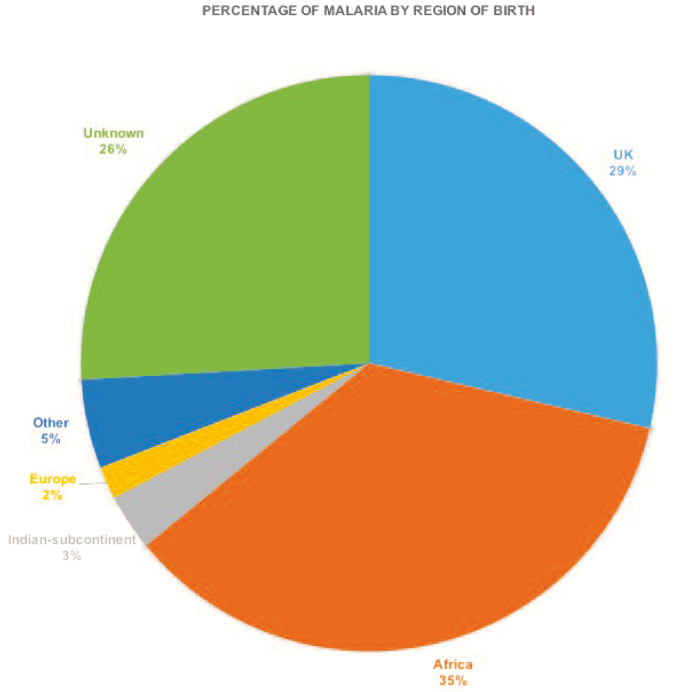
National data (Table 1) show similar trends to those seen in CUHFT, with Nigeria and Ghana also contributing the most malaria cases imported to the UK in this time period (7175 and 2567 reported cases, respectively). India had the most people coming to the UK, with >16 million people; however, it had one of the lowest rates of imported infection at 8 cases per 100 000 travelers nationally. This is in contrast to countries such as Sierra Leone, Ivory Coast, and Cameroon, which had the highest proportions of infection at 7, 8, and 5 cases per 1000 travelers, respectively, despite relatively fewer travelers. No malaria cases were recorded by the Malaria Research Laboratory nationally from Egypt, Laos, Vietnam, and Mauritius despite there being at least 1 case from each of these countries seen in CUHFT (Table 1); this disparity is unexplained. Based on country of birth, the UK and Africa accounted for 64% of all cases (Figure 5).
Table 1.
National figures for the total numbers of people traveling to malarious countries, total malaria cases in the United Kingdom, and proportions of travelers being infected. Data source: Office of National Statistics and Public Health England (PHE) Malaria Reference Laboratory, London School of Hygiene and Tropical Medicine, supplied by the Travel and Migrant Health Section, PHE National Infections Service, Colindale, London. Two cases seen in Cambridge University Hospital Foundation Trust that were recorded as “undefined Africa” (see Figure 4) have been excluded from this table. Abbreviation: CUHFT, Cambridge University Hospital Foundation Trust.
| Malaria Prophylaxis | Number of Malaria Cases |
|---|---|
| Doxycycline | 24 |
| Mefloquine | 10 |
| Proguanil | 4 |
| Chloroquine/proguanil | 4 |
| Fansidar | 4 |
| Malarone | 3 |
| Chloroquine | 3 |
| Unknown | 25 |
| None | 116 |
| No data | 32 |
Figure 5.
Malaria cases in Cambridge University Hospital Foundation Trust by patient’s region of birth.
Reasons for Travel
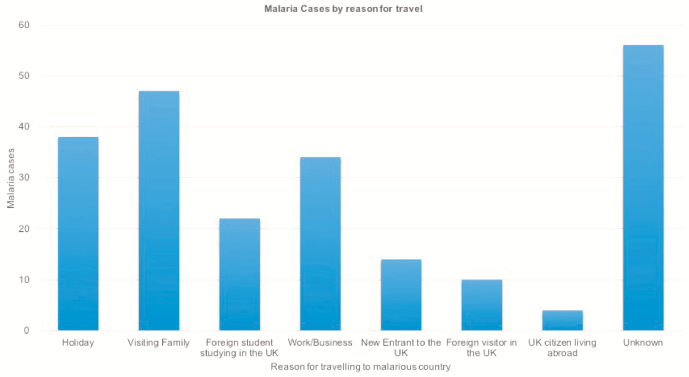
Reasons for traveling to a malarious country were documented for 169 patients. Of these, the largest group comprised those visiting family in their country of origin (27.8% [47/169]); however, a large number of patients were also UK residents visiting malarious countries for holiday (22.5% [38/169]) and/or work reasons (20.1% [34/169]) including volunteer work and military attachments. Other reasons included UK citizens living abroad (2.4% [4/169]), foreign students studying in the UK (13.0% [22/169]), new entrants to the UK (8.3% [14/169]), and foreign visitors falling ill in the UK (5.9% [10/169]) (Figure 6).
Figure 6.
Malaria cases by reason for travel, 2002–2016, Addenbrooke’s Hospital. Abbreviation: UK, United Kingdom.
Chemoprophylaxis
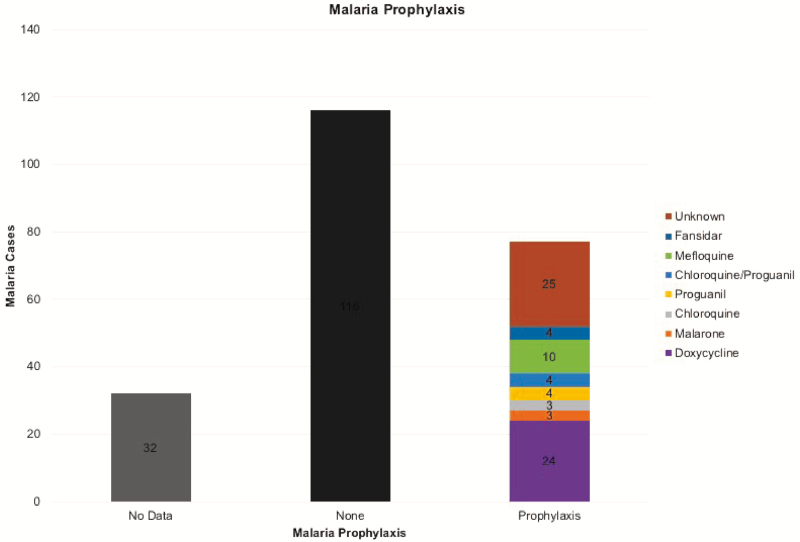
To assess the effectiveness of health messages on the importance of antimalarial chemoprophylaxis, we examined the evidence for taking and adhering to chemoprophylaxis by this population over this 15-year period. These data were available for 193 patients. Of these, a staggering 60% (116/193) of patients took no prophylaxis at all. Of the 40% (77/193) who took prophylaxis, 32.5% (25/77) did not know what they had taken (“unknown”) (Figure 7). The remaining 52 took a variety of drugs, but none consistently, and none on return to the UK. The most common identified prophylactic drug used was doxycycline.
Figure 7.
Malaria cases based on prophylaxis use, Cambridge University Hospital Foundation Trust.
Treatment and Outcomes
Parasitemia data were available for patients presenting with P. falciparum malaria in 109 of 150 patients. Most patients presented quite early on with low parasitemia levels in the range of 0.1%–0.5%, which may have contributed to the successful treatment outcomes (Supplementary Figure 1).
Heavier parasitemia was observed in patients at the extremes of age with an average of 4.3% in the age group 5–9 years and 5.35% in the age group 65–69 years (Supplementary Figure 2).
Almost all of the patients treated in Addenbrooke’s Hospital who presented with malaria between 2002 and 2016 recovered. Data on outcomes of treatment were obtained using Addenbrooke’s Epic software and was available for 202 patients. Of these, 98.5% (199/202) recovered and 1.5% (3/202) died (Supplementary Figure 3A and 3B).
UK national figures show a similar pattern of treatment outcomes as that shown in CUHFT. Most patients (99.6% [24045/24149]) who had malaria recovered, whereas 0.4% (104/24149) died.
DISCUSSION
In this study, we set out to elucidate the trends of malaria in patients presenting to Addenbrooke’s Hospital. The total malaria cases per year stayed relatively constant with an average of 15 cases every year. This is in line with data from the 2015 UK malaria report [2], which shows relatively static numbers from the year 2002 to 2015 following a steep decline from 2500 to 2050 cases annually seen between 1996 and 2001. This suggests that, since this earlier decline, current health messages about visiting malarious countries are having very little effect on combating imported malaria to the UK.
As in previous reports, most of the cases seen in hospital were P. falciparum infection. This is consistent with data across the UK as reported in the 2015 UK malaria report [2]. Most of the cases of malaria infections in this study were in young men between the ages of 15 and 40 years. It is thought that men are less compliant with chemoprophylaxis [6]; another plausible explanation is that more young men could be migrating to or emigrating from the UK for work-related reasons.
Malaria cases seen in CUHFT were acquired in a variety of countries, with the biggest contribution of malaria cases coming from West Africa, a well-documented source of imported malaria to the UK [4]. This could simply reflect the relatively large numbers of people traveling to the UK from this region, as there were >4 million visitors to the UK between 2002 and 2016 from Nigeria and Ghana alone. This is in stark contrast to a country like Ivory Coast, which had just >67 000 visitors to the UK in the same time period but had the highest proportion infected with malaria at 8 per 1000 people. No malaria cases were recorded by the Malaria Laboratory nationally from Egypt, Laos, Vietnam, and Mauritius despite there being at least 1 case from each of these countries seen in CUHFT. There are a number of possible explanations for this including inaccurate reporting/history in the case of Egypt and Mauritius. This likely also reflects significant underreporting within the UK [7], although another plausible explanation is the difficulty in assigning a country of infection to people who traveled to >1 malaria-endemic country on the same trip, as we noted in a small number of our cases.
Over the 15 years covered by this study, 52 239 837 visits were made by UK residents to malarious countries (Table 1), and 22 053 510 visits were made to the UK over the same time period from these areas. These figures are being maintained, with potential malaria exposure occurring in an average of >3 million UK residents traveling away from and >1 million visitors coming to the UK every year (Supplementary Figure 4A and 4B).
From Figures 5 and 6, it is clear that many people infected with malaria are visiting family in their own country of origin. This is a well-observed phenomenon [2, 4, 8] and is associated with poor chemoprophylaxis use (Figure 7). None of these patients reported using chemoprophylaxis as prescribed and, in particular, none reported continuing the medication upon return; where a reason was given, most of the patients cited side effects (eg, nausea) as the reasons for stopping medication. Doxycycline was the most commonly used drug in those patients in whom prophylaxis was identified, which may represent local advice as it was not the most commonly used nationally during this period. The Advisory Committee on Malaria Prevention recommends atovaquone-proguanil, doxycycline, or mefloquine for most malaria-endemic areas, especially sub-Saharan Africa [9]. Of the recommended drugs, doxycycline is the cheapest, and this may have influenced choice.
The “visiting family” group of patients has been shown to report the least use of chemoprophylaxis, which has been attributed to them visiting a familiar environment and being likely to underestimate loss of semi-immunity and the consequent risk of malaria [10]. Some travelers reported that, even if they were to get ill, they believed they would be able to easily deal with it while in their country of origin [11]. Therefore, despite much education on the importance of malarial chemoprophylaxis being targeted at this group of people, it seems these efforts, after a decline in the early years of the century [12], have now lost their impact [2]. Another potential contribution to this steep decline in cases could be more people visiting urban areas in malaria-endemic areas where transmission is likely to be lower [12]. Lack of chemoprophylaxis use and lack of adherence to chemoprophylaxis regimen likely contribute to lack of continued significant decline in malaria cases after 2001 [4, 13, 14]. Chemoprophylaxis use as low as 7% in those traveling to visit friends and family and 24% in those traveling for other reasons has been recorded [4].
The contribution of people born in the UK going to malarious regions for holidays and business is significant and has also been noted [15]. Some of the patients included in the “work” category were soldiers with the British forces. This potentially highlights 2 more groups of people in which the message of the importance of chemoprophylaxis should be stressed. The 2015 UK malaria report [2] recommends targeting Africans (ie, those traveling to visit friends and family) with pretravel advice; this has recently been confirmed [16], with highest rates of infection in those traveling to visit friends and family, black Africans in particular. Our data support this, but only in part; although the single largest group recording the highest malaria cases was the “visiting friends and family” group (27.8%), British patients traveling for work (20.1%) and holiday (22.5%) contributed significantly, with these 2 groups accounting for more malaria cases than those traveling to visit friends and family. Pretravel prophylaxis advice should thus also be targeted and emphasized toward these 2 groups. Some malaria chemoprophylaxis including Maloff Protect (atovaquone-proguanil), Avloclor (chloroquine), Paludrine (proguanil), and Paludrine/Avloclor pack are now available without prescription over the counter in the UK [17]. This is a promising endeavor to improve access to chemoprophylaxis without the need to see a doctor. The issue that remains is bringing such services to the attention of travelers and not only emphasizing the importance of taking chemoprophylaxis but adhering to the chemoprophylaxis regimen.
We noted a high average parasitemia in extremes of age (4.3% in those aged 5–9 years and 5.4% in those aged 65–69 years). At the younger age range, it has been postulated that higher intrinsic susceptibility, combined with nonspecific symptoms and potentially delayed diagnosis, could contribute to higher average parasitemia [10]. In the age group 65–69 years, factors such as comorbidities and potential immunocompromise may also contribute. This may be correlated with higher mortality rates in these 2 age groups [18].
Many patients at presentation have relatively low parasitemia levels, mostly in the 0.1%–0.5% range. This may reflect the effectiveness of the message from the 2015 UK malaria report [2], urging travelers to “take fever seriously” and visit a doctor after visiting a malarious region, and is probably reflected in the low mortality rate seen. The mortality rate at CUHFT was higher than the national average. This may reflect CUHFT being a tertiary center with a wide catchment area and a low incidence of disease. Some delay in diagnosis of some cases may have occurred, and this may explain the higher fatality rate.
This study has limitations. As a retrospective study of patient records, it was limited by lack of complete patient data in parts—in some cases data were not recorded, and in some cases data were unavailable as they were classified, in the case of the armed forces.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge and thank the UK Office of National Statistics and the Malaria Research Laboratory for providing statistical data.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tangpukdee N, Duangdee C, Wilairatana P, Krudsood S. Malaria diagnosis: a brief review. Korean J Parasitol 2009; 47:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Public Health England. Malaria imported into the UK: 2015 implications for those advising travellers (July 2016). Available at: https://www.gov.uk/government/publications/malaria-in-the-uk-annual-report.
- 3. Francis BC, Gonzalo X, Duggineni S, et al. . Epidemiology and clinical features of imported malaria in East London. J Travel Med 2016; 23. [DOI] [PubMed] [Google Scholar]
- 4. Smith AD, Bradley DJ, Smith V, et al. . Imported malaria and high risk groups: observational study using UK surveillance data 1987–2006. BMJ 2008; 337:a120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Broderick C, Nadjm B, Smith V, et al. . Clinical, geographical, and temporal risk factors associated with presentation and outcome of vivax malaria imported into the United Kingdom over 27 years: observational study. BMJ 2015; 350:h1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phillips-Howard PA, Radalowicz A, Mitchell J, Bradley DJ. Risk of malaria in British residents returning from malarious areas BMJ, 1990; 300:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cathcart SJ, Lawrence J, Grant A, et al. . Estimating unreported malaria cases in England: a capture-recapture study Epidemiol Infect, 2010; 138:1052–8. [DOI] [PubMed] [Google Scholar]
- 8. Pavli A, Maltezou HC. Malaria and travellers visiting friends and relatives. Travel Med Infect Dis 2010; 8:161–8. [DOI] [PubMed] [Google Scholar]
- 9. et al. Guidelines for malaria prevention (October 2017) Available at: https://www.gov.uk/government/publications/malaria-prevention-guidelines-for-travellers-from-the-uk.
- 10. Pinsent A, Read JM, Griffin JT, et al. . Risk factors for UK Plasmodium falciparum cases. Malar J 2014; 13:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neave PE, Behrens RH, Jones CO. “You’re losing your Ghanaianess”: understanding malaria decision-making among Africans visiting friends and relatives in the UK. Malar J 2014; 13:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Behrens RH, Carroll B, Smith V, Alexander N. Declining incidence of malaria imported into the UK from West Africa. Malar J 2008; 7:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ladhani S, Aibara RJ, Blaze M, Smith V, Shingadia DV. Trends in imported childhood malaria in the UK: 1999–2003. Arch Dis Child 2006; 91:911–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Behrens RH, Neave PE, Jones CO. Imported malaria among people who travel to visit friends and relatives: is current UK policy effective or does it need a strategic change? Malar J 2015; 14:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Unger HW, McCallum AD, Ukachukwu V, et al. . Imported malaria in Scotland—an overview of surveillance, reporting and trends Travel Med Infect Dis 2011; 9:289–97. [DOI] [PubMed] [Google Scholar]
- 16. Rees E, Saavedra-Campos, Usdin M, et al. . Trend analysis of imported malaria in London; observational study 2000 to 2014. Travel Med Infect Dis 2017; 17:35–42. [DOI] [PubMed] [Google Scholar]
- 17. Simons H, Patel D. Atovaquone/proguanil (Maloff Protect) is now available without prescription in UK pharmacies. Travel Med Infect Dis 2017; 20:67. [DOI] [PubMed] [Google Scholar]
- 18. Dhingra N, Jha P, Sharma VP, et al. . Adult and child malaria mortality in India: a nationally representative mortality survey. Lancet 2010; 376:1768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.