Abstract
Background
Visceral leishmaniasis (VL), caused by the Leishmania donovani complex, is a fatal, neglected tropical disease that is targeted for elimination in India, Nepal, and Bangladesh. Improved diagnostic tests are required for early case detection and for monitoring the outcomes of treatments. Previous investigations using Leishmania lysate antigen demonstrated that the immunoglobulin (Ig) G1 response is a potential indicator of a patient’s clinical status after chemotherapy.
Methods
IgG1 or IgG enzyme-linked immunosorbent assays (ELISAs) with rK39 or lysate antigens and novel IgG1 rK39 rapid diagnostic tests (RDTs) were assessed with Indian VL serum samples from the following clinical groups: paired pre- and postchemotherapy (deemed cured); relapsed; other infectious diseases; and endemic, healthy controls.
Results
With paired pre- and post-treatment samples (n = 37 pairs), ELISAs with rK39- and IgG1-specific conjugates gave a far more discriminative decrease in post-treatment antibody responses when compared to IgG (P < .0001). Novel IgG1 rK39 RDTs provided strong evidence for decreased IgG1 responses in patients who had successful treatment (P < .0001). Furthermore, both IgG1 rK39 RDTs (n = 38) and ELISAs showed a highly significant difference in test outcomes between cured patients and those who relapsed (n = 23; P < .0001). RDTs were more sensitive than corresponding ELISAs.
Conclusions
We present strong evidence for the use of IgG1 in monitoring treatment outcomes in VL, and the first use of an IgG1-based RDT using the rK39 antigen for the discrimination of post-treatment cure versus relapse in VL. Such an RDT may have a significant role in monitoring patients and in targeted control and elimination of this devastating disease.
Keywords: visceral leishmaniasis, IgG1, rapid diagnostic test, cure, relapse
Immunoglobulin (Ig) G1 enzyme-linked immunosorbent assays and a IgG1-based rapid diagnostic test (RDT) using rK39 antigen provide enhanced discrimination between post-treatment cure versus relapse in visceral leishmaniasis (P < .0001). This RDT may have a role in targeted disease control.
In 2012, the World Health Organization (WHO) estimated the global burden of visceral leishmaniasis (VL) to be 200 000–400 000 cases annually, with 20 000–40 000 deaths. The vast majority of cases occur within the Indian subcontinent, eastern Africa, and Brazil, with India accounting for an estimated 70% of global cases, but with a recent significant decline [1, 2]. In India, VL is caused solely by Leishmania donovani, spread by the vector Phlebotomus argentipes, and the disease is considered anthroponotic, with no proven animal reservoirs. Post–kala-azar dermal leishmaniasis (PKDL) is a non–life-threatening potential sequela of treated VL, and patients with PKDL have been shown to be readily infectious to biting sand flies of the appropriate vector species [3, 4].
Since 2005, India, Bangladesh, and Nepal have been pursuing the elimination of VL as a public health problem (<1 case per 10 000) [5]; highly endemic blocks persist in the Indian states of Bihar, Jharkhand, and West Bengal [6]. In 2016, approximately 6250 total cases were reported, representing a fall of over 50% since 2013 [6]. The elimination program focuses on early case detection, with successful treatment; improved surveillance; and integrated vector control [5]. Thus, a successful VL control program requires the implementation of specific and early diagnoses.
Clinical features of VL are prolonged fever (>14 days), hepatosplenomegaly, anemia, pancytopenia, and weight loss, which are nonspecific symptoms that prevent definitive clinical diagnoses. The parasitological diagnosis of Leishmania amastigotes is by microscopy of bone marrow or spleen aspirates, which are high-risk procedures. The detection of IgG against rK39, a fragment of the Leishmania kinesin-like gene [7], has been used with clinical presentation to diagnose VL cases; however, IgG levels may remain detectable even years after a successful cure and disease clearance, as reported from India [8, 9], Brazil [10], and Sudan [11]. Furthermore, asymptomatic individuals who are serologically positive far outnumber clinical cases [12, 13], with only a small proportion of asymptomatics progressing to active disease, thereby reducing the positive predictive value of the current rK39 rapid diagnostic test (RDT).
Studies from India and Nepal have reported postchemotherapy relapses of VL up to and beyond 12 months following the end of treatment [14, 15]. With liposomal amphotericin B, a new, first-line treatment in India, the relapse rate is an estimated 6.7%, with a significant proportion of patients relapsing between 6 and 12 months after treatment [14, 16]. To improve the monitoring of treatment outcomes of VL, and for control of the disease, the WHO has identified the vital need for a marker of postchemotherapeutic cure and the high-priority incorporation of such a biomarker into a point-of-care (POC) RDT [17].
Here, we investigated whether IgG1 detection, in combination with rK39 antigen, could improve the serological assessment of treatment outcomes in VL, particularly to discriminate cures from relapses.
METHODS
Ethics Statement
In India, sample collection was approved by the Ethics Committee of Banaras Hindu University, Varanasi. In Sudan, approval was by the Ethical Research Committee, the Medical and Health Sciences Campus, University of Khartoum, and the National Health Research Ethics Committee, Federal Ministry of Health, Sudan. Written informed consent was obtained from adult subjects or from the parents or guardians of individuals less than 18 years of age (who also gave verbal consent). This research was also approved as part of the NIDIAG (a syndromic approach to Neglected Infectious Diseases at the primary health care level) research consortium (https://cordis.europa.eu/project/rcn/97322_en.html) by the London School of Hygiene and Tropical Medicine Ethics Committee.
Samples
We selected Indian sera or plasma from archived samples that were collected after 2007 from male and female adults and children in the endemic region of Muzaffarpur, Bihar, India (Table 1). Indian VL cases had been diagnosed by positive rK39 serology and parasitologically confirmed by microscopy of splenic or bone marrow aspirates. Indian paired samples were from parasitologically confirmed VL patients at the day of diagnosis (Day 0) and when deemed cured (Month 6; n = 40 pairs). Unpaired, relapsed sera were from VL patients who had been treated, and were sampled at relapse (n = 23). As described below, not all cure pair and relapse samples were used in every assay. Control samples were from clinically confirmed tuberculosis cases (n = 10) and from people living in regions endemic and nonendemic for VL who had no clinical symptoms (endemic, healthy controls [EHCs] and nonendemic, healthy controls [NEHCs]; n = 10 in each group). We also used Sudanese serum samples collected in 2011 and 2013 from Gedaref, Sudan. In Sudan, cases of VL had been diagnosed by microscopy of bone marrow or lymph node aspirates, in conjunction with serological assays. These diagnoses were made in each location according to their respective national procedures, prior to the present study. Sera/plasma were stored at -80°C until use. Samples were previously assayed against culture-adapted promastigote lysate (Marlais et al, submitted). All patients tested negative for human immunodeficiency virus.
Table 1.
Indian Samples Used in Enzyme-linked Immunosorbent Assays and/or Rapid Diagnostic Tests
| Sample | na | Description |
|---|---|---|
| Cured, paired samples | 40 pairs | From parasitologically confirmed VL patients at day of diagnosis (Day 0) and when deemed cured (Month 6). |
| Relapsed | 23 | VL treated and subsequently relapsed. Sampled at the time of relapse diagnosis. |
| Endemic, healthy controls | 10 | Serum from patients living in regions endemic for VL, with no clinical symptoms. |
| Nonendemic, healthy controls | 10 | Serum from individuals living in regions nonendemic for VL, with no clinical symptoms. |
| Tuberculosis | 10 | Serum from patients with clinically confirmed tuberculosis. |
Abbreviation: VL, visceral leishmaniasis.
aNot all samples were used with all assays (see Results).
Antigens
Recombinant rK39 protein was obtained commercially (RAG0061, Rekom Biotech, Spain). L. donovani whole-cell lysates were derived from 2 strains: culture-adapted MHOM/IN/80/DD8 promastigote and MHOM/IN/00/BHU1 that had been cryopreserved as amastigotes. Both strains were cultured in αMEM (M0644, Sigma Aldrich, United Kingdom) and supplemented as previously described [18]. For strain BHU1, the cryopreserved amastigotes were recovered into αMEM and then passaged once into fresh medium prior to harvesting as amastigote-derived promastigotes for lysate preparation. The whole-cell lysates were prepared and sonicated as previously described [19]. Sonicates were centrifuged at 14000 x g for 10 minutes at 4°C and the supernatants containing lysate antigens were stored at -80°C with a protease inhibitor cocktail (P8340, Sigma Aldrich). Protein concentrations of these antigens were determined using the BCA Protein Assay kit against bovine serum antigen standards (23227, ThermoFisher Scientific, United Kingdom), according to manufacturer’s instructions.
Enzyme-linked Immunosorbent Assays
For optimization, we used 6 Sudanese sera (3 high, 1 low, and 2 negative titres) with titrated rK39 antigen; in subsequent assays, we used rK39 resuspended at 0.25 μg/ml in coating buffer (15 mM Na2CO3, 34 mM NaHCO3, pH 9.6).
To compare the antigenicity of rK39 and promastigote antigens using Indian sera and with separate detection of IgG1 and IgG, each ELISA plate (735–0465, VWR, United Kingdom) was divided into quadrants. The rK39 antigen at 0.25 μg/ml and culture-adapted promastigote lysates at 2 μg/ml, diluted in coating buffer, were used to coat the top and bottom halves, respectively, of the same plate at 100 μl/well and were incubated at 4°C overnight. Following 3 washes with phosphate buffered saline (PBS)/0.05% Tween 20 (PBST), 200 μl/well of blocking buffer (PBS/2% skimmed milk powder; Premier Foods, United Kingdom) was applied to the whole plate and incubated for 2 hours at 37°C. Following 3 washes, 100 μl/well of serum/plasma diluted 1:200 in PBST/2% milk was added, such that the same samples were arranged identically in each quadrant. Following incubation at 37°C for 1 hour and 6 washes in PBST, 100 μl/well of 1:5000 dilution in PBST/2% milk of horseradish peroxidase–labelled antihuman IgG1 (ab99774, Abcam, United Kingdom) or antihuman IgG (709-035-149, Jackson Immunoresearch) were added to the left and right halves of the plate, respectively. Following incubation at 37°C for 1 hour and 6 PBST washes, 100 μl/well of substrate solution (50 mM phosphate/citrate buffer, pH 5.0) containing 2 mM σ-phenylenediamine HCl (P1526, Sigma Aldrich) and 0.009% H2O2 (216763, Sigma Aldrich) was added to the entire plate and incubated in the dark. Reactions were stopped by the addition of 100 μl/well of 1M H2SO4 and absorbance was read at 490 nm. Samples were assayed on duplicate plates simultaneously.
To compare lysates, 2 µg/ml of amastigote-derived promastigote antigen was coated onto the top half of the plate at 100 µl/well, in place of rK39; otherwise, the assay was performed as described above.
Prototype Rapid Diagnostic Tests
Co-authors at Coris BioConcept manufactured the novel IgG1 rK39 RDTs described here. The RDT is composed of a nitrocellulose strip sensitized with antigen and containing antihuman IgG1-specific antibody conjugated with colloidal gold, housed within a plastic cassette with a buffer application well and a test/reading window. The antigen used was rK39 at 2 different concentrations—namely, 0.1 mg/ml (0.1rK) and 0.6 mg/ml (0.6rK)—on separate cassettes. Serum/plasma at volumes of 3.5 µl was pipetted onto the sample application zone in the test/reading window, then 120 µl of buffer solution was dispensed into the buffer application well. After 15 minutes, a test was deemed valid if a red control band was present in line with the “C” on the cassette and was deemed positive if a second band was present in line with the “T.” If no band was visible at the “T,” then the test was deemed negative. Changes in the test line intensities between paired day-0 and 6-month samples (becomes negative, decreased, no decrease) were assessed visually. The RDTs were read blind, without reference to the ELISA results.
Statistical Analyses
Statistical analyses were performed using Microsoft Excel 2016 (Microsoft Corporation), Stata 14 (StataCorp), and, for ELISA data (2-tailed, paired t-test with 95% confidence interval), using R [20]. Serum from the same EHC was included in each quadrant of each ELISA plate, from which the cut-off was established for each antigen/detection antibody combination by a mean of the EHC readings plus 3 standard deviations. The mean ELISA result for each sample was determined from the duplicate assays. Paired t-tests were used to determine the significance of differences between day-0 and 6-month samples.
RDT results were compared using a defined clinical status to establish sensitivity, with exact confidence intervals calculated with the Clopper-Pearson exact method. A 2-sided Fisher’s exact test was used to compare relapse versus 6-month post-treatment samples with both RDT types.
RESULTS
Immunoglobulin G1 Is More Discriminative Than Total Immunoglobulin G
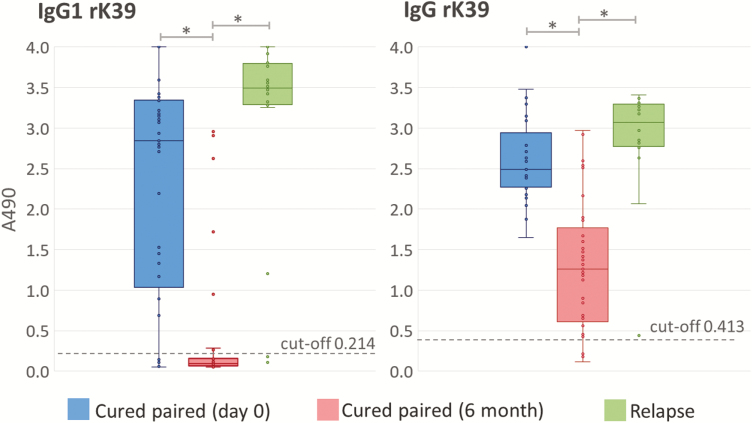
Figure 1 compares IgG1 and total IgG recognition of rK39 antigen in ELISAs, with 37 paired samples taken at Day 0 and at Month 6 (when deemed to be cured), and with 20 relapsed samples. With the same group of patients, the IgG1 titres with cured sera (at Month 6) were more discriminative of clinical status, compared to total IgG. Comparing cure and relapse data, IgG1 provided better discrimination than IgG, even when the changes from Day 0 samples were not considered. With the rK39 antigen, the ELISA readings of cured sera (Month 6) were clustered more towards low values when developed with anti-IgG1 (Figure 1): 81.2% (30/37) were below the cut-off value (A490 = 0.214), compared with only 9% (4/37) for their total IgG (cut-off A490 = 0.413). There was very strong evidence for a difference (P < .0001) between IgG1 and total IgG for 6-month, cured readings. The ELISAs using the rK39 developed more rapidly than those with promastigote antigen on the same plate and, therefore, the times for stopping the reactions across the entire plates were based on their anti-rK39 reaction intensities (Figure 2). We did not observe any significant differences in the ELISA performances using the amastigote-derived or culture-adapted promastigote lysates (Pearson correlation coefficient 0.98, P < .0001; Supplementary Figure S1).
Figure 1.
Decrease in IgG1 levels of cured patients was more evident and consistent than the decline in total IgG, by enzyme-linked immunosorbent assay (ELISA). The ELISA results for the rK39 antigen with cured visceral leishmaniasis paired samples (n = 37 pairs) and relapse samples (n = 20). Abbreviation: IgG, immunoglobulin G. *Indicates very strong evidence for a difference (paired t-test P < .0001). Strong evidence was also seen between IgG1 and IgG in 6-month cured samples (P < .0001, not depicted).
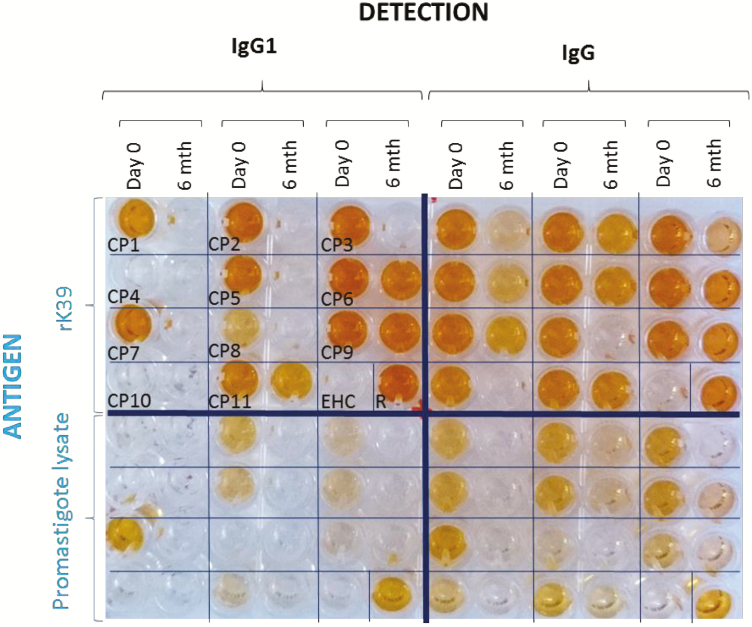
Figure 2.
Example of enzyme-linked immunosorbent assay plate quadrants. Cured paired serum samples at day 0 (pre-treatment) and at 6 months after treatment (patients deemed cured). Abbreviations: EHC, endemic, healthy control; IgG, immunoglobulin G; R, patient deemed relapsed.
Immunoglobulin G1 Rapid Diagnostic Tests Discriminate Relapse From Cure
In total, 254 RDTs were performed on 89 individual patients (Table 2). There were 10 EHCs, 10 NEHCs, and 10 confirmed tuberculosis patients who were negative with both the 0.1rK and 0.6rK RDTs.
Table 2.
Results of Indian Visceral Leishmaniasis and Control Sera With Immunoglobulin G1 rK39 Rapid Diagnostic Test
| Sample Types | Positive/Total (%) | ||||
|---|---|---|---|---|---|
| Cured VL Paired Samples (n = 38) |
Day 0 | Month 6a | 0.1rK | 0.6rK | |
| Positive | Negative | 22/38 (57.9%) |
26/38 (68.4%) |
||
| Decrease | 8/38 (21.1%) |
7/38 (18.4%) |
|||
| No decrease | 6/38 (15.8%) |
5/38 (13.2%) |
|||
| Negative | Negative | 2/38 (5.2%) |
0/38 (0%) |
||
| Positive | 0/38 (0%) |
0/38 (0%) |
|||
| Unpaired samples | |||||
| Relapse VL samples (n = 21) | 18/21 (85.7%) | 19/21 (90.5%) | |||
| Endemic, healthy control (n = 10) | 0/10 | 0/10 | |||
| Nonendemic, healthy control (n = 10) | 0/10 | 0/10 | |||
| Tuberculosis patients’ samples (n = 10) | 0/10 | 0/10 | |||
We used rK39 0.1 mg/ml (0.1rK) and 0.6 mg/ml (0.6rK).
Abbreviation: VL, visceral leishmaniasis.
aMonth 6 reading is test line intensity assessed visually compared with Day 0.
RDT sensitivity for VL (Day 0) was 94.7% (82.3–99.4) and 100% (90.7–100) for 0.1rK RDT and 0.6rK RDT, respectively. Of the 21 samples from patients at relapse, 19 were positive with 0.6rK RDT and 18 were positive with 0.1rK RDT. With both 0.6rK and 0.1rK RDTs, there was very strong evidence (P < .0001) for a difference in test positivity between Month 6 samples from individuals who relapsed versus Month 6 samples from individuals who were cured.
In comparison with the IgG1 rK39 ELISA, the 0.6rK IgG1 RDT gave the same positive result for 17/18 samples (94.4% sensitive). For the remaining sample, this RDT was positive and the ELISA reading was just below the cut-off. For the cure pairs (Day 0 and Month 6 sample pairs), 20 of the 26 patients were positive (Day 0) by both IgG1 rK39 ELISA and the 0.6rK IgG1 RDT and decreased to negative at Month 6. Of the other patients, 4 were negative by ELISA at both time points but were positive by the RDT at Day 0 and negative at Month 6; the remainder were positive by the RDT at both time points. Thus, the RDTs, which use more concentrated samples, were overall somewhat more sensitive than the corresponding ELISAs.
DISCUSSION
Improved diagnostics for VL are required to discriminate between post-treatment cure versus relapse and to predict the progression in an asymptomatic carrier to active VL. There is also a need for diagnostics to distinguish PKDL from other dermatological conditions and to detect VL in human immunodeficiency virus co-infected patients who are immunocompromised [21].
Since its early validation for VL diagnosis [22], rK39 antigen, used in either ELISA or RDT format, has been used with IgG detection. However, IgG levels can remain elevated for several years after successful treatment [8], whereas IgG1 may decline rapidly in the absence of a sustained and appropriate antigenic stimulus [23, 24]. Here, we describe the capacity of rK39 with IgG1 level detection to characterize the post-treatment clinical status of Indian VL. We demonstrated the greater discriminatory potential of IgG1, compared to IgG, as an indicator of postchemotherapeutic outcomes in VL. We have adapted the IgG1 rK39 assay to an easy-to-manufacture, POC, reproducible, rapid, and inexpensive test of cure for VL.
An ELISA comparison between IgG and IgG1 against rK39 demonstrated that with IgG1, there was a significantly greater decrease in response following a cure (P < .0001; Figure 1), supporting the continued development of IgG1-based diagnostics [19]. Paired samples from cured patients and nonpaired samples from patients who relapsed allowed evaluation of the IgG1 rK39 RDTs. In support of previous observations [19], the majority of Month 6 cured samples were negative, with a significant difference between cured and relapsed individuals (P < .0001). Thus, the IgG1 rK39 RDT provides a potential POC means of serological assessment of treatment success [25]. However, this does not obviate the need for concomitant clinical evaluations.
It is not known whether the individuals deemed to be cured at Month 6 remained free from relapse thereafter. In 1 study, most patients who relapsed did so between 6 and 12 months post-treatment [14]. Therefore, as 14 (0.1rK) and 12 (0.6rK) of 38 patients deemed cured at Month 6 were positive by IgG1, albeit the majority with decreased signal strength (Table 2), further validation of the IgG1 rK39 RDT at 12- or 18-month clinical and serological follow-ups would be required to determine the relapse rate in comparison with the rate among the RDT-negative patients deemed cured.
In terms of future application within a clinical environment, an optimum rK39 concentration will be required. The 0.1 mg/ml concentration produced some negative results and, on visual inspection, positive test bands were less clear than with the 0.6rK test; the 0.6 mg/ml concentration did not cause increased background or false positives with controls. However, considering the greater cost of manufacture involved, it would be appropriate to evaluate intermediate concentrations. Pilot trials indicate that the IgG1 RDT is directly applicable to 3.5 µl of finger-prick whole blood in the field (unpublished observations).
This is the first report of the use of rK39 with detection of IgG1. We show that this combination gives a better discrimination between cure and relapse than using IgG and that this assay can be adapted into a low-cost, POC RDT format. Similarly, POC RDTs are required to identify those asymptomatic, serologically positive individuals who are most likely to progress to active disease and those PKDL patients with nonspecific dermatological clinical presentations. The implementation of such POC RDTs within discriminative case finding initiatives would be of significant benefit in the Indian subcontinent as it prepares for a postelimination environment, in which effective diagnostic surveillance is critical.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the NIDIAG (syndromic approach to Neglected Infectious Diseases at primary health care level) network research partnership, supported by the European Commission under the Health Cooperation Work Programme of the 7th Framework Programme (Grant agreement no. 260260, http://cordis.europa.eu/fp7/home_en.html), which preceded this study, especially Marleen Boelaert. They also thank Osman Ahmed, Osama Eisa, and Alfarazdeg Saad for providing Sudanese sera and Vanessa Yardley (London School of Hygiene & Tropical Medicine) for providing L. donovani amastigotes.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed within this report are those of the authors and not necessarily those of the Sir Halley Stewart Trust.
Financial support. This work was supported by the European Commission Marie Skłodowska-Curie grant (agreement number 642609 to M. A. M.; principal coordinator Albert Picado).
Potential conflicts of interest. T. M. was supported by the Sir Halley Stewart Trust (http://www.sirhalleystewart.org.uk/) and the John Henry Memorial Fund (http://www.johnhenrymf.org/). S. S. was supported by the National Institutes of Health (grant U19 AI704321). P. M. is an employee of Coris BioConcept and B. C. B. H. was an employee of Coris BioConcept at the time the study was carried out: Coris BioConcept has several projects partially funded by the European Commission, as well as several other projects partially funded by the Région Wallonne (Belgian regional funds). G. M. received travel support from London School of Hygiene & Tropical Medicine, related to the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Control of the leishmaniases WHO technical report series 949. Geneva, Switzerland: World Health Organization, 2010. [PubMed] [Google Scholar]
- 2. Alvar J, Vélez ID, Bern C, et al. ; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLOS One 2012; 7:e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Napier L, Smith R, Das Gupta C, Mukerji S.. The infection of Phlebotomus argentipes from dermal leishmanial lesions. Ind J Med Res 1933; 21:173–8. [Google Scholar]
- 4. Molina R, Ghosh D, Carrillo E, et al. . Infectivity of post-kala-azar dermal leishmaniasis patients to sand flies: revisiting a proof of concept in the context of the kala-azar elimination program in the Indian subcontinent. Clin Infect Dis 2017; 65:150–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Regional strategy framework for elimination of kala-azar from the south-east Asia region 2011–2015. Geneva, Switzerland: World Health Organization, 2012. [Google Scholar]
- 6. National Vector Borne Disease Control Programme, India. Available at: http://nvbdcp.gov.in/. Accessed 1 August 2018.
- 7. Burns JM Jr, Shreffler WG, Benson DR, Ghalib HW, Badaro R, Reed SG.. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc Natl Acad Sci USA 1993; 90:775–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gidwani K, Picado A, Ostyn B, et al. . Persistence of Leishmania donovani antibodies in past visceral leishmaniasis cases in India. Clin Vaccine Immunol 2011; 18:346–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patil RR, Muliyil JP, Nandy A, Addy A, Maji AK, Chatterjee P.. Dynamics of the antibodies in cohorts of cured cases of visceral leishmaniasis: its implication on the validity of serological test, value in prognosis and in post therapeutic assessment. Hum Vaccin Immunother 2012; 8:725–30. [DOI] [PubMed] [Google Scholar]
- 10. De Almeida Silva L, Romero HD, Prata A, et al. . Immunologic tests in patients after clinical cure of visceral leishmaniasis. Am J Trop Med Hyg 2006; 75:739–43. [PubMed] [Google Scholar]
- 11. Zijlstra EE, Nur Y, Desjeux P, Khalil EA, El-Hassan AM, Groen J.. Diagnosing visceral leishmaniasis with the recombinant K39 strip test: experience from the Sudan. Trop Med Int Health 2001; 6:108–13. [DOI] [PubMed] [Google Scholar]
- 12. Hasker E, Kansal S, Malaviya P, et al. . Latent infection with Leishmania donovani in highly endemic villages in Bihar, India. PLOS Negl Trop Dis 2013; 7:e2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saha P, Ganguly S, Chatterjee M, et al. . Asymptomatic leishmaniasis in kala-azar endemic areas of Malda district, West Bengal, India. PLOS Negl Trop Dis 2017; 11:e0005391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burza S, Sinha PK, Mahajan R, et al. . Risk factors for visceral leishmaniasis relapse in immunocompetent patients following treatment with 20 mg/kg liposomal amphotericin B (Ambisome) in Bihar, India. PLOS Negl Trop Dis 2014; 8:e2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rijal S, Ostyn B, Uranw S, et al. . Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin Infect Dis 2013; 56:1530–8. [DOI] [PubMed] [Google Scholar]
- 16. Sundar S, Singh A, Rai M, Chakravarty J.. Single-dose indigenous liposomal amphotericin B in the treatment of Indian visceral leishmaniasis: a phase 2 study. Am J Trop Med Hyg 2015; 92:513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Research priorities for Chagas disease, human African trypanosomiasis and leishmaniasis (technical report series 975). Geneva, Switzerland: World Health Organization, 2012. [PubMed] [Google Scholar]
- 18. Nolder D, Roncal N, Davies CR, Llanos-Cuentas A, Miles MA.. Multiple hybrid genotypes of Leishmania (Viannia) in a focus of mucocutaneous Leishmaniasis. Am J Trop Med Hyg 2007; 76:573–8. [PubMed] [Google Scholar]
- 19. Bhattacharyya T, Ayandeh A, Falconar AK, et al. . IgG1 as a potential biomarker of post-chemotherapeutic relapse in visceral leishmaniasis, and adaptation to a rapid diagnostic test. PLOS Negl Trop Dis 2014; 8:e3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Available at: http://www.R-project.org/ [Google Scholar]
- 21. Singh OP, Sundar S.. Developments in diagnosis of visceral leishmaniasis in the elimination era. J Parasitol Res 2015; 2015:239469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sundar S, Reed SG, Singh VP, Kumar PC, Murray HW.. Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet 1998; 351:563–5. [DOI] [PubMed] [Google Scholar]
- 23. Pan Q, Hammarström L.. Molecular basis of IgG subclass deficiency. Immunol Rev 2000; 178:99–110. [DOI] [PubMed] [Google Scholar]
- 24. Vidarsson G, Dekkers G, Rispens T.. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014; 5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh DP, Sundar S, Mohapatra TM.. The rK39 strip test is non-predictor of clinical status for kala-azar. BMC Res Notes 2009; 2:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.