ABSTRACT
Endocrine disrupting chemicals (EDCs) can disrupt fetal developmental processes during pregnancy, leading to long-term adverse outcomes in humans. A major source of exposure to EDCs, such as phthalates and bisphenols, is the food supply, primarily due to contamination from processing and packaging. Therefore, this review aimed to 1) review food-monitoring sources of phthalates and bisphenols, and 2) evaluate methodologies and provide future directions needed to establish EDC-limiting dietary recommendations in pregnancy. Using PubMed, 10 peer-reviewed studies were found on dietary predictors of EDC exposure in pregnancy, and all were selected for review. Use of plastic containers in pregnancy was associated with higher urinary phthalate metabolites, whereas canned food consumption was associated with higher urinary bisphenol A (BPA) concentrations. Foods and dietary patterns associated with healthier food choices (e.g., organic/grown/raised/caught foods, folic acid supplements, vegetarianism) were generally associated with lower urinary phthalate metabolite and BPA concentrations. Despite the many food-monitoring studies reporting high BPA and phthalate concentrations in various foods, the designs of most studies described here were not sufficiently robust to consistently detect associations of specific foods/food groups with phthalates and BPA. Given the limitations of currently available research, future studies should incorporate more valid questionnaires to accurately assess dietary EDC exposure, strive for concurrent diet and exposure assessment, and assess whether geographical and cultural differences modify associations of diet with gestational EDC exposures. Such progress will be critical for developing dietary recommendations that ensure the safety and health of pregnant women.
Keywords: pregnancy, phthalates, bisphenols, toxicology, diet
Introduction
Many consumer products, including food contact materials, contain phthalates and bisphenols, resulting in widespread human exposure to these chemicals. Phthalates are diesters of phthalic acid (1, 2) that are classified into 2 categories based on their molecular weight: high-molecular-weight phthalates (highMWPs) and low-molecular-weight phthalates (lowMWPs) (Table 1). HighMWPs are used as plasticizers in polyvinyl chloride products to make plastics flexible for building materials, medical devices, and food processing or packaging (3–6), whereas lowMWPs are primarily used as solvents, fixatives, and adhesives in personal care products and cosmetics (3–5). Bisphenol A (BPA), and its replacements bisphenol S (BPS) and bisphenol F (BPF), are used to manufacture polycarbonate plastics and epoxy resins for consumer and food product packaging, including canned foods (4, 7–10).
TABLE 1.
Summary of reviewed phthalate parent compounds/metabolites, bisphenols, and their proposed sources1
| Compound categorization | Parent compound (name; abbreviation) | Metabolite (name; abbreviation) | Exposure sources |
|---|---|---|---|
| High-molecular-weight phthalate | Di(2-ethylhexyl) phthalate; DEHP | Mono(2-ethylhexyl) phthalate; mEHP | • PVC plastics |
| Mono(2-ethyl-5-hydroxyhexyl) phthalate; mEHHP | • Food packaging and processing | ||
| Mono(2-ethyl-5-oxohexyl) phthalate; mEOHP | • Medical devices | ||
| Mono(2-ethyl-5-carboxypentyl) phthalate; mECPP | • Pharmaceutical coatings | ||
| • Building materials | |||
| Di-isononyl phthalate; DiNP | Mono-isononyl phthalate; mNP/miNP | • PVC plastics | |
| Monooxononyl phthalate; mONP | • Food packaging | ||
| Monocarboxyoctyl phthalate; mCOP | • Building materials | ||
| • Car interiors | |||
| • Drinking straws | |||
| Di-isodecyl phthalate; DiDP | Monocarboxynonyl phthalate; mCNP | • PVC plastics | |
| • Food packaging | |||
| • Building materials | |||
| • Car interiors | |||
| • Swimming pools | |||
| Di-n-octyl phthalate; DOP/DnOP | Mono(3-carboxypropyl) phthalate; mCPP | • PVC plastics | |
| • Food packaging | |||
| • Building materials | |||
| • Adhesives | |||
| Benzylbutyl phthalate; BBzP | Monobenzyl phthalate; mBzP | • PVC plastics | |
| • Food packaging | |||
| • Car care products | |||
| • Some PCPs | |||
| Low-molecular-weight phthalate | Diethyl phthalate; DEP | Monoethyl phthalate; mEP | • Fragrant PCPs: perfumes/colognes, deodorants, soaps, shampoos, lotions |
| Di-n-butyl phthalate; DBP/DnBP | Mono-n-butyl phthalate; mBP/mnBP | • PCPs: nail polish, cosmetics | |
| Mono-hydroxybutyl phthalate; mHBP | • Printing inks | ||
| Di-iso-butyl phthalate; DiBP | Mono-isobutyl phthalate; miBP | • Pharmaceutical coatings | |
| Mono-hydroxyl-isobutyl phthalate; mHiBP | • Insecticides | ||
| Bisphenol | Bisphenol A; BPA | • Polycarbonate plastics and epoxy resins | |
| Bisphenol S; BPS | • Food packaging: lining food cans, beverage containers | ||
| Bisphenol F; BPF | • Plastic dinnerware | ||
| • Dental sealants | |||
| • Thermal receipts |
Due to their short half-lives (<24 h), exposures to phthalates and bisphenols are best characterized in urine (compared with blood) (11). Upon exposure, phthalates, specifically, are metabolized and excreted in urine, allowing for approximation of exposure by measuring urinary phthalate parent–specific metabolites (summarized in Table 1). For example, di(2-ethylhexyl) phthalate (DEHP) exposure is approximated by assessing the sum of its urinary metabolites [mono(2-ethylhexyl) phthalate, mono(2-ethyl-5-hydroxyhexyl) phthalate, mono(2-ethyl-5-carboxypentyl) phthalate, and mono(2-ethyl-5-oxohexyl) phthalate], whereas diethyl phthalate (DEP) exposure is approximated by measuring its major urinary metabolite [monoethyl phthalate (mEP)]. According to the 2013–2014 US NHANES, most women of reproductive age (15–44 y of age) (12) have urinary concentrations of these chemicals that are above the laboratory levels of detection (phthalates: 88–100%; BPA: 96%; BPS: 88%; BPF: 66%) (13). This is concerning, because phthalates and bisphenols are known endocrine disrupting chemicals (EDCs), associated with adverse health outcomes, especially in pregnancy (14). EDCs can alter, mimic, or disrupt the function of gestational hormones, such as thyroid hormone, estrogens, and androgens (15–17), making pregnancy especially sensitive to the actions of EDCs. Human epidemiological studies have shown that prenatal exposure to EDCs, specifically phthalates and bisphenols, is associated with adverse pregnancy (18, 19) and birth outcomes (20, 21), as well as childhood behavioral problems (22, 23), respiratory problems (24, 25), and obesity (26, 27). Diet is a ubiquitous source of chronic EDC exposure (28–30), because these chemicals have been shown to migrate from food contact materials (plastics, paper, metal, glass, and printing inks) that protect food from physical damage and microbial spoilage, thereby affecting human health (31). Human exposure to EDCs from food can be attributed to various factors, including animal feeding practices; food production, processing, and packaging practices; as well as food storage conditions (32).
Characterizing dietary sources of EDCs requires accurate assessment of both EDC concentrations and dietary intake history, and this aim is especially challenging during pregnancy. This is due to the many anatomical, physiological (e.g., increased renal function), and metabolic changes (33, 34) that occur in pregnancy, as well as the numerous pregnancy-related changes in dietary patterns, including diet quality and quantity (35, 36). Despite these challenges, characterizing dietary sources of these chemicals during pregnancy is important, as recommendations are needed to minimize exposure, while providing pregnant women with accessible and nutritious foods necessary to sustain a healthy pregnancy. To address these pregnancy-specific challenges, the aims of this review are to 1) briefly review food-monitoring sources of phthalates and bisphenols in the general population and 2) evaluate methodologies and provide future directions to help establish EDC-limiting dietary recommendations for pregnant women.
Methods
PubMed was searched using combinations of various keywords including diet(ary) + pregnant or pregnancy + predictor(s) or variability or determinants or distribution + endocrine disruptors or EDCs or phthalate(s) or bisphenol(s) or BPA. Studies were included if they assessed associations of consumption of foods or dietary patterns with EDC exposures in pregnancy. Based on our literature search, only 10 pregnancy cohort studies have evaluated dietary predictors of EDC exposures [summarized in Table 2; (37–46)]. Briefly, the 10 studies recruited ≥26 participants from 2003 to 2014; 6 cohorts were from the United States/Puerto Rico, 2 from Spain, 1 from the Netherlands, and 1 from Australia. The following chemicals were assessed in these studies: highMWPs [DEHP, di-isononyl phthalate (DiNP), di-isodecyl phthalate (DiDP), di-n-octyl phthalate (DOP), and benzylbutyl phthalate (BBzP)], lowMWPs [DEP, di-n-butyl phthalate (DBP), and di-iso-butyl phthalate (DiBP)], BPA, BPS, and BPF. One study investigated associations of foods with urinary paraben, benzophenone-3, triclosan, 2,4-dichlorophenol, and 2,5-dichlorophenol concentrations (37), but this review focuses on phthalates and bisphenols because diet is not a major source of exposure to these other chemicals (4, 7, 47, 48). The food categories in Supplemental Tables 1and2 were selected after abstracting all foods or dietary patterns from the 10 studies, and collapsing them across categories that were common to several studies (when possible). Additional subcategories were created when packaging or processing information was available for the same food item. For example, several studies reported on fish intake, but these studies assessed either general seafood intake, canned fish intake, or fish intake (unspecified type).
TABLE 2.
Summary of studies assessing dietary predictors of phthalate and bisphenol exposures in pregnant women1
| Study name (reference) | Recruitment, location, n | Chemicals assessed | Urine samples | Chemical analysis | Foods assessed | Dietary assessment | Urinary chemical adjustment and covariates |
|---|---|---|---|---|---|---|---|
| New Jersey Cohort (42) | • 2003–2004 • New Jersey • n = 150 |
Phthalates: • mCPP, mBzP • mEP, mBP, miBP |
1 urine • Before delivery |
• HPLC-MS/MS (CDC) | • Microwaved foods (plastic containers) • Plastic tableware • Plastic container storage |
• Questionnaire (at delivery, about pregnancy) | • None |
| Generation R Study (38) | • 2004–2005 • Netherlands • n = 642 |
Phthalates: • Sum-highMWPs = mECPP + mEHHP + mEOHP + mCMHP + mCPP + mBzP + mHxP + mHpP • Sum-DEHPs = mECPP + mEHHP + mEOHP + mCMHP • DOP, mCPP • Sum-lowMWPs = mMP + mEP + mBP + miBP • Phthalic acid Phenols: • BPA, BPS, BPF • Total bisphenols: BPA + BPS + BPF |
1 urine • First trimester |
• HPLC-ESI-MS/MS (The New York State Department of Health) | • Folic acid supplement • Vegetables • Grains • Fish/shellfish • Soft drinks • Soups and bouillon • Daily dietary caloric intake |
• 3-mo semiquantitative FFQ (first trimester) • Lifestyle questionnaire: supplements in pregnancy (first trimester) |
• Urinary creatinine (as covariate • Maternal age • Ethnicity • Prepregnancy BMI • Education • Parity • Smoking status • Folic acid supplementation • Daily dietary caloric intake |
| Infancia y Medio Ambiente Project (40) | • 2004–2006 • Spain • n = 391 |
Phthalates: • Sum-DEHPs = mEHP + mEHHP + mEOHP + mECPP + mCMHP • mBzP • mEP, miBP, mnBP |
2 urines • First trimester • Third trimester |
• UPLC-MS/MS (Bioanalysis Research Group at the Hospital del Mar Medical Research Institute) | • Bottled water • Microwaved foods (plastic containers) • Organic food • Milk, yogurt, cheese • Packed meat (sausages, pâtés) • Canned fish • Potato chips • Canned beverages (soda, beer) • Other canned food (soups, sauces) |
• 3-mo FFQ (first and third trimesters) • Whole pregnancy questionnaire: organic food, plastics (third trimester) |
• Creatinine-adjusted chemical • Maternal age • Country of origin • Prepregnancy BMI • Education • Urine collection time |
| Healthy Start Pre-Birth Cohort (37) | • 2009–2014 • Colorado • n = 446 |
Phthalates: • Sum-lowMWPs = mMP + mEP + miBP + mBP + mHiBP + mHBP • Sum-DBPs = mBP + miBP + mHBP + mHiBP • Sum-highMWPs = mBzP + mEHP + mNP + mEOHP + mEHHP + mECPP + mCOP + mCNP • Sum-DEHPs = mEHP + mEOHP + mEHHP + mECPPPhenols: • BPA, BPS • Sum-parabens = methyl + ethyl + propyl + butyl • 2,4-dichlorophenol, 2,5-dichlorophenol • Triclosan, benzophenone-3 |
1 urine • 24–32 wk |
• HPLC-MS/MS (CDC) | • Milk • Cheese • Yogurt • Ice cream • Soft drinks • Processed meat • Red meat • Seafood • Tofu • Fish oil supplements |
• 3-mo food propensity questionnaire (24–32 wk) • Questionnaire: fish oil supplement (24–32 wk) |
• Creatinine-adjusted chemical • Maternal age • Race • Prepregnancy BMI • Income • Education • Marital status • Employment status |
| Puerto Rico Testsite for Exploring Contamination Threats (41) | • 2010–2012 • Northern Puerto Rico • n = 139 |
Phthalates: • mEHP, mEHHP, mEOHP, mECPP, mCOP, mCNP, mCPP, mBzP • mEP, mnBP, miBP |
3 urines • 18 wk • 22 wk • 26 wk |
• HPLC-MS/MS (CDC) | • Milk, cheese, ice cream • Meat, chicken, fish • Cold cuts, hot dog, sausage • Microwaved foods/drinks (plastic container) • Bottled water |
• 48-h questionnaires (18, 22, and 26 wk) | • Specific gravity–adjusted chemical • Covariates that were associated with each specific chemical metabolite |
| Infant Development and Environmental Study (43) | • 2010–2012 • Minnesota, New York, Washington, California • n = 656 |
Phthalates: • Sum-DEHPs = mEHP + mEHHP + mEOHP + mECPP • mBzP • mEP • mBP • miBP |
1 urine • First trimester |
• HPLC-MS/MS (CDC) • HPLC-ESI-MS/MS (Environmental Health Laboratory at the University of Washington) |
• Peanut butter • Beef, poultry • Other meats (pork, lamb) • Oils and fats (butter, lard), spices • Soy, dairy, fast food • Bottled beverages • Organic/chemical free food • Grown/raised/caught food • Unprocessed food • Canned fruit or vegetables • Frozen fruit or vegetables |
• “Typical week” FFQ (first trimester) • General pregnancy behavior/lifestyle questionnaire (first trimester) |
• Specific gravity–adjusted chemical • Maternal age • BMI • Race • Study center • Education |
| Center for the Health Assessment of Mothers and Children of Salinas (44) | • 1999–2000 • California • n = 491 |
Phenol: • BPA |
2 urines • 5–28 wk • 18–39 wk |
• HPLC-MS/MS (CDC) | • Soda • Alcohol • Canned fruit • Bottled water • Pizza • Fish • Hamburgers |
• Alcohol and soda consumption throughout pregnancy (5–28 and 18–39 wk) • Modified 3-mo Block FFQ (18–39 wk) |
• Specific gravity–adjusted chemical • Maternal age • Years in United States • Prepregnancy BMI • Income:poverty ratio • Education • Marital status • Parity • Urine collection time • Smoke exposure • Alcohol and soda intake |
| Health Outcomes and Measures of the Environment Study (45) | • 2003–2006 • Ohio • n = 389 |
Phenol:BPA | 3 urines • 16 wk • 26 wk • Within 24 h of delivery |
• HPLC-MS/MS (CDC) | • Fresh or frozen fish (store) • Fresh fruit or vegetables (store) • Canned fruit or vegetables • Organic foods • Vegetarianism |
• Frequency of consumption questionnaire (conception to 20 wk and 20 wk to birth) | • Creatinine-adjusted chemical • Maternal age • Race • Income • Education • Marital status |
| Sabadell Birth Cohort (INMA Project) (39) | • 2004–2006 • Spain • n = 479 |
Phenol: BPA |
2 urines • 12 wk • 32 wk |
• LC-MS (The Department of Analytical Chemistry Laboratory) | • Milk, yogurt • Packaged meat (sausages, pâtés) • Nonpackaged meat (pork, chicken) • Canned fish • Noncanned fish (white fish, seafood) • Potato chips • Canned beverages (soda, beer) • Other canned foods (soups, sauces) • Fruits, vegetables (fresh) • Bottled water (consumption and cooking) • Organic food • Microwaved foods/drinks (plastic container) |
• 3-mo semiquantitative FFQ (12 and 32 wk) • Pregnancy questionnaire: water, organic foods, microwaving foods/drinks (32 wk) |
• Creatinine-adjusted chemical • Maternal age • Prepregnancy BMI • Social class • Education • Urine collection time • Smoking status • Second-hand smoke exposure |
| Australian Maternal Exposure to Toxic Substances Study (46) | • 2008–2011 • Western Australia • n = 26 |
Phenol: BPA |
1 urine • 38 wk |
• HPLC-MS/MS (The National Centre for Environmental Toxicology) | • Canned foods • Microwaved foods/drinks (plastic container) • Plastic container storage • Refillable bottles |
• Pregnancy questionnaire (38 wk) | • None |
1BPA, bisphenol A; BPF, bisphenol F; BPS, bisphenol S; DEHP, di(2-ethylhexyl) phthalate; DOP, di-n-octyl phthalate; HPLC-EIS-MS/MS, HPLC-electrospray ionization-tandem MS; HPLC-MS/MS, HPLC-tandem MS; mBP, mono-n-butyl phthalate; mBzP, monobenzyl phthalate; mCMHP, mono(2-carboxymethyl)hexyl phthalate; mCNP, monocarboxynonyl phthalate; mCOP, monocarboxyoctyl phthalate; mCPP, mono(3-carboxypropyl) phthalate; mECPP, mono(2-ethyl-5-carboxypentyl) phthalate; mEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; mEHP, mono(2-ethylhexyl) phthalate; mEOHP, mono(2-ethyl-5-oxohexyl) phthalate; mEP, monoethyl phthalate; mHBP, mono-hydroxybutyl phthalate; mHiBP, mono-hydroxyl-isobutyl phthalate; mHpP, mono-2-heptylphthalate; mHxP, mono-hexylphthalate; miBP, mono-isobutyl phthalate; mMP, mono-methylphthalate; mnBP, mono-n-butyl phthalate; mNP, mono-isononyl phthalate; sum-DBPs, sum of di-n-butyl phthalate metabolites; sum-DEHPs, sum of di(2-ethylhexyl) phthalate metabolites; sum-highMWPs, sum of high-molecular-weight phthalate metabolites; sum-lowMWPs, sum of low-molecular-weight phthalate metabolites; UPLC-MS/MS, ultra-performance LC-tandem MS.
Current Status of Knowledge
Food-monitoring studies are performed worldwide to evaluate the safety of foods and dietary patterns, including assessing exposures to environmental chemicals through certain dietary practices. These studies are performed by government agencies, as well as independent laboratories around the world. For example, the US FDA has ongoing food-monitoring programs such as the Total Diet Study (TDS) and the Chemical Contaminants Monitoring Program to examine the safety of foods on the US market (49). Through these programs, the FDA collects information about consumer food preparation and consumption practices (TDS), as well as the potential exposure to and risk of chemical contaminants found in the US food supply (TDS and Chemical Contaminants Monitoring Program) (49). Similarly, the European Food Safety Authority carries out risk assessments and food-monitoring studies within the European Union to determine the safety of chemical contaminants in foods consumed by humans and animals (50). However, many of these government food-monitoring programs have not assessed concentrations of phthalates and bisphenols in foods. Therefore, the food-monitoring studies assessing exposure to phthalates and bisphenols from the food supply (reviewed below) were conducted by independent laboratories around the world. The goal of these food-monitoring studies was to evaluate the potential for human exposure to phthalates and bisphenols through food by capturing dietary habits from various countries, including the United States, Canada, United Kingdom, France, Spain, Norway, Belgium, Tunisia, Israel, China, and Japan. There are extensive reviews in the literature summarizing food-monitoring studies that measured phthalate and BPA concentrations in foods (5, 51), and some of the results from these reviews and additional food-monitoring studies are summarized below.
Food-Monitoring Studies
Meat
Foods of animal origin, including beef, pork, and poultry, are major sources of highMWPs and BPA from processing and packaging (52), partially because highMWPs, and to a lesser extent lowMWPs, are slightly lipophilic and can bioaccumulate in fat-containing foods (53). International food-monitoring studies consistently report high detectable concentrations of highMWPs (especially DEHP) and BPA in meat and meat products (5, 54–59). Food-monitoring studies have also reported low, but detectable, concentrations of lowMWPs [compared with highMWPs (5)] in meat and meat products, suggesting that meats may also be important sources of DEP, DBP, and DiBP (54, 55).
Seafood
Numerous food-monitoring studies from the United Kingdom, Norway, Belgium, China, and the United States have reported detectable concentrations of phthalates and BPA in seafood products (54–56, 60). Similarly to other foods packaged in plastics (6, 54) and cans (59, 61), these food-monitoring studies also suggest that food packaging materials contribute to phthalate and BPA concentrations detected in seafood products. A study in Spain found that 34.7% of Spanish pregnant women reported consuming canned fish ≥1–3 times/wk, making it the most frequently consumed canned food in this population and a major source of BPA during pregnancy (62).
Fruits and vegetables
Fruit and vegetable consumption is considered a measure of healthier lifestyles associated with lower EDC exposures (59, 63). Food-monitoring studies from Belgium, France, China, and the United States report low concentrations of both highMWPs and lowMWPs in fruit and vegetable products, suggesting low likelihood for phthalate exposure from these foods (55–57, 64, 65). However, 1 study has shown that exposure to highMWPs and lowMWPs in vegetables primarily comes from ready-to-eat vegetables (e.g., lettuce, arugula, parsley, carrot, and corn salad) packaged in plastic bags (66). BPA food-monitoring studies from Norway, Canada, and the United States suggest that canned fruits and vegetables, rather than fresh, are major exposure sources (59, 67), and that overall concentrations of BPA in noncanned fruits and vegetables are relatively low (54, 58, 68).
Dairy products
Milk, yogurt, cheese, ice cream, and butter can be high in fats, making it possible for phthalates to accumulate in these foods (53). Analyses of milk in Belgium found higher concentrations of highMWPs (DEHP and BBzP) and lowMWPs (DBP and DiBP) in milk retail products than in raw cow milk, suggesting that phthalates can migrate into raw cow milk from contaminated feed ingested by cows, during the mechanical milking process, and/or from milk packaging materials used at the dairy factory (69). Food-monitoring studies reported detectable concentrations of DEHP in select cheese samples from Canada (1), DEHP/DiNP/DOP in milk and cheese from Norway (54), DEHP/DOP/BBzP in milk and other dairy from the United States (56), DEHP in milk and dairy products from Belgium (55), and BBzP in milk, butter/oil, and yogurt from Tunisia (70). In contrast, Norwegian and US food-monitoring studies reported low or undetectable concentrations of lowMWPs in milk and milk products (5, 54–56).
Similarly to highMWPs, BPA has also been detected in milk and dairy products. In Europe, higher BPA concentrations were found in canned milk and dairy products than in uncanned products (European Food Safety Authority) (71). The BPA concentrations in European dairy are consistent with those from China (72), Canada (59), and Japan (73). Detectable BPA concentrations were also found in dairy products (milk, ultra-fresh dairy products, and cheese) consumed by French pregnant women (74), suggesting that these are important sources of BPA exposure in pregnancy.
Fast food
Food-monitoring and epidemiological studies suggest that fast food and/or foods served at restaurants are likely sources of phthalate and BPA exposures (5, 53). Although phthalates and bisphenols could leach into foods during processing, they have also been shown to migrate into foods from packaging, including pizza boxes or sandwich wrappers (75). A food-monitoring study in Canada reported detectable concentrations of BPA in some fast-food products (French fries, hamburgers, and sandwiches), but not others (pizza and chicken nuggets), and concluded that hamburgers had the highest BPA concentrations of all fast foods assessed (59). An assessment of fast food intake in the general US population (NHANES 2005–2014) found that, compared with nonconsumers, both low and high consumers of food away from home had significantly higher concentrations of urinary sum of di(2-ethylhexyl) phthalate metabolites (sum-DEHPs) and monocarboxyoctyl phthalate [but not mBzP (monobenzyl phthalate), mEP, mBP (mono-n-butyl phthalate), or miBP (mono-isobutyl phthalate) (76)]. A similar study further analyzed fast food consumption in the US population by food group and found that fast-food grain, including pizza, intake was associated with urinary sum-DEHPs and sum of di-isononyl phthalate metabolites (sum-DiNPs), whereas potato chip and hamburger intakes were associated with higher urinary sum-DEHPs and sum-DiNPs (77).
Organic and environmentally friendly food and dietary patterns
Organic, chemical-free, and environmentally friendly foods are generally considered markers of healthier lifestyles, but associations of these dietary patterns with EDC exposures are not well characterized. Although the literature is limited, a dietary intervention that focused on exclusive consumption of fresh and organic foods for 3 d found that urinary DEHP metabolite concentrations decreased by 53–56% and urinary BPA concentrations decreased by 66% from pre- to during-intervention. However, foods provided to and prepared by these participants were from plastic-free packaging and nonplastic containers (52), making it difficult to determine if the decreases in urinary chemical concentrations were due to the foods’ organic status or their packaging/preparation materials. Similarly, residents of a rural vegetarian/vegan community in Israel had significantly lower urinary phthalate metabolite concentrations, but BPA concentrations in these individuals were not different from those in the general Israeli population (78). However, a US dietary intervention with fresh and organic foods prepared without plastics found significantly higher urinary DEHP metabolites after the intervention, which was due to increased intake of certain foods (e.g., spices and peanut butter) (79).
Plastic containers and tableware
Both phthalates and BPA are used to manufacture plastics for food storage and cooking containers (4). Data suggest that these are important sources of human EDC exposure, because these EDCs can migrate from plastic containers and tableware into foods and beverages, especially during heating and cooling (5, 62). For phthalates, migration levels of DBP were higher with prolonged plastic container use and longer heating time (80), and high concentrations of DEHP and DBP were found in plastic tableware at room temperature (81). BPA, however, migrates from reusable polycarbonate plastic water bottles into water at room and high temperatures (82), and the use of polycarbonate water bottles has been shown to increase urinary BPA concentrations (83), especially during the hot summer months (84).
Canned foods and beverages
BPA is used to manufacture polycarbonate and epoxy resins for metal can linings and is detectable in a variety of canned foods (54, 59, 85–87). A study characterizing dietary BPA exposure in the French population (including pregnant women) identified that canned products accounted for ∼50% of total BPA exposure (74). Phthalates are generally found in plastic food packaging materials, so their concentrations in canned products are low, but detectable (54).
Dietary Predictors of Phthalates and Bisphenols in Pregnancy
Figure 1 summarizes potential sources of phthalates and bisphenols from food packaging materials and consumer food practices. As previously mentioned, both diet and physiology are greatly modified in pregnancy (33–36). Thus, despite the many food-monitoring studies assessing dietary sources of phthalates and BPA in the general public, studies specifically in pregnancy are important for establishing dietary recommendations for this vulnerable population. In the 10 studies reviewed here, urinary concentrations of phthalates and bisphenols in women from US studies were comparable to those of women in the general US population [using data from the 2013–2014 NHANES (2)]. The studies performed outside the United States recruited cohorts to reflect their individual populations, suggesting that exposures described in these studies likely represent the general population of each country. Overall, in pregnancy, associations between use of plastic containers and increased urinary phthalate metabolite concentrations, and between consumption of canned foods and increased urinary BPA concentrations were consistent with previous food-monitoring studies. Foods and dietary patterns associated with a healthier lifestyle, such as organic foods, grown/raised/caught foods, vegetarianism, and folic acid supplementation, as well as some other dietary patterns and foods, including soups and bouillon, spices, and grains, were generally associated with lower urinary phthalate metabolite and bisphenol concentrations in pregnant women. However, despite the many food-monitoring studies reporting high phthalate and BPA concentrations in meats and dairy, the designs of most studies in pregnant women were unable to reliably detect associations of specific foods/food groups with phthalates and BPA. Some of the differences and inconsistencies in study design are highlighted in Table 2 and will be discussed in the following sections.
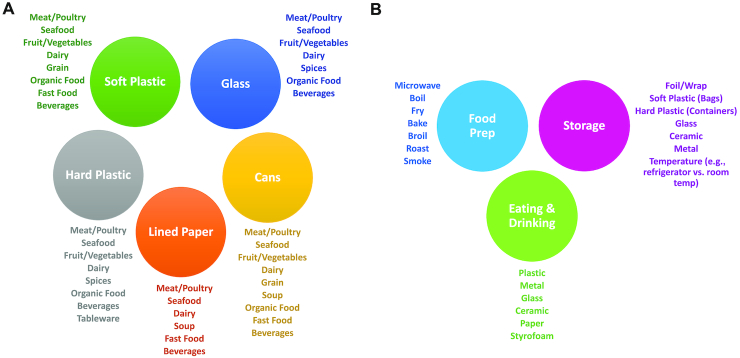
FIGURE 1.
Summary of potential food packaging materials and consumer food practices. (A) Potential determinants of phthalate and BPA exposures through food packaging materials, including soft and hard plastics, cans, lined paper, and glass. (B) Consumer food practices, including food preparation, as well as storage and consumption materials that influence exposure to phthalates and BPA. BPA, bisphenol A.
Measuring urinary phthalates and bisphenols
Some technical/analytical factors may be important to consider when evaluating currently available studies in pregnant women. First, the analytical methodologies vary slightly among the 10 studies reviewed here (Table 2), and additional studies may be needed to determine whether unifying these methods would eliminate reported inconsistencies between studies. Another important technical consideration when using urine to assess chemical exposures is establishing appropriate methods to account for urine dilution across participants. In the studies reviewed here, 2 studies did not adjust for urinary dilution, whereas 5 adjusted for creatinine and 3 adjusted for specific gravity (Table 2). Urinary density can vary greatly across pregnancy and depending on hydration status, therefore raw values of phthalate and bisphenol concentrations need to be adjusted for some measure of urine density. Furthermore, it has been suggested that creatinine in pregnancy is affected by pregnancy-related fluid dilution, making specific gravity adjustment a more accurate approach in pregnant women (88).
Evaluating exposure to phthalates and bisphenols
Most studies in pregnant women reviewed here included analyses of phthalates and bisphenols, but classification of these chemicals often varied across studies. For example, findings for associations between milk consumption and urinary mBP (metabolite of DBP) in pregnancy were conflicting. One study reported that the sum of di-n-butyl phthalate metabolite concentrations (composed of mBP, miBP, mono-hydroxybutyl phthalate, and mono-hydroxyl-isobutyl phthalate) was negatively associated with milk consumption (37), whereas other studies found no association (41) or a positive association (40) between milk consumption and urinary mBP, and no association with urinary miBP (40, 41). Similar examples were also observed for sum or individual measures of other phthalates and bisphenols (Table 2 and Supplemental Tables 1 and 2), which suggests that classifying chemicals into their predicted exposure sources (parent chemicals) may yield differing results compared with assessment of individual breakdown metabolites.
Studies in pregnant women report low reproducibility and sensitivity of urinary phthalate metabolites and bisphenols across pregnancy (89–93). To overcome this challenge, several of the studies reviewed here collected 2 (39, 40, 44) or even 3 (41, 45) urine samples across pregnancy to evaluate gestational exposure to bisphenols and phthalates. Assessing these relations at multiple times in pregnancy may be critical to account for some of the physiological and dietary shifts that occur in early compared with late pregnancy. However, aligning the timing of exposure assessment in relation to diet may be more important to accurately and consistently predict dietary sources of chemical exposure. Based on the current review of the literature, only 1 study accurately captured dietary sources of phthalates and bisphenols (41), as it assessed chemical exposure within 48 h of the dietary report, whereas other studies used surveys that assessed much broader windows of dietary intakes. This appears to be a critical difference in study design, as many observations differed between this study and others. For example, in 1 study, milk consumption assessed by a 3-mo FFQ was associated with increased urinary mBzP concentrations (40), whereas mBzP assessed within 48 h of dietary assessment was not associated with milk consumption (41). Similarly, higher cheese consumption was associated with lower urinary mBzP (41) when assessed within 48 h of the dietary survey, whereas no associations were reported with urinary mBzP in a study assessing diet using a 3-mo dietary questionnaire (40). Given that the study assessing chemical exposure within 48 h of the dietary report was limited to women in northern Puerto Rico, future studies in other pregnant populations should expand on findings from this study by assessing chemical exposure at multiple times across pregnancy and within 24–48 h of dietary assessment.
Assessing diet in pregnancy
Dietary patterns are most often assessed using FFQs, which are inexpensive, easy to use, and validated to reflect long-term dietary intake patterns in pregnant populations (94, 95). Because FFQs and other similar questionnaires are designed to assess dietary intake of nutrients, not chemicals in food, they may not accurately predict dietary sources of phthalate and BPA exposure. Especially problematic is that information collected from these questionnaires spans weeks or months, whereas the short half-lives of EDCs mean that urinary exposure assessment reflects their concentrations within (often) 24 h of assessment. Based on the aforementioned examples, assessment of diet within ∼24 h of exposure assessment will be the most appropriate approach for quantifying dietary sources of phthalates, bisphenols, and other environmental chemicals that have relatively short half-lives.
The other critical factor to consider when evaluating currently available studies in pregnancy is the broad range of dietary surveys used in these studies (Table 2). First, many studies utilized exceedingly general categories of various food types. Examples of this include using the vague category of “processed meat” (37), the term “seafood” (37, 38, 43) to refer to all types of fish or shellfish, the combined analysis of all “fruits and vegetables” (43), and the overly broad analysis of “fast food” (43) without specifying the food item or restaurant category. These general categories make it difficult to establish patterns of chemical exposures from specific foods, and to ultimately provide pregnant women with specific recommendations as they make food-purchasing decisions.
More importantly, many studies reviewed here acknowledged that their dietary surveys were unable to distinguish between various categories of food packaging, including fresh, frozen, or packaged foods. For example, in the Infancia y Medio Ambiente Project, first- and second-trimester consumption of canned fish was associated with higher urinary BPA concentrations (39), whereas 2 other studies found no associations between fresh, frozen, or uncanned fish consumption and urinary BPA (39, 45). BPA is used to manufacture polycarbonate and epoxy resins for metal can linings and is detectable in a variety of canned foods (54, 59, 85–87), so observations of higher BPA concentrations with consumption of canned (but not other) fish are consistent with food-monitoring studies. However, several other studies assessed seafood or fish intake with BPA, but the dietary questionnaires in these studies did not distinguish between fresh or canned fish, making it difficult to conclusively interpret these findings (37, 38, 44). Future studies should utilize dietary questionnaires that robustly assess both diet and the mode of food packaging/preparation by asking specifically about how each food item was packaged (e.g., fresh, canned, plastic, glass) and prepared (e.g., microwaved, steamed in plastic or glass).
Considering demographic and lifestyle factors
Another major challenge for establishing dietary correlates of EDC exposure are the cultural differences in both dietary intake and chemical production/food packaging. For example, associations of meat and dairy intake with phthalates and bisphenols differed greatly between pregnancy studies in the contiguous United States, Puerto Rico, and Spain. These differences likely reflect variability in the types and amounts of meats/dairy foods consumed in these cultures, and how these foods are processed/packaged. Although it is important to establish generalizable recommendations for pregnant women, these cultural differences may require culturally sensitive recommendations.
Most studies reviewed here acknowledged that many of the unexpected associations observed between some particular EDCs and foods may be due to other concomitant lifestyle factors. Here, and in other pregnancy cohorts, these factors included maternal age, race/ethnicity, country of origin, prepregnancy BMI, marital status, education, employment status, annual income, and personal care/household product use (37–41, 43–45, 90–93, 96). For example, mEP concentrations were higher in pregnant women who used bottled water for cooking 48 h before exposure assessment than in women who used the public water supply (41). Given that DEP (mEP's parent compound) is primarily found in personal care products, the authors of this study (41) suggest that the positive association between bottled water use and mEP is unrelated to use of plastics, and could be explained by higher urinary mEP concentrations in women reporting use of perfume/cologne and colored cosmetics. In addition to carefully controlling for other important lifestyle factors in statistical models, if dietary questionnaires fail to capture cultural or geographic differences in food packaging or processing, future studies may need to investigate these factors as modifiers of food–exposure relations. For example, annual income or employment status may affect seafood choices. Therefore, although no overall associations may be observed between fish consumption and phthalate exposure, positive associations are possible in low-income groups that tend to consume canned or packaged fish, but not in higher-income women who tend to eat fresh or unpackaged fish.
Conclusion
Maternal diet is an established determinant of a healthy pregnancy and fetal outcomes. Phthalates and BPA are known to affect pregnancy and fetal development, and the 10 studies reviewed here suggest that certain dietary components or patterns are important sources of BPA, highMWPs, and lowMWPs in pregnancy. However, consistencies in observed associations between studies were limited to long-term lifestyle choices, including those related to home food preparation (use of plastics to store, prepare, and heat foods) and choices of food types (canned, organic). Several well-designed studies in nonpregnant populations do suggest that changing dietary behaviors can limit exposure to phthalates and bisphenols. For example, a dietary intervention of 5 families in the United States (n = 20) found significant reductions in urinary BPA and DEHP metabolites after limited consumption of foods packaged and prepared in plastics and cans, and increased concentrations of these chemicals with resumption of packaged food consumption (52). Furthermore, a strict 48-h fasting study of 5 individuals from Germany observed significant reductions in urinary highMWPs from prefast to postfast, whereas urinary lowMWP concentrations stayed consistent throughout the study (97). These findings suggest that addressing the inconsistencies in study designs among the pregnancy studies described here could provide valuable insight for establishing specific EDC-limiting dietary recommendations to improve pregnancy and fetal outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—DCP: performed the literature research for this review; DCP and RSS: developed ideas for the manuscript; and all authors: wrote, read, and approved the final manuscript.
Notes
Supported by National Institute of Environmental Health Sciences (NIH/NIEHS) grant R00ES024795 (to RSS), the USDA National Institute of Food and Agriculture, and Michigan AgBioResearch.
Author disclosures: DCP, SS, and RSS, no conflicts of interest.
This publication's contents are solely the responsibility of the grantee and do not necessarily represent the official views of the NIH.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: BBzP, benzylbutyl phthalate; BPA, bisphenol A; BPF, bisphenol F; BPS, bisphenol S; DBP, di-n-butyl phthalate; DEHP, di(2-ethylhexyl) phthalate; DEP, diethyl phthalate; DiBP, di-iso-butyl phthalate; DiNP, di-isononyl phthalate; DOP, di-n-octyl phthalate; EDC, endocrine disrupting chemical; highMWP, high-molecular-weight phthalate; lowMWP, low-molecular-weight phthalate; mBP, mono-n-butyl phthalate; mBzP, monobenzyl phthalate; mEP, monoethyl phthalate; miBP, mono-isobutyl phthalate; sum-DEHPs, sum of di(2-ethylhexyl) phthalate metabolites; sum-DiNPs, sum of di-isononyl phthalate metabolites; TDS, Total Diet Study.
References
- 1. Cao XL, Zhao W, Churchill R, Hilts C. Occurrence of di-(2-ethylhexyl) adipate and phthalate plasticizers in samples of meat, fish, and cheese and their packaging films. J Food Prot. 2014;77(4):610–20. [DOI] [PubMed] [Google Scholar]
- 2. CDC. Fourth national report on human exposure to environmental chemicals, updated tables (March 2018). Atlanta (GA): US Department of Health and Human Services, Centers for Disease Control and Prevention; 2018. [Google Scholar]
- 3. Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect. 2014;122(3):235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CDC. Biomonitoring summaries. [Internet] Atlanta (GA): CDC; 2017; [cited 2019 Jan 13]. Available from: https://www.cdc.gov/biomonitoring/biomonitoring_summaries.html. [Google Scholar]
- 5. Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health. 2014;13(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Erythropel HC, Maric M, Nicell JA, Leask RL, Yargeau V. Leaching of the plasticizer di(2-ethylhexyl) phthalate (DEHP) from plastic containers and the question of human exposure. Appl Microbiol Biotechnol. 2014;98(24):9967–81. [DOI] [PubMed] [Google Scholar]
- 7. Philippat C, Wolff MS, Calafat AM, Ye X, Bausell R, Meadows M, Stone J, Slama R, Engel SM. Prenatal exposure to environmental phenols: concentrations in amniotic fluid and variability in urinary concentrations during pregnancy. Environ Health Perspect. 2013;121(10):1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rochester JR, Bolden AL. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect. 2015;123(7):643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lorber M, Schecter A, Paepke O, Shropshire W, Christensen K, Birnbaum L. Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ Int. 2015;77:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noonan GO, Ackerman LK, Begley TH. Concentration of bisphenol A in highly consumed canned foods on the U.S. market. J Agric Food Chem. 2011;59(13):7178–85. [DOI] [PubMed] [Google Scholar]
- 11. Calafat AM, Longnecker MP, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, Rudel RA, Engel SM, Teitelbaum SL et al.. Optimal exposure biomarkers for nonpersistent chemicals in environmental epidemiology. Environ Health Perspect. 2015;123(7):A166–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat 23. 2005(25):1–160. [PubMed] [Google Scholar]
- 13. CDC. National Health and Nutrition Examination Survey data. National Center for Health Statistics , editor. Hyattsville (MD): US Department of Health and Human Services, Centers for Disease Control and Prevention; 2013–2014. [Google Scholar]
- 14. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: the Endocrine Society's second Scientific Statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36(6):E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society Scientific Statement. Endocr Rev. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aker AM, Watkins DJ, Johns LE, Ferguson KK, Soldin OP, Anzalota Del Toro LV, Alshawabkeh AN, Cordero JF, Meeker JD. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ Res. 2016;151:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang PC, Tsai CH, Liang WY, Li SS, Huang HB, Kuo PL. Early phthalates exposure in pregnant women is associated with alteration of thyroid hormones. PLoS One. 2016;11(7):e0159398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toft G, Jonsson BA, Lindh CH, Jensen TK, Hjollund NH, Vested A, Bonde JP. Association between pregnancy loss and urinary phthalate levels around the time of conception. Environ Health Perspect. 2012;120(3):458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, Herrick R, Swan SH. Maternal urinary metabolites of di-(2-ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol. 2009;169(8):1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karwacka A, Zamkowska D, Radwan M, Jurewicz J. Exposure to modern, widespread environmental endocrine disrupting chemicals and their effect on the reproductive potential of women: an overview of current epidemiological evidence. Hum Fertil (Camb). 2019 Apr;22:(1):2–25..doi: 10.1080/14647273.2017.1358828. Epub 2017 Jul 31. [DOI] [PubMed] [Google Scholar]
- 21. Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168(1):61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Findlay LC, Kohen DE. Bisphenol A and child and youth behaviour: Canadian Health Measures Survey 2007 to 2011. Health Rep. 2015;26(8):3–9. [PubMed] [Google Scholar]
- 23. Philippat C, Nakiwala D, Calafat AM, Botton J, De Agostini M, Heude B, Slama R; Eden Mother–Child Study Group. Prenatal exposure to nonpersistent endocrine disruptors and behavior in boys at 3 and 5 years. Environ Health Perspect. 2017;125(9):097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vernet C, Pin I, Giorgis-Allemand L, Philippat C, Benmerad M, Quentin J, Calafat AM, Ye X, Annesi-Maesano I, Siroux V et al.. In utero exposure to select phenols and phthalates and respiratory health in five-year-old boys: a prospective study. Environ Health Perspect. 2017;125(9):097006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buckley JP, Quiros-Alcala L, Teitelbaum SL, Calafat AM, Wolff MS, Engel SM. Associations of prenatal environmental phenol and phthalate biomarkers with respiratory and allergic diseases among children aged 6 and 7 years. Environ Int. 2018;115:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhandari R, Xiao J, Shankar A. Urinary bisphenol A and obesity in U.S. children. Am J Epidemiol. 2013;177(11):1263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deierlein AL, Wolff MS, Pajak A, Pinney SM, Windham GC, Galvez MP, Rybak M, Calafat AM, Kushi LH, Biro FM et al.. Phenol concentrations during childhood and subsequent measures of adiposity among young girls. Am J Epidemiol. 2017;186(5):581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lakind JS, Naiman DQ. Daily intake of bisphenol A and potential sources of exposure: 2005–2006 National Health and Nutrition Examination Survey. J Expo Sci Environ Epidemiol. 2011;21(3):272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fromme H, Gruber L, Schlummer M, Wolz G, Bohmer S, Angerer J, Mayer R, Liebl B, Bolte G. Intake of phthalates and di(2-ethylhexyl)adipate: results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data. Environ Int. 2007;33(8):1012–20. [DOI] [PubMed] [Google Scholar]
- 30. Muncke J. Endocrine disrupting chemicals and other substances of concern in food contact materials: an updated review of exposure, effect and risk assessment. J Steroid Biochem Mol Biol. 2011;127(1–2):118–27. [DOI] [PubMed] [Google Scholar]
- 31. Muncke J, Backhaus T, Geueke B, Maffini MV, Martin OV, Myers JP, Soto AM, Trasande L, Trier X, Scheringer M. Scientific challenges in the risk assessment of food contact materials. Environ Health Perspect. 2017;125(9):095001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wittassek M, Koch HM, Angerer J, Bruning T. Assessing exposure to phthalates – the human biomonitoring approach. Mol Nutr Food Res. 2011;55(1):7–31. [DOI] [PubMed] [Google Scholar]
- 33. Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2012;51(6):365–96. [DOI] [PubMed] [Google Scholar]
- 34. Tasnif Y, Morado J, Hebert MF. Pregnancy-related pharmacokinetic changes. Clin Pharmacol Ther. 2016;100(1):53–62. [DOI] [PubMed] [Google Scholar]
- 35. Chen LW, Low YL, Fok D, Han WM, Chong YS, Gluckman P, Godfrey K, Kwek K, Saw SM, Soh SE et al.. Dietary changes during pregnancy and the postpartum period in Singaporean Chinese, Malay and Indian women: the GUSTO birth cohort study. Public Health Nutr. 2014;17(9):1930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Kleinman KP, Oken E, Gillman MW. Changes in dietary intake from the first to the second trimester of pregnancy. Paediatr Perinat Epidemiol. 2006;20(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Polinski KJ, Dabelea D, Hamman RF, Adgate JL, Calafat AM, Ye X, Starling AP. Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the Healthy Start Study. Environ Res. 2018;162:308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Philips EM, Jaddoe VWV, Asimakopoulos AG, Kannan K, Steegers EAP, Santos S, Trasande L. Bisphenol and phthalate concentrations and its determinants among pregnant women in a population-based cohort in the Netherlands, 2004–5. Environ Res. 2018;161:562–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Casas M, Valvi D, Luque N, Ballesteros-Gomez A, Carsin AE, Fernandez MF, Koch HM, Mendez MA, Sunyer J, Rubio S et al.. Dietary and sociodemographic determinants of bisphenol A urine concentrations in pregnant women and children. Environ Int. 2013;56:10–18. [DOI] [PubMed] [Google Scholar]
- 40. Valvi D, Monfort N, Ventura R, Casas M, Casas L, Sunyer J, Vrijheid M. Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. Int J Hyg Environ Health. 2015;218(2):220–31. [DOI] [PubMed] [Google Scholar]
- 41. Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, Calafat AM, Crespo N, Jimenez-Velez B, Padilla IY et al.. Urinary phthalate metabolite concentrations among pregnant women in northern Puerto Rico: distribution, temporal variability, and predictors. Environ Int. 2014;62:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yan X, Calafat A, Lashley S, Smulian J, Ananth C, Barr D, Silva M, Ledoux T, Hore P, Robson MG. Phthalates biomarker identification and exposure estimates in a population of pregnant women. Hum Ecol Risk Assess. 2009;15(3):565–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Serrano SE, Karr CJ, Seixas NS, Nguyen RH, Barrett ES, Janssen S, Redmon B, Swan SH, Sathyanarayana S. Dietary phthalate exposure in pregnant women and the impact of consumer practices. Int J Environ Res Public Health. 2014;11(6):6193–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quiros-Alcala L, Eskenazi B, Bradman A, Ye X, Calafat AM, Harley K. Determinants of urinary bisphenol A concentrations in Mexican/Mexican–American pregnant women. Environ Int. 2013;59:152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, Barr DB, Sathyanarayana S, Lanphear BP. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119(1):131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Callan AC, Hinwood AL, Heffernan A, Eaglesham G, Mueller J, Odland JO. Urinary bisphenol A concentrations in pregnant women. Int J Hyg Environ Health. 2013;216(6):641–4. [DOI] [PubMed] [Google Scholar]
- 47. Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ Health Perspect. 2010;118(5):679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Perspect. 2008;116(3):303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. FDA. Science & research (food). [Internet] Silver Spring (MD): US Food & Drug Administration; 2018; [cited 13 January, 2019]. Available from: https://www.fda.gov/food/foodscienceresearch/default.htm. [Google Scholar]
- 50. EFSA. Chemical contaminants. [Internet] Parma: European Food Safety Authority; 2018; [cited 2019 Jan 13]. Available from: http://www.efsa.europa.eu/en/topics/topic/chemical-contaminants. [Google Scholar]
- 51. Careghini A, Mastorgio AF, Saponaro S, Sezenna E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: a review. Environ Sci Pollut Res Int. 2015;22(8):5711–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, Rizzo J, Nudelman JL, Brody JG. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119(7):914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cao XL. Phthalate esters in foods: sources, occurrence, and analytical methods. Compr Rev Food Sci Food Saf. 2010;9(1):21–43. [DOI] [PubMed] [Google Scholar]
- 54. Sakhi AK, Lillegaard IT, Voorspoels S, Carlsen MH, Loken EB, Brantsaeter AL, Haugen M, Meltzer HM, Thomsen C. Concentrations of phthalates and bisphenol A in Norwegian foods and beverages and estimated dietary exposure in adults. Environ Int. 2014;730:259–69. [DOI] [PubMed] [Google Scholar]
- 55. Van Holderbeke M, Geerts L, Vanermen G, Servaes K, Sioen I, De Henauw S, Fierens T. Determination of contamination pathways of phthalates in food products sold on the Belgian market. Environ Res. 2014;134:345–52. [DOI] [PubMed] [Google Scholar]
- 56. Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, Hommel M, Imran N, Hynan LS, Cheng D et al.. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ Health Perspect. 2013;121(4):473–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guo Y, Zhang Z, Liu L, Li Y, Ren N, Kannan K. Occurrence and profiles of phthalates in foodstuffs from China and their implications for human exposure. J Agric Food Chem. 2012;60(27):6913–9. [DOI] [PubMed] [Google Scholar]
- 58. Liao C, Kannan K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem. 2013;61(19):4655–62. [DOI] [PubMed] [Google Scholar]
- 59. Cao XL, Perez-Locas C, Dufresne G, Clement G, Popovic S, Beraldin F, Dabeka RW, Feeley M. Concentrations of bisphenol A in the composite food samples from the 2008 Canadian total diet study in Quebec City and dietary intake estimates. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28(6):791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bradley EL, Burden RA, Bentayeb K, Driffield M, Harmer N, Mortimer DN, Speck DR, Ticha J, Castle L. Exposure to phthalic acid, phthalate diesters and phthalate monoesters from foodstuffs: UK total diet study results. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30(4):735–42. [DOI] [PubMed] [Google Scholar]
- 61. Schecter A, Malik N, Haffner D, Smith S, Harris TR, Paepke O, Birnbaum L. Bisphenol A (BPA) in U.S. food. Environ Sci Technol. 2010;44(24):9425–30. [DOI] [PubMed] [Google Scholar]
- 62. Mariscal-Arcas M, Rivas A, Granada A, Monteagudo C, Murcia MA, Olea-Serrano F. Dietary exposure assessment of pregnant women to bisphenol-A from cans and microwave containers in southern Spain. Food Chem Toxicol. 2009;47(2):506–10. [DOI] [PubMed] [Google Scholar]
- 63. Bai PY, Wittert GA, Taylor AW, Martin SA, Milne RW, Shi Z. The association of socio-demographic status, lifestyle factors and dietary patterns with total urinary phthalates in Australian men. PLoS One. 2015;10(4):e0122140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mervish N, McGovern KJ, Teitelbaum SL, Pinney SM, Windham GC, Biro FM, Kushi LH, Silva MJ, Ye X, Calafat AM et al.. Dietary predictors of urinary environmental biomarkers in young girls, BCERP, 2004–7. Environ Res. 2014;133:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Martine B, Marie-Jeanne T, Cendrine D, Fabrice A, Marc C. Assessment of adult human exposure to phthalate esters in the urban centre of Paris (France). Bull Environ Contam Toxicol. 2013;90(1):91–6. [DOI] [PubMed] [Google Scholar]
- 66. Cacho JI, Campillo N, Vinas P, Hernandez-Cordoba M. Determination of alkylphenols and phthalate esters in vegetables and migration studies from their packages by means of stir bar sorptive extraction coupled to gas chromatography–mass spectrometry. J Chromatogr A. 2012;1241:21–7. [DOI] [PubMed] [Google Scholar]
- 67. Cao XL, Corriveau J, Popovic S. Bisphenol A in canned food products from Canadian markets. J Food Prot. 2010;73(6):1085–9. [DOI] [PubMed] [Google Scholar]
- 68. Lu J, Wu J, Stoffella PJ, Wilson PC. Analysis of bisphenol A, nonylphenol, and natural estrogens in vegetables and fruits using gas chromatography-tandem mass spectrometry. J Agric Food Chem. 2013;61(1):84–9. [DOI] [PubMed] [Google Scholar]
- 69. Fierens T, Van Holderbeke M, Willems H, De Henauw S, Sioen I. Transfer of eight phthalates through the milk chain—a case study. Environ Int. 2013;51:1–7. [DOI] [PubMed] [Google Scholar]
- 70. Beltifa A, Feriani A, Machreki M, Ghorbel A, Ghazouani L, Di Bella G, Van Loco J, Reyns T, Mansour HB. Plasticizers and bisphenol A, in packaged foods sold in the Tunisian markets: study of their acute in vivo toxicity and their environmental fate. Environ Sci Pollut Res Int. 2017;24(28):22382–92. [DOI] [PubMed] [Google Scholar]
- 71. EFSA. Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015;13(1):3978. [Google Scholar]
- 72. Shao B, Han H, Tu X, Huang L. Analysis of alkylphenol and bisphenol A in eggs and milk by matrix solid phase dispersion extraction and liquid chromatography with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;850(1–2):412–16. [DOI] [PubMed] [Google Scholar]
- 73. Sajiki J, Miyamoto F, Fukata H, Mori C, Yonekubo J, Hayakawa K. Bisphenol A (BPA) and its source in foods in Japanese markets. Food Addit Contam. 2007;24(1):103–12. [DOI] [PubMed] [Google Scholar]
- 74. Bemrah N, Jean J, Riviere G, Sanaa M, Leconte S, Bachelot M, Deceuninck Y, Bizec BL, Dauchy X, Roudot AC et al.. Assessment of dietary exposure to bisphenol A in the French population with a special focus on risk characterisation for pregnant French women. Food Chem Toxicol. 2014;72:90–7. [DOI] [PubMed] [Google Scholar]
- 75. Rosenmai AK, Bengtstrom L, Taxvig C, Trier X, Petersen JH, Svingen T, Binderup ML, Barbara Medea Alice VV, Dybdahl M, Granby K et al.. An effect-directed strategy for characterizing emerging chemicals in food contact materials made from paper and board. Food Chem Toxicol. 2017;106(Pt A):250–9. [DOI] [PubMed] [Google Scholar]
- 76. Varshavsky JR, Morello-Frosch R, Woodruff TJ, Zota AR. Dietary sources of cumulative phthalates exposure among the U.S. general population in NHANES 2005–2014. Environ Int. 2018;115:417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zota AR, Phillips CA, Mitro SD. Recent fast food consumption and bisphenol A and phthalates exposures among the U.S. population in NHANES, 2003–2010. Environ Health Perspect. 2016;124(10):1521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tordjman K, Grinshpan L, Novack L, Goen T, Segev D, Beacher L, Stern N, Berman T. Exposure to endocrine disrupting chemicals among residents of a rural vegetarian/vegan community. Environ Int. 2016;97:68–75. [DOI] [PubMed] [Google Scholar]
- 79. Sathyanarayana S, Alcedo G, Saelens BE, Zhou C, Dills RL, Yu J, Lanphear B. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J Expo Sci Environ Epidemiol. 2013;23(4):378–84. [DOI] [PubMed] [Google Scholar]
- 80. Moreira MA, Andre LC, Cardeal ZL. Analysis of phthalate migration to food simulants in plastic containers during microwave operations. Int J Environ Res Public Health. 2013;11(1):507–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li C, Xu J, Chen D, Xiao Y. Detection of phthalates migration from disposable tablewares to drinking water using hexafluoroisopropanol-induced catanionic surfactant coacervate extraction. J Pharm Anal. 2016;6(5):292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cooper JE, Kendig EL, Belcher SM. Assessment of bisphenol A released from reusable plastic, aluminium and stainless steel water bottles. Chemosphere. 2011;85(6):943–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, Ye X, Calafat AM, Michels KB. Polycarbonate bottle use and urinary bisphenol A concentrations. Environ Health Perspect. 2009;117(9):1368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Makris KC, Andra SS, Jia A, Herrick L, Christophi CA, Snyder SA, Hauser R. Association between water consumption from polycarbonate containers and bisphenol A intake during harsh environmental conditions in summer. Environ Sci Technol. 2013;47(7):3333–43. [DOI] [PubMed] [Google Scholar]
- 85. Braunrath R, Podlipna D, Padlesak S, Cichna-Markl M. Determination of bisphenol A in canned foods by immunoaffinity chromatography, HPLC, and fluorescence detection. J Agric Food Chem. 2005;53(23):8911–17. [DOI] [PubMed] [Google Scholar]
- 86. Geens T, Apelbaum TZ, Goeyens L, Neels H, Covaci A. Intake of bisphenol A from canned beverages and foods on the Belgian market. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010;27(11):1627–37. [DOI] [PubMed] [Google Scholar]
- 87. Tzatzarakis MN, Karzi V, Vakonaki E, Goumenou M, Kavvalakis M, Stivaktakis P, Tsitsimpikou C, Tsakiris I, Rizos AK, Tsatsakis AM. Bisphenol A in soft drinks and canned foods and data evaluation. Food Addit Contam Part B Surveill. 2017;10(2):85–90. [DOI] [PubMed] [Google Scholar]
- 88. Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, Nelson H, Bhat HK, Perera FP, Silva MJ et al.. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116(4):467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fisher M, Arbuckle TE, Mallick R, LeBlanc A, Hauser R, Feeley M, Koniecki D, Ramsay T, Provencher G, Berube R et al.. Bisphenol A and phthalate metabolite urinary concentrations: daily and across pregnancy variability. J Expo Sci Environ Epidemiol. 2015;25(3):231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wenzel AG, Brock JW, Cruze L, Newman RB, Unal ER, Wolf BJ, Somerville SE, Kucklick JR. Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere. 2018;193:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Weiss L, Arbuckle TE, Fisher M, Ramsay T, Mallick R, Hauser R, LeBlanc A, Walker M, Dumas P, Lang C. Temporal variability and sources of triclosan exposure in pregnancy. Int J Hyg Environ Health. 2015;218(6):507–13. [DOI] [PubMed] [Google Scholar]
- 92. Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, Ye X, Ford J, Keller M, Meeker JD et al.. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect. 2012;120(11):1538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, Ye X, Anzalota Del Toro LV, Crespo-Hernandez N, Jimenez-Velez B et al.. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ Sci Technol. 2013;47(7):3439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. McGowan CA, Curran S, McAuliffe FM. Relative validity of a food frequency questionnaire to assess nutrient intake in pregnant women. J Hum Nutr Diet. 2014;27(Suppl 2):167–74. [DOI] [PubMed] [Google Scholar]
- 95. Venter C, Higgins B, Grundy J, Clayton CB, Gant C, Dean T. Reliability and validity of a maternal food frequency questionnaire designed to estimate consumption of common food allergens. J Hum Nutr Diet. 2006;19(2):129–38. [DOI] [PubMed] [Google Scholar]
- 96. Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A, Gaudreau E, Foster WG, Choeurng V, Fraser WD et al.. Phthalate and bisphenol A exposure among pregnant women in Canada—results from the MIREC study. Environ Int. 2014;68:55–65. [DOI] [PubMed] [Google Scholar]
- 97. Koch HM, Lorber M, Christensen KL, Palmke C, Koslitz S, Bruning T. Identifying sources of phthalate exposure with human biomonitoring: results of a 48 h fasting study with urine collection and personal activity patterns. Int J Hyg Environ Health. 2013;216(6):672–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.