Abstract
Animal-source foods (ASFs) are a food group of interest for interventions aimed at reducing stunting and other inadequate growth measures in early childhood. The aim of this systematic review was to examine the relation between ASF consumption and stunting in children aged 6–60 mo in low- and middle-income countries (LMICs). The secondary aim was to examine the relation between ASF consumption and other indicators of growth and development (length/height, weight, head circumference, and anemia). A search of the peer-reviewed and grey literature published from January 1980 to June 2017 was conducted. Databases searched included CINAHL, Embase, Global Index Medicus, PubMed, and Web of Science. There were 14,783 records and 116 full text articles dual screened; 21 studies were included in the review and were dual evaluated for risk of bias (RoB). The relation between ASF and stunting (length- or height-for-age z-score←2) was examined in randomized-controlled trials [(RCTs), n = 3] and cross-sectional studies (n = 4) only; ASF reduced stunting in 1 RCT and was associated with reduced stunting in 1 cross-sectional study. We did not identify any longitudinal cohorts that examined this relation. The relation between ASF and secondary indicators length/height, weight, head circumference, and anemia were largely nonsignificant across study designs. The intervention/exposure, comparator, outcome measures, methods, and analyses were highly heterogeneous. Although we did not find a consistent relation between ASF consumption and our primary and secondary outcomes, this may have been a function of inconsistencies in study design. Foods in the whole diet, particularly combination dishes, are inherently difficult to assess. To quantitatively assess the relation between ASF and stunting and other indicators of growth and iron status in early childhood, future research should provide consistency in the definition and quantification of the exposure and outcomes allowing for interstudy quantitative comparisons.
Keywords: animal-source food, children, stunting, growth, height, weight
Introduction
Stunting is broadly defined as restricted linear growth. The WHO classifies stunting as mild [length-for-age/height-for-age z-score (LAZ/HAZ) −1 to > −2], moderate (LAZ/HAZ −2 to > −3), or severe (LAZ/HAZ ≤ −3) (1). As an indicator used to assess population-level linear growth, stunting is a proxy marker of chronic undernutrition (2). It is associated with impaired cognitive development, increased morbidity and mortality risk in early life, and increased noncommunicable disease risk in later life (3). Poor educational outcomes, reduced earnings, and lower national economic productivity have been linked to stunting (4). Among females, stunting also leads to decreased birthweight among their offspring (4). Worldwide, an estimated 151 million, or 22% of children <5 y, are stunted (5). In 2012, the World Health Assembly endorsed a target of a 40% reduction by 2025 in the number of children aged <5 y who are stunted (4, 6). Reducing the prevalence of stunting requires improving the quantity and quality of foods consumed by women during pregnancy and breastfeeding, as well as the foods fed to infants and young children in the first 5 y of life.
Animal-source foods (ASFs) have been proposed as a vehicle to improve macro- and micronutrient consumption in early childhood (7). As a class of foods, they are particularly rich in micronutrients such as iron, vitamins A and B, zinc, calcium, and iodine. ASFs are also rich in amino acids that are often deficient in many children in low- and middle-income countries (LMICs) (7). Broadly, ASFs include dairy, flesh foods, eggs, insects, and seafood. These foods are key components of dietary diversity, which acts as a proxy measure for assessing dietary quality and micronutrient sufficiency.
Current Status of Knowledge
The WHO has long advised that children aged >6 mo should consume varied and adequate quantities of locally produced meat, poultry, fish or eggs, as well as vitamin-A-rich fruits and vegetables (8, 9), but recent evidence from LMICs suggests ASFs may play a greater and more nuanced role in reducing malnutrition than previously thought, particularly early in life (10). For instance, in a recent meta-analysis of data from over 112,000 children aged 6–23 mo from 46 countries, Headey et al. (10) found that differences between children who did, and did not, consume ASFs became increasingly significant as children aged, with the largest differences around 15–18 mo.
Many studies have evaluated the impact of ASF or ASF supplements on growth outcomes. For instance, Roberts and Stein (11) recently found that 4 animal protein-based interventions had predominantly positive but nonsignificant results on linear growth in children aged <2 y. A recent systematic review hasbegun to delve further into this association (12). In late 2017, Eaton et al. (12) published a protocol in the Cochrane Database of Systematic Reviews with the intent of conducting subgroup analyses, by 1) age (6–23 mo compared with 24–59 mo compared with mixed age groups) and 2) type of ASF (eggs compared with meat compared with fish compared with dairy compared with mixed foods). The current study is the first systematic review examining the relation between ASF consumption and stunting from randomized controlled trials (RCTs), longitudinal cohorts, and cross-sectional studies. Therefore, our systematic review aimed to answer the following 2 research questions:
How does ASF consumption relate to stunting in children 6–60 mo in LMICs?
How does ASF consumption relate to the secondary outcomes of length/height including LAZ/HAZ, weight [including weight for age z-score (WAZ) and weight for length/height z-score (WLZ/WHZ)], head circumference [including head circumference z-score (HCz)], and iron status [blood hemoglobin and prevalence of iron deficiency anemia (IDA)] among children 6–60 mo in LMICs?
Methods
To examine the relation between ASF consumption, growth, and nutritional outcomes, a protocol outlining the systematic review objectives and search with a priori inclusion and exclusion criteria (Table 1) was submitted to PROSPERO (CRD42016043998) (13). For the purposes of this review, we used the term “stunting” to include both moderate (LAZ/HAZ −2 to > −3) and severe (LAZ/HAZ ≤ 3) stunting (14–16). A search for peer-reviewed literature in English, French, Portuguese, and Spanish was conducted for articles published between January 1980 and June 2017. The following databases were searched: CINAHL, The Cochrane Library Collection (Cochrane Database of Systematic Reviews), Cochrane Central Register of Controlled Trials (CENTRAL), Embase, Global Index Medicus [including African Index Medicus, Index Medicus for the Eastern Mediterranean Region, Index Medicus for the South-East Asian Region, Latin American and Caribbean Health Sciences Literature (Literatura Latino Americana em Ciências da Saúde), Western Pacific Index Medicus, and the WHO Department of Knowledge Management and Sharing], PubMed, and Web of Science. The full search terms were developed by the study authors and reviewed by a medical-reference librarian (Supplemental Material 1). Searches in the grey literature included the following sources: International Food Policy Research Institute, The New York Academy of Medicine Grey Literature Report, OpenGrey, and Proquest Digital Dissertations and Theses (restricted to dissertations). Hand searching was conducted in the proceedings of key nutrition conferences: the American Society for Nutrition Annual Meeting, the International Congress of Nutrition, the Micronutrient Forum, and the Nestlé Nutrition Workshop Series. A snowball search was conducted via hand searching the reference lists of all included articles and consultation with experts in the field. Study teams were contacted for additional data and analysis if the article had the exposure/intervention and outcome(s) but they were not analyzed in a manner applicable to this review.
TABLE 1.
Inclusion and exclusion criteria for this review1
| Criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Study design | Quantitative studies including but not limited to randomized controlled trials, longitudinal, cohort studies, cross-sectional studies, and case-control studies | Studies that are entirely qualitative, such as ethnographic studies, and studies performed on a single individual, such as case studies |
| Population | Children aged 6–60 mo in low- and middle-income countries (as classified by the World Bank (18) at the time of data collection, or at the time of publication if the data collection dates were not reported) with height or length or head circumference and ASF consumption assessments. ASF consumption assessment including frequency and/or quantity of consumption | Studies conducted entirely in high-income countries or without a subgroup of participants from low- and middle-income countries analyzed independently from high-income country subjects. Children with congenital disorders or intellectual disabilities that impede eating. Children on parenteral nutrition. Children with sickle-cell anemia. Studies that do not contain assessments of height or length or head circumference of subjects or information on ASF consumption. ASF derivatives including powders, isolated proteins, or individual composite foods made with a minority percentage of ASF |
| Intervention/exposure | The exposure of interest in this review is consumption of ASFs. Data on the consumption of whole ASF or composite dishes or foods where ASF comprise the majority of the dish must include either frequency of consumption, quantity of consumption (precise measures), or both | Data on ASF consumption that is not at the level of the individual, e.g., household consumption, expenditure on ASF |
| Comparator | In studies where a comparator or control group is presented, the comparator or control group may involve a nonASF, such as a PSF, or no intervention | Not applicable |
| Outcome measures | The primary outcome measure was the proportion of stunting among children aged 6–60 mo as defined by a LAZ/HAZ ←2 according to the following growth standards: the 1978 WHO/National Center for Health Statistics Growth References (14), the 2000 Centers for Disease Control Growth Charts (15), or the 2006 WHO Growth Standards (16) | Other measures of arm anthropometry including upper-arm muscle area and upper-arm fat area, and other measures of iron status including ferritin, transferrin, total iron-binding capacity, and erythrocyte protophyrin were not abstracted for this review |
| The secondary outcomes, either point estimates or changes in outcomes over the study period, included in this review were: length/height including LAZ/HAZ, weight including weight-for-age z-score, weight-for-length/weight-for-height z-score, BMI, BMI z-score, MUAC, MUAC z-score, head circumference including z-scores, hemoglobin concentration, prevalence of anemia | — |
1ASF, animal-source food; HAZ, height for age z-score; LAZ, length for age z-score; MUAC, midupper arm circumference; PSF, plant-source food.
References obtained from the peer-reviewed databases were dual screened at 2 levels: title and abstract (reviewed simultaneously), and full text. Conflicts were resolved by the 2 screeners or, when necessary, a third study coauthor. Data was abstracted by 1 study author and abstractions were reviewed and verified by a coauthor. Statistical significance was set at P < 0.05.
The risk of bias (RoB) assessment was conducted independently by 2 coauthors using quality assessment tools from the NHLBI (17) that assess the overall quality of individual studies as good, fair, or poor. The NHLBI Quality Assessment of Controlled Intervention Studies (17) tool was used for RCTs. Question #4 related to blinding (participants and providers) to treatment group assignment and was not assessed for this review because the interventions were food based (Supplemental Table 1). The NHLBI Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (17 was used for these study designs. Two questions were excluded: #7 due to insufficient available evidence in the nutrition literature to assess this question and #12 because the exposure status was usually part of a more inclusive dietary data collection tool. Five questions were modified. Modifications included providing further guidance to assessors and splitting questions for enhanced specificity (Supplemental Table 2). Details on dietary data collection tools used within the included observational studies were also provided to assessors (Supplemental Table 3).
The nature of this systematic review resulted in vastly heterogeneous variables. Intervention foods (Table 2 and Supplemental Table 4) and exposure and outcome measures in observational studies (Table 3 and Supplemental Table 3) varied throughout included studies. The heterogeneity in the intervention foods and exposure and outcome measures prevented us from being able to conduct a meta-analysis. Therefore, results were synthesized in a narrative form.
TABLE 2.
Study details for randomized controlled trials
| Reference | Study country | Sample size, n | Study duration, mo | Child age at assessment, mo | Female participants, % | Animal-source foods | Comparators |
|---|---|---|---|---|---|---|---|
| Bauserman, 2015 (19) | Democratic Republic of the Congo (DRC) | 175 | 12 | 6–18 | 49 | Caterpillar cereal | Usual diet |
| Iannotti, 2017 (20) | Ecuador | 148 | 6 | 6–9; 12–15 | 46 | Egg | Usual diet |
| — | — | — | — | — | — | — | |
| Krebs, 2012 (21) | DRC, Guatemala, Pakistan, Zambia | 1602 | 12 | 6–18 | 51 | Beef (lyophilized) | Rice-soy cereal (micronutrient fortified) |
| Lartey, 1999 (22) | Ghana | 190 | 6 | 6–12 | 53 | 1. Weanimix (maize, soybeans, peanuts) plus fish powder (anchovy) | 3. Weanimix alone |
| — | — | — | — | — | 2. Koko (fermented maize dough powder) plus fish powder | 4. Weanimix plus vitamins and minerals | |
| Lin, 2008 (23) | Malawi | 211 | 12 | 6–18 | Not reported (NR) | Fish-fortified maize porridge | Micronutrient-fortified soy/peanut spread |
| Long, 2012 (24)1 | Kenya | 274 | 5 | Baseline: 11–40 | 53 | 1. Millet porridge with minced meat (beef) | Millet porridge alone |
| — | — | — | Endpoint: 16–45 | — | 2. Millet porridge with milk | — | |
| Tang, 2014 (39) | China | 1471 | 12 | 6–18 | NR | Pork | 1. Multiple-micronutrient-fortified cereal |
| — | — | — | — | — | — | 2. Nonfortified cereal quantities not reported |
The age at end of follow-up assessment was not reported in the publication; for the review it was extrapolated from the reported baseline age range and study duration.
TABLE 3.
Study details for longitudinal cohort and cross-sectional studies
| Reference and study design | Study country | Sample size, n | Study duration, mo | Child age at assessment, mo | Female participants,% | Animal-source food (ASF) variables | ASF quantification |
|---|---|---|---|---|---|---|---|
| Allen, 1992 (25) longitudinal cohort (LC) | Mexico | 67 | 12 | 18–30 | 52 | ASF: meats, fish, eggs, dairy products, and fast and processed foods predominantly made of ASF | Continuous: ASF energy in MJ/d and MJ/kg bodyweight, ASF protein in g/d and g per kg bodyweight, percent of total energy from ASF, and percent of total daily protein from ASF |
| Campbell, 2016 (26) LC | Bangladesh | 1267 | 12 | 6, 12, 15, | 51 | Dairy: formula, milk, suji or payesh, yogurt1 | Binary: yes if at least one of the foods in the category were consumed at the time of the 24-h recall, no if not |
| — | — | — | 18 | — | Eggs: eggs, mixed dishes containing egg | — | |
| — | — | — | — | — | Meat/fish: beef, chicken, fish, goat, liver | — | |
| Diana, 2017 (27) LC | Indonesia | 190 | 3 | 9, 12 | 54 | ASF: dairy products (milk, yogurt, cheese, infant formula), eggs, flesh foods | Binary: yes if at least one of the foods defined in the variable was recorded at least once in the 48-h dietary data collected, no if not. |
| — | — | — | — | — | Eggs | — | |
| — | — | — | — | — | Flesh foods: meat, fish, poultry, liver/organ meat | — | |
| Leonard, 2000 (28) LC | Ecuador | 91 | 6 | 12–60 | Not reported (NR) | ASF energy: exact foods NR | Continuous: ASF energy in kcal/d, ASF protein in g/d |
| — | — | — | — | — | ASF protein: exact foods NR | — | |
| Marquis, 1997 (29) LC | Peru | 107 | 3 | 12–15 | NR | ASF: any cow milk, meat, organ meats, any eggs, fish | Categorical: 3 categories were “not consumed, infrequently consumed, or consumed >2 times/wk.” |
| Miller, 2016 (30) LC | Nepal | varies | 48 | 6–60 | NR | ASF: milk or other dairy products, eggs, fish, meat or offal | Binary: 1–2 ASF consumed in the previous 24 h vs. 2 or more |
| — | — | — | — | — | — | Continuous: aggregated number of ASFs consumed in previous 24 h (values 0–4) | |
| Ntab, 2005 (31) LC | Senegal | 500 | 7 | 8–422 | NR | Fish | Continuous: frequency of consumption (d/wk) |
| — | — | — | — | — | Meat | — | |
| — | — | — | — | — | Milk: animal milks | — | |
| Aramburú, 2014 (38) cross-sectional (CS) | Peru | 435 | Not applicable (NA) | 6–30 | 50 | Dairy: milk, yogurt, cheese | Binary: yes if at least one of the foods in the category were consumed at the time of the 24-h recall, no if not |
| — | — | — | — | — | Meat: meat, fish, birds, liver, organ meats | — | |
| — | — | — | — | — | Eggs | — | |
| Darapheak, 2013 (32) CS | Cambodia | 1907 | NA | 12–59 | NR | ASF: beef, pork, lamb, goat, rabbit, deer, chicken, duck, other birds, liver, kidney, heart, other organs, eggs, fish, shellfish | Binary: yes if at least one of the foods in the category were consumed at the time of the 24-h recall, no if not |
| — | — | — | — | — | Milk products: milk (tinned/powder or fresh milk), infant formula | — | |
| Fierstein, 2017 (33) CS | Uganda | NR | NA | 6– < 24 | NR | Dairy: milk, yogurt, cheese, infant formula | Binary: yes if at least one of the foods in the category were consumed at the time of the 24 h recall, no if not |
| Jin and Iannotti, 2014 (34) CS | Kenya | 183 | NA | 6–60 | 46 | ASF: poultry, meat, eggs, fish, milk, dairy products | Continuous: frequency of consumption in the previous 7 d |
| Semba, 2011 (35) CS | Indonesia | 295,841 | NA | 6–59 | NR | Fortified milk products | Binary: yes if consumed in the previous 7 d, no if not |
| Walker, 1990 (36) CS | Jamaica | 191 | NA | 9–24 | NR | Dairy products | Continuous: number of items consumed in each food group per d |
| — | — | — | — | — | Meat, fish, and eggs | — | |
| Zhao, 2016 (37) CS | Myanmar | 807 | NA | 6–36 | 47 | Eggs | Binary: yes if ever consumed in the child's life, no if not |
| — | — | — | — | — | Meat | — |
Complete list of foods included in each food group was not reported. The foods included in this cell were obtained from the top 3 most frequently consumed in each food group at ages 6, 9, 12, 15, and 18 mo.
For age at data collection, the study states: “A cross-sectional food consumption and anthropometric survey was conducted in April–May 2003 within a sample of 543 children aged 8–42 mo and nested into the randomized [Intermittent Preventive malaria Treatment in children (IPTc)] trial, which included measurements of height and weight in September 2002, thus allowing for computation of height increments over the 7 mo before the survey.”
Results
Overview of included articles
The total number of records screened from the database searches, after removing duplicates, was 14,783; 115 full texts were screened. Twenty-one studies published between 1990 and 2017 were included in this review (Figure 1). Nineteen of the included studies were in English and published in peer-reviewed journals (19–24, 25–31, 32–37). The data included in this review for 1 study (26) was obtained from study authors (P Christian, Johns Hopkins Bloomberg School of Public Health, personal communication, 2018). One included study was in Spanish and indexed on Global Index Medicus/LILACS as a thesis (38). One article was identified through hand searching (39). The top reasons for exclusion were an exposure/intervention or outcome that did not meet our inclusion criteria. Seven included studies were RCTs, 7 were longitudinal cohorts, and 7 were cross-sectional studies.
FIGURE 1.
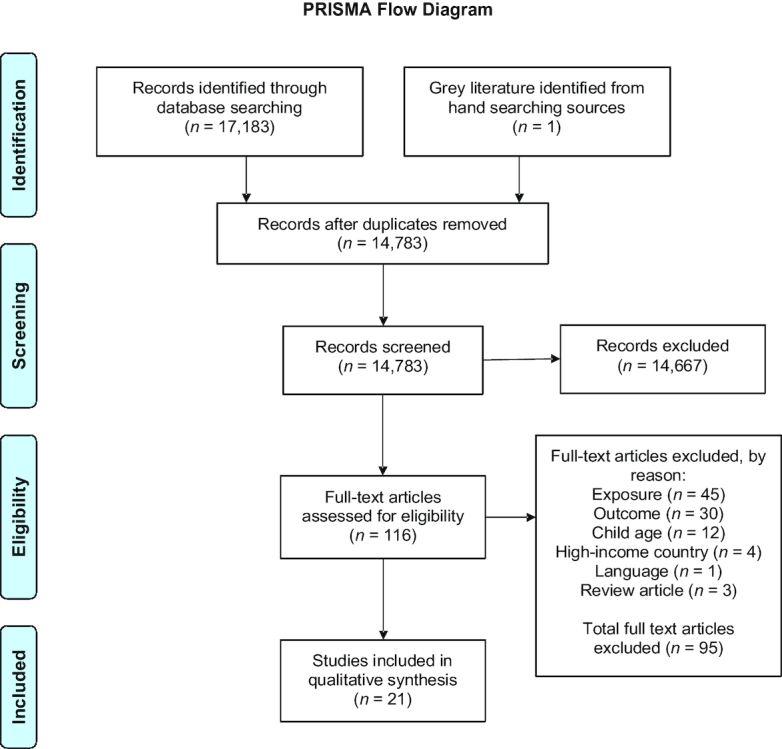
PRISMA flow diagram.
Included study details and demographics
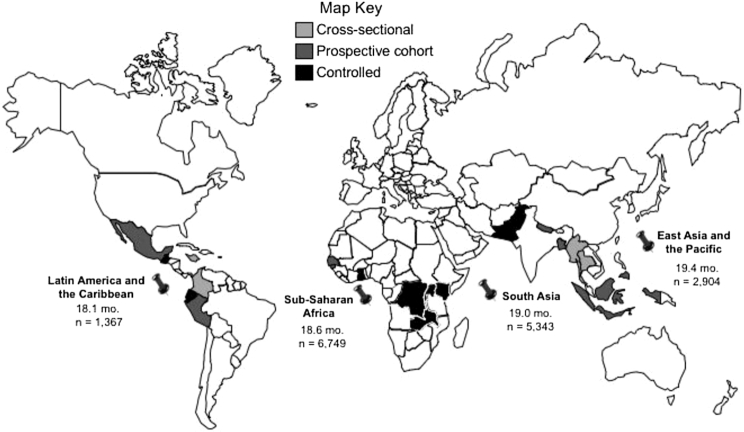
Among the 21 included studies, the data reported were collected in 18 countries (see map in Figure 2). Of the20 studies conducted in 1 country, 7 were conducted in sub-Saharan Africa (19, 22–24, 31, 33, 34), 4 in South America (20, 28, 29, 38), 5 in South East Asia ( 27, 32, 35, 37, 39) , 2 in the Indian subcontinent (26, 30), and 1 each in the Caribbean (36) and Central America (25). Ten studies were conducted in low-income countries (19, 22–24, 26, 30, 31, 32–34), 7 in low-middle income countries (25, 27–29, 35–37), and 3 in upper-middle income countries (20, 38, 39). One article reported on a multi-site trial conducted in 4 countries, 1 low- and 3 middle-income countries (Democratic Republic of the Congo [DRC], Guatemala, Pakistan, and Zambia) (21).
FIGURE 2.
World map of included studies, by region, median age, and average sample size. Note: age listed is the median average age for all countries in the specified region. Sample size is the total number of participants in each region. Countries represented by >1 study in this review include: 2 in the Democratic Republic of the Congo (2 controlled interventions); 2 each in Indonesia and Peru (cross-sectional and prospective cohort); and 4 in Kenya (2 cross-sectional, 1 controlled, and 1 prospective cohort).
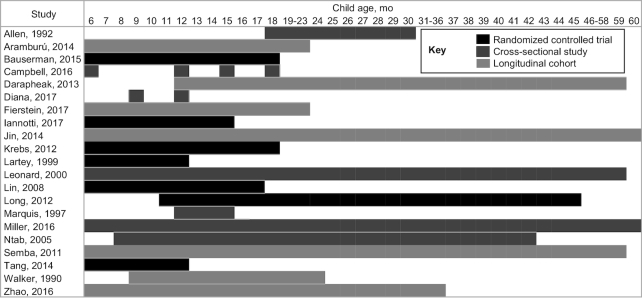
Among the 7 RCTs, 5 had sample sizes between 100 and 200 participants at the end of follow-up (EoF) (Table 2). The age of the participants at baseline ranged from 6 to 9 mo in 6 of the 7 RCTs (19–23, 39) (Figure 3), and study duration ranged from 5 to 12 mo. The sample sizes of the longitudinal studies were more heterogeneous, ranging from 67 to 1267 (25, 26), whereas study duration ranged from 3 to 48 mo (27, 30) (Table 3). Four studies had a determinate, homogeneous participant age at baseline and reported at EoF (25–27, 30), whereas in 3 an overall range was reported without specific measurement points (28, 30, 31). By far the largest age range was found in cross-sectional studies (6–60 mo) as was the largest sample size; Semba et al. (35) reported n = 95,841.
FIGURE 3.
Child age at assessment in months.
Interventions and comparators in RCTs
ASF interventions in the RCTs included fish (22, 23), beef (21, 24), insects (19), milk (24), pork (39), and eggs (20). The comparator interventions also varied. Some included ASF compared with usual diet (19, 20), whereas others included 1 or more groups of ASF compared with plant-source food (PSF) (21–24, 39). The strongest compliance measures were supervised feeding with weighed leftovers (24), whereas the weakest were either not reported (39) or were study staff reminders during weekly home visits (20).
ASF exposures in longitudinal cohorts
In the 7 longitudinal cohort studies, 6 studies provided descriptions of specific ASFs analyzed (25–27, 29–31). Overall, 5 studies contained an ASF composite variable (25, 27–30). Other variables included eggs (26, 27), animal milks (31), dairy (26), fish (31), meat, liver, organ meats, and offal (26, 27, 29, 30). Dietary data collection methods included 24-h recall (26, 28, 30), FFQ (29), 24-h recall and FFQ (31), food weighing and maternal report (27), and food weighing, observation, maternal recall, and food record (25). Dietary data was collected at multiple time points in 4 studies (25, 26, 29, 30) and at a single time point in 3 studies (27, 28, 31). The exposures were quantified as binary (26, 27, 30), categorical (29), and continuous (25, 28, 31).
ASF exposures in cross-sectional studies
In the 7 cross-sectional studies, milk-based foods were the exposures most widely assessed. Darapheak et al. (32) specified the widest variety of mammals, birds, organ meat, eggs, and seafood in their ASF variable. The dietary data collection instruments were single 24-h recall (38, 32, 33), 24-h recall over 2 d (36), and FFQs (34, 35, 37). Two studies using an FFQ collected dietary data for the previous 7 d (34, 35) and 1 did not specify the time period (37). The studies used binary (38, 32, 33, 35) and continuous (34, 36, 37) quantification for the exposure variables.
Outcome variables
The outcome indicators were highly heterogenous. Six studies examined stunting (RCTs, 20, 26, 39; cross-sectional, 22, 33, 35; and no cohorts). The length/height indicators are listed in Table 5, and the weight indicators in Tables 6 and 7. The relation between ASF and LAZ/HAZ was investigated in 6 RCTs (19–22, 24, 39), 5 longitudinal cohorts (25–28, 31), and 2 cross-sectional studies (33, 34). The relation between ASF and head circumference was examined in 4 RCTs (21, 22, 24, 39), 1 longitudinal cohort (30), and 1 cross-sectional study (39). The relation between ASF and iron status indicators was only examined in 3 RCTs (19, 21, 22).
TABLE 5.
Relation between animal-source food intake and length/height indicators in included studies1
| Reference and study design | Animal- source food (ASF) | Comparator | Child age at assessment, mo2 | Length/height, cm | Length/height gain, cm | Length/height growth slope | Linear growth velocity | Length/height for age | Length-for-age z-score (LAZ)/height-for-age z-score (HAZ) | Change in LAZ/HAZ | LAZ/HAZ growth slope |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bauserman, 2015 (19) randomized controlled trial (RCT) | Caterpillar cereal | Usual diet | 6, 9, 12, 18 | — | — | — | — | — | Not significant (NS) | — | — |
| — | — | 6–183 | — | — | — | NS | — | — | — | — | |
| Iannotti, 2017 (20) RCT | Eggs | Usual diet | 15 (model not age adjusted) | — | — | — | — | — | ß (95% CI): 0.64 (0.4, 0.89)§ | — | — |
| — | — | 15 (age adjusted model) | — | — | — | — | — | ß (95% CI): 0.63 (0.38–0.88)§ | — | — | |
| Krebs, 2012 (21) RCT | Beef | Fortified rice-soy cereal | 6, 9, 12, 18 | — | — | — | — | — | NS | — | — |
| — | — | 6–183 | — | — | — | NS | — | — | — | — | |
| Lartey, 1999 (22) RCT4 | 1. Wean-imix (maize, soy-beans, peanuts) plus fish powder (anchovy) | 3. Wean-imix mix alone | 6, 7, 8, 9, 10, 11, 12 | — | — | — | — | — | NS | — | — |
| 2. Koko (fermented maize dough powder) plus fish powder | 4. Wean-imix plus vitamins and minerals | 6–123 | — | NS | — | — | — | — | — | — | |
| Lin 2008 (23) RCT | Fish-fortified maize porridge | Micro-nutrient-fortified soy/peanut spread | 6–123 | — | NS | — | — | — | — | — | — |
| — | — | 12–183 | — | NS | — | — | — | — | — | — | |
| Long, 2012 (24) RCT5 | Millet w/meat | Millet porridge | Baseline: 11–40 | — | — | NS | — | — | — | — | NS |
| — | — | Endpoint: 16–453 | — | — | — | — | — | — | — | — | |
| Millet porridge w/milk | Millet porridge | Baseline: 11–40 | — | — | NS | — | — | — | — | NS | |
| — | — | Endpoint: 16–453 | — | — | — | — | — | — | — | — | |
| Tang, 2014 (39) RCT | Pork | Cereal | 6–183 | — | Mean SD pork: 13.01 (1.9); PSF, mean (SD): 12.75 (1.8)† | — | — | — | — | Mean (SD) pork: −0.43 (0.72); PSF, mean (SD): −0.54 (0.67)‡ | — |
| Allen, 1992 (25) longitudinal cohort (LC) | ASF energy, MJ/d | Not applicable (NA), examined correlations | 30 | — | — | NS | — | — | Pearson Correlation (PCorr): 0.36‡; PCorr: 0.24‡ | — | — |
| ASF energy, MJ/kg body weight | — | 30 | — | — | — | — | — | — | — | — | |
| ASF protein, g/d | — | 30 | — | — | NS | — | — | PCorr: 0.33‡ | — | — | |
| ASF protein, g/kg body weight | — | 30 | — | — | — | — | — | NS | — | — | |
| Daily energy from ASF, % | — | 30 | — | — | NS | — | — | PCorr: 0.38‡ | — | — | |
| Daily protein from ASF, % | — | 30 | — | — | NS | — | — | PCorr: 0.35‡ | — | — | |
| Campbell, 2016 (26) LC | Dairy | Consumed food group in previous 24 h (yes vs. no) | 6 | — | — | — | — | — | NS | — | — |
| — | — | 12 | — | — | — | — | — | Mean (SD) yes: −1.53 (1.01) vs. no: −1.67 (1.03)† | — | — | |
| — | — | 15 | — | — | — | — | — | NS | — | — | |
| — | — | 18 | — | — | — | — | — | NS | — | — | |
| Eggs | — | 6 | — | — | — | — | — | NS | — | — | |
| — | — | 12 | — | — | — | — | — | Mean (SD) yes: −1.52 (1.03) vs. no: −1.68 (1.03)† | — | — | |
| — | — | 15 | — | — | — | — | — | Mean (SD) yes: −1.69 (0.95) vs. no: −1.83 (1.07)† | — | — | |
| — | — | 18 | — | — | — | — | — | NS | — | — | |
| Meat/fish | — | 6 | — | — | — | — | — | Mean (SD) yes: −1.13 (0.94) vs. no: −1.36 (1.04)† | — | — | |
| — | — | 12 | — | — | — | — | — | Mean (SD) yes: −1.55 (1.02) vs. no: −1.71 (1.03)† | — | — | |
| — | — | 15 | — | — | — | — | — | NS | — | — | |
| — | — | 18 | — | — | — | — | — | Mean (SD) yes: −1.84 (1.00) vs. no: −2.00 (1.05)† | — | — | |
| Diana, 2017 (27) LC | Flesh foods | Consumed food (yes or no) during 2-d food record at 9 mo6 | 12 | — | — | — | — | — | NS | — | — |
| Eggs | — | 12 | — | — | — | — | — | NS | — | — | |
| ASF | — | 12 | — | — | — | — | — | NS | — | — | |
| Leonard, 2000 (28) LC7 | ASF protein | NA, examined correlations | Coast: 12–35.9 | — | — | — | — | — | — | NS | — |
| — | — | Coast: 36–59.9 | — | — | — | — | — | — | NS | — | |
| — | — | Highland: 12–35.9 | — | — | — | — | — | — | NS | — | |
| — | — | Highland: 36–59.9 | — | — | — | — | — | — | NS | — | |
| ASF energy | — | Coast: 12–35.9 | — | — | — | — | — | — | NS | — | |
| — | — | Coast: 36–59.9 | — | — | — | — | — | — | NS | — | |
| — | — | Highland: 12–35.9 | — | — | — | — | — | — | NS | — | |
| — | — | Highland: 36–59.9 | — | — | — | — | — | — | NS | — | |
| Marquis, 1997 (29) LC | ASF | 3 categories8 | 15 | NS | — | — | — | — | — | — | — |
| Ntab 2005 (31) LC9 | Milk | Number of d/wk food group was | 12–42, not age adjusted | — | NS | — | — | — | — | — | — |
| Fish | Consumed: 0 d vs. 1–3 vs. ≥4 d | 12–42, age adjusted | — | NS | — | — | — | — | — | — | |
| — | — | 12–42, not age adjusted | — | Mean (SD): 0 d: 7.3 (2.4), 1–3 d: 6.5 (1.8), ≥4 d: 6.0 (1.9)† | — | — | — | — | — | — | |
| — | — | 12–42, age adjusted | — | NS | — | — | — | — | — | — | |
| Meat | — | 12–42, not age adjusted | — | 0 d: 6.2 (1.9), 1–3 d: 5.6 (2.0), ≥4 d: 6.1 (1.8)† | — | — | — | — | — | — | |
| — | — | 12–42, age adjusted | — | NS | — | — | — | — | — | — | |
| Fish/meat combined | Number of d/wk food group was consumed: 0–2 d vs. ≥3 d | 9–23 mo, not age adjusted | — | — | — | — | — | NS | — | — | |
| — | — | 9–23 mo, age adjusted | — | — | — | — | NS | — | — | — | |
| Milk | — | 9–23 mo, not age adjusted | — | — | — | — | — | NS | — | — | |
| — | — | 9–23 mo, age adjusted | — | — | — | — | NS | — | — | — | |
| Fierstein, 2017 (33) cross-sectional (CS) | Dairy | Consumed dairy in previous 24 h: yes vs. no | 6 to <2410 | — | — | — | — | — | NS | — | — |
| Jin and Iannotti, 2014 (34) CS | ASF | Frequency of consumption in previous wk | 6–6010 | — | — | — | — | — | NS | — | — |
Results in this table are limited to those that were reported via tests of statistical significance. Statistical significance: † = P < 0.05; ‡ = P < 0.01; § = P < 0.001; Not significant = P ≥ 0.05.
Single digits separated by commas, e.g., 6, 9, 12 mo, indicates that the associations reported were for the measures taken at each time point.
Change from baseline to end of follow-up.
All 4 food groups were compared against each other.
The age at end of follow-up assessment was not reported in the publication; for the review it was extrapolated from the reported baseline age range and study duration.
Diet data collected at age 9 mo and LAZ/HAZ at age 12 mo.
Data was collected in 2 waves 6 mo apart. Age groups are based on child's age at first wave. Change in LAZ/HAZ represents the change from the first to second wave of data collection.
The categorical assessment was described in the study as: “Food categories were recorded as not consumed, infrequently consumed, or consumed >2 times/wk.”
For age at data collection, the study states: “A cross-sectional food consumption and anthropometric survey was conducted in April–May 2003 within a sample of 543 children aged 8–42 mo and nested into the randomized IPTc trial, which included measurements of height and weight in September 2002, thus allowing for computation of height increments over the 7 mo before the survey.”
Ages pooled in analysis.
TABLE 6.
Relation between animal-source food intake and weight indicators, part 11
| Reference and study design | Animal- source food (ASF) | Comparator | Child age at assessment, mo2 | Weight gain, g | Weight growth slope | Weight-for-length z-score (WLZ)/weight-for-height z-score (WHZ) | Change in WLZ/WHZ | WLZ/WHZ growth slope | Wasting prevalence | Wasting odds ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| Bauserman, 2015 (19) randomized controlled trial (RCT) | Caterpillar cereal | Usual diet | 6, 9, 12, 18 | — | — | NS | — | — | NS | — |
| Iannotti, 2017 (20) RCT | Eggs | Usual diet | 15, not age adjusted 15, age adjusted | — | — | ß (95% CI): 0.42 (0.20, 0.65)§ | — | — | — | — |
| — | — | — | — | — | ß (95% CI): 0.33 (0.14, 0.51)§ | — | — | — | — | |
| Krebs, 2012 (21) RCT | Beef | Fortified rice-soy cereal | 6, 9, 12, 18 | — | — | NS | — | — | NS | — |
| Lartey, 1999 (22) RCT3 | 1. Weanimix (maize, soybeans, peanuts) plus fish powder (anchovy) | 3. Weanimix alone | 6–124 | NS | — | — | — | — | — | — |
| 2. Koko (fermented maize dough powder) plus fish powder | 4. Weanimix plus vitamins and minerals | — | — | — | — | — | — | — | — | |
| Lin, 2008 (23) RCT | Fish-fortified maize porridge | Micro-nutrient fortified soy/peanut spread | 6–124 | NS | — | — | — | — | — | — |
| — | — | 12–184 | Mean (SD) fish- fortified thickened maize porridge: 1,000 g (430), micro-nutrient-fortified peanut/soy spread: 1,110 g (440)† | — | — | — | — | — | — | |
| Long, 2012 (24) RCT | Millet porridge w/meat | Millet porridge | 12–184 | — | NS | — | — | NS | — | — |
| Millet porridge w/milk | Millet porridge | 16–454 | — | NS | — | — | NS | — | — | |
| Tang, 2014 (39) RCT | Pork | Cereal | 6–184 | NS | — | — | NS | — | — | — |
| Allen, 1992 (25) longitudinal cohort (LC) | ASF energy, MJ/d | Not applicable (NA), examined correlations | 30 | — | NS | — | — | — | — | — |
| ASF energy, MJ/kg body weight | — | 30 | — | NS | — | — | — | — | — | |
| ASF protein, g/d | — | 30 | — | Pearson Correlation (PCorr): 0.29† | — | — | — | — | — | |
| ASF protein, g/kg body weight | — | 30 | — | PCorr: 0.26† | — | — | — | — | — | |
| Energy, % | — | 30 | — | NS | — | — | — | — | — | |
| Protein, % | — | 30 | — | NS | — | — | — | — | — | |
| Diana, 2017 (27) LC | Flesh foods | — | 12 | — | — | NS | — | — | — | — |
| Eggs | — | 12 | — | — | NS | — | — | — | — | |
| ASF | — | 12 | — | — | NS | — | — | — | — | |
| Darapheak, 2013 (32) cross-sectional (CS) | ASF | — | 12–595 | — | — | — | — | — | — | NS |
| Milk products | — | 12–595 | — | — | — | — | — | — | NS | |
| Jin and Iannotti, 2014 (34) CS | ASF | — | 6–605 | — | — | NS | — | — | — | — |
| Zhao, 2016 (37) CS | Eggs | — | 6–365; not age adjusted | — | — | — | — | — | — | NS |
| Meat | — | 6–365; age adjusted | — | — | — | — | — | — | NS |
Results in this table are limited to those that were reported via tests of statistical significance. Statistical significance: † = P < 0.05; ‡ = P < 0.01; § = P < 0.001; Not significant = P ≥ 0.05.
Single digits separated by commas, e.g., 6, 9, 12 mo, indicates that the associations reported were for the measures taken at each time point.
All 4 food groups were compared against each other.
Change from baseline to end of follow-up.
Ages pooled in analysis.
TABLE 7.
Relation between animal-source food intake and weight indicators, part 21
| Reference | Animal-source food (ASF) | Comparator | Child age at assessment, mo2 | Weight-for-age z-score (WAZ) | WAZ growth slope | Under-weight (UW)3 odds ratio | UW prevalence ratio (PR) | BMI z-score | Odds of mid-upper arm circum-ference z-score←2 |
|---|---|---|---|---|---|---|---|---|---|
| Bauserman, 2015 (19) randomized controlled trial (RCT) | Caterpillar cereal | Usual diet | 6, 9, 12, 18 | NS | — | — | — | — | — |
| Iannotti, 2017 (20) RCT | Eggs | Usual diet | 15, not age adjusted | ß (95% CI): 0.71 (0.53, 0.90)§ | — | — | NS | ß (95% CI): 0.45 (0.20, 0.70)§ | — |
| — | — | 15, age adjusted | ß (95% CI): 0.61 (0.45, 0.77)§ | — | — | PR (95% CI): 0.26 (0.10, 0.70)‡ | PR (95% CI): 0.29 (0.08, 0.49)‡ | — | |
| Krebs, 2012 (21) RCT | Beef | Fortified rice-soy cereal | 6, 9, 12, 18 | NS | — | — | — | — | — |
| Lartey, 1999 (22) RCT4 | 1. Weanimix (maize, soybeans, peanuts) plus fish powder (anchovy) | 3. Weanimix alone | 6, 7, 8, 9, 10, 11, 12 | NS | — | — | — | — | — |
| 2. Koko (fermented maize dough powder) plus fish powder | 4. Weanimix plus vitamins and minerals | — | — | — | — | — | — | — | |
| Long, 2012 (24) RCT | Millet porridge w/meat | Millet porridge | 16–455 | — | NS | — | — | — | — |
| Millet porridge w/milk | Millet porridge | 16–455 | — | NS | — | — | — | — | |
| Tang, 2014 (39) RCT | Pork | Cereal | 6–185 | NS | — | — | — | — | — |
| Longitudinal cohort (LC) | ASF energy, MJ/d | Not applicable (NA), examined correlations | 30 | 0.30† | — | — | — | — | — |
| ASF energy, MJ/kg body weight | — | 30 | NS | — | — | — | — | — | |
| ASF protein, g/d | — | 30 | 0.33‡ | — | — | — | — | — | |
| ASF protein, g/kg body weight | — | 30 | NS | — | — | — | — | — | |
| Energy, % | — | 30 | 0.34‡ | — | — | — | — | — | |
| Protein, % | — | 30 | 0.32‡ | — | — | — | — | — | |
| Diana, 2017 (27) LC | Flesh foods | Consumed food (yes or no) during 2-d food record at 9 mo6 | 12 | NS | — | — | — | — | — |
| Eggs | — | 12 | NS | — | — | — | — | — | |
| ASF | — | 12 | NS | — | — | — | — | — | |
| Leonard, 2000 (28) LC7 | ASF protein | NA, examined correlations | Coast: 12–35.9 | NS | — | — | — | — | — |
| — | — | Coast: 36–59.9 | NS | — | — | — | — | — | |
| — | — | Highland: 12–35.9 | NS | — | — | — | — | — | |
| — | — | Highland: 36–59.9 | NS | — | — | — | — | — | |
| ASF energy | — | Coast: 12–35.9 | NS | — | — | — | — | — | |
| — | — | Coast: 36–59.9 | NS | — | — | — | — | — | |
| — | — | Highland: 12–35.9 | NS | — | — | — | — | — | |
| — | — | Highland: 36–59.9 | NS | — | — | — | — | — | |
| Darapheak, 2013 (32) Cross-sectional | ASF | Consumed food group in previous 24 h (yes vs. no) | 12–598 | — | — | OR (95% CI): 0.74 (0.57, 0.96)† | — | — | — |
| Milk products | — | 12–598 | — | — | NS | — | — | — | |
| Jin and Iannotti, 2014 (34) CS | ASF | Frequency of consumption in previous week | 6–608 | ß = 0.04‡ | — | — | — | — | — |
| Zhao, 2016 (37) CS | Eggs | Consumed food group in previous 24 h (yes vs. no) | 6–368; not age adjusted | — | — | NS | — | — | NS |
| Meat | — | 6–368; age adjusted | — | — | NS | — | — | NS |
Results in this table are limited to those that were reported via tests of statistical significance. Statistical significance: † = P < 0.05; ‡ = P < 0.01; § = P < 0.001; Not significant = P ≥ 0.05.
Single digits separated by commas, e.g., 6, 9, 12 mo, indicates that the associations reported were for the measures taken at each time point.
Underweight defined as WAZ < –2.
All 4 food groups were compared against each other.
Change from baseline to end of follow-up.
Diet data collected at age 9 mo and WAZ at age 12 mo.
Data was collected in 2 waves 6 mo apart. Age groups are based on child's age at first wave. Change in WAZ represents the change from the first to second wave of data collection.
Ages pooled in analysis.
Relation between ASF and outcome variables
Stunting
Table 4 presents the relation between ASF and stunting of the included studies. Consistent relations between ASF intake and reduced stunting were found by Semba et al. (35) and Iannotti et al. (20), after adjustment for age. The remaining studies showed either a reduction in stunting associated with increased ASF intake among select subgroups (32, 35) or exclusively nonsignificant results (19, 21, 37).
TABLE 4.
Relation between animal-source food intake and stunting in included studies1
| Reference and study design | Animal- source food (ASF) | Comparators | Child age at assessment, mo2 | Stunting prevalence | Stunting prevalence ratio3 | Stunting rate | Odds of stunting |
|---|---|---|---|---|---|---|---|
| Bauserman, 2015 (19) randomized controlled trial (RCT) | Caterpillar cereal | Usual diet | 6, 9, 12, 18 | Not significant (NS) | — | — | — |
| Iannotti, 2017 (20) RCT | Eggs | Usual diet | 15 (model not age adjusted) | — | NS | — | — |
| — | — | 15 (age adjusted model) | — | PR (95% CI): 0.26 (0.10, 0.70)‡ | — | — | |
| Krebs, 2012 (21) RCT | Beef | Fortified rice-soy cereal | 6, 9, 12, 18 | — | — | NS | — |
| Darapheak, 2013 (32) cross-sectional (CS) | ASF | Consumed food group in previous 24 h (yes vs. no) | 12–594 | — | — | — | Odds ratio (OR) (95% CI): 0.69 (0.54, 0.89)‡ |
| Milk products | — | 12–594 | — | — | — | NS | |
| Semba, 2011 (35) CS | Fortified milk powder | Consumed food group in previous 24 h (yes vs. no) | 6–594 (rural participants) | 43.4% vs. 56.2%§ | — | — | OR (95% CI): 0.87 (0.85, 0.90)§ |
| — | — | 6–594 (urban participants) | 42.8% vs. 53.7%§ | — | — | OR (95% CI): 0.80 (0.76, 0.85)§ | |
| Walker, 1990 (36) CS | Dairy | Number of items consumed by stunted vs. nonstunted children | 9–244 | Median (range) stunted 1.5 (0, 4.0) vs. nonstunted 2.0 (0.5, 4.0) § | — | — | — |
| Meat, fish, and eggs | — | 9–244 | NS | — | — | — | |
| Zhao, 2016 (37) CS | Eggs | Consumed food group in previous 24 h (yes vs. no) | 6–364 (model not age adjusted) | — | — | — | NS |
| — | — | 6–364 (age adjusted model) | — | — | — | NS | |
| Meat | — | 6–364 (model not age adjusted) | — | — | — | NS | |
| — | — | 6–364; (age adjusted model) | — | — | — | NS |
Associations in this table are limited to those that were reported via tests of statistical significance. Statistical significance: ‡ = P < 0.01; § = P < 0.001; Not significant (NS) = P ≥ 0.05.
Single digits separated by commas, e.g., 6, 9, 12 mo, indicates that the associations reported were for the measures taken at each time point.
Stunting prevalence ratio is the prevalence of stunting in the intervention group divided by the prevalence of stunting in the control group.
Ages pooled in analysis.
Length/height
Ten of the 15 studies that examined the relation between ASF interventions and length/height outcomes reported significant results, including 5 RCTs (19, 21–24), 3 longitudinal (27, 28, 24), and 2 cross-sectional (21, 33) studies (Table 5). Two RCTs (20, 39) showed an increase in length/height compared with comparison groups and 5 studies did not have significant results (19, 21–24). Among the longitudinal studies, 3 had mixed results, e.g., a mix of increased length/height gains with increased ASF intake and nonsignificant results (25, 26, 31).
Weight
Of the 13 studies that examined the relation between ASF interventions and weight outcomes, 9 showed largely nonsignificant results, including 5 RCTs (19, 21, 22, 24, 39), 3 longitudinal (25, 27, 28), and 1 cross-sectional study (37) (Tables 6 and 7 ). The RCT by Iannotti et al. (20) was unique as they reported that the ASF intervention had positive effects on WLZ, WAZ, BMIz, and the underweight prevalence ratio (prevalence of underweight in the intervention group divided by prevalence of underweight in the control group) in an age-adjusted model. Comparing an ASF intervention to a PSF comparator, Lin et al. (23) reported that the PSF had a greater effect on weight gain than the ASF.
TABLE 8.
Relation between ASF, head circumference, and iron status indicators in included studies1
| Reference and Study Design | Animal-source food (ASF) | Comparator | Child age at assessment, mo2 | Head circumference (HC) | HC growth slope | HC z-score (HCz) | Odds of HCz←2 z-score | Hemo-globin, g/dl | Iron deficiency anemia3 prevalence |
|---|---|---|---|---|---|---|---|---|---|
| Bauserman, 2015 (19) randomized controlled trial (RCT) | Caterpillar cereal | Usual diet | 18 | — | — | — | — | Mean (SD) caterpillar cereal: 10.7 (1.6), usual diet:10.1 (1.8)† | Caterpillar cereal: 26%, usual diet control: 50%‡ |
| Krebs, 2012 (21) RCT | Beef | Fortified rice-soy cereal | 6 | — | — | NS | — | — | — |
| — | — | 9 | — | — | NS | — | — | — | |
| — | — | 12 | — | — | NS | — | — | — | |
| — | — | 18 | — | — | Mean (SD) beef: −0.69 (0.99), rice-soy cereal: −0.50 (1.43)† | — | NS | — | |
| Lartey, 1999 (22) RCT4 | 1. Weanimix (maize, soybeans, peanuts) plus fish powder (anchovy) | 3. Weanimix alone | 6–125 | NS | — | — | — | NS | — |
| 2. Koko (fermented maize dough powder) plus fish powder | 4. Weanimix plus vitamins and minerals | 12 | — | — | — | — | NS | — | |
| Long, 2012 (24) RCT | Millet porridge w/meat | Millet porridge | 16–455 | — | NS | — | — | — | — |
| Millet porridge w/milk | Millet porridge | 16–455 | — | NS | — | — | — | — | |
| Tang, 2014 (39) RCT | Pork | Cereal | 6–185 | NS | — | NS | — | — | — |
| Miller, 2016 (30) longitudinal cohort | ASF | Continuous, total number consumed in previous 24 h | 6 to <366 | — | — | ß (SE): 0.26 (0.09)‡ | — | — | — |
| ASF | Categorial, 0–1 vs. ≥2 consumed in previous 24 h | 6–366 | — | — | Mean (SE) 0–1 ASF: −2.08 (0.10), ≥2 ASF: −1.69 (0.05)‡ | — | — | — | |
| — | — | 6–126 | — | — | NS | — | — | — | |
| — | — | 13–246 | — | — | Mean (SE) 0–1 ASF: −2.25 (0.19), ≥2 ASF: −1.79 (0.10)† | — | — | — | |
| — | — | 25–366 | — | — | 0–1 ASF: −1.92 (0.12), ≥2 ASF: −1.59 (0.06)† | — | — | — | |
| — | — | 37–606 | — | — | NS | — | — | — | |
| Zhao, 2016 (37) cross-sectional | Eggs | — | 6–366; not age adjusted | — | — | — | NS | — | — |
| Meat | — | 6–366; age adjusted | — | — | — | NS | — | — |
Results in this table are limited to those that were reported via tests of statistical significance. Statistical significance: † = P < 0.05; ‡ = P < 0.01; § = P < 0.001; Not significant = P ≥ 0.05.
Single digits separated by commas, e.g., 6, 9, 12 mo, indicates that the associations reported were for the measures taken at each time point.
Iron deficiency anemia is defined as Hb ≤10.0 g/dl.
All 4 food groups were compared against each other.
Change from baseline to end of follow-up.
Ages pooled in analysis.
Head circumference
Seven studies examined head circumference outcomes, with the majority producing nonsignificant results— including 3 RCTs (22, 24, 39) and 1 cross-sectional study (39) (Table 8). Krebs et al. (21) reported that the intervention group had significantly lower head circumference z-scores than the comparison group at EoF. Miller et al. (30) examined continuous and categorical ASF exposures and reported mixed significant and nonsignificant results.
Iron status
Only 3 RCTs examined the relation between ASF consumption and iron status; 1 reported an improvement in iron status (19) and the other 2 reported no effect (21, 22) (Table 8). Bauserman et al. (19) reported that mean hemoglobin was higher and IDA prevalence was lower in the caterpillar cereal group compared with usual diet at age 18 mo.
The role of breastfeeding and infant formula in included studies
In the global guidelines for the assessment of infant and young child feeding, a current topic of controversy is how breast and formula milks should be assessed in relation to ASFs (40). Therefore, in this review breast and formula milks are reported as a special consideration. Eighteen of the studies included in this review collected data on breastfeeding: 6 of the 7 RCTs collected data on breastfeeding (19, 21–24, 39), 6 of the 7 longitudinal cohort studies (25–28, 31), and 6 of the 7 cross-sectional studies (38, 32–35). Five of the studies in this review collected data on formula consumption: 3 longitudinal cohort studies (26, 27, 29), and 2 cross-sectional studies (32, 33).
Exclusive breastfeeding rates among study groups were compared at baseline in 2 RCTs (19, 21) whereas a third enrolled only exclusively breastfed infants (39). Although 6 longitudinal cohort studies collected data on breastfeeding, the practice was analyzed as an independent variable in only 1 (29). For the 6 cross-sectional studies that collected breastfeeding data, it was not included as a covariate in any of the studies.
Among RCTs, Lin et al. (23) reported that almost all the subjects, except for 1, were breastfeeding at the time of enrollment and throughout the duration of the study. Lartey et al. (22) reported data on the contribution of breastfeeding to daily energy intake during the study, and Long et al. (24) reported breast milk intake as part of total daily energy intake.
Although formula feeding was not reported in the RCTs, 2 RCTs excluded participants who received (21) or were likely to receive (19) free or subsidized infant formula. Three longitudinal cohort studies included formula in the ASF variable (26, 27, 29) and a fourth study investigated “bottle feeding” but did not explicitly examine formula (31). Formula was also included in ASF variables in 2 cross-sectional studies (32, 33). It was unclear if formula was included in the fortified powdered milk investigated by Semba et al. (35).
RoB results
RoB in RCTs
Three of the 7 RCTs were rated as good (19, 21, 23), 2 were rated as fair (20, 22), and 2 were rated as poor (24, 39). The studies rated as good all had strong methodologies and comprehensive reporting, with the exception that none reported using an intent-to-treat analysis (19, 21, 23). Two studies were rated as fair because we could not determine whether there was high adherence to the treatment protocol (20, 22). The studies rated as poor failed to report on key methodological aspects including treatment allocation concealment, adherence to the intervention, and sample size calculation (24, 39). Tang et al. (39) failed to report on randomization, did not account for differences among groups at baseline in the analysis of secondary outcomes, and the most important reporting failure was that there were 2 comparator groups at baseline (micronutrient-fortified cereal group; nonfortified cereal group) that were combined during analysis without justification (39).
RoB in cohort studies
Three of the cohort studies were rated as good (26, 27, 30), 2 as fair (25, 29), and 2 as poor (28, 31). The studies rated as good had strong reporting and methodology. We assessed the article by Campbell et al. (26) by applying the questions in the tool to the control group only; the analyses obtained for this review via author contact were performed on the control group only and were treated as observational cohort results (P Christian, Johns Hopkins Bloomberg School of Public Health,personal communication, 2018). The study conducted by Allen et al. (25) was rated as fair because despite strong methodology, including dietary assessment and inclusion/exclusion criteria, sample size justification was not reported. Marquis et al. (29) employed less rigorous dietary data collection tools and did not provide a sample size justification. For the studies assessed as poor, the critical flaw in the Leonard et al. (28) study was that they did not report how they selected the focal child within each recruited household. The data collection by Ntab et al. (31) was embedded within a malaria treatment survey and the possible relation between the observational data and the concurrent intervention was not reported.
RoB in cross-sectional studies
Two of the cross-sectional studies were assessed as good (35, 38), 3 as fair (33, 36, 37), and 2 as poor (20, 32). The studies assessed as good had strong methodology and reporting. For the studies assessed as fair, none reported a sample size justification (33, 36, 37), 1 lacked reporting on subject selection (26), and 2 did not control for sufficient confounders (36, 37).
Discussion
The primary relation we examined was between ASF consumption and stunting in children aged 6–60 mo in LMICs. The secondary set of relations examined were ASF consumption and indicators of height, weight, head circumference, and iron status. We did not find a consistent relation between ASF consumption and stunting in RCTs and cross-sectional studies. The relation between ASF and the secondary outcomes of length/height, weight, head circumference, and iron status were largely nonsignificant across study designs.
Among the RCTs, only 1 intervention had positive effects on multiple indicators of growth (20). It was also the only RCT that compared a single ASF to usual diet; all of the other interventions either combined ASF with PSF, i.e. ASF and porridge or cereal (19, 22–24), or used a single ASF with PSF comparator (21, 39). It is possible that the PSF components of the interventions may have had an association with the outcomes, particularly in studies where the PSF comparator was micronutrient fortified (21, 22, 39). However, there may have been community requests or ethical issues that prevented using a usual diet comparator. Long et al. (24) stated, “Due to strong community sentiment, there was no nonsupplemented control group.” Further justification and reporting related to the selection of the PSF comparator, particularly fortified comparators, would have aided our assessment of the results. Global ASF interventions need to use locally available foods from the current diet, including combination foods. Although combination foods deserve a special focus, the challenge is in isolating and distinguishing the treatment effect of ASF.
Additionally, methods accounting for breast and formula milks were inconsistent across RCTs and may not have been adequately addressed. Breast and formula milks may have played a role in the relation between the interventions and outcomes in the included RCTs but the reporting was insufficient. How breast and formula milks are accounted in Infant and Young Child Feeding (IYCF) Indicators was a topic of discussion in a 2017 WHO meeting on reconsidering, refining, and extending the WHO IYCF indicators (40). The role of breast and formula milks in calculating minimum diet diversity (MDD) was discussed including updating the indicator food groups to include breast milk (40). The possible exclusion of formula milk in MDD calculations was also discussed. Since breast and formula milks are a source of controversy in the calculation of MDD and an important part of IYCF, they should be accounted for in studies examining ASF consumption.
Among the longitudinal cohort studies, the largest body of evidence that we captured were analyses that found nonsignificant relations between ASF consumption and indicators of height and weight. The only other indicator investigated in the longitudinal cohorts was head circumference (30). In this body of evidence there was a large degree of heterogeneity in the methods used to collect dietary data, quantify ASF consumption, and model the relations between dietary ASF and the outcomes. Data collection methods ranged from 24-h recall (26, 28) to food weighing (25, 27), and the construction and quantification of the ASF variables ranged from continuous quantities of ASF energy and protein (25, 28) to binary quantification of single food exposures in the previous 24 h (26, 32). Analyses ranged from unadjusted correlations (25) to multivariate linear regressions (27, 29). Across a variety of data collection methods, variable constructions, and analyses, nonsignificant relations between ASF exposures and height and weight outcomes were consistently reported in the body of evidence. Using standardized data collection and analysis tools that are widely available could help to homogenize the research to a degree that allows for interstudy comparisons across sites.
The largest evidence base examining the relation between ASF and stunting we identified was in the cross-sectional literature. Four of the 5 studies we identified with an ASF exposure and stunting outcome had a statistically significant relation (32, 35–37). Although Semba et al. (35) reported consistent relations between fortified milk consumption and reduced stunting prevalence in a large cohort of Indonesian children, the composition of the fortified milk was not reported. Therefore, it wasn't possible to make the distinction between fortified milks and formula designed as a breast milk substitute. The other studies found either a combination of significant and nonsignificant (32, 35) or solely nonsignificant (37) relations. In contrast to the cohort studies, in the cross-sectional studies there were more single food variables reported; only 2 studies reported constructing ASF composite variables (20, 32). There was also a greater degree of consistency in data collection methods, only 24-h recall and FFQs were used, and the exposure variables tended to binary quantification. Similarly, the studies largely employed multiple linear or logistic regression for the analyses. There was more homogeneity in the methods for the cross-sectional studies than in the longitudinal cohorts. However, the exposures examined had a greater degree of heterogeneity and, in turn, the results were more heterogeneous. The heterogeneity of the exposures in the cross-sectional studies made extensive interstudy comparisons not possible and we were unable to draw conclusions on the relation between ASF and stunting and other indicators of growth from the cross-sectional data.
The strengths of this study include the extensive search for peer-reviewed and grey literature with an exhaustive list of search terms. The peer-reviewed literature captured a large number of records that were screened with a rigorous dual-screening methodology. The dual RoB assessment comprehensively examined the bias in the included studies. One weakness of this study was that, because of the heterogeneity of the exposures and outcomes in the included studies, we were unable to conduct a meta-analysis to quantitatively examine the results. A second weakness of this study is found in the literature search strategy as it relates to the secondary outcome of anemia examined in this review. We constructed the search to include terms related to growth disorders (this included height and weight), stunting, and head circumference. We were unable to include terms related to anemia because the number of records captured would have been exponentially greater than those in which our team were able to screen for this review. Therefore, this review examined anemia as a secondary outcome among all studies retrieved that examined growth disorders (including height and weight), stunting, and head circumference in the specified population. We believe the number of records examined in this review (n = 14,783) was sufficiently large to provide a thorough examination of the relation between ASF consumption and anemia within the context of this review.
Overall, we did not find strong relations between ASF and growth outcomes. The studies were largely heterogeneous in their exposures and outcomes, which limited the interstudy comparisons. To quantitatively elucidate the relation between ASF and indicators of growth during early childhood in LMICs, future research should differentiate age groups to determine the effects of ASF consumption during different periods of growth. Future studies should also provide consistency in the definition and quantification of ASF exposure and outcomes that will facilitate interstudy comparisons. For example, the MDD food groups with their standardized definition could be used for defining food groups. Public health practitioners and researchers who collect data in LMICs that contain ASF exposure and growth outcomes could improve future research in this field by making these datasets accessible to researchers through interinstitutional collaborations.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—SMD, KK, KK, and JF: conceived the project; MJS, SMD, HJS, and QD: conducted the review; MJS, SMD, and HJS: prepared the manuscript; MP, QD, KK, KK, KPW, and JF: provided feedback on the manuscript; and all authors: read and approved the final manuscript.
The authors thank Andrea Louise Falzon for her collaboration in writing the search terms and Alex Schmall for her assistance in RoB assessment.
Notes
This work was supported by PATH and the Sight and Life Foundation.
Author disclosures: MJS, SMD, HJS, MP, DQ, KK, KK, KPW Jr., and JF, no conflicts of interest.
Supplemental Material 1 and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: ASF, animal-source food; BMIz, body mass index z-score; DRC, Democratic Republic of the Congo; EoF, end of follow-up; HAZ, height for age z-score; HCz, head circumference z-score; IDA, iron deficiency anemia; IYCF, Infant and Young Child Feeding Indicators; LAZ, length for age z-score; LMIC, low- and middle-income country; MDD, minimum diet diversity; MUACz, midupper arm circumference z-score; PSF, plant-source food; RoB, risk of bias; RCT, randomized controlled trial; WAZ, weight for age z-score; WHZ, weight for height z-score; WLZ, weight for length z-score.
References
- 1. Stevens GA, Finucane MM, Paciorek CJ, Flaxman SR, White RA, Donner AJ, Ezzati M, on behalf of Nutrition Impact Model Study Group (Child Growth). Trends in mild, moderate, and severe stunting and underweight, and progress towards MDG 1 in 141 developing countries: a systematic analysis of population representative data. Lancet. 2012;380(844):824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perumal N, Bassani DG, Roth DE. Use and misuse of stunting as a measure of child health. J Nutr. 2018;148(3):311–5. [DOI] [PubMed] [Google Scholar]
- 3. Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J;. Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–60. [DOI] [PubMed] [Google Scholar]
- 4. WHO. Global nutrition targets 2025: stunting policy brief (WHO/NMH/NHD/14.3). Geneva: WHO; 2014. [Google Scholar]
- 5. UNICEF, WHO, and the World Bank. Joint child malnutrition estimates - levels and trends: 2018 edition. May 2018. [Internet]. World Health Organization: Geneva: Available from: http://www.who.int/nutgrowthdb/2018-jme-brochure.pdf?ua=1, [updated 2018; cited 3 July 2018]. [Google Scholar]
- 6. Global Health Observatory. WHO. 2018. Global Health Observatory Data. [Internet]. Available from: http://www.who.int/gho/child-malnutrition/en/, [updated 2018; cited 3 July 2018].
- 7. Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev H; Maternal and Child Undernutrition Study Group Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO. Indicators for assessing infant and young child feeding practices: conclusions of a consensus meeting held 6–8 November 2007 in Washington D.C., USA. Geneva: WHO; 2008. [Google Scholar]
- 9. WHO. Global strategy for infant and young child feeding. Geneva: WHO; 2003. [PubMed] [Google Scholar]
- 10. Headey D, Hirvonen K, Hoddinott J. Animal sourced foods and stunting. Washington (DC): International Food Policy Research Institute (IFPRI) IFPRI Discussion Paper 01695. [Google Scholar]
- 11. Roberts JL, Stein AD. The impact of nutritional interventions beyond the first 2 years of life on linear growth: a systematic review and meta-analysis. Adv Nutr. 2017;8(2):323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eaton JC, Rothpletz-Puglia P, Dreker MR, Kaganda J, Iannotti L, Lutter C, Rayco-Solon P. Effectiveness of provision of animal-source foods for supporting optimal growth and development in children 6 to 59 months of age. Cochrane Database Syst Rev. 2017;10:1465–1858. Art. No.: CD012818 doi:10.1002/14651858.CD012818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shapiro M, Downs S, Fanzo J, Kreis K, Kraemer K, Quelhas D, Rajagopal S, Swartz H. The association between consumption of animal source food and stunting in children aged 6 months to 5 years: a systematic review. [Internet]. PROSPERO. 2016; CRD42016043998 Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016043998, [updated 3 April 2018; cited 3 July 2018]. [Google Scholar]
- 14. Hamill PVV, Drizd TA, Johnson CL, Reed RB, Roche AF. Growth curves for children, birth–18 years. 1977. National Center for Health Statistics. Vital and Health Statistics, Series 11 No. 165. [PubMed]
- 15. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC growth charts for the United States: methods and development. 2002. National Center for Health Statistics. Vital and Health Statistics, Series 11 No. 246. [PubMed]
- 16. WHO Multicentre Growth Reference Study Group. WHO child growth standards. Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Department of Nutrition Health and Development. Geneva: World Health Organization; 2006. [Google Scholar]
- 17. NHLBI. Study Quality Assessment Tools. Bethesda (MD): National Heart, Lung, and Blood Institute; 2017. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools, [updated 2017; cited 3 July 2018]. [Google Scholar]
- 18. World Bank. 2017. Historical classification by income in XLS format. Country and lending groups, country classification, Data Help Desk of the World Bank. [Internet]. Available from: http://databank.worldbank.org/data/download/site-content/OGHIST.xls, [updated 2017; cited 3 July 2018].
- 19. Bauserman M, Lokangaka A, Gado J, Close K, Wallace D, Kodondi KK, Tshefu A, Bose C. A cluster-randomized trial determining the efficacy of caterpillar cereal as a locally available and sustainable complementary food to prevent stunting and anaemia. Public Health Nutr. 2015;18(10):1785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iannotti LL, Lutter CK, Stewart CP, Gallegos Riofrío CA, Malo C, Reinhart G, Palacios A, Karp C, Chapnick M, Cox K et al.. Eggs in early complementary feeding and child growth: a randomized controlled trial. Pediatrics. 2017;140(1). [DOI] [PubMed] [Google Scholar]
- 21. Krebs NF, Mazariegos M, Chomba E, Sami N, Pasha O, Tshefu A, Carlo WA, Goldenberg RL, Bose CL, Wright LL et al.. Randomized controlled trial of meat compared with multimicronutrient-fortified cereal in infants and toddlers with high stunting rates in diverse settings. Am J Clin Nutr. 2012;96(4):840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lartey A, Manu A, Brown KH, Peerson JM, Dewey KG. A randomized, community-based trial of the effects of improved, centrally processed complementary foods on growth and micronutrient status of Ghanaian infants from 6 to 12 mo of age. Am J Clin Nutr. 1999;70(3):391–404. [DOI] [PubMed] [Google Scholar]
- 23. Lin CA, Manary MJ, Maleta K, Briend A, Ashorn P. An energy-dense complementary food is associated with a modest increase in weight gain when compared with a fortified porridge in Malawian children aged 6–18 months. J Nutr. 2008;138(3):593–8. [DOI] [PubMed] [Google Scholar]
- 24. Long JK, Murphy SP, Weiss RE, Nyerere S, Bwibo NO, Neumann CG. Meat and milk intakes and toddler growth: a comparison feeding intervention of animal-source foods in rural Kenya. Public Health Nutr. 2012;15(6):1100–7. [DOI] [PubMed] [Google Scholar]
- 25. Allen LH, Backstrand JR, Stanek EJ 3rd, Pelto GH, Chávez A, Molina E, Castillo JB, Mata A. The interactive effects of dietary quality on the growth and attained size of young Mexican children. Am J Clin Nutr. 1992;56(2):353–64. [DOI] [PubMed] [Google Scholar]
- 26. Campbell RK, Hurley KM, Shamim AA, Shaikh S, Chowdhury ZT, Mehra S, de Pee S, Ahmed T, West KP Jr, Christian P. Effect of complementary food supplementation on breastfeeding and home diet in rural Bangladeshi children. Am J Clin Nutr. 2016;104(5):1450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diana A, Mallard SR, Haszard JJ, Purnamasari DM, Nurulazmi I, Herliani PD, Nugraha GI, Gibson RS, Houghton L. Consumption of fortified infant foods reduces dietary diversity but has a positive effect on subsequent growth in infants from Sumedang district, Indonesia. PLoS One. 2017;12(4):e0175952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leonard WR, Dewalt KM, Stansbury JP, McCaston MK. Influence of dietary quality on the growth of highland and coastal Ecuadorian children. Am J Hum Biol. 2000;12(6):825–37. [DOI] [PubMed] [Google Scholar]
- 29. Marquis GS, Habicht JP, Lanata CF, Black RE, Rasmussen KM. Breast milk or animal-product foods improve linear growth of Peruvian toddlers consuming marginal diets. Am J Clin Nutr. 1997;66(5):1102–9. [DOI] [PubMed] [Google Scholar]
- 30. Miller LC, Joshi N, Lohani M, Singh R, Bhatta N, Rogers B, Griffiths JK, Ghosh S, Mahato S, Singh P et al.. Head growth of undernourished children in rural Nepal: association with demographics, health and diet. Paediatr Int Child Health. 2016;36(2):91–101. [DOI] [PubMed] [Google Scholar]
- 31. Ntab B, Simondon KB, Milet J, Cissé B, Sokhna C, Boulanger D, Simondon F. A young child feeding index is not associated with either height-for-age or height velocity in rural Senegalese children. J Nutr. 2005;135(3):457–64. [DOI] [PubMed] [Google Scholar]
- 32. Darapheak C, Takano T, Kizuki M, Nakamura K, Seino K. Consumption of animal source foods and dietary diversity reduce stunting in children in Cambodia. Int Arch Med. 2013;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fierstein JL, Eliasziw M, Rogers BL, Forrester JE. Nonnative cattle ownership, diet, and child height-for-age: evidence from the 2011 Uganda demographic and health survey. Am J Trop Med Hyg. 2017;96(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jin M, Iannotti LL. Livestock production, animal source food intake, and young child growth: the role of gender for ensuring nutrition impacts. Soc Sci Med. 2014;105:16–21. [DOI] [PubMed] [Google Scholar]
- 35. Semba RD, Moench-Pfanner R, Sun K, de Pee S, Akhter N, Rah JH, Campbell AA, Badham J, Bloem MW, Kraemer K. Consumption of micronutrient-fortified milk and noodles is associated with lower risk of stunting in preschool-aged children in Indonesia. Food Nutr Bull. 2011;32(4):347–53. [DOI] [PubMed] [Google Scholar]
- 36. Walker SP, Powell CA, Grantham-McGregor SM. Dietary intakes and activity levels of stunted and non-stunted children in Kingston, Jamaica. Part 1. Dietary intakes. Eur J Clin Nutr. 1990;44(7):527–34. [PubMed] [Google Scholar]
- 37. Zhao A, Gao H, Li B, Zhang J, Win NN, Wang P, Li J, Zhang Y. Inappropriate feeding behavior: one of the important causes of malnutrition in 6- to 36-month-old children in Myanmar. Am J Trop Med Hyg. 2016;95(3):702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aramburú La Torre AM. Diversidad alimentaria y su asociación con el retraso del crecimiento en niños de 6–23 meses. Perú, 2008–2010/Dietary diversity and its association with growth retardation in children aged 6–23 months. Peru, 2008–2010. Rio de Janeiro; s.n;2014.
- 39. Tang M, Sheng XY, Krebs NF, Hambidge NM. Meat as complementary food for older breastfed infants and toddlers: a randomized, controlled trial in rural China. Food Nutr Bull. 2014;35(4 Suppl):S188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. WHO. Meeting report on reconsidering, refining, and extending the World Health Organization Infant and Young Child Feeding Indicators. [Internet]. 20–22 June, 2017. New York: World Health Organization: Geneva: Available from: http://www.who.int/nutrition/events/2017-team-technicalconsultation-iycf-indicators-meetingreport.pdf?ua=1 [cited 3 July 2018]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.