Abstract
Background
The development of strategies to accelerate disease resolution and shorten antibiotic therapy is imperative in curbing the global tuberculosis epidemic. Therapeutic application of novel vaccines adjunct to antibiotics represents such a strategy.
Methods
By using a murine model of pulmonary tuberculosis (TB), we have investigated whether a single respiratory mucosal therapeutic delivery of a novel chimpanzee adenovirus-vectored vaccine expressing Ag85A (AdCh68Ag85A) accelerates TB disease control in conjunction with antibiotics and restricts pulmonary disease rebound after premature (nonsterilizing) antibiotic cessation.
Results
We find that immunotherapy via the respiratory mucosal, but not parenteral, route significantly accelerates pulmonary mycobacterial clearance, limits lung pathology, and restricts disease rebound after premature antibiotic cessation. We further show that vaccine-activated antigen-specific T cells, particularly CD8 T cells, in the lung play an important role in immunotherapeutic effects.
Conclusions
Our results indicate that a single-dose respiratory mucosal immunotherapy with AdCh68Ag85A adjunct to antibiotic therapy has the potential to significantly accelerate disease control and shorten the duration of conventional treatment. Our study provides the proof of principle to support therapeutic applications of viral-vectored vaccines via the respiratory route.
Keywords: antibiotics, immunotherapy, respiratory mucosal immunization, tuberculosis, viral-vectored vaccines
Using a murine model of pulmonary TB, a single respiratory mucosal delivery of chimpanzee adenoviral-based TB vaccine in conjunction with antibiotic therapy markedly improved disease treatment and restricted its relapse after antibiotic cessation.
Pulmonary tuberculosis (TB) remains the leading global infectious cause of death, claiming 1.6 million lives yearly [1]. Although antibiotics are available for treating TB, their success relies on patient adherence to treatment guidelines. Given that effective therapy is complex, requiring the use of multiple antibiotics for a minimum 6 months, many patients fail to properly follow and complete antibiotic therapy [2, 3]. This leads to the relapse of active disease and is a major reason for the emergence of multidrug-resistant and extensively drug-resistant TB cases that are increasingly more difficult to treat and account for significantly higher mortality rates [4, 5].
The development and implementation of effective vaccination strategies is central in combatting the TB epidemic. These vaccines are classified as prophylactic, postexposure (latent infection), and therapeutic vaccines [6–8]. Over the last 2 decades, significant progress has been made mostly in the development of prophylactic vaccines and, to a lesser extent, postexposure vaccine strategies [9]. However, the development of effective TB vaccines for therapeutic applications has lagged far behind.
Therapeutic vaccines are designed to be administered in conjunction with conventional TB antibiotic therapy to hosts with active disease. This strategy aims to boost or redirect the host anti-TB immune response to better control TB disease and/or shorten antibiotic therapy [8, 10–12]. In this regard, a limited number of subunit protein- and viral-based approaches have been explored for their therapeutic application [10, 13–16]. Although these studies have provided insights into the applicability of therapeutic vaccine strategies, they led to either no efficacy [15] or variable efficacies in limiting infection and lung pathology [10, 13, 14, 16]. Of note, all of these therapeutic vaccines had to be given repeatedly via the parenteral route.
We and others have developed viral-vectored TB vaccines for prophylactic applications after parenteral and respiratory mucosal (RM) routes of immunization [17–25]. Via these studies, it has been well established that RM immunization is superior to the parenteral route via positioning protective T cell immunity in the respiratory mucosa before Mycobacterium tuberculosis (M.tb) exposure. In a recent study, we showed that prophylactic vaccination with a chimpanzee adenovirus serotype-68 vectored vaccine expressing M.tb antigen Ag85A (AdCh68Ag85A) via the RM route provides robust immunity against M.tb challenge by reducing bacterial burden and lung pathology [26]. However, it has remained unclear whether such vaccine strategies can be applied therapeutically via the RM route, in conjunction with antibiotic therapy, to treat established pulmonary TB disease.
In the current study, we have investigated the therapeutic potential of AdCh68Ag85A. For the first time, we demonstrate that a single delivery of RM TB vaccine in conjunction with conventional antibiotic therapy can significantly improve disease treatment and restrict disease relapse after antibiotic cessation. Our findings thus suggest that viral-vectored TB vaccines designed for prophylactic respiratory immunization can also be used for effective immunotherapeutic application.
METHODS
Detailed methods are provided in the online Supplement.
Animal Models
Female BALB/c mice (6–8 weeks old) were housed and used within the biosafety level 3 facility in accordance with guidelines of institutional Animal Research Ethics Board.
Mycobacterium tuberculosis Infection and Antibiotic Therapy
Mycobacterium tuberculosis H37Rv was prepared for infection via the RM route as described [21, 26]. Animals were treated with a triple antibiotic cocktail of rifampicin, isoniazid, and pyrazinamide via drinking water. The nonsterilizing antibiotic regimens were chosen in this immunotherapeutic investigation.
AdCh68Ag85A Immunotherapy
AdCh68Ag85A was administered intranasally (I.N.) or intramuscularly (I.M.) as described [18, 23, 26] to infected animals. Some animals received an empty AdCh68 control vector.
In Vivo T Cell Depletion
CD4 and CD8 T cells were depleted by intraperitoneal (I.P.) injection of anti-CD4 (clone GK1.5) and anti-CD8 (clone 2.43) monoclonal antibodies (mAbs) [27].
Tumor Necrosis Factor α Protein Levels
Tumor necrosis factor α (TNFα) was measured in bronchoalveolar lavage (BAL) fluids and sera using enzyme-linked immunosorbent assay (ELISA).
Mononuclear Cell Isolation and Intracellular Cytokine Staining
Lung mononuclear cells were isolated using our previously published protocol [20, 21, 26]. Intracellular cytokine staining was performed after stimulation with antigens (Ags). Stained cells were acquired on a BD LSRFortessa cytometer, and data were analyzed using FlowJo software, version 10 (TreeStar, Ashland, OR).
Tuberculosis Disease Indices
Lung bacterial burden was evaluated by colony-forming unit (CFU) assay [26]. Lung sections were hematoxylin and eosin-stained for histopathological analysis and quantification or Ziehl Neelsen-stained for visualization of acid-fast bacilli (AFB) [20].
T Cell and Macrophage Distribution by Immunohistochemistry
Immunohistochemical staining of CD4, CD8, and F4/80 was performed on deparaffinized sections using anti-mouse CD4, CD8, and F4/80 mAbs.
Statistical Analysis
Two-tailed Student t tests were used for comparison between 2 groups. One-way analysis of variance was used followed by posttest Tukey analysis for multiple-group comparison using GraphPad Prism 8 software. Results were considered significant for P values ≤.05. Area-under-the-curve (AUC) analysis was done to summate changes in bacterial burden over time. Unpaired t tests were performed in AUC analysis.
RESULTS
AdCh68Ag85A Respiratory Mucosal Immunotherapy Improves Pulmonary Tuberculosis Disease Control During Antibiotic Therapy
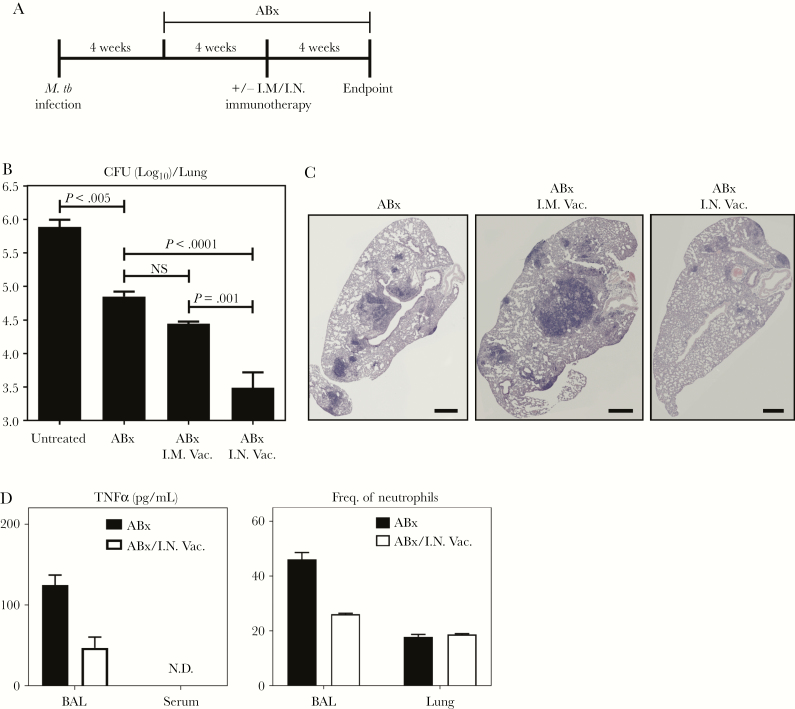
With its demonstrated prophylactic efficacy [26], we investigated whether AdCh68Ag85A could be used as a therapeutic vaccine adjunctive to a triple antibiotic therapy in treating pulmonary TB. To this end, mice infected with M.tb for 4 weeks were treated with antibiotics alone (ABx) or in conjunction with intramuscular (ABx I.M. Vac.) or RM (ABx I.N. Vac.) immunotherapy with a single dose of AdCh68Ag85A at 4 weeks postinitiation of antibiotic treatment (Figure 1A). A set of mice was left untreated. All groups of mice were sacrificed at 12 weeks postinfection and were assessed for TB disease indices. As expected, antibiotic treatment significantly reduced pulmonary bacterial burden compared with untreated animals (Figure 1B). Parenteral immunotherapy (ABx I.M.Vac.) failed to significantly further enhance bacterial control. However, RM administration of AdCh68Ag85A (ABx I.N.Vac.) provided further significant reduction in bacterial burden by 1.25 log, reducing pulmonary mycobacterial burden to 3.5 log. Histological analysis revealed markedly reduced lung pathology in ABx I.N.Vac. animals compared with those treated with ABx or in conjunction with parenteral immunotherapy (ABx I.M.Vac.) (Figure 1C).
Figure 1.
AdCh68Ag85A respiratory mucosal immunotherapy improves tuberculosis (TB) disease control during antibiotic therapy. (A) Experimental schema. At 4 weeks post-Mycobacterium tuberculosis (M.tb) infection, mice were started on an oral antibiotic (antibiotics alone [ABx]) therapy of rifampicin, isoniazid, and pyrazinamide. A group of these mice was treated either intramuscularly (ABx I.M Vac.) or intranasally (ABx I.N. Vac.) with AdCh68Ag85A at 4 weeks after the initiation of antibiotic therapy. All mice were sacrificed 12 weeks postinfection for assessment of TB disease indices. A set of M.tb-infected animals were left untreated as controls (untreated). (B) Bar graph comparing bacterial burden assessed by colony-forming unit (CFU) assay in the lungs of 4 groups of mice. (C) Representative micrographs of lung sections stained with hematoxylin and eosin, comparing the extent of lung inflammation and granulomatous lesions. Scale bar indicates 500 µm. (D) Bar graphs showing levels of tumor necrosis factor α (TNFα) protein in the bronchoalveolar lavage (BAL) fluid and in sera, and frequencies of neutrophils (CD11b+Ly6G+) in the BAL and lung at 72 hours post-I.N. immunotherapy. Data are expressed as the mean ± standard error of the mean of 6–10 mice/group, representative of 3 independent experiments.
Given that adjunct RM immunotherapy can significantly improve pulmonary TB disease, we assessed whether such improvement might have been rendered at the expense of overzealous acute tissue inflammation, which could pose a potential safety concern. For this, mice infected with M.tb for 4 weeks were treated with ABx or in conjunction with RM AdCh68Ag85A (ABx/I.N.Vac.) and sacrificed 72 hours postimmunotherapy. Bronchoalveolar lavage, lung tissue, and sera were examined for proinflammatory TNFα levels and neutrophil infiltration. Collectively, in ABx/I.N.Vac. animals, BAL and serological concentrations of TNFα and neutrophil recruitment to the airway and lung did not increase over those treated with ABx (Figure 1D). On the contrary, they appeared even lower in the BAL of ABx/I.N.Vac. animals.
The above data suggest that compared with antibiotic therapy alone, a single dose of AdCh68Ag85A delivered via the RM route, but not via parenteral route, in conjunction with antibiotic therapy significantly accelerates bacterial clearance and reduces lung pathology.
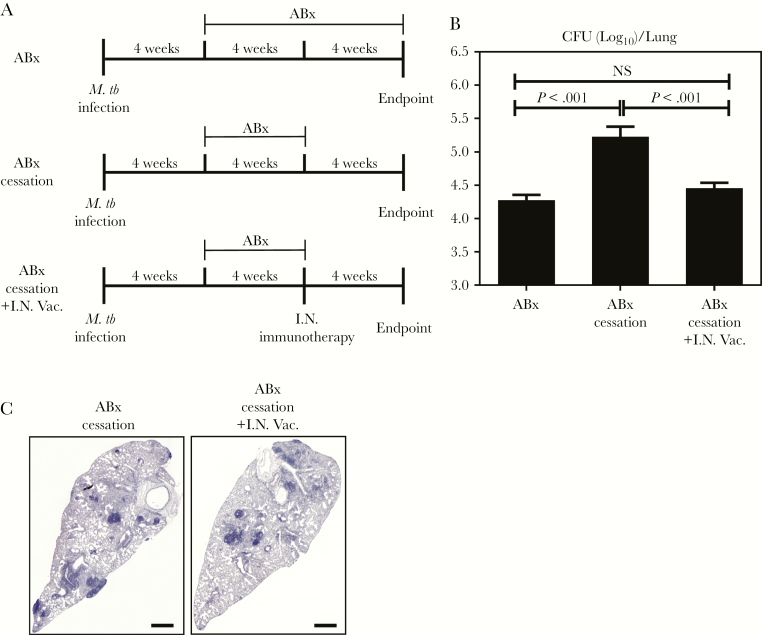
AdCh68Ag85A Respiratory Mucosal Immunotherapy Controls Bacterial Infection in the Lung After Premature Antibiotic Therapy Cessation
Having established the potency of RM immunotherapy adjunctive to continuing antibiotic treatment (Figure 1), we next set out to address whether RM immunotherapy can shorten antibiotic therapy and control antibiotic cessation-associated infection rebound. To this end, animals were infected with M.tb and at week 4 postinfection, all animals were treated with triple antibiotics. In a set of animals, antibiotic therapy continued for a total of 8 weeks (ABx). In another set of animals, antibiotic therapy was prematurely stopped at 4 weeks (ABx cessation). A group of animals received a single dose of RM immunotherapy at the time when antibiotic therapy was stopped (ABx cessation+I.N.Vac) (Figure 2A). All animals were sacrificed 12 weeks postinfection and lungs were assessed for TB disease indices. Premature cessation of antibiotic therapy (ABx cessation) led to a significant rebound of bacterial burden in the lung as indicated by a 1 log increase in CFU compared with animals receiving continuing antibiotic therapy (ABx) (Figure 2B). In contrast, lung bacterial burden in animals treated with RM immunotherapy at the time of antibiotic cessation (ABx cessation+I.N.Vac) did not undergo rebound with CFU kept similar to that in ABx animals with continuing antibiotic therapy. In support, the lungs of ABx cessation+I.N.Vac animals had reduced granulomatous lesions both in size and number compared with ABx cessation animals (Figure 2C). These data suggest that RM immunotherapy helps control TB disease in the lung even after premature antibiotic cessation.
Figure 2.
Respiratory mucosal immunotherapy controls bacterial infection in the lung after antibiotic cessation. (A) Experimental schema. At 4 weeks post-Mycobacterium tuberculosis (M.tb) infection, mice were started on oral antibiotic therapy. Groups of mice were treated for 4 (antibiotics alone [ABx] cessation) or 8 (ABx) weeks. In a set of animals, antibiotic therapy was ceased after 4 weeks and a single dose of AdCh68Ag85A was administered intranasally (ABx cessation+I.N.Vac.). All mice were sacrificed 12 weeks postinfection for assessment of tuberculosis disease indices. (B) Bar graph comparing bacterial burden in the lungs of various groups of mice. (C) Representative micrographs of lung sections stained with hematoxylin and eosin, comparing the extent of lung inflammation and granulomatous lesions. Scale bar indicates 500 µm. Data are expressed as the mean ± standard error of the mean of 6–10 mice/group. CFU, colony-forming units.
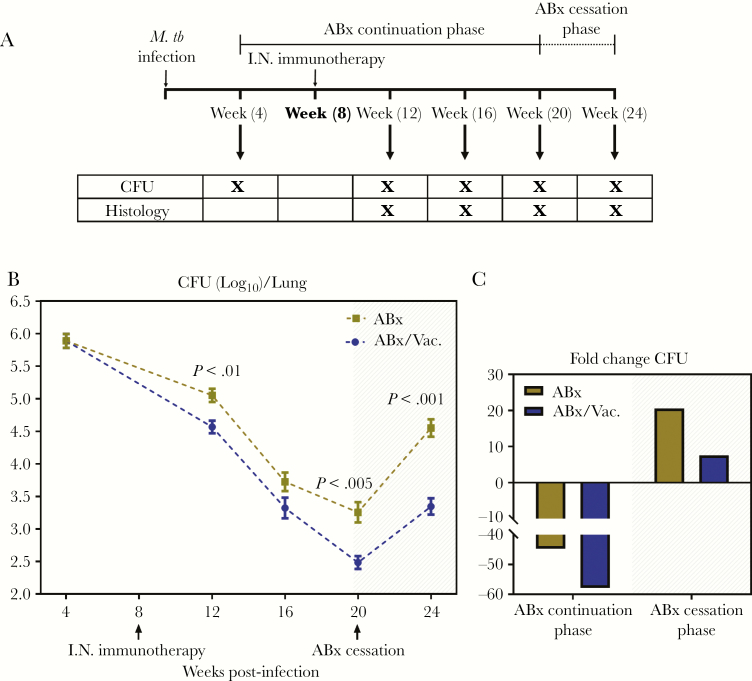
Adjunct Respiratory Mucosal Immunotherapy Accelerates Bacterial Clearance and Curbs Bacterial Rebound During Chronic Pulmonary Tuberculosis
Having established that adjunct AdCh68Ag85A RM immunotherapy is able to help control TB disease in a 12-week infection model (Figures 1 and 2), we next investigated the utility of this immunotherapeutic strategy in a protracted model of chronic TB disease. To this end, 4-week M.tb-infected mice were treated with ABx or ABx plus a single RM immunotherapy performed at week 8 (ABx/Vac.) and were sacrificed at 4, 12, 16, and 20 weeks postinfection (ABx continuation phase) (Figure 3A). We observed that both ABx and ABx/Vac. led the bacterial burden in the lungs to continue to decrease (Figure 3B). However, the bacterial clearance in animals with adjunct RM immunotherapy (ABx/Vac.) was significantly accelerated (AUC = 9.16 vs 9.635, P = .001), resulting in a significantly lower bacterial burden at 12 and 20 weeks postinfection compared with ABx animals (Figure 3B). Thus, whereas ABx alone reduced the bacterial burden by 40-fold over the antibiotic continuation phase, conjunctive ABx/Vac. therapy brought it down by 60-fold (Figure 3C).
Figure 3.
Adjunct respiratory mucosal immunotherapy accelerates bacterial clearance and curbs bacterial rebound during chronic pulmonary tuberculosis. (A) Experimental schema. At 4 weeks postinfection, mice were started on an oral antibiotic therapy. Groups of mice were treated for 16 weeks. In a set of animals, a single dose of AdCh68Ag85A was administered intranasally (I.N.) at 4 weeks after the initiation of antibiotic therapy. Animals were sacrificed for analysis at specified time points. In some animals, antibiotic therapy was ceased at 20 weeks postinfection. Tuberculosis disease indices were assessed in these animals 4 weeks after the cessation of antibiotic therapy (week 24). (B) Line graph showing kinetic changes in bacterial burden in the lung. Unshaded area indicates antibiotic continuation phase and shaded area indicates antibiotic cessation phase. (C) Bar graph showing the mean fold changes in lung bacterial burden during the antibiotic continuation phase and subsequently during the antibiotic cessation phase. Data are expressed as the mean ± standard error of the mean of 10–12 mice/group, representative of 1 to 3 independent experiments (depending on the time point). ABx, antibiotics alone; CFU, colony-forming units; M.tb, Mycobacterium tuberculosis.
We next determined whether adjunct immunotherapy could control disease rebound after premature antibiotic cessation in this protracted TB model. Thus, by the experimental design described above, antibiotic therapy was stopped in a group of animals at 20 weeks postinfection, and animals were sacrificed and examined for the extent of disease rebound 4 weeks later (antibiotic cessation phase) (Figure 3A). The ABx animals showed a rapid bacterial rebound (1.5 log increase from the time of antibiotic cessation) equating to a 20-fold increase in bacterial burden (Figure 3B and C). In contrast, ABx/Vac. animals showed a significantly restricted bacterial rebound (0.75 log, AUC = 3.324 vs 4.137, P < .0001), equating to only a 10-fold increase (Figure 3B and C).
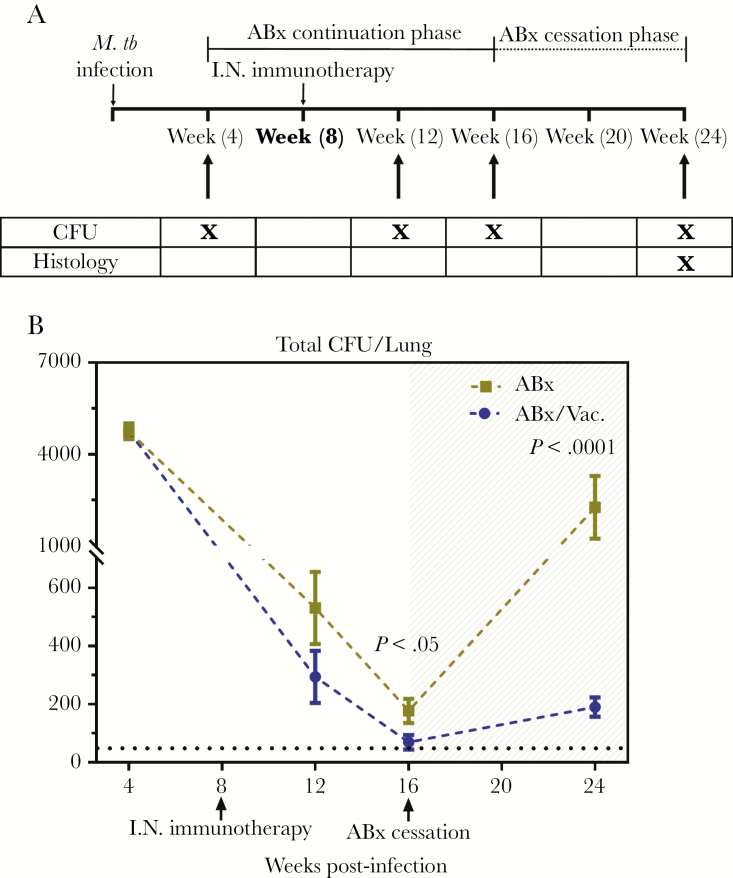
Because it is well documented that natural M.tb infection occurs after exposure to very few bacilli [28, 29], we further determined the efficacy of AdCh68Ag85A RM immunotherapy in an infection model set up by a much reduced dose of M.tb (100 CFU). Infected animals were subsequently treated with ABx or in conjunction with RM immunotherapy (ABx /I.N. Vac.) similarly as described above except that they were sacrificed at 12 and 16 weeks postinfection (ABx continuation phase) (Figure 4A). In agreement with the earlier findings (Figure 3), both ABx and ABx /I.N.Vac. led to a sharp decline in lung bacterial burden over time (Figure 4B). However, ABx/I.N.Vac. animals had the most significant CFU reduction in comparison to ABx-treated animals (AUC = 2704 vs 2995, P = .01), even with some animals with below-limit of CFU detection at 16 weeks postinfection (Figure 4B, dotted black line). Furthermore, when assessed at 8 weeks (24 weeks postinfection) after antibiotic cessation (Figure 4A), ABx/I.N.Vac. animals showed a remarkably limited rebound as indicated by minimal detectable CFU (AUC = 129.6 vs 1220, P < .005) (Figure 4B) and markedly reduced lung pathology (Supplementary Figure 1) in comparison to ABx animals.
Figure 4.
Efficacy of AdCh68Ag85A respiratory mucosal immunotherapy in a low infection dose model. (A) Experimental schema. Mice were infected and treated as described in Figure 3A (schema) but with a low dose of Mycobacterium tuberculosis (M.tb) infection (100 colony-forming units [CFU]), and antibiotic therapy ceased at week 16 postinfection. At specified time points, tuberculosis disease indices were assessed. (B) Line graph showing kinetic changes in bacterial burden in the lung. Unshaded area indicates antibiotic continuation phase and shaded area indicates antibiotic cessation phase. Dotted horizontal line represents the limit of detection (50 CFU). Data are expressed as the mean ± standard error of the mean of 6–12 mice/group, representative of 1 to 3 independent experiments (depending on the time point). ABx, antibiotics alone; I.N., intranasal.
The above data together indicate an even greater protective role by adjunct RM immunotherapy in a more clinically relevant low-dose infection model.
Adjunct Respiratory Mucosal Immunotherapy Reduces Tuberculosis-Associated Tissue Pathology and Enhances CD8 T Cell Infiltration in the Lung
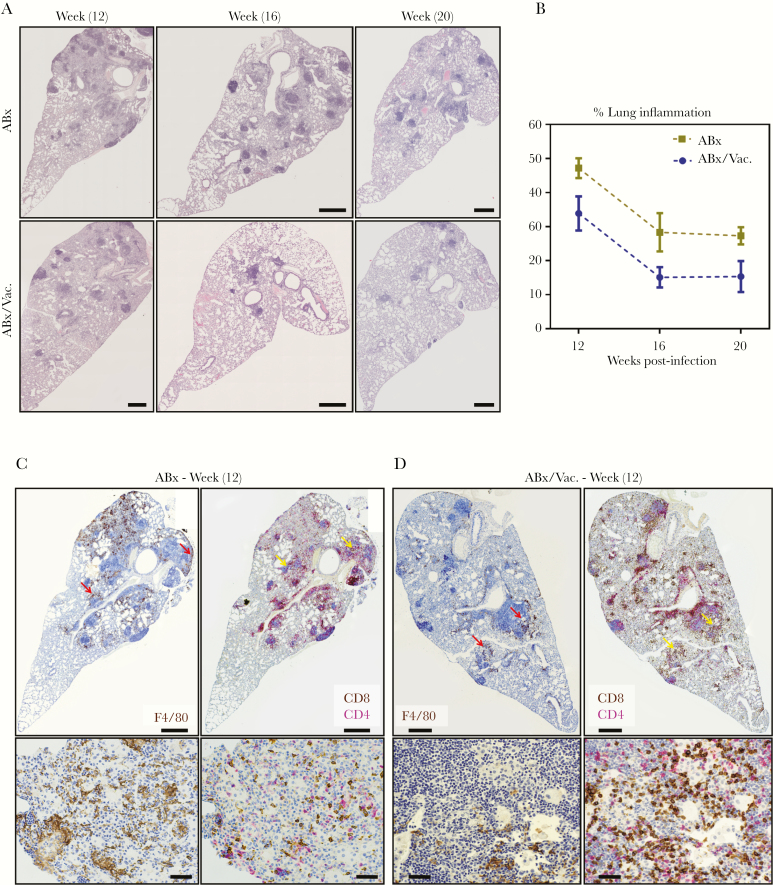
The extent of lung immunopathology is a critical index in assessing TB disease and vaccine-induced protection and even more so in the setting of TB immunotherapy [16, 18, 30]. Thus, besides quantifying bacterial burden, we performed an in-depth histopathological examination of the lungs from our protracted TB disease model illustrated in Figure 3A. Antibiotic therapy alone reduced TB-associated lung pathology shown by decreasing granulomatous regions and inflammation over time (Figure 5A, B). In comparison, adjunct RM immunotherapy (ABx/Vac.) significantly accelerated the resolution of lung pathology as indicated by major reductions in the severity and number of granulomatous lesions and overall reduced pulmonary inflammation at 12, 16 and 20, weeks postinfection (Figure 5A and B). Using 2-color immunohistochemistry, we examined the distribution of macrophages (F4/80+) and CD4+/CD8+ T cell subsets in consecutive lung sections obtained at 12 weeks postinfection. We found abundant presence of macrophages in the regions outside granulomatous lesions of ABx animals (Figure 5C, red arrows). In comparison, fewer macrophages were detected and were mostly localized within granulomatous regions in ABx/Vac. animals (Figure 5D, red arrows). On the other hand, considerably more CD8 T cells were found around and within granulomatous lesions in the lung of ABx/Vac. animals (Figure 5D, yellow arrows). This contrasted greatly with a high CD4 versus CD8 T cell ratio and much less intensity of CD8 T cells in lung lesions of ABx animals (Figure 5C, yellow arrows).
Figure 5.
Adjunct respiratory mucosal immunotherapy reduces tuberculosis-associated tissue pathology and enhances CD8 T cell infiltration in the lung during antibiotic continuation phase. Experiments were set up as depicted in experimental schema Figure 3A. (A) Representative micrographs of lung sections stained with hematoxylin and eosin, comparing the extent of lung inflammation and granulomatous lesions at weeks 12, 16, and 20 weeks postinfection. Scale bars indicate 500 µm. (B) Line graph showing semiquantified area of lung inflammation. Displayed values are averages from 3 micrographs per mouse. (C and D) Representative micrographs of immunohistochemically stained lung sections at 12 weeks postinfection visualizing the spatial distribution of F4/80 macrophages (brown stain) and of CD4 (red stain) and CD8 (brown stain) T cells costained in consecutive sections. Red arrows highlight macrophage-rich areas. Yellow arrows highlight T cell-rich areas. Top panel scale bars indicate 500 µm; bottom panel scale bars indicate 50 µm. ABx, antibiotics alone.
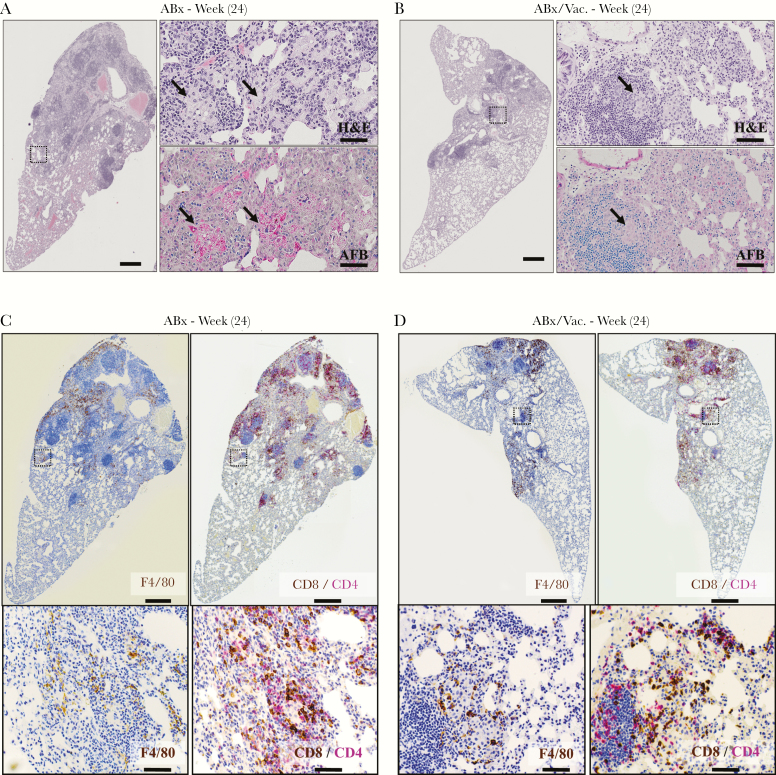
We also assessed lung histopathology upon disease rebound at 4 weeks postantibiotic cessation (24 weeks postinfection) (Figure 3A). In ABx animals, antibiotic cessation led to much worsened immunopathology as shown by large consolidated granulomatous lesions in the lung (Figure 6A). At higher magnification, these lesions were characterized by islands of lipid-laden foamy macrophages surrounded by mononuclear cells (Figure 6A, black arrows). Upon Ziehl-Neelsen staining for identifying AFB, these macrophages were densely packed with mycobacterial AFB and their products (Figure 6A, black arrows and red bacilli). In contrast, the lungs of ABx/Vac. animals showed very limited pathological rebound with much less granulomatous lesion, few foamy macrophages, and minimally detectable AFB (Figure 6B, black arrows), consistent with at least 10-fold fewer CFU in the lungs of ABx/Vac. animals in the ABx cessation phase (Figure 3C). Immunohistochemistry provided similar findings as in Figure 5C with macrophages encasing granulomatous lesions largely void of CD4 and CD8 T cells in ABx animals (Figure 6C, dotted black box). In contrast, the lungs of ABx/Vac. animals had fewer macrophages and a CD8 T cell-dominant response within the lesions (Figure 6D, dotted black box).
Figure 6.
Adjunct respiratory mucosal immunotherapy reduces tuberculosis-associated tissue pathology in the lung during antibiotic cessation phase. Experiments were set up as depicted in experimental schema Figure 3A. (A and B) Representative micrographs of lung sections stained with hematoxylin and eosin (H&E), comparing the extent of lung inflammation and granulomatous lesions at 4 weeks postantibiotic cessation (24 weeks). Scale bars indicate 500 µm. Higher magnifications of H&E micrographs depict abundant foamy macrophages (H&E) colocalized with acid-fast bacilli (AFB) (black arrows). Scale bars indicate 50 µm. (C and D) Representative micrographs of immunohistochemically stained lung sections at 4 weeks postantibiotic cessation (24 weeks) visualizing the spatial distribution of F4/80 macrophages (brown stain) and CD4 (red stain) and CD8 (brown stain) T cells costained in consecutive sections. Scale bars indicate 500 µm. ABx, antibiotics alone.
Taken together, the above data suggest that adjunct RM immunotherapy effectively controls TB disease even after premature antibiotic cessation, not only via reducing mycobacterial bacillary burden but, importantly, via mitigating immunopathology in the lung. This vaccine-induced immunotherapeutic effect is closely associated with CD8 T cell infiltration in the lung.
Adjunct Respiratory Mucosal Immunotherapy Enhances Antigen-Specific CD8 T Cell Responses in the Lung and Confers Protection in a T Cell-Dependent Manner
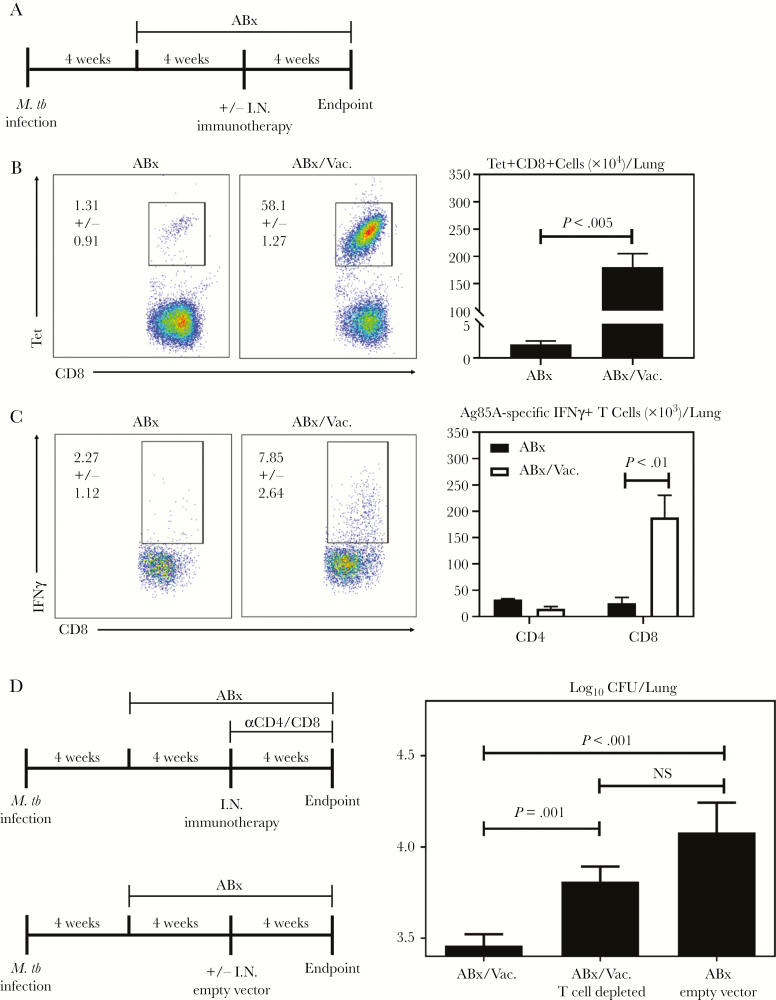
Our results thus far have suggested a role of vaccine-induced CD8 T cells in accelerated resolution of TB disease and limiting disease rebound by adjunct AdCh68Ag85A immunotherapy. To investigate the mechanisms underlying the therapeutic efficacy of AdCh68Ag85A, we first examined T cell responses in the lung of animals treated with ABx or in conjunction with RM immunotherapy (ABx/Vac.) by flow cytometry (Figure 7A). Immunotherapy caused a shift from an overall CD4 T cell-dominated response in the lung of ABx animals to a CD8 T cell-dominated response in ABx/Vac. animals (Supplementary Figure 2). By Ag-specific Ag85A CD8 tetramer (Tet) immunostaining or by specific Ag-stimulated intracellular cytokine straining of total lung mononuclear cells, we found that although there was a very small frequency of CD8 T cells specific for Ag85A in ABx lungs, more than 50% of the CD8 T cells in the lung of ABx/Vac. animals were Ag85A-specific, resulting in a much greater number of Tet+ CD8 T cells (Figure 7B). These Ag-specific cells were functionally activated as indicated by their ability to produce interferon-γ upon stimulation (Figure 7C, dot plots). In comparison, Ag85A-specific CD4 T cell responses were negligible (Figure 7C, bar graph), thus supporting the immunohistochemical findings (Figure 5D). Of interest, although CD4 T cells dominated the lung in ABx animals, only a very small number of them were M.tb-specific, comparable to those in ABx/Vac. animals (Supplementary Figure 3). On the other hand, M.tb-specific CD8 T cell responses were markedly increased in ABx/Vac. animals compared with ABx animals.
Figure 7.
Adjunct respiratory mucosal (RM) immunotherapy enhances antigen (Ag)-specific CD8 T cell responses in the lung and confers protection in a T cell-dependent manner. (A) Experimental schema. Mice were set up as depicted in experimental schema Figure 3A. (B) Representative dot plots depicting the frequencies and bar graph depicting absolute numbers of Ag85A-specific CD8 T cells as assessed by tetramer immunostaining. (C) Representative dot plots depicting the frequencies and bar graph depicting absolute numbers of Ag85A-specific IFNγ + T cells as assessed by intracellular cytokine staining after recombinant Ag85A stimulation of lung mononuclear cells. (D) Experimental schema. Mice were infected and treated with antibiotics as per Figure 1A. One set of animals received RM immunotherapy 4 weeks after the initiation of antibiotics (ABx/Vac.). T cells were subsequently depleted weekly after immunotherapy (ABx/Vac. T cell depleted). A set of animals received a single dose of AdCh68 empty control vector in place of AdCh68Ag85A (ABx/empty vector). Data are expressed as the mean ± standard error of the mean of 3 mice/group for B or C and 6–10 mice/group for D. ABx, antibiotics alone; CFU, colony-forming units.
We next further investigated the role of T cells in protective immunotherapeutic effects of AdCh68Ag85A. To this end, mice infected with M.tb for 4 weeks started on antibiotics in conjunction with RM immunotherapy with AdCh68Ag85A (ABx/Vac.). A subset of ABx/Vac. animals received weekly I.P. injections of anti-CD4/CD8 antibodies until experimental endpoint (ABx/Vac. T cell depleted), and an additional subset of animals received RM empty viral vector lacking the Ag85A gene (ABx/Empty vector) instead of AdCh68Ag85A. All animals were sacrificed 12 weeks postinfection and assessed for TB disease indices (Figure 7D). We found that T cell ablation in ABx/Vac. animals was associated with a significant increase in pulmonary bacterial burden in comparison to their undepleted counterparts. On the other hand, the animals receiving only a control viral vector (ABx empty vector) also had heightened bacterial burden in the lung (Figure 7D). Taken together, the above data suggest that RM immunotherapy-induced Ag85A-specific T cells contribute directly to the protective therapeutic efficacy of AdCh68Ag85A.
DISCUSSION
The emergence of multidrug-resistant to total drug-resistant strains of M.tb and the paucity of new drugs call for developing new therapeutic strategies [1, 3]. In this study, we show that a single dose of adjunct AdCh68Ag85A RM immunotherapy accelerates the resolution of pulmonary TB disease and limits disease rebound after premature antibiotic cessation in a model of chronic TB. Respiratory mucosal immunotherapy-induced Ag-specific T cells contribute to its protective therapeutic efficacy. These data indicate that virus-based TB vaccines designed for prophylactic RM immunization have the potential for therapeutic applications.
Our study represents the first to show the potency of respiratory route of immunotherapy and its superiority over the parenteral route. Failure of parenteral immunotherapy to enhance TB disease control by antibiotics supports the importance of quantity and quality of Ag-specific T cells in the lung [7, 19, 31]. A previous study has also shown the inability of repeated parenteral immunotherapies with adenoviral (Ad26/Ad35)-vectored TB vaccines to enhance protection [15]. High Ag exposure associated with repeated parenteral immunotherapies and ongoing TB infection may lead to impaired T cell functions [32] and severe local adverse effects [9]. Such situations may likely be worsened during antibiotic therapy. By comparison, besides its potency in directly furnishing infected lungs with T cells, adenoviral-based RM immunotherapy may train/activate lung macrophages such that they not only have enhanced mycobactericidality but also become better responders to Ag-specific T cells [7, 27]. Indeed, we show here that a single dose of adjunct mucosal immunotherapy markedly reduces both bacterial burden and pathology in the lung. This approach appears advantageous over a repeated parenteral immunotherapeutic strategy that significantly reduced lung pathology but had a much less effect on bacterial control [16].
Consideration of the timing of immunotherapy during antibiotic therapy is also of importance, in particular to the safety and efficacy of RM immunotherapy [33]. In our study, RM immunotherapy was carried out until after 4-week antibiotic therapy. This was to ensure that antibiotic therapy had markedly reduced bacterial infection and antigenic load and associated inflammation in the lung. This helps avoid not only the morbidity resulting from vaccine-enhanced inflammation [16, 30] but also T cell exhaustion [32].
The protective correlates of anti-TB immunity have remained to be established. Our current study provides the evidence that RM immunotherapeutic vaccine-induced infiltration of T cells, particularly CD8 T cells, in TB lesions plays an important protective role. A recent study showed that M.tb-specific CD8 T cells recognize and inhibit M.tb growth in infected macrophages [34]. Our data suggest that inducing Ag85A-specific CD8 T cells is a useful criterion for immunotherapeutic development. In addition to T cells, it is likely that vaccine-trained innate immune cells may also play a role because we have recently shown that adenoviral infection in the lung induces memory macrophages with enhanced antimicrobial activities [27].
The success of current TB antibiotic regimens is challenged by the length of therapy required to cure disease without rebound. Even with the implementation of Direct Observed Treatment, short course, treatment failures spike as high as 40% for individuals with drug-resistant TB [2, 35, 36]. Treatment-shortening regimens are therefore highly sought after to improve antibiotic treatment success. Different from other immunotherapeutic studies published to-date, we show here that a single-dose immunotherapy with AdCh68Ag85A not only enhances infection control by antibiotics, but it also restrains disease rebound after antibiotic stoppage in a protracted TB disease model.
Our study establishes the therapeutic efficacy of AdCh68Ag85A in the context of pulmonary TB. Although this is the most prevalent form of the disease, TB in humans can manifest in extrapulmonary sites, and it would be relevant to examine the relationship of its local therapeutic effects to extrapulmonary infection in future studies [37]. Furthermore, future work should also investigate whether our therapeutic vaccine strategy differentially affects replicating and dormant/persistent M.tb bacilli. This is of particular relevance because the latter underinvestigated mycobacterial population is thought to be a major contributor to the disease relapse post-antibiotic therapy [33, 38–40].
CONCLUSIONS
In conclusion, we have provided strong evidence to support the safe and effective immunotherapeutic application, via the respiratory route, of a recombinant chimpanzee adenovirus-based prophylactic TB vaccine. Further clinical translation shall help develop strategies to shorten antibiotic therapy and curb the emergence of drug-resistant disease.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are grateful for the technical assistance from Xueya Feng, Mary Jo Smith, and the biosafety level 3 animal care staff and the provision of Mycobacterium tuberculosis (M.tb) antigens and MHC tetramers by the BEI Resources and NIH Tetramer Core, respectively.
Financial support. This study was funded by the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Canadian Foundation for Innovation, and Ontario Government.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. Global Tuberculosis Report 2018. World Health Organization, Geneva, Switzerland; 2018. [Google Scholar]
- 2. Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. Rylko-Bauer B, editor. PLoS Med 2007; 4:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Guidelines for treatment of tuberculosis and patient care. WHO 2017; 1:85–97. [Google Scholar]
- 4. Zumla A, Memish ZA, Maeurer M, et al. Emerging novel and antimicrobial-resistant respiratory tract infections: new drug development and therapeutic options. Lancet Infect Dis 2014; 14:1136–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bloemberg GV, Keller PM, Stucki D, et al. Acquired resistance to bedaquiline and delamanid in therapy for tuberculosis. N Engl J Med 2015; 373:1986–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andersen P, Kaufmann SH. Novel vaccination strategies against tuberculosis. Cold Spring Harb Perspect Med 2014; 4:a018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jeyanathan M, Yao Y, Afkhami S, Smaill F, Xing Z. New tuberculosis vaccine strategies: taking aim at un-natural immunity. Trends Immunol 2018; 39:419–33. [DOI] [PubMed] [Google Scholar]
- 8. Kaufmann SH, Weiner J, von Reyn CF. Novel approaches to tuberculosis vaccine development. Int J Infect Dis 2017; 56:263–7. [DOI] [PubMed] [Google Scholar]
- 9. Thienemann F, Innes JC, Khatoon Azam A, et al. Phase 2b controlled trial of M72/AS01 E vaccine to prevent tuberculosis. N Engl J Med 2018; 379:1621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coler RN, Bertholet S, Pine SO, et al. Therapeutic immunization against Mycobacterium tuberculosis is an effective adjunct to antibiotic treatment. J Infect Dis 2013; 207:1242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schön T, Lerm M, Stendahl O. Shortening the ‘short-course’ therapy- insights into host immunity may contribute to new treatment strategies for tuberculosis. J Intern Med 2013; 273:368–82. [DOI] [PubMed] [Google Scholar]
- 12. Cardona PJ. The progress of therapeutic vaccination with regard to tuberculosis. Front Microbiol 2016; 7:1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coupet C, Gouanvic M, Hoffmann C, et al. A multi-antigenic MVA vaccine increases efficacy of combination chemotherapy against Mycobacterium tuberculosis. PLoS One 2018; 13: e0196815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aagaard C, Hoang T, Dietrich J, et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med 2011; 17:189–94. [DOI] [PubMed] [Google Scholar]
- 15. Alyahya SA, Nolan ST, Smith CM, Bishai WR, Sadoff J, Lamichhane G. Immunogenicity without efficacy of an adenoviral tuberculosis vaccine in a stringent mouse model for immunotherapy during treatment. PLoS One 2015; 10: e0127907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larsen S, Baldwin S, Orr M, et al. Enhanced anti-Mycobacterium tuberculosis immunity over time with combined drug and immunotherapy treatment. Vaccines 2018; 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeyanathan M, Shao Z, Yu X, et al. AdHu5Ag85A respiratory mucosal boost immunization enhances protection against pulmonary tuberculosis in BCG-primed non-human primates. PLoS One 2015; 10:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao Y, Lai R, Afkhami S, et al. Enhancement of antituberculosis immunity in a humanized model system by a novel virus-vectored respiratory mucosal vaccine. J Infect Dis 2017; 216:135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beverley PC, Sridhar S, Lalvani A, Tchilian EZ. Harnessing local and systemic immunity for vaccines against tuberculosis. Mucosal Immunol 2014; 7:20–6. [DOI] [PubMed] [Google Scholar]
- 20. Santosuosso M, McCormick S, Zhang X, Zganiacz A, Xing Z. Intranasal boosting with an adenovirus-vectored vaccine markedly enhances protection by parenteral Mycobacterium bovis BCG immunization against pulmonary tuberculosis. Infect Immun 2006; 74:4634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santosuosso M, Zhang X, McCormick S, Wang J, Hitt M, Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J Immunol 2005; 174:7986–94. [DOI] [PubMed] [Google Scholar]
- 22. Santosuosso M, McCormick S, Roediger E, et al. Mucosal luminal manipulation of T cell geography switches on protective efficacy by otherwise ineffective parenteral genetic immunization. J Immunol 2007; 178:2387–95. [DOI] [PubMed] [Google Scholar]
- 23. Wang J, Thorson L, Stokes RW, et al. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol 2004; 173:6357–65. [DOI] [PubMed] [Google Scholar]
- 24. Forbes EK, Sander C, Ronan EO, et al. Multifunctional, high-level cytokine-producing th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2014; 181:4955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guérin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol 2003; 171:1602–9. [DOI] [PubMed] [Google Scholar]
- 26. Jeyanathan M, Thanthrige-Don N, Afkhami S, et al. Novel chimpanzee adenovirus-vectored respiratory mucosal tuberculosis vaccine: overcoming local anti-human adenovirus immunity for potent TB protection. Mucosal Immunol 2015; 8:1373–87. [DOI] [PubMed] [Google Scholar]
- 27. Yao Y, Jeyanathan M, Haddadi S, et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell 2018; 175:1634–1650.e17. [DOI] [PubMed] [Google Scholar]
- 28. Roederer M. Parsimonious determination of the optimal infectious dose of a pathogen for nonhuman primate models. PLoS Pathog 2015; 11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simmons JD, Stein CM, Seshadri C, et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat Rev Immunol 2018; 18:575–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taylor JL, Turner OC, Basaraba RJ, Belisle JT, Huygen K, Orme IM. Pulmonary necrosis resulting from DNA vaccination against tuberculosis. Infect Immun 2003; 71:2192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Afkhami S, Yao Y, Xing Z. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol Ther Methods Clin Dev 2016; 3:16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Billeskov R, Lindenstrøm T, Woodworth J, et al. High antigen dose is detrimental to post-exposure vaccine protection against tuberculosis. Front Immunol 2018; 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buccheri S, Reljic R, Caccamo N, et al. Prevention of the post-chemotherapy relapse of tuberculous infection by combined immunotherapy. Tuberculosis 2009; 89:91–4.0 [DOI] [PubMed] [Google Scholar]
- 34. Yang JD, Mott D, Sutiwisesak R, et al. Mycobacterium tuberculosis-specific CD4+ and CD8+ T cells differ in their capacity to recognize infected macrophages. PLoS Pathog 2018; 14:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nellums LB, Rustage K, Hargreaves S, Friedland JS. Multidrug-resistant tuberculosis treatment adherence in migrants: a systematic review and meta-analysis. BMC Med 2018; 16:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karumbi J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev 2015; CD003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Venkataprasad N, Ledger P, Ivanyi J. The effect of glucosaminylmuramyl dipeptide injection to mice on the course of tuberculous infection and in vitro superoxide anion production. Int Arch Allergy Immunol 1997; 114:23–9. [DOI] [PubMed] [Google Scholar]
- 38. Connolly LE, Edelstein PH, Ramakrishnan L. Why is long-term therapy required to cure tuberculosis? PLoS Med 2007; 4:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Defraine V, Fauvart M, Michiels J. Fighting bacterial persistence: current and emerging anti-persister strategies and therapeutics. Drug Resist Updat 2018; 38:12–26. [DOI] [PubMed] [Google Scholar]
- 40. Mukamolova GV, Turapov O, Malkin J, Woltmann G, Barer MR. Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am J Respir Crit Care Med 2010; 181:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.