Abstract
Intracranial calcifications in the pediatric population can have many etiologies including neoplastic, infectious, neurodegenerative, metabolic, or cerebrovascular abnormalities. We present the case of a 2-year-old boy with vein of Galen malformation, a rare cause of intracranial calcifications with a review of literature.
Keywords: Intracranial calcifications, vein of Galen aneurysm, pediatric imaging, plain radiography, ultrasonography, computed tomography, magnetic resonance imaging, angiography
CASE REPORT
A two-year-old male patient presented with new onset seizures and significant developmental delay. History went back to the age of 3 months with failure to meet global milestones and progressive hypotonia, for which investigations showed Vein of Galen aneurysmal malformation (VGAM), treated with posterior cerebral artery coiling. Otherwise, birth, family, and medical history were unremarkable, and vaccinations were up to date. Physical examination was significant for macrocephaly and neurological examination revealed a total absence of interaction with the environment, failure to follow objects, and severe muscle weakness.
Multi-slice CT of the brain was done and showed VGAM for which coiling of bilateral posterior cerebral artery feeders was previously performed, numerous tortuous venous collaterals involving the deep cerebral veins extending into the 4th ventricle, 3rd ventricle, prepontine and quadrigeminal cisterns, prominence of the posterior arterial circulation including the basilar and vertebral arteries, subcortical, periventricular and basal ganglia symmetric and extensive white matter calcifications (Figure 1). The presence of calcifications prompted further laboratory investigations which showed a negative TORCH (Toxoplasmosis, Other Agents, Rubella, Cytomegalovirus, and Herpes Simplex) panel, and negative genetic and metabolic studies. Final diagnosis was extensive dystrophic calcifications secondary to chronic venous hypertension and ischemia from VGAM.
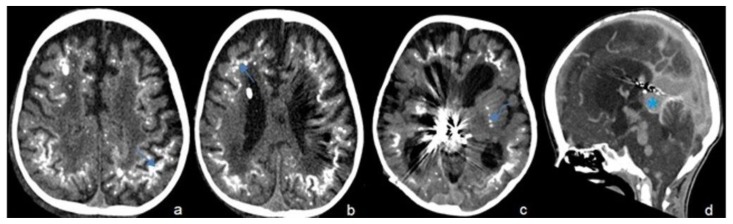
Figure 1.
Two-year-old male with vein of Galen aneurysmal malformation.
Findings: Axial cuts of non-enhanced CT scan of the brain (a–c) shows extensive calcifications in the subcortical white matter (arrow) and basal ganglia (curved arrow) which are secondary to chronic venous hypertension and ischemia from vein of Galen malformation (asterisk) that is seen on the sagittal view of enhanced CT scan of the brain (d) along with multiple venous collaterals
Technique: Axial and sagittal CT, 450 mAs, 120 kV, 0.8 mm slice thickness, 25ml Omnipaque
The patient subsequently underwent further endovascular VGAM embolization and insertion of right occipital ventriculo-peritoneal shunt (Figure 2).
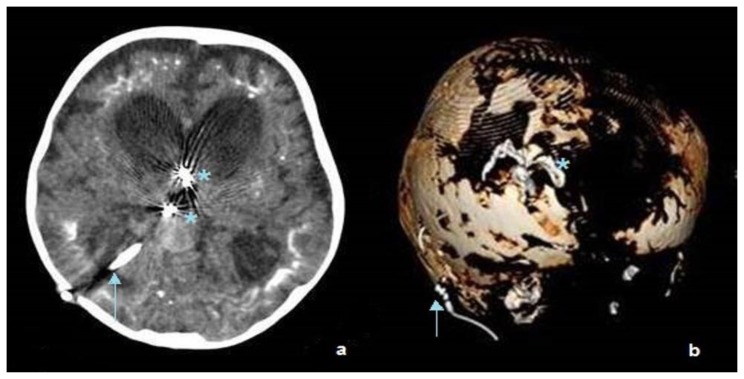
Figure 2.
Two-year-old male with vein of Galen aneurysmal malformation
Findings: Axial cut of the brain (a) and 3D reformation (b) show coiling of the vascular feeders at the level of the third ventricle (asterisk) and insertion of right posterior temporal ventriculoperitoneal shunt (arrows).
Technique: Axial CT, 450 mAs, 120 kV, 0.8 mm slice thickness, 25ml Omnipaque
DISCUSSION
Intracranial calcifications in pediatric neuroimaging most often hint to a damaged, neoplastic, or malformed brain [1] as physiologic calcifications are almost never seen under 6 years of age [2]. Identification of characteristic patterns of calcification allows a diagnosis to be made in many cases [3]. Overall, half of all cases of focal calcification occur in neoplastic brain tissue. Other causes include congenital brain infections such as cytomegalovirus (CMV) and toxoplasmosis, as well as genetic neurodegenerative diseases, hypoparathyroidism, Fahr disease, neurocutaneous syndromes, and cerebrovascular disorders such as vein of Galen aneurysm malformation (VGAM).
Etiology & Demographics
Vein of Galen aneurysmal malformation is a rare cerebral vascular abnormality that represents 1% of the abnormalities of the fetal cerebral arteriovenous system. It consists of multiple arteriovenous shunts usually established between the choroidal arteries and the embryonic precursor of the vein of Galen, the median prosencephalic vein of Markowski which fails to regress between 6 and 11 weeks of gestation [4]. It has a male predominance.
Clinical & Imaging Findings
Symptoms vary based on age and anatomy of the malformation. In the neonatal age group, the choroidal type dominates where multiple feeders located in the subarachnoid space in the choroidal fissure converge on a fistula site at the anterior aspect of the median prosencephalic vein. Evidence of high output heart failure dominates leading up to multiorgan failure. In infancy, as in the presented case, the mural type dominates with fistulae in the subarachnoid space in the wall of the median prosencephalic vein. Features include hydrocephalus, macrocrania, and developmental retardation. Seizures, as seen in this patient, are rarely seen in VGAMs.
Untreated VGAMs result in chronic venous ischemia with secondary development of dystrophic subcortical white matter calcifications and subependymal atrophy with ventricular dilatation [5]. Calcifications are secondary to deep hydrovenous watershed failure and occur when the compliance of the medullary veins loses its normal ventricular-cortical gradient. They are usually bilateral and symmetrical, but may be asymmetrical and are mostly unilateral in shunted children (often on the side opposite to the shunt). Subependymal atrophy is primarily seen in the occipital regions. It may be severe and result in spontaneous thromboses of isolated cortical veins.
The pattern of periventricular calcifications due to VGAM has rarely been described in the literature [6]. As such, VGAM should be included in the differential diagnosis of periventricular calcifications especially when other causes such as cytomegalovirus infection have been ruled out. Moreover, the presence of calcifications in patients with VGAM is a negative prognostic factor as it represents irreversible brain damage with a poor neurological outcome. Overall, less than 10 cases of calcifications due to VGAM have been described in the literature. In a cohort of 317 patients described by Lasjaunias et al., only 2 patients developed multiple intracranial calcifications, and they were of the older age group [7].
VGAM has characteristic imaging findings [8,9]. Plain radiography of the skull may demonstrate a rim of calcification within the wall of the aneurysmal sac while chest radiographs may reveal features of congestive heart failure. Antenatal ultrasound scans demonstrate the venous sac as a sonolucent mass located posterior to the third ventricle. Demonstration of pulsatile flow within it helps in differentiating VGAMs from other midline cystic lesions. Associated venous anomalies are often visualized. Evidence of hydrocephalus and cardiac dysfunction may be obtained on antenatal ultrasonography. On contrast-enhanced computed tomography, it appears as a well-defined, multilobulated, intensely enhancing lesion, located within the cistern of velum interpositum, along with dilatation of the ventricular system, periventricular white matter hypodensities and calcifications, diffuse cerebral atrophy. On magnetic resonance imaging, the dilated feeding and draining vessels appear as flow-voids on T2. MRI can demonstrate the location of fistula, presence of any nidus, the arterial components, the venous sac and the status of venous drainage, and allows adequate depiction of thrombosis of the venous sac. Angiography is the gold standard for the evaluation of VGAMs. It catheterizes small feeders supplying the fistula, and evaluates the dynamic aspects of the venous drainage of the normal brain, and arterio-venous shunt.
Treatment & Prognosis
Endovascular embolization results in a good clinical outcome, an acceptable mortality and complications. Prior to endovascular intervention, the prognosis was poor, with 100% mortality without treatment. The prognosis depends highly on the presence or absence of cardiac failure in-utero, as such detectable signs may indicate non-response to therapy [10]. The size of the shunt is another prognostic factor as larger shunts present with earlier deterioration into cardiac and multi-organ failure in comparison to smaller shunts which may present later in life with mild heart failure and failure to thrive [4]. There are no reports of spontaneous regression or size change over time.
Differential Diagnoses
Congenital Cytomegalovirus (CMV)
Presents with microcephaly, jaundice, hepatosplenomegaly, blueberry muffin rash, and periventricular calcifications on imaging [3].
Hypoparathyroidism and pseudohypoparathyroidism
These entities can be primary or transient in neonates, or due to sepsis or asphyxia. Symptoms are due to hypocalcemia and include hypotonia, seizures, apnea, poor feeding, and cardiac failure. CT/MRI demonstrate symmetrical basal ganglia, thalamic calcifications and deep gyral calcifications [3,11,12].
Cockayne Syndrome
A rare neurodegenerative disorder characterized by microcephaly, nervous system abnormalities, growth failure, photosensitivity, and premature aging. CT shows rock or spot basal ganglia calcifications, with or without gyral calcifications, while MRI demonstrates severe hypomyelination, cerebral atrophy, early cerebellar atrophy [13].
Fahr Syndrome
A rare inherited or sporadic progressive degenerative neurological disorder characterized by abnormal deposits of calcium in areas of the brain that control movement with absence of biochemical abnormalities and somatic features suggestive of a mitochondrial or metabolic disease or other systemic disorder and absence of an infectious, toxic, or traumatic cause [3, 14,15].
TEACHING POINT
The rare occurrence of intracranial calcifications due to a rare entity like Vein of Galen aneurysmal malformation emphasizes the need for physicians to include VGAM in the differential diagnosis of brain calcifications in children.
Table 1.
Summary table of Vein of Galen Malformation.
| Etiology | Congenital anomaly with no known etiology. |
| Incidence | Very rare. 1% of the abnormalities of the fetal cerebral arteriovenous system. Some sources report 1:25000 births. |
| Gender ratio | Literature reports increased male predilection. |
| Age predilection | Congenital. |
| Risk factors | No clear risk factors. |
| Treatment | Endovascular embolization. |
| Prognosis | Endovascular embolization results in a good clinical outcome, an acceptable mortality and complications. Prior to endovascular intervention, the prognosis was poor, with 100% mortality without treatment. The prognosis also depends highly on the presence or absence of cardiac failure in-utero, as such detectable signs may indicate non-response to therapy. |
| Findings on imaging | Sonolucent mass located posterior to the third ventricle with pulsatile flow. Associated venous anomalies are often visualized along with evidence of hydrocephalus, periventricular white matter hypodensities and calcifications, diffuse cerebral atrophy. |
Table 2.
Differential diagnoses table for Vein of Galen Aneurysmal Malformation.
| Differential | Radiograph/CT | Ultrasound | MRI |
|---|---|---|---|
| Vein of Galen aneurysmal malformation |
|
|
|
| Congenital Cytomegalovirus (CMV) |
|
|
|
| Hypoparathyroidism and pseudohypo-parathyroidism |
|
Suboptimal imaging for this disease |
|
| Cockayne syndrome | Rock or spot basal ganglia calcifications, with or without gyral calcifications | Suboptimal imaging for this disease |
|
| Fahr Disease | Abnormal deposits of calcium in bilateral basal ganglia, subcortical white matter, thalami, dentate nuclei, cerebral cortex, cerebellum and hippocampus. | Suboptimal imaging for this disease |
|
ABBREVIATIONS
- CMV
Cytomegalovirus
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- TORCH
Toxoplasmosis, Other Agents, Rubella, Cytomegalovirus, and Herpes Simplex
- US
Ultrasound
- VGAM
Vein of Galen aneurysmal malformation
REFERENCES
- 1.Kendall B, Cavanagh N. Intracranial calcification in paediatric computed tomography. Neuroradiology. 1986;28(4):324–330. doi: 10.1007/BF00333438. [DOI] [PubMed] [Google Scholar]
- 2.Schey WL. Intracranial Calcifications in Childhood. AJR Am J Roentgenol. 1974;122(3):495–502. doi: 10.2214/ajr.122.3.495. [DOI] [PubMed] [Google Scholar]
- 3.Livingston JH, Stivaros S, Warren D, Crow YJ. Intracranial calcification in childhood: a review of aetiologies and recognizable phenotypes. Dev Med Child Neurol. 2014;56(7):612–626. doi: 10.1111/dmcn.12359. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya JJ, Thammaroj J. Vein of Galen Malformations. J Neurol Neurosurg Psychiatry. 2003;74(1):i42–i44. doi: 10.1136/jnnp.74.suppl_1.i42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez H, Monaco RG, Rodesch G, Sachet M, Krings T, Lasjaunias P. Vein of Galen Aneurysmal Malformations. Neuroimaging Clin N Am. 2007;17(2):189–206. doi: 10.1016/j.nic.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Perez Fontan JJ, Herrera M, Fina A, Peguero G. Periventricular calcifications in a newborn associated with aneurysm of the great vein of Galen. Pediatr Radiol. 1982;12(5):249–251. doi: 10.1007/BF00971773. [DOI] [PubMed] [Google Scholar]
- 7.Lasjaunias PL, Chng SM, Sachet M, Alvarez H, Rodesch G, Garcia-Monaco R. The management of vein of Galen aneurysmal malformations. Neurosurgery. 2006;59(suppl_5):S3–184. doi: 10.1227/01.NEU.0000237445.39514.16. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A, Varma D. Vein of galen malformations: Review. Neurol India. 2004;52(1):43–53. [PubMed] [Google Scholar]
- 9.Kothari SS, Naik N, Juneja R, Saxena A. Aneurysm of the vein of galen in neonates: report of four cases. Indian Heart J. 2001;53(4):499–502. [PubMed] [Google Scholar]
- 10.Yan J, Wen J, Gopaul R, Zhang CY, Xiao SW. Outcome and complications of endovascular embolization for vein of Galen malformations: a systematic review and meta-analysis. J Neurosurg. 2015 Oct;123(4):872–890. doi: 10.3171/2014.12.JNS141249. [DOI] [PubMed] [Google Scholar]
- 11.Margolin D, Hamerstad J, Orwoll E, McClung M, Calhoun D. Intracranial calcification in hyperparathyroidism associated with gait apraxia and parkinsonism. Neurology. 1980;30:1005–7. doi: 10.1212/wnl.30.9.1005. [DOI] [PubMed] [Google Scholar]
- 12.Kahloul N, Chaari W, Boughamoura L, Charfeddine L, Khammeri S, Amri F. Pseudohypoparathyroidism revealed by Fahr syndrome. Arch Pediatr. 2009;16:444–8. doi: 10.1016/j.arcped.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Koob M, Laugel V, Durand M, et al. Neuroimaging in Cockayne syndrome. AJNR Am J Neuroradiol. 2010;31:1623–30. doi: 10.3174/ajnr.A2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manyam BV. What is and what is not ‘Fahr’s disease’? Parkinsonism Relat Disord. 2005;11:73–80. doi: 10.1016/j.parkreldis.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Orinya OJ. Relationship Between Intracranial Calcifications in Vein of Galen Malformations and Fahr’s Syndrome: A Case Report and Review of the Literature. Journal of Surgery. 2016;4(2):13. doi: 10.11648/j.js.20160402.12. [DOI] [Google Scholar]