Abstract
目的
探讨晚期糖基化终末产物受体(RAGE)信号对甲苯二异氰酸脂(TDI)哮喘小鼠黏蛋白MUC5AC表达及气道黏液分泌的影响。
方法
在体内水平上建立TDI小鼠哮喘模型,实验分4组:对照组、TDI溶剂(AOO)致敏激发组、TDI致敏激发组、RAGE抑制剂处理组。采用糖原染色评估各组间气道黏液分泌情况,Western blotting及免疫组化法检测MUC5AC表达水平。同时通过Western blotting检测各组间细胞外调节蛋白激酶(ERK)通路磷酸化水平。在体外水平配制TDI-人血清白蛋白(TDIHSA)复合物,刺激人支气管上皮细胞(16HBE)及经慢病毒转染空载体和shRNA-RAGE载体的人支气管上皮细胞,检测各组MUC5AC及ERK通路分子表达情况,加入ERK抑制剂U0126后, 进一步评估MUC5AC mRNA表达情况。
结果
与对照组相比,TDI哮喘组小鼠糖原染色阳性率及MUC5AC表达升高(P < 0.05),p-ERK表达增多(P < 0.05),RAGE抑制剂处理组糖原染色阳性率及MUC5AC表达较TDI组减少(P < 0.05)。同时,p-ERK表达下降(P < 0.05)。体外水平,与对照组相比,TDI-HSA处理组MUC5AC mRNA及p-ERK表达增多(P < 0.05),sh RNA-RAGE组MUC5AC mRNA及p-ERK表达下调(P < 0.05),ERK抑制剂预处理组,MUC5AC mRNA表达下调(P < 0.05)。
结论
TDI小鼠哮喘模型下RAGE可能通过激活ERK通路促进黏蛋白MUC5AC的表达及黏液高分泌的发生。
Keywords: 甲苯二异氰酸酯哮喘, 晚期糖基化终末产物受体, MUC5AC, p-ERK
Abstract
Objective
To explore the role of the receptor for advanced glycation end products (RAGE) in regulating the expression of MUC5AC and mucus production in a mouse model of toluene diisocyanate (TDI)-induced asthma.
Methods
BALB/c mice were randomly divided into control group, vehicle (AOO) group, TDI-induced asthma group and RAGE inhibitor (FPS-ZM1) group. PAS staining, Western blotting, and immunohistochemistry were used to analyze the changes in mucus production and MUC5AC expression in the airway of the mice, and the expression of p-ERK was detected with Western blotting. In vitro cultured human bronchial epithelial cell line 16HBE was transfected with lentiviral vector carrying short hairpin RNA targeting RAGE (shRNA-RAGE) and subsequently challenged with a TDI-human serum albumin (TDIHSA) conjugate, and the changes in cellular MUC5AC mRNA expression as detected using RT-PCR; the protein expressions of ERK and p-ERK in the cells were examined with Western blotting. The effect of ERK inhibitor U0126 pretreatment on MUC5AC mRNA expression was also analyzed in the cells.
Results
Compared with the control mice, TDI-induced asthmatic mice showed significantly higher rates of PAS positivity and increased MUC5AC and p-ERK expressions in the airway (P < 0.05). Treatment with FPS-ZM1 significantly decreased PAS positivity and lowered MUC5AC and p-ERK expressions in the airway of the asthmatic mice (P < 0.05). Exposure of 16HBE cells to TDI-HSA caused a significant increase in MUC5AC mRNA expression and p-ERK protein expression (P < 0.05), while RAGE knockdown obviously suppressed TDI-HSAinduced upregulation of p-ERK and MUC5AC mRNA (P < 0.05). Treatment with the ERK inhibitor U0126 also lowered TDI-HSA-induced up-regulation of MUC5AC mRNA in the cells (P < 0.05).
Conclusion
RAGE signaling induces MUC5AC expression via extracellular signalregulated kinase pathway to promote mucus overproduction in mice with TDI-induced asthma.
Keywords: toluene diisocyanate, asthma, receptor for advanced glycation end products, MUC5AC, p-ERK
支气管哮喘(简称哮喘)是一种常见的慢性气道疾病,全球约有3亿哮喘患者,每年约25万人因哮喘导致死亡[1]。气道黏液高分泌是支气管哮喘重要病理生理特征之一。正常生理状况下,气道黏液的分泌是呼吸道防御的一种自身保护机制。气道黏液由多种组分混合而成,其中黏蛋白是其主要成分。在已知的21种黏蛋白基因中,MUC5AC编码的蛋白是呼吸道黏液最丰富的组分,同样也是哮喘患者气道表达最丰富的黏蛋白[2-3]。其与哮喘气道黏液的过度分泌和杯状化生过程密切相关[4-5]。气道分泌的含MUC5AC丰富的大量黏液甚至可形成黏液栓,过多的黏液可造成哮喘患者的气道细菌定植、气流受限及通气功能障碍,甚至造成哮喘相关的致死性的危害[6]。因此,抑制气道的黏液高分泌可能成为哮喘治疗的靶点。
甲苯二异氰酸脂(TDI)是工业环境中常见的一种小分子过敏原,广泛存在于油漆、聚氨酯泡沫、涂料之中,其是诱发职业性哮喘最为常见的原因之一[7]。TDI诱发的哮喘病理生理特征与普通哮喘相似,具有气道炎症、气道高反应及气道重塑等特征。晚期糖基化终末产物受体(RAGE)是一种模式识别受体,可通过识别多种配体,介导下游多种信号通路,其与许多慢性气道疾病密切相关,近年来在哮喘的作用备受关注[8-10]。本课题组前期研究发现在TDI介导的小鼠哮喘模型中RAGE表达明显升高,且阻断RAGE信号可显著减轻TDI哮喘小鼠的气道炎症反应及气道高反应性[11],但其是否也能影响气道黏液的分泌尚不明确。因此本实验采用TDI小鼠哮喘模型及气道上皮细胞模型,通过干预RAGE信号来探讨其对TDI哮喘模型黏液分泌及MUC5AC表达的影响及可能的机制。
1. 材料和方法
1.1. 试剂
丙酮、橄榄油、TDI(sigma);RAGE抑制剂FPSZM1(Millipore),RAGE一抗(abcam)、MUC5AC一抗(abcam)、ERK p-ERK(cst);ERK抑制剂U0126(sellneck),HE染液,DAB显色试剂盒。
1.2. 实验动物及TDI哮喘动物模型建立
SPF级雄性BALB/c小鼠30只,购自南方医科大学实验动物中心,6~8周龄,体质量22~22 g,随机分为4组:对照组(Control组)、AOO(TDI溶剂,丙酮和橄榄油混合液)致敏激发组、哮喘组(TDI组)、RAGE抑制剂组。对照组为未经过致敏激发处理的同批小鼠。TDI组:致敏阶段:第1天及第8天采用0.3% TDI(溶剂为丙酮与橄榄油混合物)20 μL滴到小鼠耳背,40 μL/只;激发阶段:第15、18、21天给予3% TDI雾化吸入,TDI持续雾化3 h。每次激发前1 h按150 mg/kg剂量腹腔注射生理盐水。AOO组:操作同实验组,但致敏及激发采用TDI溶剂。RAGE抑制剂组:操作过程同实验组,但每次激发前1 h按150 mg/kg剂量腹腔注射RAGE抑制剂FPS-ZM1。
1.3. 淋巴细胞培养、肺组织糖原染色、免疫组化
动物模型建立3周后处死小鼠,取小鼠颈部淋巴结组织,经细胞筛处理后细胞计数,以1×107/mL接种于含3.5 mg/mL刀豆素A的完全培养基中培养48 h,留上清液。取右肺组织制作石蜡切片,常规脱蜡至水,做HE染色,糖原染色及气道MUC5AC的免疫组化。详见相关资料。
1.4. TDI-人血清白蛋白(TDI-HSA)复合物的配制
TDI-HSA复合物配制方法如文献[12]中所述,TDI与HSA摩尔比大约为4.5。
1.5. 细胞培养、shRNA-RAGE慢病毒转染及筛选
人支气管上皮细胞16HBE购自中科院,其采用含10%胎牛血清的RPMI 1640培养基,在37 ℃、5% CO2培养箱中传代培养,细胞生长状态良好时用于实验。敲低RAGE基因表达的慢病毒包装系统由南京爱必梦生物材料有限公司完成,构建RAGE基因干扰重组质粒,将重组后的质粒与慢病毒转染试剂混合转染293T细胞,72 h收集病毒上清,感染16HBE细胞。空载体质粒同上操作感染的16HBE细胞作为阴性对照,未感染慢病毒的16HBE细胞作为空白对照。将细胞扩大培养后经流式细胞仪分选富集GFP阳性细胞,Western blotting检测RAGE干扰效率。
1.6. Western blotting及ELISA
采用凯基蛋白提取试剂盒抽提细胞及肺组织总蛋白,Bradford法进行蛋白浓度测定。取等量蛋白样品进行SDS-PAGE电泳,恒压将蛋白转至PVDF膜,5%脱脂奶粉封闭1 h,孵一抗4 ℃过夜,TBST洗膜30 min,二抗孵育1 h,TBST洗膜30 min,LI-COR红外荧光扫描成像检测。ELISA操作参见具体说明书。
1.7. 荧光定量RT-PCR
检测细胞中MUC5AC mRNA的表达。采用RNAiso Reagent试剂(TaKaRa)参照说明书提取各组细胞内总RNA,取1 μg RNA逆转录(逆转录试剂盒购自TaKaRa),按试剂盒说明书合成cDNA。MUC5AC及GAPDH引物序列如下:MUC5AC的上下游引物分别是5'-CTGCCAAGTGGTCAGAGGG-3'和5'-TGTCCAGGAAGGTGTAGTAGGTG-3'内参GAPDH上下游引物分别是5'-AGTGGATATTGTTGCCATCA-3'和5'-GAAGATGGTGATGGGATTTC-3',通过实时定量PCR扩增目的基因,PCR反应条件为:95 ℃预变性30 s 95 ℃变性5 s 60 ℃退火、延伸20 s,反应40个循环。采用2-△△Ct法分析目的基因mRNA的相对表达水平。
1.8. 统计学分析
实验数据以均数±标准差表示,采用SPSS 20.0统计软件分析处理,比较采用单因素方差分析,符合方差齐性检验者,使用Bonferonni方法分析,不符合方差齐性检验者,使用Dunnett's T3分析,以P < 0.05表示差异具有统计学意义。
2. 结果
2.1. RAGE抑制剂减少TDI哮喘模型黏液分泌
TDI小鼠哮喘模型建立后,采用ELISA法检测各组间小鼠血清IgE及淋巴上清液中Th2型细胞因子IL-4的水平,结果发现TDI组小鼠IgE及IL-4明显释放增多(图 1A、B)。肺组织糖原染色结果显示:相比Control组及AOO组,TDI哮喘组小鼠糖原染色评分升高(图 1C,P < 0.05)。气道上皮杯状细胞显著增生,黏液分泌增多(图 1D)。与单纯TDI哮喘组相比,采用RAGE抑制剂FPS-ZMI处理的小鼠,糖原染色评分减少(图 1C,P < 0.05),气道上皮杯状细胞数量及黏液分泌减少(图 1D)。
1.
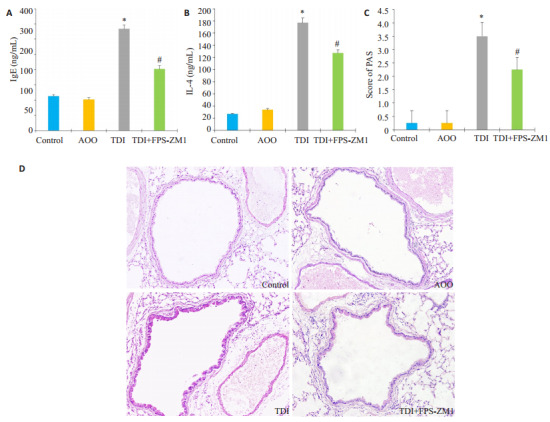
RAGE抑制剂对于小鼠TDI哮喘模型气道黏液分泌的影响
Effects of RAGE antagonists on mucus production in mice with TDI-induced asthma. A, B: Serum levels of total IgE and IL-4 in auricular lymph node cells, respectively, measured using ELISA; C: PAS score for assessing mucus production in different groups; D: PAS stained of the lung sections in different groups (Original magnification, ×200). *P < 0.05 vs: AOO group; #P < 0.05 vs: TDI group.
2.2. RAGE抑制剂减少TDI哮喘模型MUC5AC的表达
采用免疫组化及Western blotting法进一步检测MUC5AC在肺部的分布及表达。结果显示:TDI组气道上皮MUC5AC染色阳性率较对照组(Control组)及AOO组有明显增多,而RAGE抑制剂组较TDI组有所下降(图 2A)。Western blotting结果显示:与AOO组相比,TDI哮喘组RAGE的表达显著增高(图 2B,P < 0.05),与此同时,TDI哮喘组MUC5AC的表达较AOO也显著增多(图 2B,P < 0.05),采用RAGE抑制剂组MUC5AC表达出现下降,与TDI哮喘组相比差异具有统计学意义(图 2B,P < 0.05)。
2.
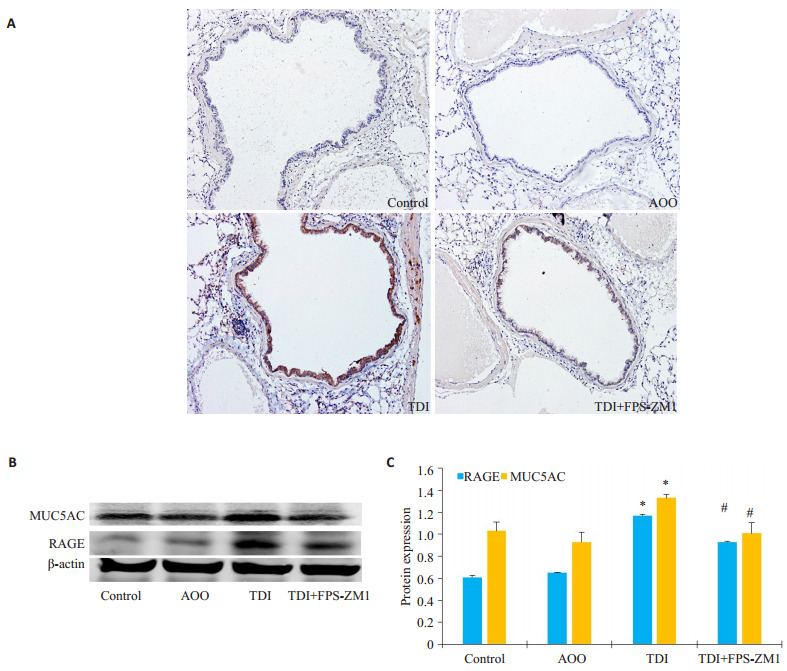
RAGE抑制剂对于TDI哮喘小鼠MUC5AC表达的影响
Effects of RAGE antagonists on MUC5AC expression in mice with TDI-induced asthma. A: Representative immunohistochemical staining of MUC5AC in each group (×200); B: Western blotting of MUC5AC in the lungs in different groups; C: Densitometric analysis of the blots. *P < 0.05 vs: AOO group; #P < 0.05 vs: TDI group.
2.3. RAGE抑制剂可抑制TDI哮喘模型的ERK信号通路
采用Western blotting法比较各组间ERK通路的变化情况,Western blotting统计结果显示:与AOO组相比,TDI组的p-ERK蛋白水平表达显著升高(图 3B,P < 0.05),而RAGE抑制剂组较TDI哮喘组p-ERK表达明显下降,差异具有统计学意义(P < 0.05)。
3.
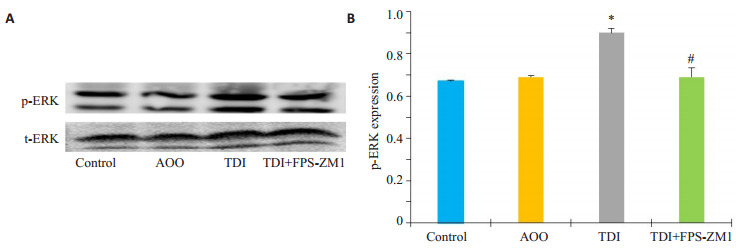
RAGE抑制剂对TDI哮喘模型ERK磷酸化水平的影响
Effects of RAGE antagonists on ERK signaling pathway in mice with TDI-induced asthma. A: Western blotting for p-ERK in different groups; B: Densitometric analysis of the blots. *P < 0.05 vs: AOO group, #P < 0.05 vs: TDI group.
2.4. 敲低RAGE表达下调TDI-HSA诱导的16HBE的MUC5AC的表达
体外配制TDI-HSA复合物刺激16HBE细胞,并且构建RAGE敲低的稳定细胞株,比较各组间MUC5AC的表达情况。Western blotting结果显示,RAGEshRNA稳转细胞株的RAGE表达水平较空载体组明显降低(P < 0.05,图 4A)。采用80 μg/mL TDI-HSA复合物处理16HBE细胞24 h,比较敲低RAGE表达后MUC5AC mRNA的改变情况。运用2-△△Ct法计算出各组MUC5AC mRNA水平相对于空白对照组的表达量。结果显示TDI-HSA处理组(2.31±0.13)与TDIHSA处理空载体组(2.24±0.04)较空白对照组(0.8±0.06)MUC5AC表达水平明显升高(图 4B,P < 0.05),而TDI-HSA处理shRNA-RAGE组(1.71±0.09)MUC5AC表达水平下调,其与TDI-HSA组比较,差异有统计学意义(图 4B,P < 0.05)。
4.
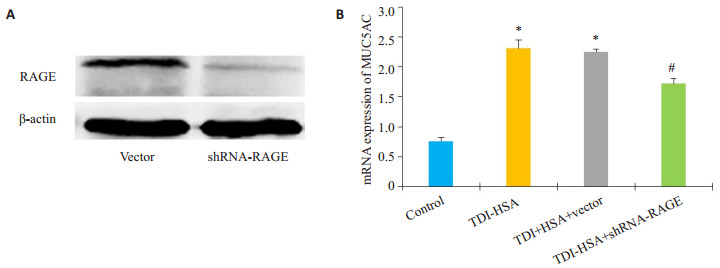
敲低RAGE表达对TDI-HSA处理的16HBE的MUC5AC表达的影响
ffect of RAGE knockdown on TDI-HSA-induced upregulation of MUC5AC gene in 16HBE cells. A: Expression of RAGE in different group; B: Quantitative analysis of relative MUC5AC mRNA levels in different groups. *P < 0.05 vs: control, #P < 0.05 vs: TDI-HSA group.
2.5. 敲低RAGE表达抑制TDI-HSA诱导的16HBE的ERK通路所介导的MUC5AC的表达
采用Western blotting法检测各组间ERK通路的变化情况,结果显示:与空白对照组相比,p-ERK在经80 μg/mL TDI-HSA处理的16HBE细胞及空载体细胞中表达显著增多(图 5B,P < 0.05),而shRNA-RAGE组的p-ERK表达显著下调,与TDI-HSA组相比,差异有统计学意义(P < 0.05)。运用ERK抑制剂10 μmol/L U0126预处理16HBE 2 h后,加80 μg/mL TDI-HSA处理24 h,采用RT-PCR法检测各组间MUC5AC的表达水平,结果显示:单用U0126对照组与空白对照组的MUC5AC的表达无统计学差异,与空白对照组相比,TDI-HSA组的MUC5AC mRNA的表达显著升高,而与TDI-HSA组(2.96±0.10)相比,ERK抑制剂预处理组(1.99±0.03)能减少MUC5AC的表达,差异有统计学意义(图 5C,P < 0.05)。
5.
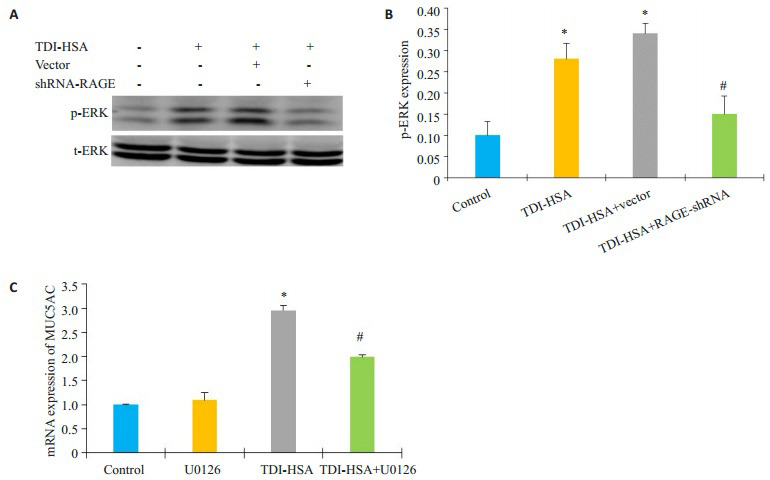
抑制ERK通路对TDI-HSA诱导的MUC5AC表达的影响
Effects of ERK inhibitor on TDI-HSAinduced upregulation of MUC5AC expression. A: Expression of p-ERK in different groups; B: Densitometric analysis of the blots; C: Quantitative analysis of relative MUC5AC mRNA levels in different groups. *P < 0.05 vs: control group, #P < 0.05 vs: TDI-HSA group.
3. 讨论
本研究首次发现TDI哮喘小鼠气道黏膜MUC5AC显著上调表达,RAGE抑制剂FPS-ZM1能显著减少TDI哮喘气道的黏液分泌及MUC5AC的表达。气道黏液主要由气道上皮杯状细胞和黏液下腺体所分泌的黏蛋白,水,脂质及无机盐组成,其中黏蛋白构成了黏液最主要的成分。而MUC5AC作为呼吸道表达最主要的黏蛋白,其多由气道表面的杯状细胞所分泌。已有多项研究表明哮喘患者气道上皮及诱导痰中MUC5AC的表达较健康受试者明显增多,且随哮喘严重程度增加而升高[13-14],其水平与气道高反应性、杯状化生以及黏液栓的形成密切相关[15]。卵蛋白或屋尘螨致敏的小鼠哮喘模型中也同样有MUC5AC的增多[16-17]。本研究首先建立TDI哮喘小鼠哮喘模型,与前期研究[18]一致的是,TDI诱发的哮喘小鼠表现为杯状上皮化生显著增多,进一步检测发现,相比对照组,哮喘组小鼠气道上皮内MUC5AC的表达显著增多。
大量体外研究证实多种促炎介质包括多种炎症因子、细菌病毒产物以及某些化学成分均能刺激气道上皮细胞上调黏蛋白基因转录。IL-13在气液培养的人支气管气道上皮细胞中诱导杯状细胞化生,上调MUC5AC的表达[19]。大肠杆菌、香烟萃取物等也被报道能上调气道上皮细胞MUC5AC的表达[20]。本实验研究发现在体外16HBE细胞研究模型中,TDI-HSA复合物也可显著增加气道上皮细胞中MUC5AC基因的表达,提示TDI能够诱导哮喘小鼠的黏液高分泌及气道上皮细胞MUC5AC的表达。
研究提示RAGE与哮喘的发生发展密切相关,敲低RAGE小鼠可明显减轻卵蛋白、屋尘螨等多种过敏原诱导的气道炎症反应及气道高分应性[10, 21]。前期研究同样支持RAGE信号的激活参与了TDI哮喘的气道炎症过程[11]。本研究在TDI致敏的小鼠哮喘模型中发现,RAGE抑制剂可显著改善TDI诱导的杯状上皮化生及MUC5AC的表达及分布。进一步在体外16HBE敲低RAGE的表达,同样发现可降低TDI-HSA诱导的MUC5AC的表达。既往有研究在体外水平上发现RAGE配体钙结合蛋白S100蛋白能诱导气道上皮细胞MUC5AC的表达[22],这与本研究结果一致。课题组前期研究亦发现,TDI小鼠哮喘模型中RAGE配体包括钙结合蛋白S100蛋白,高迁移率族蛋白1(HMGB1)均显著上调表达[11]。上述研究结果提示RAGE参与了哮喘气道上皮黏液高分泌及MUC5AC的表达。
那么,RAGE信号是如何调控MUC5AC的表达呢?MUC5AC的表达可受多种刺激物的调控,这些刺激物通过激活多种胞内下游通路实现MUC5AC基因表达的调控,多项研究证实ERK通路的活化可能在MUC5AC的调控中占有重要地位[19-20, 22-23]。前期研究也显示TDI哮喘模型中ERK通路明显活化,且参与了屏障功能破坏[24]。本研究设想ERK也参与TDI哮喘模型MUC5AC的表达,与此一致,在哮喘模型及16HBE模型中,RAGE抑制剂或者敲低RAGE的表达均能减少TDI所诱导的ERK的磷酸化水平,进一步抑制ERK通路能显著下调气道上皮细胞中TDI所诱导的MUC5AC的表达,支持RAGE信号可能通过促进下游ERK通路的活化,从而调控气道上皮MUC5AC的表达。然而,ERK通路是如何激活MUC5AC的机制尚不清楚,有研究表明MUC5AC基因的启动子序列包含多个Sp1转录因子的连接位点,而其多受上游ERK通路的调控[25-26],这可能是机制之一,值得深入探讨。
综上所述,本实验证实RAGE信号可调控TDI哮喘模型气道黏液的分泌及MUC5AC的表达,RAGE信号可能通过激活ERK通路介导了气道上皮内MUC5AC的表达及气道黏液高分泌的发生。本研究为治疗TDI哮喘的黏液高分泌提供了新的理论依据。
Biography
熊婧,在读硕士研究生,E-mail:312295231@qq.com
Funding Statement
国家自然科学基金(81670026);国家重点研发计划精准医学研究发展计划(2016YFC0905800);广东省自然科学基金(2015A030313236);广东省科技计划项目(2016A020215117)
Supported by National Natural Science Foundation of China (81670026)
Contributor Information
熊 婧 (Jing XIONG), Email: 312295231@qq.com.
赵 海金 (Haijin ZHAO), Email: zhjin@smu.edu.cn.
蔡 绍曦 (Shaoxi CAI), Email: caishaox@smu.edu.cn.
References
- 1.D'Amato G, Vitale C, Molino A, et al. Asthma-related deaths. Multidiscip Respir Med. 2016;11(1):37. doi: 10.1186/s40248-016-0073-0. [D'Amato G, Vitale C, Molino A, et al. Asthma-related deaths[J]. Multidiscip Respir Med, 2016, 11(1):37.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimizu S, Kouzaki H, Ogawa T, et al. Eosinophil-epithelial cell interactions stimulate the production of MUC5AC mucin and profibrotic cytokines involved in airway tissue remodeling. Am J Rhinol Allergy. 2014;28(2):103–9. doi: 10.2500/ajra.2014.28.4018. [Shimizu S, Kouzaki H, Ogawa T, et al. Eosinophil-epithelial cell interactions stimulate the production of MUC5AC mucin and profibrotic cytokines involved in airway tissue remodeling[J]. Am J Rhinol Allergy, 2014, 28(2):103-9.] [DOI] [PubMed] [Google Scholar]
- 3.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86(1):245–78. doi: 10.1152/physrev.00010.2005. [Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease[J]. Physiol Rev, 2006, 86(1):245-78.] [DOI] [PubMed] [Google Scholar]
- 4.Izuhara K, Ohta S, Shiraishi H, et al. The mechanism of mucus production in bronchial asthma. Curr Med Chem. 2009;16(22):2867–75. doi: 10.2174/092986709788803196. [Izuhara K, Ohta S, Shiraishi H, et al. The mechanism of mucus production in bronchial asthma[J]. Curr Med Chem, 2009, 16(22):2867-75.] [DOI] [PubMed] [Google Scholar]
- 5.Young HW, Williams OW, Chandra D, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5' elements. Am J Respir Cell Mol Biol. 2007;37(3):273–90. doi: 10.1165/rcmb.2005-0460OC. [Young HW, Williams OW, Chandra D, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5' elements[J]. Am J Respir Cell Mol Biol, 2007, 37(3):273-90.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonser LR, Zlock L, Finkbeiner W, et al. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J Clin Invest. 2016;126(6):2367–71. doi: 10.1172/JCI84910. [Bonser LR, Zlock L, Finkbeiner W, et al. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma[J]. J Clin Invest, 2016, 126(6):2367-71.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Moual N, Siroux V, Pin I, et al. Asthma severity and exposure to occupational asthmogens. Am J Respir Crit Care Med. 2005;172(4):440–5. doi: 10.1164/rccm.200501-111OC. [Le Moual N, Siroux V, Pin I, et al. Asthma severity and exposure to occupational asthmogens[J]. Am J Respir Crit Care Med, 2005, 172(4):440-5.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akirav EM, Henegariu O, Preston-Hurlburt P, et al. The receptor for advanced glycation end products (RAGE) affects T cell differentiation in OVA induced asthma. PLoS One. 2014;9(4):e95678. doi: 10.1371/journal.pone.0095678. [Akirav EM, Henegariu O, Preston-Hurlburt P, et al. The receptor for advanced glycation end products (RAGE) affects T cell differentiation in OVA induced asthma[J]. PLoS One, 2014, 9(4):e95678.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sukkar MB, Ullah MA, Gan WJ, et al. RAGE:a new frontier in chronic airways disease. Br J Pharmacol. 2012;167(6):1161–76. doi: 10.1111/j.1476-5381.2012.01984.x. [Sukkar MB, Ullah MA, Gan WJ, et al. RAGE:a new frontier in chronic airways disease[J]. Br J Pharmacol, 2012, 167(6):1161-76.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milutinovic PS, Alcorn JF, Englert JM, et al. The receptor for advanced glycation end products is a central mediator of asthma pathogenesis. Am J Pathol. 2012;181(4):1215–25. doi: 10.1016/j.ajpath.2012.06.031. [Milutinovic PS, Alcorn JF, Englert JM, et al. The receptor for advanced glycation end products is a central mediator of asthma pathogenesis[J]. Am J Pathol, 2012, 181(4):1215-25.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao L, Zhao H, Tang H, et al. The receptor for advanced glycation end products is required for β-catenin stabilization in a chemicalinduced asthma model. Br J Pharmacol. 2016;173(17):2600–13. doi: 10.1111/bph.v173.17. [Yao L, Zhao H, Tang H, et al. The receptor for advanced glycation end products is required for β-catenin stabilization in a chemicalinduced asthma model[J]. Br J Pharmacol, 2016, 173(17):2600-13.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, Peng H, Cai SX, et al. Toluene diisocyanate enhances human bronchial epithelial cells' permeability partly through the vascular endothelial growth factor pathway. Clin Exp Allergy. 2009;39(10):1532–9. doi: 10.1111/cea.2009.39.issue-10. [Zhao H, Peng H, Cai SX, et al. Toluene diisocyanate enhances human bronchial epithelial cells' permeability partly through the vascular endothelial growth factor pathway[J]. Clin Exp Allergy, 2009, 39(10):1532-9.] [DOI] [PubMed] [Google Scholar]
- 13.Lachowicz-Scroggins ME, Yuan S, Kerr SC, et al. Abnormalities in MUC5AC and MUC5B protein in airway mucus in asthma. Am J Respir Crit Care Med. 2016;194(10):1296–9. doi: 10.1164/rccm.201603-0526LE. [Lachowicz-Scroggins ME, Yuan S, Kerr SC, et al. Abnormalities in MUC5AC and MUC5B protein in airway mucus in asthma[J]. Am J Respir Crit Care Med, 2016, 194(10):1296-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–47. doi: 10.1056/NEJMra0910061. [Fahy JV, Dickey BF. Airway mucus function and dysfunction[J]. N Engl J Med, 2010, 363(23):2233-47.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans CM, Raclawska DS, Ttofali F, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. http://adsabs.harvard.edu/abs/2015NatCo...6E6281E. Nat Commun. 2015;6(3):6281. doi: 10.1038/ncomms7281. [Evans CM, Raclawska DS, Ttofali F, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity[J]. Nat Commun, 2015, 6(3):6281.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Li Y, Luo D, et al. Lyn regulates mucus secretion and MUC5AC via the STAT6 signaling pathway during allergic airway inflammation. https://www.researchgate.net/publication/313801407_Lyn_regulates_mucus_secretion_and_MUC5AC_via_the_STAT6_signaling_pathway_during_allergic_airway_inflammation. Sci Rep. 2017;7(2):42675. doi: 10.1038/srep42675. [Wang X, Li Y, Luo D, et al. Lyn regulates mucus secretion and MUC5AC via the STAT6 signaling pathway during allergic airway inflammation[J]. Sci Rep, 2017, 7(2):42675.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li G, Fox J, Liu Z, et al. Lyn mitigates mouse airway remodeling by downregulating the TGF-β3 isoform in house dust mite models. J Immunol. 2013;191(11):5359–70. doi: 10.4049/jimmunol.1301596. [Li G, Fox J, Liu Z, et al. Lyn mitigates mouse airway remodeling by downregulating the TGF-β3 isoform in house dust mite models[J]. J Immunol, 2013, 191(11):5359-70.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao L, Zhao H, Tang H, et al. Phosphatidylinositol 3-Kinase mediates β-Catenin dysfunction of airway epithelium in a toluene Diisocyanate-Induced murine asthma model. Toxicol Sci. 2015;147(1):168–77. doi: 10.1093/toxsci/kfv120. [Yao L, Zhao H, Tang H, et al. Phosphatidylinositol 3-Kinase mediates β-Catenin dysfunction of airway epithelium in a toluene Diisocyanate-Induced murine asthma model[J]. Toxicol Sci, 2015, 147(1):168-77.] [DOI] [PubMed] [Google Scholar]
- 19.Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures:MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L730–9. doi: 10.1152/ajplung.00089.2003. [Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures:MAP kinase and phosphatidylinositol 3-kinase regulation[J]. Am J Physiol Lung Cell Mol Physiol, 2003, 285(3):L730-9.] [DOI] [PubMed] [Google Scholar]
- 20.Bae CH, Choi YS, Song SY, et al. Escherichia coli-derived and Staphylococcus aureus-derived extracellular vesicles induce MUC5AC expression via extracellular signal related kinase 1/2 and p38 mitogen-activated protein kinase in human airway epithelial cells. Int Forum Allergy Rhinol. 2017;7(1):91–8. doi: 10.1002/alr.2017.7.issue-1. [Bae CH, Choi YS, Song SY, et al. Escherichia coli-derived and Staphylococcus aureus-derived extracellular vesicles induce MUC5AC expression via extracellular signal related kinase 1/2 and p38 mitogen-activated protein kinase in human airway epithelial cells[J]. Int Forum Allergy Rhinol, 2017, 7(1):91-8.] [DOI] [PubMed] [Google Scholar]
- 21.Ullah MA, Loh Z, Gan WJ, et al. Receptor for advanced glycation end products and its ligand high-mobility group box-1 mediate allergic airway sensitization and airway inflammation. J Allergy Clin Immunol. 2014;134(2):440. doi: 10.1016/j.jaci.2013.12.1035. [Ullah MA, Loh Z, Gan WJ, et al. Receptor for advanced glycation end products and its ligand high-mobility group box-1 mediate allergic airway sensitization and airway inflammation[J]. J Allergy Clin Immunol, 2014, 134(2):440.] [DOI] [PubMed] [Google Scholar]
- 22.Kang JH, Hwang SM, Chung IY. S100A8, S100A9 and S100A12 activate airway epithelial cells to produce MUC5AC via extracellular signal-regulated kinase and nuclear factor-κB pathways. Immunology. 2015;144(1):79–90. doi: 10.1111/imm.2014.144.issue-1. [Kang JH, Hwang SM, Chung IY. S100A8, S100A9 and S100A12 activate airway epithelial cells to produce MUC5AC via extracellular signal-regulated kinase and nuclear factor-κB pathways[J]. Immunology, 2015, 144(1):79-90.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song KS, Lee WJ, Chung KC, et al. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinasesMSK1-CREB activation in human airway epithelial cells. J Biol Chem. 2003;278(26):23243–50. doi: 10.1074/jbc.M300096200. [Song KS, Lee WJ, Chung KC, et al. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinasesMSK1-CREB activation in human airway epithelial cells[J]. J Biol Chem, 2003, 278(26):23243-50.] [DOI] [PubMed] [Google Scholar]
- 24.Song J, Zhao H, Dong H, et al. Mechanism of E-cadherin redistribution in bronchial airway epithelial cells in a TDI-induced asthma model. Toxicol Lett. 2013;220(1):8–14. doi: 10.1016/j.toxlet.2013.03.033. [Song J, Zhao H, Dong H, et al. Mechanism of E-cadherin redistribution in bronchial airway epithelial cells in a TDI-induced asthma model[J]. Toxicol Lett, 2013, 220(1):8-14.] [DOI] [PubMed] [Google Scholar]
- 25.Barbier D, Garcia-Verdugo I, Pothlichet J, et al. Influenza a induces the major secreted airway mucin MUC5AC in a protease-EGFRextracellular regulated kinase-Sp1-dependent pathway. Am J Respir Cell Mol Biol. 2012;47(2):149–57. doi: 10.1165/rcmb.2011-0405OC. [Barbier D, Garcia-Verdugo I, Pothlichet J, et al. Influenza a induces the major secreted airway mucin MUC5AC in a protease-EGFRextracellular regulated kinase-Sp1-dependent pathway[J]. Am J Respir Cell Mol Biol, 2012, 47(2):149-57.] [DOI] [PubMed] [Google Scholar]
- 26.Shin IS, Shin NR, Park JW, et al. Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of ErkSp1 signaling. J Pineal Res. 2015;58(1):50–60. doi: 10.1111/jpi.2014.58.issue-1. [Shin IS, Shin NR, Park JW, et al. Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of ErkSp1 signaling[J]. J Pineal Res, 2015, 58(1):50-60.] [DOI] [PubMed] [Google Scholar]


