Abstract
Purpose:
Recent data indicate consolidative radiation therapy improves progression-free survival (PFS) for patients with oligometastatic non-small cell lung cancer (NSCLC). Data on long-term outcomes are limited.
Methods and Materials:
This prospective, multicenter, single-arm, phase 2 trial was initiated in 2010 and enrolled patients with oligometastatic NSCLC. Oligometastatic disease was defined as a maximum of 5 metastatic lesions for all disease sites, including no more than 3 active extracranial metastatic lesions. Limited mediastinal lymph node involvement was allowed. Patients achieving a partial response or stable disease after 3 to 6 cycles of platinum-based chemotherapy were treated with CRT to the primary and metastatic sites of disease, followed by observation alone. The primary endpoint was PFS, with secondary endpoints of local control, overall survival (OS), and safety.
Results:
Twenty-nine patients were enrolled between October 2010 and October 2015, and 27 were eligible for consolidative radiation therapy. The study was closed early because of slow accrual but met its primary endpoint for success, which was PFS >6 months (P < .0001). The median PFS (95% confidence interval) was 11.2 months (7.6–15.9 months), and the median OS was 28.4 months (14.5–45.8 months). Survival outcomes were not significantly different for patients with brain metastases (P = .87 for PFS; P = .12 for OS) or lymph node involvement (P = .74 for PFS; P = .86 for OS).
Conclusions:
For patients with oligometastatic NSCLC, chemotherapy followed by consolidative radiation therapy without maintenance chemotherapy was associated with encouraging long-term outcomes.
Summary
This phase a study of patients with oligometastatic non-small cell lung cancer highlights the clinical outcomes and toxicity profile of consolidative radiotherapy to all known sites of disease. We included patients with mediastinal disease and a history of brain metastases. Additional chemotherapy was only used at the time of progression. Our results suggest that a cohort of patients can be treated with consolidative radiotherapy, have limited toxicity, and experience encouraging durable control.
Introduction
Metastatic non-small cell lung cancer (NSCLC) encompasses a wide group of patients in terms of disease burden, underlying health, and treatment options. Historically, patients with very limited metastatic disease (eg, a solitary brain metastasis, unilateral adrenal gland metastasis, single lung metastasis) have been treated with surgical resection with the potential for durable disease control (1–3). The development of highly conformal and potent stereotactic body radiation therapy (SBRT) has unlocked the potential for effective use of radiation treatments in the oligometastatic setting. Small prospective and retrospective studies suggested both clinical efficacy and a reasonable safety profile for patients with oligometastatic cancers who receive SBRT (4–7). Similar outcomes have been demonstrated in small studies specific to oligometastatic NSCLC, suggesting a need to integrate this consolidative radiation therapy approach into larger trials (8–10).
A retrospective analysis of patients treated at the University of Colorado with chemotherapy for metastatic NSCLC demonstrated that, for patients who would have been eligible for consolidative radiation therapy (but did not receive it), progression after first-line chemotherapy most often occurred at sites of known disease rather than at new sites (11). This finding complements data from the University of Rochester that suggested that the outcome for patients with oligometastatic stage IV NSCLC who received consolidative radiation therapy (CRT) is very similar to the outcome for patients with stage III disease (12).
We report the results of a multi-institutional phase 2 study evaluating the efficacy and safety of CRT after the completion of first-line chemotherapy for oligometastatic stage IV NSCLC.
This phase 2 study of patients with oligometastatic NSCLC highlights the clinical outcomes and toxicity profile of CRT to all known sites of disease. We included patients who had mediastinal disease and history of brain metastases. Additional chemotherapy was used only at the time of progression. Our results suggest that a cohort of patients can be treated with CRT, have limited toxicity, and experience encouraging durable control.
Materials and Methods
Study design
Patients with biopsy proven stage IV oligometastatic NSCLC amenable to conventional radiation or stereotactic radiation were enrolled in the study. Patients were eligible for enrollment either before or after the completion of first-line systemic chemotherapy for 3 to 6 cycles, at the discretion of the treating medical oncologist. Patients who progressed on first-line chemotherapy for metastatic disease were excluded. Patients with brain metastasis, either single or multiple, could be enrolled if they had a magnetic resonance imaging scan of the brain within 80 days before study enrollment that showed no active lesions. This caveat was allowed because these patients commonly would be treated to the brain before first-line chemotherapy. Additional patients may have required palliative radiation therapy to a metastatic site before enrollment; they remained eligible if they had untreated thoracic disease. A performance status of ≤2 was required. Patients were required to be at least 18 years old and to have adequate lung function based on pulmonary function testing. Patients were excluded if they had received treatment for another primary cancer within the last 2 years (except nonmelanoma skin cancer, low-risk prostate cancer, noninvasive bladder cancer, and cervical carcinoma in situ) or if they had received prior upper abdominal radiation and required radiation treatment for liver metastases. Patients also were excluded if they had untreated contralateral hilar or mediastinal nodal disease (N3). However, ipsilateral supraclavicular disease (N3) was allowable. Pregnant and lactating women were excluded. This multicenter study was approved by the institutional review board at each participating center and registered on ClinicalTrials.gov as . All patients were required to have signed a study-specific consent form.
Restaging computed tomography (CT) or positron emission tomography/CT imaging was obtained after completion of chemotherapy and before delivery of CRT. Patients with either a partial response or stable disease, as defined by the Response Evaluation Criteria in Solid Tumors guideline (version 1.1), were eligible to proceed to local radiation therapy to the remaining sites of disease within 28 days after the last cycle of chemotherapy (13). Radiographic eligibility was confirmed retrospectively by a faculty radiologist (H.C.) who reviewed imaging before and after initial chemotherapy. Patients who demonstrated progressive disease after first-line chemotherapy did not receive CRT and were excluded from statistical analyses.
In the initial study design, maintenance chemotherapy was not allowed. When maintenance chemotherapy became a standard of care, the study was amended to allow maintenance chemotherapy, at the treating medical oncologist’s discretion. However, no patients on the study received maintenance chemotherapy.
Oligometastatic selection criteria
Given the potential for complex combinations of oligometastatic disease in terms of location and number of lesions, several parameters were used to subdivide and include a heterogeneous group of patients. Oligometastatic sites were divided into the categories of lung, brain, liver, spine/paraspinal, and other. Oligometastatic disease was generally defined as no more than 5 lesions spread across 3 disease sites other than the primary tumor and any hilar or mediastinal lymph nodes. Patients were included if they developed metastatic disease after treatment of prior stage I to III NSCLC. In an extracranial site, the number of lesions could not exceed 3. Patients with a single lung metastasis and no other metastatic disease were not eligible because of concerns for misdiagnosis of a second primary cancer rather than a true metastatic lesion. The cumulative long-axis diameter of all lung lesions could not exceed 7 cm (excluding lymph nodes). For liver lesions, the cumulative size limit was 6 cm.
Nodal metastases were considered part of the primary site and were not included in the calculation of metastatic sites. Untreated N2 nodal disease was allowed if the patient had not received prior radiation therapy to the mediastinum. There was no restriction on initial T or N stage for patients who developed M1 disease after initial diagnosis of stage I to III lung cancer and treatment.
Bilateral adrenal metastases were excluded. Bone lesions at any site were permissible, at the discretion of the treating radiation oncologist, as long as acceptable dosimetric standards for the target and normal tissues at risk were safely met. A maximum of 3 involved vertebral or paraspinal sites was allowed (each involved vertebral body or paraspinal site was scored as 1 site of disease). Patients with clinical or radiographic evidence of spinal cord compression were not eligible. If spinal metastases were within a previously irradiated field, a 6-month minimum interval was required between prior radiation course and study registration. Prior spinal cord maximum dose at level of vertebral disease could not exceed 50 Gy. This scenario might come into play if, for example, the patient had prior treatment for a stage III lung cancer and then developed a spinal column metastasis.
Radiation therapy
Radiation treatment was initiated within 28 days after completion of the last cycle of chemotherapy. Treatment planning was performed with CT imaging with or without contrast, using 4-dimensional respiratory timed imaging for all sites except spine, paraspinal, and bone. For each lesion, the gross tumor volume (GTV) was defined. If 4-dimensional imaging was used, an internal target volume was created to encompass the internal motion of the target lesion. The clinical target volume was identical to the GTV or internal target volume, as appropriate for each case. A subsequent expansion by 3 to 5 mm was used to generate the planning target volume. Treatment was delivered via linear accelerator using coplanar or noncoplanar 6 to 10 MV photons.
The prescribed radiation therapy dose was left to the discretion of the treating radiation oncologist to respect normal tissue tolerances. For SBRT, 54 Gy in 3 fractions or 50 Gy in 5 fractions was recommended for lung, liver, axial skeleton, and adrenal tumors, if the GTV was ≤5 cm in maximum diameter. If normal tissue constraints could not be met, or for tumors >5 cm, 50 Gy in 10 fractions was recommended. Spinal tumors received either 24 Gy in 1 fraction or 27 Gy in 3 fractions for de novo and reirradiation, respectively. For non-SBRT treatments, 60 Gy in 30 fractions was encouraged. The dose of radiation therapy was prescribed such that 95% of the planning target volume was encompassed by the prescription dose. For SBRT targets, the maximum dose to the GTV was encouraged to be 120% to 130% of the prescription dose. Normal tissue constraints were based on institutional standards and specified within the protocol. Dose constraints are outlined in Tables 1 and 2.
Table 1.
Dose constraints for stereotactic body radiation therapy/Stereotactic radiosurgery regimens
| Serial tissue | Volume, (cm3) | Maximum volume (Gy) | Maximum point dose (Gy) | Endpoint (Grade ≥3) |
|---|---|---|---|---|
| 3 fractions | ||||
| Spinal cord | <0.5 | 18 (6 Gy/fx) | 22 (7.33 Gy/fx) | Myelitis |
| Cauda equina | <5 | 21.9 (7.3 Gy/fx) | 24 (8 Gy/fx) | Neuritis |
| Sacral plexus | <3 | 22.5 (7.5 Gy/fx) | 24 (8 Gy/fx) | Neuropathy |
| Esophagus | <5 | 21 (7 Gy/fx) | 27 (9 Gy/fx) | Stenosis/fistula |
| Ipsilateral brachial plexus | <3 | 22.5 (7.5 Gy/fx) | 24 (8 Gy/fx) | Neuropathy |
| Heart/pericardium | <15 | 24 (8 Gy/fx) | 30 (10 Gy/fx) | Pericarditis |
| Great vessels | <10 | 39 (13 Gy/fx) | 45 (15 Gy/fx) | Aneurysm |
| Trachea and ipsilateral bronchus | <4 | 15 (5 Gy/fx) | 30 (10 Gy/fx) | Stenosis/fistula |
| Skin | <10 | 22.5 (7.5 Gy/fx) | 24 (8 Gy/fx) | Ulceration |
| Stomach | <10 | 21 (7 Gy/fx) | 24 (8 Gy/fx) | Ulceration/fistula |
| Duodenum | <5 | 15 (5 Gy/fx) | 24 (8 Gy/fx) | Ulceration |
| Jejunum/ileum | <5 | 16.2 (5.4 Gy/fx) | 27 (9 cGy/fx) | Enteritis/obstruction |
| Colon | <20 | 20.4 (6.8 Gy/fx) | 30 (10 Gy/fx) | Colitis/fistula |
| Rectum | <20 | 20.4 (6.8 Gy/fx) | 30 (10 Gy/fx) | Proctitis/fistula |
| Bladder wall | <20 | 15 (5 Gy/fx) | 30 (10 Gy/fx) | Cystitis/fistula |
| Femoral heads (right and left) | <10 | 21.9 (7.3 Gy/fx) | - | Necrosis |
| Renal hilum/vascular trunk | <2/3 volume | 18.6 (6.2 Gy/fx) | - | Malignant Hypertension |
| Five fractions | ||||
| Spinal cord | <0.5 | 22.5 (4.5 Gy/fx) | 30 (6 Gy/fx) | Myelitis |
| Cauda equina | <5 | 30 (6 Gy/fx) | 34 (6.4 Gy/fx) | Neuritis |
| Sacral plexus | <3 | 30 (6 Gy/fx) | 32 (6.4 Gy/fx) | Neuropathy |
| Esophagus | <5 | 27.5 (5.5 Gy/fx) | 35 (7 Gy/fx) | Stenosis/fistula |
| Ipsilateral brachial plexus | <3 | 30 (6 Gy/fx) | 32 (6.4 Gy/fx) | Neuropathy |
| Heart/pericardium | <15 | 32 (6.4 Gy/fx) | 38 (7.6 Gy/fx) | Pericarditis |
| Great vessels | <10 | 47 (9.4 Gy/fx) | 53 (10.6 Gy/fx) | Aneurysm |
| Trachea and ipsilateral bronchus | <4 | 18 (3.6 Gy/fx) | 38 (7.6 Gy/fx) | Stenosis/fistula |
| Skin | <10 | 30 (6 Gy/fx) | 32 (6.4 Gy/fx) | Ulceration |
| Stomach | <10 | 28 (5.6 Gy/fx) | 32 (6.4 Gy/fx) | Ulceration/fistula |
| Duodenum | <5 | 18 (3.6 Gy/fx) | 32 (6.4 Gy/fx) | Ulceration |
| Jejunum/ileum | <5 | 19.5 (3.9 Gy/fx) | 35 (7 Gy/fx) | Enteritis/obstruction |
| Colon | <20 | 25 (5 Gy/fx) | 38 (7.6 Gy/fx) | Colitis/fistula |
| Rectum | <20 | 25 (5 Gy/fx) | 38 (7.6 Gy/fx) | Proctitis/fistula |
| Bladder wall | <20 | 18.3 (3.65 Gy/fx) | 38 (7.6 Gy/fx) | Cystitis/fistula |
| Femoral heads (right and left) | <10 | 30 (6 Gy/fx) | - | Necrosis |
| Renal hilum/vascular trunk | <2/3 volume | 23 (4.6 Gy/fx) | - | Malignant hypertension |
| Ten fractions | ||||
| Spinal cord | <0.5 | 32 (3.2 Gy/fx) | 36 (3.6 Gy/fx) | Myelitis |
| Cauda equina | <5 | 36 (3.6 Gy/fx) | 40 (4.0 Gy/fx) | Neuritis |
| Sacral plexus | <3 | 37 (3.7 Gy/fx) | 44 (4.4 Gy/fx) | Neuropathy |
| Esophagus | <5 | 40 (4 Gy/fx) | 50 (5.0 Gy/fx) | Stenosis/fistula |
| Ipsilateral brachial plexus | <3 | 37 (3.7 Gy/fx) | 44 (4.4 Gy/fx) | Neuropathy |
| Heart/pericardium | <15 | 39 (3.9 Gy/fx) | 45 (4.5 Gy/fx) | Pericarditis |
| Great vessels | <10 | 58 (5.8 Gy/fx) | 64 (6.4 Gy/fx) | Aneurysm |
| Trachea and ipsilateral bronchus | <4 | 32 (3.2 Gy/fx) | 50 (5.0 Gy/fx) | Stenosis/fistula |
| Skin | <10 | 38 (3.8 Gy/fx) | 45 (4.5 Gy/fx) | Ulceration |
| Stomach | <10 | 35 (3.5 Gy/fx) | 45 (4.5 Gy/fx) | Ulceration/fistula |
| Duodenum | <5 | 32 (3.2 Gy/fx) | 45 (4.5 Gy/fx) | Ulceration |
| Jejunum/ileum | <5 | 32 (3.2 Gy/fx) | 45 (4.5 Gy/fx) | Enteritis/obstruction |
| Colon | <20 | 36 (3.6 Gy/fx) | 50 (5.0 Gy/fx) | Colitis/fistula |
| Rectum | <20 | 38 (3.8 Gy/fx) | 52 (5.2 Gy/fx) | Proctitis/fistula |
| Bladder wall | <20 | 32 (3.2 Gy/fx) | 54 (5.4 Gy/fx) | Cystitis/fistula |
| Femoral heads (right and left) | <10 | 38 (3.8 Gy/fx) | - | Necrosis |
| Renal hilum/vascular trunk | <2/3 volume | 35 (3.5 Gy/fx) | - | Malignant hypertension |
Table 2.
Spine radiosurgery (special case)
| Organ at risk | Dose constraint | Margin | Rank |
|---|---|---|---|
| Three fractions (prior radiation therapy <50 Gy) | |||
| Cord | 13.5 Gy | 10% | 1 |
| Esophagus | 15 Gy max pt | 0 mm | 1 |
| Single fraction | |||
| Cord | 10 Gy | 10% | 1 |
| Esophagus | 15 Gy 2 cm3 | 0 mm | 1 |
| Esophagus | 20 Gy 2 cm3 | 3 mm | 2 |
Study assessments
The primary endpoint of this study was determination of progression-free survival (PFS). Secondary endpoints included local control of oligometastatic sites after irradiation, overall survival (OS), and safety. PFS was defined as the time from chemotherapy initiation to progression or death from any cause; OS was defined as time from chemotherapy initiation toil death from any cause. After radiation therapy was completed, tumor response was assessed by the investigator every 3 months for 5 years or until progression. Safety was assessed by means of evaluations of the incidence of adverse events attributed to CRT, graded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Statistical analysis
A 2-stage design was used so that the study could be terminated prematurely if there were no evidence of improvement in PFS. A maximum sample size of 57 evaluable patients was planned, with 22 of these patients accrued during the first phase and, if necessary, the remaining 35 accrued in the second stage. Our power calculation was performed based on historical and hypothesized probability of 6-month PFS. The goal of demonstrating a median PFS >6 months was thought to be clinically meaningful because this represented roughly a 2-month improvement on the PFS typically achieved with platinum-based chemotherapy alone (14–17). At the interim analysis, the outcomes observed in the first stage were considered sufficiently encouraging to proceed to the second stage. However, in October 2015, after ongoing monitoring, the study was closed because of slow accrual. PFS and OS were determined with the Kaplan-Meier product-limit method and are reported with 95% confidence intervals (CIs).
All analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC.) A 2-tailed alpha level of 0.05 was used throughout.
Results
Population
Between October 2010 and October 2015, 29 patients were enrolled. The median duration of follow-up for patients on the study was 24.2 months. Of the patients enrolled, 2 demonstrated progression after receiving first-line chemo-therapy. These 2 patients were excluded from statistical analyses. Table 3 lists the clinical characteristics of the remaining 27 patients who were enrolled and received CRT.
Table 3.
Patient characteristics
| Patient characteristics | n (%) |
|---|---|
| Median age (range), y | 65 (49–83) |
| Sex | |
| Male | 16 (59) |
| Female | 11 (41) |
| Performance status | |
| 0 | 1 (4) |
| 1 | 22 (81) |
| 2 | 4 (15) |
| Histology | |
| Adenocarcinoma | 17 (63) |
| Squamous | 6 (22) |
| Adenosquamous | 1 (4) |
| Not otherwise specified | 3 (11) |
| T stage | |
| Tl | 8 (30) |
| T2 | 10 (37) |
| T3 | 4 (15) |
| T4 | 5 (19) |
| N stage | |
| N0 | 13 (48) |
| Nl | 3 (11) |
| N2 | 9 (33) |
| N3 | 2 (7) |
| Number of lesions (all sites) | |
| 1 | 3 (11) |
| 2 | 14 (52) |
| 3 | 7 (26) |
| 4 | 3 (11) |
| Location of metastatic sites | |
| Lung | 8 (30) |
| Bone | 5 (19) |
| Brain | 11 (41) |
| Liver | 3 (11) |
| Adrenal | 3 (11) |
| Spine/paraspinal | 4 (15) |
| Other | 3 (11) |
| Chemotherapy | |
| Carboplatin/pemetrexed | 14 (52) |
| Carboplatin/paclitaxel | 8 (30) |
| Carboplatin/pemetrexed/avastin | 2 (7) |
| Cisplatin/pemetrexed | 2 (7) |
| Carboplatin/gemcitabine | 1 (4) |
Adverse events
Adverse events attributed to CRT were uncommon. No grade 3 or greater adverse events, at least possibly related to CRT, were observed. One patient experienced a grade 2 rib fracture that was likely related to CRT with stereotactic radiation to the primary lung tumor.
Survival
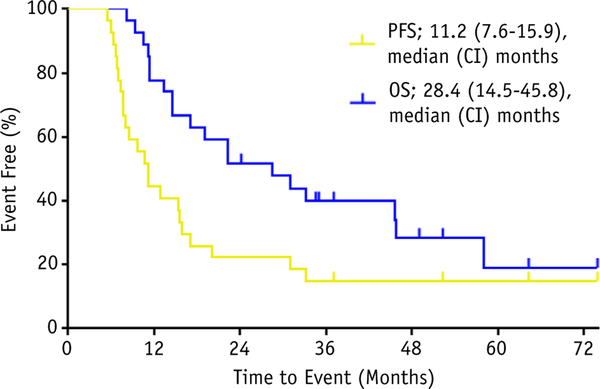
For PFS, 21 patients progressed over the course of the study, 2 patients died without progression, and the remaining 4 patients were censored at 37, 52, 64, and 74 months. For OS, 17 patients died, and the remaining 10 were censored. Figure 1 shows the Kaplan Meyer curves for PFS and OS.
Fig. 1.
PFS and OS for the study population. Abbreviations: CI = confidence interval; OS = overall survival; PFS = progression-free survival.
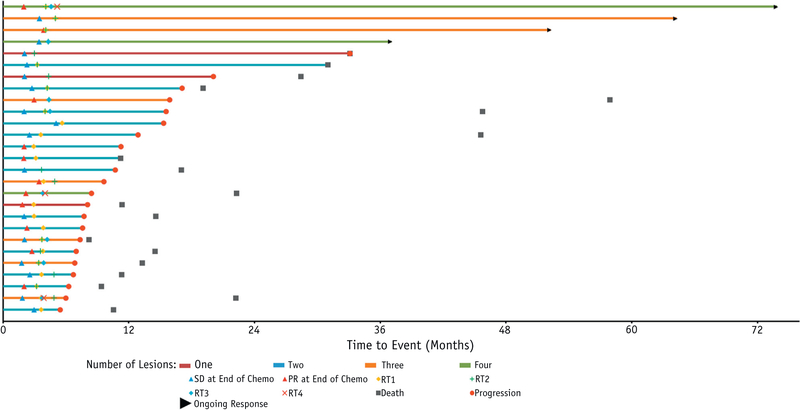
The primary endpoint for the study was a median PFS >6 months because this was considered representative of a clinically meaningful improvement over historical controls of platinum-based chemotherapy alone. Despite early closure, the study met its primary endpoint for success, with a median PFS of 11.2 months (95% CI, 7.6–15.9 months), which was significantly greater than 6 months (P < .0001). The median OS observed in this study was 28.4 months (95% CI, 14.5–45.8 months). In Figure 2, a swimmer plot shows, on an individual patient basis, the relationship among the number of tumors, time to progression, and OS.
Fig. 2.
Swimmer plot showing the treatment course and outcomes for evaluable patients. Abbreviations: PR = partial response; SD = stable disease; RT1, 2, 3, 4 defines end of radiotherapy for sites treated.
Subgroup analyses
Several clinical characteristics were expected to predict poor outcomes, including presence of brain metastases or presence of nodal metastases. To evaluate this possibility, log rank comparisons of PFS and OS survival curves were performed based on these variables. No significant differences were identified for median PFS (P = .87) or median OS (P = .12) among patients with brain metastases compared with patients without brain metastases. A nonsignificant trend toward improved OS was observed for patients with brain metastases at baseline. Similarly, no significant differences were identified for median PFS (P = .74) or median OS (P = .86) when comparing patients with or without lymph node involvement.
Patterns of failure
At the time of progression, disease progression was categorized as local, distant, or both. Distant failures were subclassified as occurring in the brain or in other sites. Table 4 shows the type of disease progression observed for patients who experienced progression before death or study completion. The progression site for 1 patient is categorized as unknown because the patient demonstrated local progression but did not undergo restaging to assess the status of systemic disease.
Table 4.
Site of progression
| Site of progression | n (%) |
|---|---|
| Total | 20 |
| Local | 1 (5) |
| Distant | 10 (50) |
| Both local and distant | 2 (10) |
| Brain | 6 (30) |
| Unknown | 1 (5) |
Local failure was uncommon, and none of the examined variables predicted risk for local failure. Progression in the brain was significantly more common for patients with brain metastases at baseline compared with patients without brain metastases at baseline (45% vs 6%, P = .016).
Discussion
Our results are consistent with other recently reported prospective trials. Our results would be stronger if the trial had been randomized. However, we can make comparisons to other contemporaneous studies while acknowledging obvious inherent limitations in such comparisons.
We reported a median PFS of 11.2 months (95% CI, 7.6–15.9 months) and a median OS of 28.4 months (95% CI,14.5–45.8 months). De Ruysscher et al reported a phase 2 trial of 39 patients with synchronous oligometastatic NSCLC (8). Patients on this trial had fairly advanced thoracic disease with relatively low volume metastatic disease; 74.4% had stage 3 disease (ignoring the M1 status) and 87.2% had a single metastasis. With definitive radiation therapy to all sites, the patients demonstrated a median PFS of 12.1 months and a median OS of 13.5 months. Patients did not receive additional chemotherapy until progression.
Collen et al completed a phase 2 trial of 26 patients with oligometastatic NSCLC showing a median PFS of 11.2 months using a consistent radiation therapy dose of 50 Gy in 10 fractions (18). About half of the patients had more than 1 metastasis, and these patients were not treated with additional chemotherapy after CRT.
In the oligo-progressive setting, Iyengar et al demonstrated that SBRT could be effectively used as a salvage setting to shift the pattern of failure from known sites to new sites of disease, albeit after a prolonged disease-free interval (19). More recently, Iyengar et al reported a small randomized phase 2 trial of patients randomized to maintenance chemotherapy or maintenance chemotherapy plus CRT for nonprogressive oligometastatic NSCLC (20). With only 29 patients enrolled, they showed a statistically significant improvement in PFS from 3.5 to 9.7 months with the addition of radiation therapy (P = .01). Consistent with their earlier study in oligo-progressive NSCLC, the addition of radiation therapy again shifted the pattern of failure from local to distant sites (19).
A randomized trial by Gomez et al reported improvement in PFS for CRT that was both statistically significant and clinically meaningful (21). In their control group, which received standard chemotherapy with maintenance chemotherapy, the median PFS was 3.9 months. In their experimental group, which also received CRT, the median PFS was a remarkable 11.9 months (P = .0054). Although the study was slow to accrue, it was stopped early because of the large PFS benefit seen on an interim analysis. Both of these studies had a relatively small sample size and short duration of follow-up. Our single-arm trial compares favorably with a PFS of 11.2 months, and comparison of our study and the studies by Gomez et al (21) and Iyengar et al (19) is shown in Table 5.
Table 5.
Study comparison
| Patient and treatment characteristics | Gomez et al study (21) | Iyengar et al study (20) | Current study | ||
|---|---|---|---|---|---|
| Treatment | No CRT | CRT | No CRT | CRT | CRT |
| N | 24 | 25 | 15 | 14 | 27 |
| Median follow-up, mo | 11.32 | 13.44 | 9.6 | 9.6 | 24.1 |
| EGFR or Alk positive | 12% | 20% | 0% | 0% | 0% |
| Median PFS (95% CI), mo | 3.9 (2.30–6.64) | 11.93 (5.72–10.90) | 3.5 (not reported) | 9.7 (not reported) | 11.2 (7.6–15.9) |
| Median OS (95% CI), mo | Not reached | Not reached | 17 (not reported) | Not reached | 28.4 (14.5–40.8) |
Abbreviations: CI = confidence interval; CRT = consolidative radiation therapy; OS = overall survival; PFS = progression-free survival.
All of these studies are small and ultimately hypothesis generating. There are also some differences in the timing of endpoints, such as whether PFS is measured from the start of all therapy or the start of radiation therapy. In any event, the relatively long duration of control reported in these studies is encouraging. Local failure was uncommon in our study, with only 1 isolated local failure. All of the phase 2 and randomized phase 2 studies report remarkably similar PFS results, despite differences in the definitions of oligometastatic disease and treatment regimens.
The hypothesis of our study was that the addition of CRT would improve PFS from <6 months to beyond that threshold. Despite a small sample size, we exceeded our expectations in that regard. Maintenance chemotherapy trials have commonly included a broad range of patients with no restriction for oligometastatic disease. These trials typically have shown a PFS of 2 to 4 months with maintenance chemotherapy (22). In the highly selective oligometastatic trials by Iyengar (19, 20) and Gomez (21), the control arms showed a respective PFS of 3.5 and 3.9 months among patients who did not receive CRT. In context with the shift in patterns of failure to distant sites, it is intriguing that the oligometastatic setting did not select for better outcomes than when maintenance chemotherapy was used alone. It seems clear that the impact of chemo-therapy is on micrometastatic disease with limited durability of visible tumors. Our study reports a PFS to the treatment arms similar to that of these 2 studies, despite the lack of maintenance chemotherapy. This result is consistent with the earlier reported trials by Collen (18) and De Ruysscher et al (8); these trials also did not use maintenance chemotherapy. Although not directly addressed, a possible hypothesis is that there is minimal benefit to maintenance chemotherapy in this highly favorable subset and that additional chemotherapy could be withheld until the salvage setting. This suggests the possibility of reducing health care costs and patient toxicities, including patient financial toxicities.
A coherent definition of oligometastatic disease is critical for future clinical research and implementation of CRT for metastatic NSCLC. The definitions in our study have both similarities to and differences from the definitions in the studies by De Ruysscher et al (8), Collen et al (18), Gomez et al (21), and Iyengar et al (19, 20). Our study was more permissive than those studies in its inclusion of patients with nodal disease and brain metastases. First, our study allowed limited mediastinal nodal disease and did not count these as a separate region of metastatic disease. In the study by De Ruysscher et al, for example, each nodal station was counted as a disease site. Second, our study allowed multiple brain metastases and grouped them as a single site of disease. The study by Colleen et al, for example, included brain metastasis, but the ongoing randomized NRG LU002 excludes them. In our study, subgroup analyses failed to detect a negative impact of nodal disease or brain metastases on PFS or OS. The lack of difference in PFS seen across studies suggests that the brain does not need to be treated as a privileged organ site. Taken together, our results suggest that our more permissive inclusion of patients is reasonable for future study designs.
Immunotherapy has become a standard treatment for metastatic NSCLC and is becoming a component in the treatment of earlier stage disease (23–26). With oligometastatic lung cancer representing (at least in part) an intermediate state between localized lung cancer and widespread metastatic disease, the issue of how to interdigitate CRT into the use of immunotherapy will be critical. Possible paradigms would be completion of radiation therapy before the initiation of immunotherapy to try to take advantage of any possible potentiation by radiation therapy and abscopal effects or a delayed approach at the time of progression as a means to repotentiate the immune system (27). None of the currently reported phase 2 and randomized phase 2 trials accounts for the paradigm shift that has occurred with the addition of immunotherapy to the treatment of metastatic NSCLC. The ongoing randomized NRG LU002 trial () evaluates maintenance chemotherapy versus maintenance chemotherapy plus CRT and is undergoing a major amendment to allow for immunotherapy. How this will factor into the results is clearly unknown but is a critically important adjustment in the trial design. Our results raise the question of whether maintenance chemotherapy is necessary with CRT. However, the immune-stimulatory effects of radiation therapy will suggest synergy could be obtained by combining immunotherapy and CRT for patients with oligometastatic disease.
Delivery of CRT after chemotherapy for patients with oligometastatic NSCLC appears to improve long-term outcomes compared with historical controls. Clinical trials for this small proportion of patients with metastatic NSCLC have been challenged by slow accrual. Given the degree of potential benefit for this population, trials comparing or combining modern chemotherapy and immunotherapy with CRT should be a priority.
Acknowledgments
This work was supported by the Comprehensive Cancer Center of Wake Forest University (grant no. NCI-P30-CA012197).
Footnotes
This protocol () is registered with Clinical Trials.gov, and may be viewed online at https://clinicaltrials.gov/ct2/show/NCT01185639.
Conflict of interest: none.
References
- 1.Billing PS, Miller DL, Allen MS, et al. Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg 2001;122:548–553. [DOI] [PubMed] [Google Scholar]
- 2.Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37–49. [DOI] [PubMed] [Google Scholar]
- 3.Tanvetyanon T, Robinson LA, Schell MJ, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: A systematic review and pooled analysis. J Clin Oncol 2008;26:1142–1147. [DOI] [PubMed] [Google Scholar]
- 4.Milano MT, Katz AW, Muhs AG, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 2008;112:650–658. [DOI] [PubMed] [Google Scholar]
- 5.Okunieff P, Petersen AL, Philip A, et al. Stereotactic body radiation therapy (SBRT) for lung metastases. Acta Oncol 2006;45: 808–817. [DOI] [PubMed] [Google Scholar]
- 6.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009;27:1579–1584. [DOI] [PubMed] [Google Scholar]
- 7.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572–1578. [DOI] [PubMed] [Google Scholar]
- 8.De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: Long-term results of a prospective phase II trial (). J Thorac Oncol 2012;7:1547–1555. [DOI] [PubMed] [Google Scholar]
- 9.Hasselle MD, Haraf DJ, Rusthoven KE, et al. Hypofractionated image-guided radiation therapy for patients with limited volume metastatic non-small cell lung cancer. J Thorac Oncol 2012;7:376–381. [DOI] [PubMed] [Google Scholar]
- 10.Khan AJ, Mehta PS, Zusag TW, et al. Long term disease-free survival resulting from combined modality management of patients presenting with oligometastatic, non-small cell lung carcinoma (NSCLC). Radiother Oncol 2006;81:163–167. [DOI] [PubMed] [Google Scholar]
- 11.Rusthoven KE, Hammerman SF, Kavanagh BD, et al. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol 2009;48:578–583. [DOI] [PubMed] [Google Scholar]
- 12.Cheruvu P, Metcalfe SK, Metcalfe J, et al. Comparison of outcomes in patients with stage III versus limited stage IV non-small cell lung cancer. Radiat Oncol 2011;6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 14.Kelly K, Crowley J, Bunn PA Jr., et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: A Southwest Oncology Group trial. J Clin Oncol 2001;19:3210–3218. [DOI] [PubMed] [Google Scholar]
- 15.Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 2002;20:4285–4291. [DOI] [PubMed] [Google Scholar]
- 16.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–3551. [DOI] [PubMed] [Google Scholar]
- 17.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small cell lung cancer. N Engl J Med 2002;346:92–98. [DOI] [PubMed] [Google Scholar]
- 18.Collen C, Christian N, Schallier D, et al. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol 2014;25: 1954–1959. [DOI] [PubMed] [Google Scholar]
- 19.Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol 2014;32:3824–3830. [DOI] [PubMed] [Google Scholar]
- 20.Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small cell lung cancer: A phase 2 randomized clinical Trial. JAMA Oncol 2017;4:e173501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez DR, Blumenschein GR Jr., Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerber DE. Maintenance therapy for advanced lung cancer: Who, what, and when? J Clin Oncol 2013;31:2983–2990. [DOI] [PubMed] [Google Scholar]
- 23.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 24.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016; 387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 25.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17:1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 27.Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: A new systemic therapy for solid tumors? Cancer Immunol Res 2014;2:831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]