Abstract
The blood coagulation protein fibrinogen is deposited in the brain in a wide range of neurological diseases and traumatic injuries with blood–brain barrier (BBB) disruption. Recent research has uncovered pleiotropic roles for fibrinogen in the activation of CNS inflammation, induction of scar formation in the brain, promotion of cognitive decline and inhibition of repair. Such diverse roles are possible in part because of the unique structure of fibrinogen, which contains multiple binding sites for cellular receptors and proteins expressed in the nervous system. The cellular and molecular mechanisms underlying the actions of fibrinogen are beginning to be elucidated, providing insight into its involvement in neurological diseases, such as multiple sclerosis, Alzheimer disease and traumatic CNS injury. Selective drug targeting to suppress the damaging functions of fibrinogen in the nervous system without affecting its beneficial effects in haemostasis opens a new fibrinogen therapeutics pipeline for neurological disease.
As we strive to understand the biological complexity of neurological diseases, it becomes increasingly clear that we cannot study neurons and glia in isolation from their extracellular environment. Instead, we need to understand how changes in the brain microenvironment trigger, enable, amplify and contribute to neuronal dysfunction, glial activation and inflammatory activity in neuronal tissues. Identifying extrinsic molecular determinants of neurological disease not only deepens our understanding of the mechanisms of communication between the brain, the immune system and the vasculature but also reveals a unique niche for novel targets for therapeutic intervention in neurological disease. In the majority of neurological diseases, there is a dramatic transformation of the neurovascular interface from a safeguarding niche essential for physiological CNS functioning to a pro-inflammatory niche where an influx of plasma proteins and immune cells contributes to — and sometimes drives—the neurodegenerative process. Studying neurological diseases through the multidisciplinary prism of vascular biology, immunology and neuroscience could be critical for identifying novel mechanisms of disease pathogenesis, developing new animal models, detecting biomarkers and discovering therapeutic treatments (FIG. 1). However, the vasculature, immune responses and neurodegeneration have been mostly studied individually or in pairs in the context of cerebrovascular biology or neuroimmunology, and mainly in stroke and multiple sclerosis (MS), respectively. As a result, the sequence of events and causative relationships between alterations in the vasculature, the immune system and the nervous system that lead to disease remain elusive. New evidence of immune gene associations and vascular pathology with Alzheimer disease (AD) pathogenesis has led to a reassessment of the aetiology and mechanisms of neurodegeneration and has underscored the need for targeting immune and vascular pathways to develop new therapies for neurodegeneration1–4. Furthermore, common threads among different neurological diseases that share similar vascular and immune abnormalities have been overlooked.
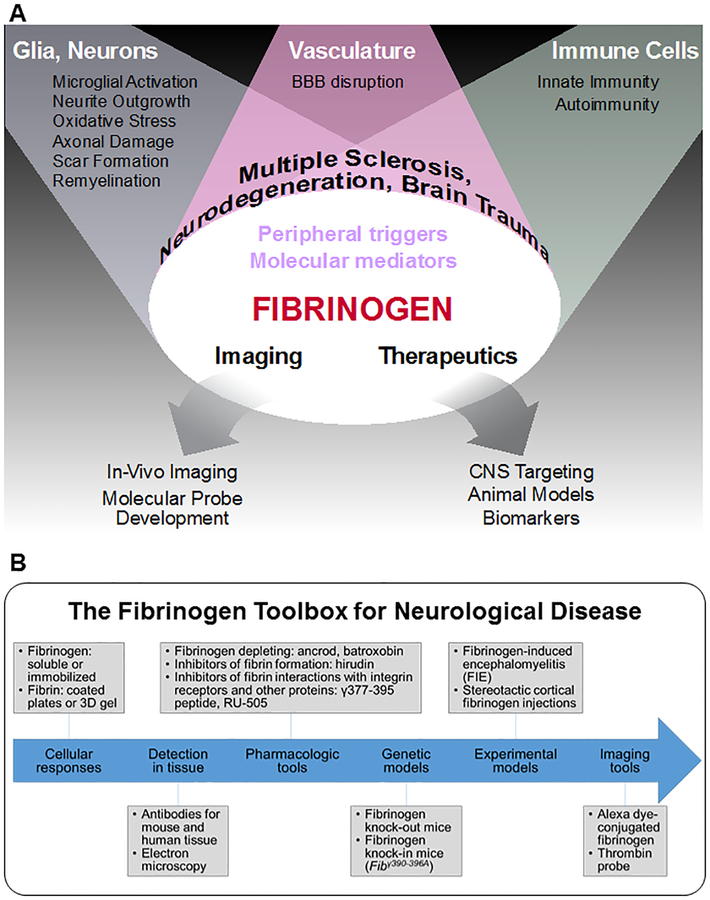
Figure 1 |. Fibrinogen at the nexus of the brain–vascular–immune axis.
a | Fibrinogen is a molecular mediator entering the CNS after blood–brain barrier (BBB) disruption that is causally linked with neuroinflammation, neuronal damage and immune cell recruitment in the nervous system. As a key component of the pathological lesion environment, fibrinogen is uniquely positioned to serve as an imaging and fluid biomarker as well as a therapeutic target for neurological diseases. b | A ‘fibrinogen toolbox’ of experimental models, pharmacological and genetic tools, molecular imaging probes and novel therapeutics has been developed to study fibrinogen’s contribution to neurovascular pathology in CNS disease. To study cellular responses and dissect molecular signalling mechanisms in vitro, fibrinogen and fibrin can be added to culture media, coated onto plates or formed into 3D gels8,12,13,36,75,142,158. Fibrin and/or fibrinogen can be detected in the diseased or injured nervous system with specific antibodies and through electron microscopy9,194 (TABLE 1). A number of pharmacological agents and genetic tools have been used to test the causal role of fibrinogen in neurological disease, including fibrinogen-depleting drugs8,13,75,86,142,195,196, inhibitors of fibrin formation9,65, inhibitors of fibrin interactions with integrin receptors and other proteins8,111,124 and fibrinogen-knockout and knock-in mice8,9–13,86. Stereotactic fibrinogen injections and infusions into the CNS provide experimental models to test fibrinogen-induced cellular responses and signalling pathways in vivo9,12,75,116,142. Novel imaging tools also allow for real-time analysis of fibrinogen and coagulation dynamics at the neurovascular unit in health and disease9,166,168. Altogether, the fibrinogen toolbox can be employed to determine the role of fibrinogen in any neurological disease with BBB disruption and a dysfunctional brain–vascular–immune axis.
A fundamental change at the neurovascular interface during disease is the influx of plasma proteins into the CNS due to increased blood–brain barrier (BBB) permeability that disrupts the homeostasis between the vasculature and the CNS5. A potential framework for a new approach to understanding the role of cerebrovascular dysfunction across neurological diseases could include addressing the following key questions. What are the molecular links between increased BBB leakage, immune activation and neurodegeneration? Is there bidirectional crosstalk between the vasculature and the immune system that influences neuronal functions? Does leakage of plasma proteins in the CNS play a causative role in neurological disease? Are there cellular targets for plasma proteins in the nervous and the immune system? Does leakage of plasma proteins into the nervous system contribute to neuronal dysfunction? Do plasma proteins affect nervous system functions directly by acting on neurons, glia and vascular cells or indirectly by activating the immune response?
A key change in the molecular composition of the extracellular microenvironment in neurodegeneration, neuroinflammation and traumatic injury is the abundant extravasation of the plasma protein fibrinogen into the CNS through a leaky BBB6. Fibrinogen is a pleiotropic protein with essential roles in coagulation, inflammation and tissue repair7. Although fibrinogen is undetectable in the healthy CNS, it is abundantly deposited in a wide range of neurological diseases and traumatic injuries associated with BBB disruption. As such, it has been extensively used as a reliable marker of BBB disruption in human tissue and relevant animal models. Emerging studies using genetic or pharmacological depletion of fibrinogen have shown that fibrinogen is not only a marker of BBB disruption but also plays a causative role in a wide range of animal models of neurological diseases, highlighting the potential role of fibrinogen in MS8,9, AD10,11, brain trauma12 and nerve injury13. The neuropathological effects of fibrinogen in the nervous system are numerous and include the following: microglial activation, axonal damage, inhibition of Schwann cell and oligodendrocyte progenitor cell (OPC) differentiation and remyelination, binding of amyloid-β (Aβ), opening of the BBB via direct actions on brain endothelial cells, induction of astrocyte scar formation and inhibition of neurite outgrowth. Fibrinogen is unique among plasma proteins owing to its molecular structure, which contains binding sites for receptors expressed by nervous system cells and for proteins that regulate key nervous system functions. The inflammatory functions of fibrinogen mediated primarily via binding to the CD11b/CD18 integrin receptor (also known as αMβ2 or complement receptor 3) in microglia and macrophages contribute to neurological deficits in neuroinflammatory disease. At the same time, fibrinogen binding to proteins such as latent transforming growth factor-β (TGFβ) or Aβ contributes to brain trauma and AD, respectively. Thus, fibrinogen is at the nexus of crosstalk between neurons and glia, the vasculature and immune cells and is a key molecular integrator of neurological, cerebrovascular and immune mechanisms of CNS injury and disease (FIG. 1).
The goal of this Review is to discuss the pleiotropic functions of fibrinogen in neurological disease with an emphasis on the cellular targets of fibrinogen in the nervous system and the fibrinogen-induced signal transduction pathways involved in disease pathogenesis. First, we highlight the unique evolution and structural characteristics of fibrinogen that enable it to become an active participant in many different biological processes and pathological settings and include a summary of the many cellular targets and receptors of fibrinogen in the nervous system. Next, we discuss the mechanisms by which fibrinogen contributes to inflammation, neurodegeneration and repair in MS, AD and traumatic CNS injury. We then examine biomarkers present in body fluids and the use of novel imaging probes for the detection of fibrinogen and increased coagulation activity in neurological diseases. Finally, we discuss the potential for nervous-system-specific targeting of fibrinogen in neurological disease without affecting its beneficial effects in haemostasis. Overall, we propose that fibrinogen leakage upon BBB disruption is a global mediator of neurodegeneration and activation of innate immunity in the CNS that may be a promising therapeutic target for neurological diseases with BBB disruption.
Fibrinogen enters the diseased CNS
Fibrinogen, a protein synthesized by hepatocytes in the liver and circulating in the bloodstream, is not present in the healthy brain owing to an intact BBB. The German physician Paul Ehrlich made the astute observation in the early 1900s that an injected dye stains all organs except the brain, illuminating the existence of the BBB14. The BBB is a key component of the neurovascular unit, the dynamic structural and functional unit of the CNS composed of highly regulated interactions between vascular cells, glial cells and neurons15,16. With an intact BBB, endothelial cells are connected by tight-junction and adherens-junction proteins and contain specialized transport systems to regulate paracellular and transcellular movements of molecules and fluid between the brain and blood17,18. Pericytes and astrocyte end feet surround the endothelial cells and provide physical contacts, secreted ligands and matrix proteins critical for the development and maintenance of the BBB19,20. Thus, the BBB provides a dynamic physical and metabolic barrier between the CNS and systemic circulation, protecting the neural microenvironment from the influx of potentially harmful substances in the blood, such as plasma proteins and immune cells, while promoting the efflux of toxins and waste products that may disturb normal neuronal functions19,20. Therefore, the tightly regulated structure and function of the BBB are necessary to maintain CNS homeostasis, and, when disturbed, have the potential to promote neuropathology.
After BBB disruption, fibrinogen enters the nervous system parenchyma and is converted into insoluble fibrin by perivascular tissue factor and procoagulant proteins abundant after injury21,22. Disruption of the BBB is a common feature of many CNS pathologies, including traumatic brain and spinal cord injury, stroke, MS, HIV encephalitis, age-related dementia, Parkinson disease, amyotrophic lateral sclerosis (ALS), cerebral malaria, Huntington disease, AD and neuropsychiatric disorders such as schizophrenia and bipolar disease15,19,23–25. Accumulating evidence indicates that BBB disruption and fibrin deposition precede demyelination and neuronal damage in many of these conditions, suggesting that neurovascular disruption contributes to disease initiation and/or progression5,15. Therefore, understanding the mechanisms of action of fibrinogen in the CNS may potentially open therapeutic avenues targeting the initial pathological changes in nervous system disease rather than later, downstream effectors.
Biology of fibrinogen
What makes fibrinogen stand out among other plasma proteins that leak into the CNS after BBB disruption? The answer lies in the unique molecular structure of fibrinogen, which contains multiple binding sites for receptors and proteins, resulting in a pluralistic signalling network and pleiotropic functions in neurological disease (FIG. 2). Fibrinogen is a 340 kDa glycoprotein secreted by the liver that circulates in the blood as a soluble homodimer26,27. Fibrinogen’s two identical disulfide-linked subunits are each composed of three polypeptide chains, designated Aα, Bβ, and γ28 (FIG. 2). Upon activation of the coagulation cascade, fibrinogen is converted into insoluble fibrin by thrombin, which enzymatically removes fibrinopeptide A and fibrinopeptide B from fibrinogen to expose polymerization sites that facilitate clot formation27,29,30.
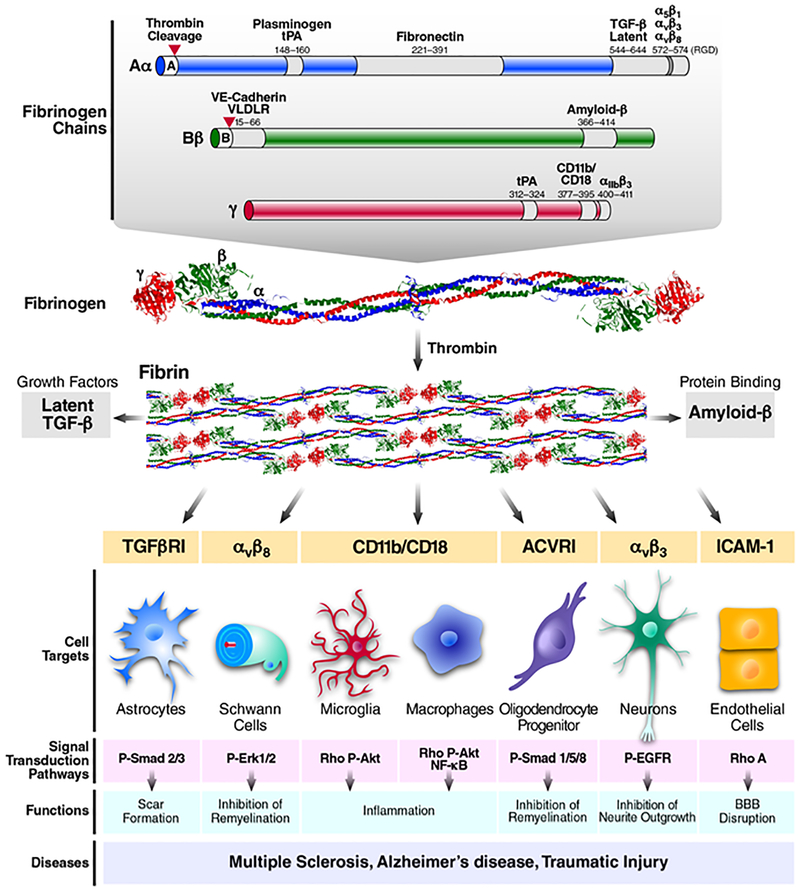
Figure 2 |. Fibrinogen structure, cellular targets and signalling networks in the nervous system.
Fibrin and fibrinogen interact with receptors on nervous system cells to activate downstream signalling, regulate basic cellular functions and influence inflammatory, neurodegenerative and repair processes in disease6,7,24,34. Fibrinogen is composed of three distinct polypeptides, designated Aα, Bβ and γ28, which contain multiple binding sites for cellular receptors and proteins. Grey-shaded areas designate the location of binding sites, with fibrinogen amino acid sequence numbers and the corresponding receptor or bound protein listed above the site. Fibrinogen chain Aα (blue) binds plasminogen/tissue-type plasminogen activator (tPA)197, fibronectin198 and latent transforming growth factor-β (TGFβ)12 and interacts with the integrin receptors α5β1 (REF. 199), αvβ3 (REF. 200) and αvβ8 (REF. 201) through its Arg–Gly–Asp (RGD) motif. Fibrinogen chain Bβ (green) binds vascular endothelial cadherin (VE-cadherin; also known as CDH5)202, very low-density lipoprotein receptor (VLDLR)203 and amyloid-β120. Fibrinogen chain γ (red) binds tPA197, the integrin receptors CD11b/CD1876,77 and αIIbβ3 (REF. 27); it also binds intercellular adhesion molecule 1 (not shown on figure)204. Upon activation of the coagulation, fibrinogen is converted into insoluble fibrin by thrombin, which cleaves fibrinopeptide A and B from fibrinogen (red triangles) to expose polymerization sites that facilitate clot formation29. Fibrinogen, fibrin and fibrin degradation products produce different responses from CNS cells. Soluble fibrinogen can induce growth factor receptor pathway activation in astrocytes, oligodendrocyte progenitor cells (OPCs) and neurons to regulate scar formation, cell differentiation and inhibition of neurite outgrowth, respectively12,142,158. Fibrinogen and fibrin also exert biological effects on mature oligodendrocytes and pericytes (see note added in proof). Conversion of fibrinogen into fibrin or immobilization of fibrinogen to a substrate exposes cryptic epitopes, such as the γ377–395 epitope, which is the binding site for the CD11b/CD18 integrin receptor76,77. Indeed, immobilized fibrinogen or fibrin activates microglia and macrophages8,9,75. Similar to soluble fibrinogen, immobilized fibrinogen inhibits OPC maturation142. 3D fibrin gels have also been utilized for testing Schwann cell differentiation and migration13,157. 3D fibrin gels are ideally suited for the study of mechanisms of fibrin degradation and the discovery of novel mechanisms in the CNS that regulate fibrinolysis. Indeed, the discovery of the low-affinity neurotrophin receptor p75NTR (NGFR) as a regulator of PLAT and plasminogen activator inhibitor 1 (PAI1) transcriptional regulation was facilitated by the culture of Schwann cells on 3D fibrin gels13,36. Studies to methodically compare the responses of CNS cells to all the potential forms of fibrinogen, fibrin and fibrin degradation products are required to associate fibrinogen structure and function with an observed biological outcome. These studies can be influenced by the experimental setting, the purity of fibrinogen preparations, the methods of primary cell isolation and the stage of cell differentiation at the time of fibrinogen or fibrin treatment. ACVR1, activin A receptor type 1; P-AKT, phosphorylated serine/threonine-protein kinase; P-EGFR, phosphorylated epidermal growth factor receptor; P-SMAD2/3, phosphorylated MAD homologue 2/3; RHOA, transforming protein RhoA; ROS, reactive oxygen species; TGFR1, TGFβ receptor type 1.
As the major protein component of blood clots, fibrin binds to activated platelets and acts as a molecular bridge to enable platelet aggregation and haemostasis31,32. Lysis of the fibrin clot is mediated by plasmin, which is generated after tissue-type plasminogen activator (tPA) or urokinase-type plasminogen activator (uPA) activates the zymogen plasminogen33,34. Plasminogen activator inhibitor 1 (PAI1) and neuroserpin inhibit tPA and uPA, resulting in decreased fibrinolysis33–35. Increased expression of the low-affinity neurotrophin receptor p75NTR (NGFR) also suppresses tPA and increases PAI1, resulting in decreased fibrinolysis36. Disruption of the coagulation–fibrinolysis balance can lead to pathology. For example, fibrin is an important part of the provisional matrix in cutaneous wounds that contributes to haemostasis and cellular organization37. However, excessive or prolonged fibrin deposition in plasminogen-deficient mice inhibits wound repair38–40. Therefore, proteolysis is a key mechanism for fibrin clearance, and impaired fibrinolysis results in excessive fibrin deposition associated with reduced repair. In addition to proteolytic degradation, a CC-chemokine receptor 2 (CCR2) macrophage endocytic pathway has been proposed as a clearance mechanism at sites of inflammation41 (BOX 1).
Box 1 |. Fibrinogen frequently asked questions.
Is it fibrinogen or fibrin?
Converting fibrinogen into fibrin exposes cryptic epitopes that promote inflammation. Fibrinogen converted into fibrin by thrombin or immobilized on a substrate binds to CD11b/CD18 and activates microglia and macrophages. Fibrinogen, fibrin and their degradation products contribute to neurological disease via multiple mechanisms.
Do fibrin and complement bind the same receptor?
Yes, both bind the CD11b‑I domain of complement receptor 3 (CR3) and induce pro‑inflammatory signal transduction. Studies in Itgam−/− mice in disease models with blood–brain barrier (BBB) leakage must be cautiously interpreted to attribute CD11b/CD18 binding to either ligand.
Will blocking fibrin in the CNS inhibit repair?
Inhibiting fibrin promotes repair in the nervous system. Excessive fibrin deposition in Plg−/− mice, which cannot degrade fibrin, delays wound healing and remyelination, an effect rescued in Plg−/−Fga−/− mice. Fibrinogen depletion enhances repair in mice after sciatic nerve crush injury and chemical demyelination.
How is fibrin removed from the brain?
Proteolysis via tissue‑type plasminogen activator (tPA) and plasmin is the main mechanism. Plasminogen‑deficient humans have fibrin deposition in tissues similar to Plg−/− mice, which are rescued by fibrinogen deficiency. Fibrin is internalized in cells, possibly by phagocytosis. The balance between coagulation and degradation could determine fibrin’s contribution to disease.
Is fibrin expressed in brain and is it genetically linked with neurological diseases?
Fibrinogen is not expressed in brain and must cross a disrupted BBB from the circulation to be found in the nervous system. Fibrinogen is highly polymorphic with over 300 single-nucleotide polymorphisms. Metagenome-wide association studies are needed to test for a genetic link with disease.
What is the evolutionary benefit of fibrin?
Fibrinogen is essential for clotting and reproduction. Pro‑inflammatory functions of clotting could be beneficial if fibrin is cleared. In neurological diseases, there is impaired fibrin clearance by plasminogen activator inhibitor 1 (PAI1) upregulation or binding of fibrin to amyloid‑β. Chronic fibrin deposition contributes to chronic inflammation and neurodegeneration.
The polymerization of fibrinogen to fibrin is mediated by highly conserved domains in the carboxy-terminal globular region of the fibrinogen molecule. These domains, first recognized in vertebrate fibrinogens, are termed fibrinogen-related domains (FReDs)42. The carboxy-terminal FReDs on the fibrinogen Bβ and γ-chains form a binding-site ‘hole’ that interacts with ‘knobs’ protruding from the central amino-terminal region of adjacent fibrinogen molecules, facilitating the formation of a double-stranded protofibril28,43 (FIG. 2). Molecules containing FReDs have been found throughout the animal kingdom; however, only the FReDs on vertebrate fibrinogen are known to mediate coagulation43. Interestingly, many FReD-containing molecules in vertebrates, such as fibroleukin, the tenascins and the ficolins, do not polymerize like fibrinogen but play important roles in innate immunity and the inflammatory response by directly interacting with surface components of pathogens and host cells44–46. In invertebrates, the vast majority of FReD-containing proteins are involved in pathogen pattern recognition and the host defence response47. In addition, some FReD-containing molecules, such as the scabrous gene product from Drosophila, bind directly to host cell receptors and mediate signalling events48. Thus, the FReD proteins contribute to a variety of biological processes that rely on specific interactions with the fibrinogen-related domains; however, from an evolutionary standpoint, fibrinogen’s role in coagulation is a recent development and unique among FReD proteins. Given that fibrinogen-related proteins display diverse functions that often include direct cell signalling and host defence, it reasons that fibrinogen may play a role in the response to injury or disease that goes beyond coagulation and reflects its ancestral functions. Many pathological conditions involve vascular disruption and leakage of fibrinogen into the tissue, where it directly interacts with cells and their receptors. Studies of fibrin deposition in human disease and in mice with genetic or pharmacological manipulation of the coagulation and fibrinolytic pathways have revealed a wide range of physiological and pathological conditions that are affected by fibrin, such as inflammation and tissue repair (FIG. 3; TABLE 1). As a component of the perivascular extracellular matrix19, fibrinogen directly interacts with all cellular components of the neurovascular unit, binding receptors on nervous system cells to influence inflammatory, neurodegenerative and repair processes in the injured CNS6,7. Fibrinogen in the CNS binds both integrin and non-integrin receptors to directly regulate many basic functions of glia, neurons and endothelial cells (FIG. 2). In addition, fibrinogen acts as a carrier of diverse proteins that include a number of growth factors as well as components of the coagulation and fibrinolytic cascade24. Fibrinogen, fibrin and fibrin degradation products elicit different responses from CNS cells owing to different epitope exposure and different biochemical properties of soluble molecules and insoluble 3D matrices (detailed description in FIG. 2). Studies of the cellular and molecular mechanisms of fibrinogen functions in neurological diseases, as outlined in detail below, have shown that fibrinogen is not merely a marker of vascular compromise in pathological conditions but rather a critical modulator of disease that serves as a molecular link between coagulation, inflammation and tissue regeneration.
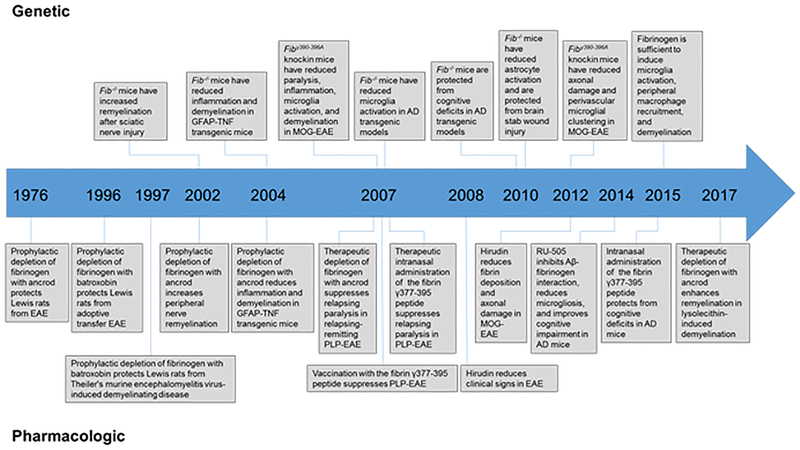
Figure 3 |. Timeline of in vivo genetic and pharmacological evidence showing a causal role for fibrin and/or fibrinogen in the development of neurological disease.
Pharmacological depletion of fibrinogen with the defibrinogenating agents ancrod or batroxobin protected Lewis rats in multiple sclerosis (MS) models85,195,196; this finding was later confirmed in mouse MS models8,75,86. Akassoglou et al. provided the first genetic proof for a causal role for fibrin in a nervous system experimental model of nerve regeneration13. Later studies demonstrated fibrin to be a critical determinant of neuroinflammation as fibrinogen-knockout mice showed reduced microglial activation and improved neurological function in animal models of MS8,86 and protection from neuropathology and cognitive deficits in Alzheimer disease (AD)10,11. In addition, blocking the conversion of fibrinogen to fibrin with the thrombin inhibitor hirudin protected from experimental autoimmune encephalitis (EAE)9,65. To selectively inhibit the inflammatory actions of fibrin in the CNS while preserving coagulation, Adams et al. blocked fibrin’s interaction with its high-affinity receptor CD11b/CD18 genetically by using Fggγ390−396A mice, in which fibrinogen has been mutated to lack the CD11b/CD18-binding motif88, and pharmacologically by administering the γ377–395 peptide8. Genetic and pharmacological inhibition of fibrin–CD11b/CD18 reduced paralysis, inflammation, microglia activation, axonal damage and demyelination in EAE8,9. In AD mice, administration of a compound that specifically inhibits fibrin–amyloid-β (Aβ) interaction reduced vascular pathology, blocked neuroinflammation and protected from cognitive decline111. Altogether, the genetic and pharmacological evidence point to a causal role for fibrin and/or fibrinogen in the CNS and may represent a novel target for therapeutic intervention in neurological disease. GFAP, glial fibrillary acidic protein; MOG, myelin-oligodendrocyte glycoprotein; PLP, myelin proteolipid protein; TNF, tumour necrosis factor.
Table 1 |.
Fibrin(ogen) deposition in neurological diseases
| Multiple sclerosis | Comment | Refs |
|---|---|---|
| Progressive (primary and/or secondary) | ||
| NAWM | Occasional fibrillary pattern of fibrin centred around vessels in areas of intact myelin | 62,63,207 |
| Pre-active NAWM | Fibrin centred around vessels with clusters of microglia with enhanced MHCII+ CD45+ cells and CD68+ cells in areas of intact myelin | 62 |
| Active | Fibrin throughout the demyelinated lesion parenchyma but centred around vessels along with MHCII+ Oil Red O-stained macrophages | 62–64,68, 207,208 |
| Chronic active | Fibrin throughout the demyelinated lesion, colocalized with astrocytic and axonal processes; lesion borders with MHCII+ macrophages | 62 |
| Chronic inactive | Fibrin throughout the demyelinated lesion, colocalized with astrocytic and axonal processes; hypocellular lesions with widespread astrogliosis | 62–64, 142,207 |
| EM detection of parenchymal fibrin deposition in the perivascular space associated with gliosis | 194 | |
| Fibrin deposition in the MS motor cortex correlated with decreased neuronal density | 66 | |
| Acute | ||
| Active | Fibrin deposition present in all active lesions correlating with microglia and/or macrophages and perivenous demyelination |
69,142, 208,210 |
| Pre-active NAWM | Fibrin deposition in areas of intact myelin with microglial activation, iNOS+ cells and APP+ neurons, but no parenchymal T cell infiltration | 69 |
| Other neurological diseases | ||
| Alzheimer disease | Perivascular fibrin correlating with monocyte and/or macrophages, COX2+ cells and interruptions of ZO1 | 102 |
| ELISA detection of insoluble fibrinogen–albumin complexes | 211 | |
| Fibrin in the perivascular area with amyloid plaques, microglial activation, astrogliosis and HLA-DR+ cells | 101 | |
| Fibrin deposition in the vessels with cerebral amyloid angiopathy | 11 | |
| Fibrin deposition in the vessels associated with cerebral amyloid angiopathy and increased perivascular macrophages | 100 | |
| Perivascular fibrin deposits correlated with loss of pericyte coverage of brain vessels | 105 | |
| Fibrin detection in areas with amyloid plaques and dystrophic neurites | 104 | |
| Fibrin accumulation in the parietal cortex correlated with PDGFRβ loss and fibrillary Aβ accumulation | 103 | |
| Amyotrophic lateral sclerosis | Fibrin accumulations found in motor-neuron-dense regions in the cervical spinal cord anterior horn grey matter | 129 |
| PD | Extravascular fibrin increased 9.4-fold in the striatum of PD patients compared with controls; all 12 PD patients showed fibrin extravasation | 133 |
| HD | Extravascular fibrin increased 2.5-fold in the putamen of HD patients compared with controls | 25 |
| Traumatic brain injury | Widespread perivascular fibrin deposition in both acute and chronic phases after traumatic brain injury | 138 |
| Cerebral malaria | Widespread fibrin deposition associated with perivascular cell processes | 212 |
| Brain tumour | Fibrinogen and/or fibrin found in primary (glioblastoma) and metastatic human brain tumours in close association with macrophages | 213 |
Aβ, amyloid-β; APP, Aβ precursor protein; COX2, cyclooxygenase 2 (also known as PTGS2); EM, electron microscope; ELISA, enzyme-linked immunosorbent assay; HD, Huntington disease; HLA-DR, human leukocyte antigen-DR; iNOS, inducible NO synthase (also known as NOS2); MHCII, major histocompatibility complex class II; MS, multiple sclerosis; NAWM, normal-appearing white matter; PD, Parkinson disease; PDGFRβ, platelet-derived growth factor receptor-β; ZO1, zonula occludens protein 1.
MS and neuroinflammation
MS is a chronic inflammatory disease of the nervous system characterized by BBB disruption, perivascular inflammation, demyelination and axonal damage, which can lead to permanent functional impairments for those individuals affected by this devastating disease49–51. In 1863, Eduard Rindfleisch, a German pathologist who analysed post-mortem brain tissue from MS patients, provided the first descriptions suggesting that inflammation centred around the blood vessels in MS lesions is involved in the underlying aetiology of the disease52. He wrote: “if one looks carefully at freshly altered parts of the white matter in the brain, one perceives already with the naked eye a red point or line in the middle of each individual focus, … the lumen of a small vessel engorged with blood. … All this leads us to search for the primary cause of the disease in an alteration of individual vessels and their ramifications; an assumption which is completely confirmed by microscopic examination. All vessels running inside the foci, but also that traverse the immediately surrounding but still intact parenchyma are in a state characteristic of chronic inflammation.”52 Now, 150 years later, we have begun to delineate how BBB disruption, one of the earliest events in MS pathology, is linked to the inflammation and white matter injury that define this neuroinflammatory disease.
BBB disruption and fibrin deposition.
Breakdown of the BBB is a classic hallmark of MS white matter lesions53. MRI has been used clinically for decades as the gold standard for identifying BBB disruption in active MS lesions by visualizing the leakage of intravenously administered contrast agents such as gadolinium54,55. MRI studies show that BBB breakdown in MS patients is an early event in active lesion formation and actually occurs before the onset of clinical symptoms56,57. Serial MRI scans obtained with dynamic contrast enhancement on high-resolution (3T) and 7T scanners show that most active enhancing lesions are perivascular and appear to grow outward from a central vein58,59. Studies that have linked MRI findings to underlying lesion histopathology reveal extensive leakage of plasma proteins and prominent perivascular inflammation in these contrast-enhancing lesions60,61. Indeed, fibrin deposition is a prominent feature of MS pathology and is present throughout the course of the disease (TABLE 1). Fibrin is found not only in active lesions but also in chronic plaques, where it persists despite a lack of contrast enhancement on standard MRI62–64. Proteomic analysis of laser-microdissected human MS lesions reveals dysregulation of several proteins of the coagulation cascade, such as tissue factor and protein C inhibitor, within chronic active MS lesions65. Fibrin is also deposited in the cortex in patients with progressive MS and correlates with neuronal loss66. In addition, MS lesions appear to have an impaired capacity to degrade fibrin owing to an increase in PAI166–68. Interestingly, fibrinogen was detected not only in the parenchyma but also intracellularly as also documented in prior studies66,69. The mechanisms of intracellular accumulation of fibrinogen and its potential role in disease pathogenesis remain unknown. Dysregulation of the coagulation and fibrinolytic pathways likely contributes to the excessive and prolonged deposition of fibrin observed in MS.
Fibrinogen and the pathways that control fibrin formation and degradation are considered among the triggers that could contribute to the initiation and course of MS53. Fibrin deposition in MS lesions correlates with early lesions and areas of demyelination and, importantly, is found in close association with inflammation and damaged axons67,69–71 (TABLE 1). The breakdown of the BBB and activation of the innate immune system appears to be an early event in MS pathology. In 1952, Adams and Kubik provided the first pathological description of an early MS lesion in which clusters of microglia, the resident innate immune cells of the nervous system, were found in areas of morphologically intact myelin72. This finding is now referred to as a pre-demyelinating or pre-active lesion and is identified by the characteristic activation of microglia in otherwise normal-appearing white matter62,69,73. Pre-demyelinating lesions display BBB disruption and fibrin deposition that colocalize with areas of microglial activation62,63,69. Of note, fibrin deposition and microglial activation in MS lesions precede T cell infiltration, suggesting that fibrin serves as a critical molecular signal in MS pathology and plays a role in the initiation and/or progression of inflammation in MS69. This notion is further supported by longitudinal MRI studies co-registered with histopathology in marmoset experimental autoimmune encephalomyelitis (EAE), an established autoimmune animal model of MS, which identified disruption of the BBB 4 weeks before T cell infiltration and demyelination74. Overall, these studies suggest that BBB leakage and fibrin deposition are early events in the genesis of MS lesions that precede demyelination and neuronal damage.
Fibrin and neuroinflammation.
Is fibrin merely a marker of BBB disruption or does it have a role in the onset and progression of neuroinflammatory diseases? Fibrin, a potent inducer of microglial activation, is uniquely positioned to orchestrate immune and oxidative stress responses at sites of BBB disruption (FIG. 4). Importantly, studies using fibrinogen-mutant mice and anticoagulants strongly support a causal role for fibrin in neuroinflammation (FIG. 3). Fibrin induces neuroinflammation by activating microglia and promoting the recruitment and activation of peripheral inflammatory macrophages into the CNS (FIG. 4)8,9,75. Fibrinogen conversion into fibrin also exposes a cryptic epitope within the carboxy-terminus of the fibrinogen γ-chain (amino acid sequence γ377–395), which binds with high affinity to the CD11b-I domain of CD11b/CD1876,77. Binding of fibrin to the CD11b/CD18 receptor on microglia and infiltrating macrophages activates multiple signal transduction pathways to promote inflammatory responses7. Fibrin induces nuclear factor-κB (NF-κB), mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways to mediate adhesion, migration, chemotaxis and phagocytosis22. Fibrin also activates the serine/threonine-protein kinase AKT and Rho-family GTPases in microglia via CD11b/CD18, resulting in an increase in cell body size, rearrangements of the actin cytoskeleton and increased phagocytosis8. In addition to morphological changes and phagocytosis, fibrin induces a unique transcriptional M1-like activation of microglia and macrophages that is associated with induction of antigen presentation, release of reactive oxygen species (ROS) and secretion of the leukocyte-recruiting chemokines monocyte chemoattractant protein 1 (MCP1; also known as CCL2) and CXC-chemokine ligand 10 (CXCL10)9,75. Studies in macrophages isolated from Toll-like receptor 4 (TLR4)-knockout mice have also indicated an involvement of TLR4 in fibrinogen-induced activation of NF-κB and cytokine gene expression78,79. Although there is no evidence to suggest that fibrinogen directly binds TLR4, TLR4 signalling may be involved in fibrin-induced inflammation. Indeed, crosstalk between TLR4 and CD11b/CD18 signalling is well established80–83, and fibrin might be a CD11b/CD18 ligand that modulates TLR4-mediated signalling in innate immune cells in the CNS84. Future studies will be required to determine the relative contribution of known inflammatory receptors to fibrin and/or fibrinogen signalling and to discover novel cellular receptors for fibrin and/or fibrinogen in the CNS.
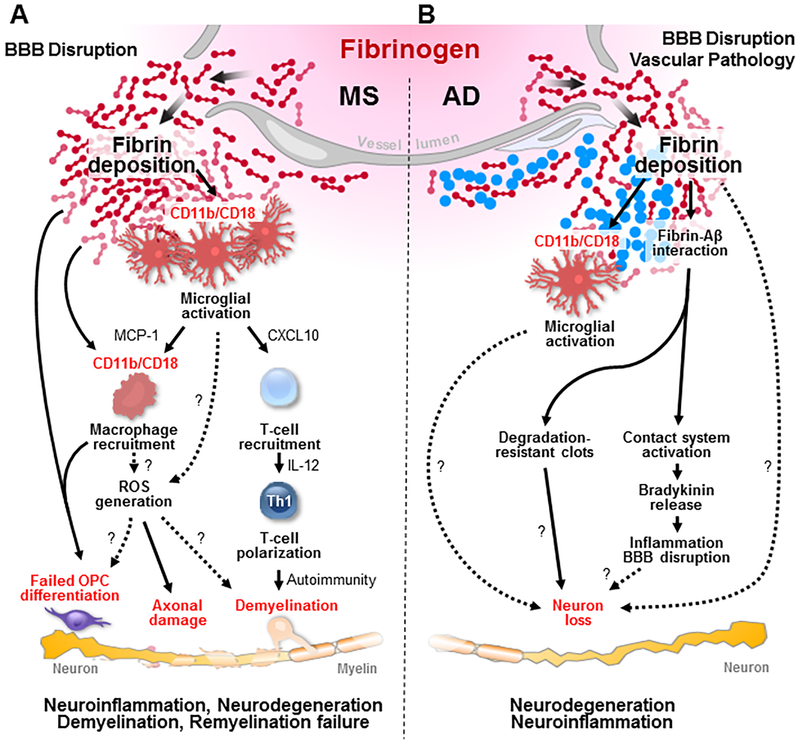
Figure 4 |. Fibrinogen at the helm of CNS innate immune activation and neurodegeneration.
Fibrin is a key contributor to inflammatory demyelination, neurodegeneration and inhibition of CNS repair. a | In multiple sclerosis animal models, blood–brain barrier (BBB) disruption leads to increased coagulation activity and fibrin deposition in the brain, which binds to the CD11b/CD18 integrin receptor, thus driving early microglial activation, recruitment of peripheral macrophages and T cells and leading to demyelination and axonal damage8, 9,75. Fibrin-induced activation induces a gene transcription signature characterized by increased secretion of chemokines (such as CXC-chemokine ligand 10 (CXCL10) and monocyte chemoattractant protein 1 (MCP1)) to promote recruitment of T cells, increased antigen presentation and release of instructive signals (such as interleukin 12 (IL-12)) for inducing T helper 1 (TH1) cell differentiation to promote autoimmunity and demyelination75. In parallel, perivascular microglia cluster at sites of fibrin deposition, which correlate with areas of reactive oxygen species (ROS) generation and axonal damage in vivo9. Mechanisms and consequences of fibrin-induced ROS release in the CNS are not known (dotted lines). Fibrinogen also blocks oligodendrocyte progenitor cell (OPC) differentiation to myelinating cells by direct and immune-mediated mechanisms142. b | In animal models of Alzheimer disease (AD), fibrin binds to amyloid-β (Aβ), which results in degradation-resistant clots11,120,122. Aβ further drives fibrin formation and the release of pro-inflammatory molecules121,193. The mechanisms by which fibrin–Aβ interaction contributes to neuronal loss in AD models are not known (question marks and dotted lines). In addition, whether fibrin-induced microglia activation contributes to amyloid-driven neurodegeneration is unknown (question marks and dotted lines).
Davalos et al. used two-photon laser scanning microscopy (2pLSM) to study the in vivo response of microglia to fibrinogen in real time9. Fibrinogen injections into the healthy brain induced rapid (within ~30 minutes) microglial process extension that was sustained9. These effects were specific for fibrinogen as they were not detected with injections of other blood proteins or plasma from fibrinogen-knockout mice, which contains all the plasma proteins except fibrinogen9. Serial in vivo imaging in animals with EAE revealed that fibrinogen-induced microglial clustering is one of the earliest events in inflammatory demyelination9. Interestingly, a single stereotactic injection of fibrinogen in the corpus callosum was sufficient to induce microglial activation and local chemokine secretion, followed by recruitment and local differentiation of myelin antigen-specific T helper 1 (TH1) cells, which lead to demyelination75. This fibrin-driven, autoimmune-mediated model of inflammatory demyelination has been termed fibrin-induced encephalomyelitis (FIE)75 and has been proposed as a novel experimental setting to study fibrin-induced microglia-driven demyelination. Induction of FIE in CD11b-knockout mice or after pharmacological treatment with anti-CD11b neutralizing antibodies ameliorates microglia activation, chemokine and cytokine release and demyelination75, indicating the requirement of CD11b/CD18 for fibrin-induced immune activation in the CNS in vivo.
Causal role for fibrin in MS pathology.
Genetic depletion of fibrinogen and pharmacological studies targeting the coagulation cascade and subsequent fibrin deposition have shown a causal role for fibrin in neuro-inflammatory disease (FIG. 3). The first evidence that fibrin depletion may be beneficial in inflammatory demyelination came from a study of EAE in rats over 40 years ago85. In this study, perivascular deposition of fibrin was found in conjunction with paralytic episodes. EAE animals that had circulating levels of fibrinogen pharmacologically reduced with ancrod, a thrombin-like serine protease derived from the Malayan pit viper that causes the abnormal cleavage and degradation of fibrinogen24, demonstrated reduced fibrin deposition and decreased paralysis. Studies in multiple laboratories using EAE, tumour necrosis factor (TNF)-transgenic and viral models of MS have confirmed the protective and therapeutic effects of genetic or pharmacological depletion of fibrinogen on inflammatory demyelination8,86,87. Similar to the fibrinogen-reducing effects of ancrod, blocking the conversion of fibrinogen into insoluble fibrin with the thrombin inhibitor hirudin was effective in reducing the severity of EAE9,65. Importantly, therapeutically targeting the coagulation cascade in EAE not only reduces inflammatory lesions but also protects from axonal damage9, an increasingly appreciated contributor to MS pathology.
The fibrin γ377–395 epitope binds the CD11b/CD18 integrin receptor on microglia and macrophages to induce innate inflammatory responses, and, as noted above, fibrin’s effects in vivo are attenuated in CD11b-knockout mice8,75,76. However, CD11b/CD18 is a pleiotropic receptor with several endogenous ligands. Therefore, results in CD11b-knockout mice need to be cautiously interpreted and not biasedly associated with a specific ligand, especially in disease models, such as EAE, in which CD11b/CD18 is exposed to multiple ligands. To overcome this caveat, the fibrin–CD11b/CD18 interaction can be selectively disrupted using Fggγ390–396A knock-in mice that express a mutant fibrinogen that cannot bind CD11b/CD18 (REF. 88) without affecting the binding of other ligands to CD11b/CD18 (FIG. 4). Microglial responses after injection of Fggγ390–396A plasma into the corpus callosum are significantly lower than the responses to wild-type plasma75. Fggγ390–396A mice with EAE demonstrate reduced microglial activation, decreased axonal damage, less demyelination and reduced paralysis8,9. Fggγ390–396A mice are also protected in models of peripheral inflammation, including rheumatoid arthritis, colitis and muscle degeneration7. The interaction of fibrin with CD11b can also be disrupted pharmacologically using the fibrin γ377–395 peptide76. Therapeutic administration of the γ377–395 peptide via intranasal delivery or the vaccination of mice with the γ377–395 peptide also protects from EAE8. Therefore, fibrinogen entry into the CNS and subsequent CD11b-mediated microglial activation may be key upstream molecular events that drive inflammatory demyelination75. Importantly, genetically or pharmacologically inhibiting the fibrin–CD11b/CD18 interaction does not affect fibrin’s clotting functions8,89; therefore, inhibitory peptides or antibodies that target the γ377–395 peptide may selectively reduce the pathological effects of fibrin in the CNS while preserving the benefits of fibrin in blood coagulation. Given the inflammatory and autoimmune-promoting functions of fibrin in the CNS, fibrin may serve as a therapeutic target that could potentially modify the pathological responses at the onset and progression of MS.
Alzheimer disease neurodegeneration
AD is a progressive neurodegenerative disorder that results in profound impairments in learning and memory90. Genetic studies have strongly implicated immune genes, and in particular pathways regulating microglial functions, in AD1,3,4. Along with microglial activation and neuronal cell death, the neuropathological hallmarks of AD include the extracellular deposition of Aβ in senile plaques and blood vessel walls and the intracellular accumulation of neurofibrillary tangles containing phosphorylated tau34,90. Vascular causes of dementia have been considered to be critical for AD pathology15,91. However, there is considerable overlap between these two entities15,91. Cerebrovascular lesions coexist in ~50% of patients with clinically diagnosed AD92,93, suggesting that vascular factors play an important role in AD pathogenesis. In patients with AD, BBB disruption correlates with disease progression94, and in some AD animal models, BBB permeability actually precedes other neuropathological alterations in the brain95, suggesting that BBB breakdown has a causative role in AD initiation and progression. Indeed, microhaemorrhages are frequently observed in the brains of humans with AD96, and they contribute to the conversion of mild cognitive impairment into AD97. Microhaemorrhages colocalize with amyloid plaques in the brains of individuals with AD98, further indicating that BBB disruption or blood itself may be relevant to plaque deposition.
Accumulating evidence points to the coagulation cascade as a molecular link between vascular-related and Aβ-related pathology in AD34,99. Fibrin deposition in the CNS is a prominent feature of cerebrovascular pathology in human AD (TABLE 1). Fibrin accumulations in AD occur within the CNS vessels, especially in conjunction with cerebral amyloid angiopathy11,100, and in the perivascular brain parenchyma, where it colocalizes with Aβ plaques, perivascular macrophages, areas of pericyte loss and dystrophic neurites101–105. CNS fibrin deposits are also increased in patients with AD with two alleles of apolipoprotein E (Apo-E) ε4 (REF. 100), the strongest genetic risk factor for AD91. Furthermore, elevated plasma fibrinogen levels are associated with cognitive decline and an increased risk of AD106,107. Consistent with the human data, CNS fibrin deposition has been extensively reported in animal models of AD. Transgenic mice with mutations in the human Aβ precursor protein (APP) gene develop AD-like pathology, including accumulations of Aβ, neurodegeneration and evidence of BBB breakdown108. Perivascular fibrin deposits have been detected in the brains of AppSw/0 (Tg2576)10,109, Appsw/0 crossed with the presenilin (Psen)-mutant PsenΔE9 (REF. 110), App V717F (PDAPP)10, AppSwl (TgCRND8)10 and AppSwFlLo; Psen1M146L/L28 (5×FAD)111 mice. BBB breakdown and CNS fibrin deposition are also prominent features of the progressive neurodegeneration that occurs in pericyte-deficient112,113 and APOE-transgenic mice114,115.
Causal role for fibrin in AD pathology.
Given fibrin’s prominence in the brain of people with AD, studies have explored whether fibrin is causally linked to neurodegeneration in animal models of AD (FIG. 3). In Aβ injection models, neuroinflammation and AD-like neurodegeneration are exacerbated when fibrinogen is co-injected and significantly reduced when fibrinogen is pharmacologically depleted with ancrod or upon administration of an anti-CD11b neutralizing antibody116. In transgenic AD mice, genetic or pharmacological fibrinogen depletion attenuates disease pathology, reduces microglial activation and improves cognitive function10,11,104. Similarly, blocking the conversion of fibrinogen into fibrin with anticoagulants is protective in AD mice117–119. Interestingly, fibrin can physically interact with Aβ to promote Aβ fibril formation11,120. In turn, Aβ can activate the coagulation factor XII (FXII)-mediated contact pathway to further drive the conversion of fibrinogen into fibrin121. Fibrin–Aβ interactions also alter fibrin clot structure and block plasminogen binding to fibrin, which results in degradation-resistant clots and may further enhance fibrin-induced AD pathology122. Indeed, Aβ plaque deposition and cognitive impairment are more severe in AD mice lacking one allele for tPA123, which may be owing to impaired fibrin degradation. Administration of a small molecule that specifically inhibits fibrin–Aβ interaction in AD mice reduces vascular pathology, blocks neuro-inflammation and protects from cognitive decline111. Similar to EAE8, therapeutic intranasal administration of the γ377–395 peptide leads to cognitive improvement and reduction of brain parenchyma Aβ deposition in AβPP/PS1 AD mice124. Recently, Chen et al. demonstrated that depleting FXII also ameliorates brain pathology and cognitive impairment in AD mice99,125. These studies suggest that the coagulation system and its end product fibrin represent a pathogenic constituent of the underlying vascular damage in AD that promotes inflammatory processes important for disease onset and progression.
Numerous independent studies by multiple laboratories have consistently demonstrated increased fibrin deposition and BBB permeability in human patients with AD and in AD animal models (FIG. 5a; TABLE 1). As recently reviewed108, these studies employ a variety of methods to detect BBB disruption, including fibrin immunodetection and high-resolution confocal or multiphoton microscopy. A recent study using a single systemic administration of tracers and antibodies did not detect BBB breakdown in three transgenic AD mouse models: PS2-APP, TauP301S and APOE126. This study may appear inconsistent with previous work that showed fibrin deposition in these models10,114. However, it could potentially be explained by the focal nature and temporal features of BBB disruption. Fibrinogen immunohistochemistry is an excellent tool to detect the cumulative effect of BBB disruption over time because fibrinogen clots and accumulates in the brain as fibrin, an insoluble macromolecular deposit that can be removed from the brain only upon proteolytic degradation34. As such, fibrin can accumulate in the CNS over a long period of time; therefore, fibrin immunohistochemistry is indicative of long-term leakage. By contrast, a single injection of exogenous tracers documents BBB disruption only on the specific day of administration. Although a single administration of tracer can be informative in acute models with a defined time point of onset (for example, EAE immunization or trauma), it may be inadequate to determine BBB integrity in a transgenic neurodegeneration model that develops pathology over several months. High-resolution confocal and multiphoton imaging of fibrin may also improve the sensitivity of detecting subtle or focal disruptions of the BBB that may develop over time in AD animal models9,10,112,114. Overall, combining complementary methods to assess BBB integrity, adapting techniques to account for the spatiotemporal differences of BBB disruption in acute injury and neuroinflammation models versus chronic models of neurodegeneration and implementing fibrinogen immunohistochemistry to detect cumulative BBB disruption over time are essential considerations to rigorously assess BBB integrity in animal models of neurodegeneration.
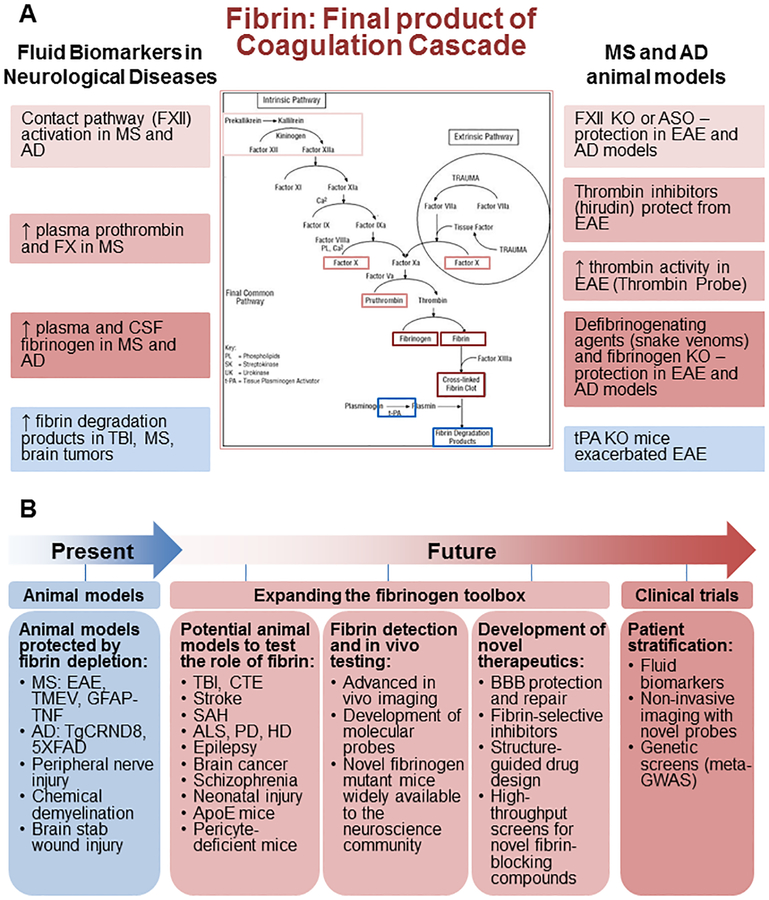
Figure 5 |. The coagulation cascade and its final product fibrin as clinically relevant biomarkers and potential therapeutic targets for neurological disease.
a | Fibrinogen and components of the coagulation cascade could serve as clinically relevant plasma and cerebrospinal fluid (CSF) biomarkers to diagnose and track disease progression in neurological disease. Upstream components of the coagulation cascade, including the contact system, coagulation factor X and prothrombin, are elevated in multiple sclerosis (MS)177,178 and/or Alzheimer disease (AD)179. Fibrinogen itself is elevated in the plasma or CSF from patients with MS176 and AD106,107,169–173 and often correlates with disease severity. Fibrin degradation products are also found in the CSF of traumatic brain injury (TBI) patients175. Studies of animal models of AD and MS highlight the therapeutic potential of targeting coagulation for CNS disease. Coagulation factor XII depletion protects mice in the experimental autoimmune encephalitis (EAE) MS model178 and in AD models125. The thrombin inhibitor hirudin blocks the conversion of fibrinogen into fibrin and is protective in EAE9,65. Genetic and/or pharmacological depletion of fibrinogen is protective in animal models of MS8,75,85–87,195,196, AD10,101 and nervous system injury13,205. By contrast, tissue-type plasminogen activator (tPA)-knockout (KO) mice, which have impaired fibrin degradation, show increased severity of EAE and delayed recovery206. Together, human biomarker and animal studies highlight the potential utility of monitoring and perhaps targeting the coagulation cascade in neurological disease. b | In the future, expanding the ‘fibrinogen toolbox’ will enable translation of novel therapeutics, biomarkers and imaging probes to human clinical trials to test the ‘fibrin hypothesis’ of neurological disease. ALS, amyotrophic lateral sclerosis; Apo-E, apolipoprotein E; ASO, antisense oligonucleotide; BBB, blood–brain barrier; CTE, chronic traumatic encephalopathy; GFAP, glial fibrillary acidic protein; GWAS, genome-wide association studies; HD, Huntington disease; PD, Parkinson disease; SAH, subarachnoid haemorrhage; TMEV, Theiler murine encephalomyelitis virus; TNF, tumour necrosis factor; 5×FAD and TgCRND8 are both transgenic mouse models of AD that develop severe amyloid pathology.
Other neurodegenerative diseases.
In addition to a potential pathogenic role in AD, fibrinogen may also contribute to the pathogenesis of other neurodegenerative diseases with vascular dysfunction (TABLE 1). Indeed, BBB alterations have been reported in patients with frontotemporal dementia127,128, ALS129,130, Parkinson disease131–133 and Huntington disease25. Similarly, acute traumatic injuries to the nervous system result in BBB breakdown, inflammation and neurodegeneration134,135. Of interest, some patients with traumatic brain injury display continued disruption of the BBB for months or even years after the initial insult136–138. Chronic disruption of the BBB, as seen in transgenic animals with deficient endothelial transport or aberrant pericyte signalling, is associated with increased perivascular deposition of fibrin and progressive neurodegeneration109,112. Alterations in the expression of endothelial tight-junction proteins and/or enhanced bulk-flow transcytosis may both contribute to the accumulation of CNS fibrin deposits in these animals109,112,139. Fibrin deposition precedes neuronal degenerative changes and behavioural deficits in pericyte-mutant mice112, suggesting that fibrin entry into the CNS is a critical factor that initiates or potentiates neurodegenerative processes after vascular disruption. Unlike animal models of MS, AD and traumatic injury7, pericyte-deficient mice lack a significant neuroinflammatory response until late in the disease course after considerable neuronal loss is already established112. Therefore, fibrin and/or fibrinogen may have direct effects on CNS cells, such as neurons, oligodendrocytes and pericytes, that promote neurodegenerative and cognitive changes independent from and/or synergistic with the pathology induced by fibrin-mediated inflammation. Studies with genetic or pharmacological manipulation of fibrinogen in pericyte-deficient mice and other neurodegenerative disease models are needed to explore the potential causative role of fibrinogen in these models and to determine the neuronal, oligodendrocyte and pericyte responses to fibrinogen that may contribute to neurodegeneration after chronic BBB disruption (see note added in proof).
Regeneration and repair
Disruption of the BBB and fibrinogen deposition in the CNS affects not only underlying inflammation and neurodegeneration but also the regenerative capacity of the injured tissue. A growing body of work demonstrates that fibrinogen is a key extrinsic inhibitor of nervous system repair by regulating growth factor receptor signalling and inflammatory responses after CNS injury and disease. Fibrinogen activates bone morphogenetic protein (BMP), TGFβ and epidermal growth factor receptor (EGFR) signalling in OPCs, astrocytes and neurons, respectively, and influences the polarization of microglia and macrophages to promote inflammation and inhibit regeneration. As such, fibrinogen plays a critical role in the inhibition of remyelination, impairment of neuronal regeneration and formation of the glial scar and serves as a critical environmental signal that transactivates signalling pathways that limit nervous system repair (FIG. 4).
Inhibition of remyelination.
After demyelinating injury in the CNS, myelin sheaths can be regenerated from OPCs, which are recruited to sites of damage and differentiate into myelin-producing oligodendrocytes in a process called remyelination140. Remyelination is critical for proper axonal function and recovery after injury but often fails in a number of neurological diseases141. Petersen et al. showed that fibrinogen is a key component of the CNS lesion microenvironment that inhibits remyelination after vascular damage142. Fibrinogen activates BMP receptor signalling in OPCs142, an important developmental pathway that regulates oligodendrocyte regeneration after injury143. The BMP–TGFβ superfamily is an evolutionarily conserved set of ligands, serine/threonine kinase cell surface receptors and intracellular signalling cascades, including the canonical SMAD pathway, that together control basic cellular functions, such as proliferation, survival and differentiation144. BMP signalling coordinates processes throughout the body, from developmental patterning and organ formation to tissue homeostasis and repair, often through the regulation of stem cells145. Indeed, the BMP–TGFβ superfamily controls the survival and cell fate determination of many stem cell and progenitor cell populations throughout the body, including embryonic, mesenchymal, neural and cancer stem cells145,146. In CNS development, BMP signalling suppresses oligodendrogenesis and promotes astrogliogenesis from neural stem cells147,148. After demyelinating CNS injury, Petersen et al. found that fibrinogen is an extrinsic activator of BMP receptor signalling and inhibits OPC differentiation into myelinating oligodendrocytes while promoting an astrocyte-like cell fate142. Fibrinogen activates the BMP receptor activin A receptor type I (ACVR1) and downstream BMP-specific SMAD proteins in OPCs independent of free BMP ligands to inhibit myelin production142. In addition, fibrin induces M1-like activation of microglia and macrophages9,75, which can be toxic to OPCs and impair remyelination149–151. Indeed, conditioned medium from fibrin-treated macrophages also inhibits OPC differentiation142, indicating that fibrin and/or fibrinogen may inhibit remyelination through both immune and non-immune mechanisms (FIG. 4). In a demyelinating injury model, therapeutic depletion of fibrinogen decreases BMP pathway activation, increases the number of mature oligodendrocytes within lesions and enhances remyelination142. Therefore, fibrinogen is a newly recognized blood-derived regulator of BMP receptor signalling and pro-inflammatory innate immune responses that changes the molecular composition of the stem cell niche after vascular disruption to direct stem cell fate and function152,153. Fibrinogen-induced signalling pathways may be important for neurological diseases in which remyelination fails, such as MS, neonatal brain injury and stroke, and in conditions with aberrant cell fate determination or disrupted repair, such as brain and spinal cord injury, cancer, cardiovascular disease and fibrosis.
Fibrinogen’s inhibitory role in remyelination is not limited to the CNS. After peripheral nerve injury, fibrinogen infiltrates the nerve through a disrupted blood–nerve barrier and is deposited as fibrin13,154,155. Mice deficient in tPA and/or plasminogen, which have an impaired ability to degrade fibrin, display increased fibrin deposition and exacerbation of axonal degeneration after experimental sciatic nerve crush injury154. Genetic or pharmacological depletion of fibrinogen or exogenous administration of tPA attenuates this injury and promotes regeneration and functional recovery, revealing an important role for fibrin in peripheral nerve damage and repair13,154,156. Fibrin-activated signalling pathways in Schwann cells play a central role in inhibiting nerve regeneration by regulating Schwann cell migration and remyelination13,157. Fibrin induces phosphorylation of extracellular signal-regulated kinase 1 (ERK1; also known as MAPK3) and ERK2 and production of NGFR in Schwann cells and maintains them in a proliferating, nonmyelinating state13. Genetic or pharmacological depletion of fibrinogen after experimental sciatic nerve injury enhances peripheral nerve remyelination through a faster transition of the Schwann cells to a myelinating state13. Although through different signalling pathways, fibrinogen impairs the formation of mature myelinating cells in both the peripheral nervous system and CNS to impair remyelination.
Inhibition of neurite outgrowth.
In addition to its effect on remyelination, fibrinogen also regulates growth factor receptor signalling in neurons and astrocytes to inhibit neurite outgrowth and induce glial scar formation, two key features of CNS pathology that limit regeneration12,158. Fibrinogen inhibits neurite outgrowth by binding the αVβ3 integrin and transactivating the EGFR in neurons, producing an inhibition of neurite outgrowth as robust as myelin158. In agreement with these findings, three different in vivo models of spinal cord injury demonstrate extensive fibrinogen deposition in the CNS that spatially correlates with axonal damage and phosphorylated EGFR158. Fibrinogen also inhibits axonal regeneration indirectly by inducing astrocytosis and stimulating the production of inhibitory proteoglycans that form the glial scar. In vitro, fibrinogen-bound latent TGFβ is activated by primary astrocytes and stimulates the production of neurocan, a potent inhibitor of neurite outgrowth12. In vivo, fibrinogen-bound latent TGFβ circulating in the blood enters the CNS after BBB disruption and interacts with perivascular astrocytes to form active TGFβ. Mice with genetic or pharmacological depletion of fibrinogen display significantly reduced levels of active TGFβ and dramatic reductions in astrocytosis and glial scar formation after spinal cord injury as compared with control mice12. These studies again highlight fibrinogen’s role as an extracellular regulator of growth factor receptor signalling and a critical molecular link between vascular disruption and failure of CNS regeneration. Given its pleiotropic functions, fibrinogen may be an apical signal that orchestrates the molecular and cellular composition of the CNS after vascular damage. As such, the persistence of fibrinogen in the CNS may be an important indicator of a dysfunctional injury response, and therapeutic strategies that target fibrinogen may tip the balance from a dysregulated environment, which often occurs in CNS diseases with BBB disruption, to one that promotes regeneration and repair.
Fibrinogen pathway as a biomarker
Immunohistochemical detection of fibrin deposition has been extensively used as a marker of BBB dysfunction in diverse CNS pathological conditions5,6,34. Fibrin deposition in the CNS has several advantages as a marker of BBB dysfunction and CNS pathology. First, fibrin deposition is an early event in CNS disease that often precedes demyelination or neuronal loss. Second, fibrin deposition is sustained in many CNS lesions; therefore, it can be used to identify sites of BBB dysfunction even after the barrier is restored. Finally, fibrin deposition is specific among blood proteins as a driver of cellular injury responses; therefore, it is not only a marker of BBB disruption but also a marker of CNS pathology. Therefore, detection of fibrin in the CNS provides several advantages over other molecular tracers or contrast agents that detect only active BBB leakage at the time of administration. Fibrin detection has the potential to become a valuable diagnostic tool in human CNS disease but requires advancing from static, immunohistochemical detection of fibrin in post-mortem tissues to real-time, in vivo monitoring of fibrin formation in animals and humans.
Conjugated molecular probes have been developed to specifically image fibrin and/or fibrinogen in vivo. Anti-fibrin antibodies or fibrin and/or fibrinogen itself has been fluorescently labelled, injected into mice and imaged in real time using confocal microscopy or 2pLSM to study the mechanisms and kinetics of fibrin clearance from the vasculature and tissue41,159,160. Taking advantage of the high subcellular resolution (<1 μm) of 2pLSM, Davalos et al. used intravenous injections of fluorescently labelled human fibrinogen to monitor BBB disruption and fibrin deposition in the spinal cord of living mice during the course of EAE9. The parenchymal fibrin deposition in the CNS correlated with perivascular microglial clustering, formation of ROS and axonal damage, highlighting the potential use of fibrin-specific probes to identify areas of pathology9. Tsai et al. developed a novel fibrin-affinity peptide coupled to a fluorescent near-infrared dye to detect endogenous fibrin deposition in mice using noninvasive whole-body scans161. To date, fibrin-specific probes that can be imaged noninvasively with computed tomography (CT)162, MRI163 or positron emission tomography (PET)164 have been primarily used to monitor intravascular thrombosis in animal models of cardiovascular disease or stroke. Although these fibrin-specific peptides can identify large clots in the vasculature, it is unknown whether these probes are sensitive enough to detect perivascular fibrin deposition in the brain parenchyma after BBB disruption.
An alternative to directly imaging fibrin in the CNS is to utilize probes that can monitor the activity of upstream components of the coagulation cascade (FIG. 5a). The serine protease thrombin catalyses the conversion of fibrinogen into insoluble fibrin. Thrombin-specific activatable cell-penetrating peptides (ACPPs) deliver fluorescent and MRI agents specifically to areas of high thrombin activity, such as acute blood clots, atherosclerotic plaques and ischaemic areas of the brain165–167. A thrombin-specific ACPP has also been used to detect coagulation activity in EAE in vivo168. In EAE, thrombin activation is an early event that precedes demyelination, correlates with disease progression and is strongly associated with BBB disruption, microglial activation and axonal damage168. The cellular uptake of the ACPP allows concentration of the probe specifically at sites of increased protease activity; therefore, ACPP-based probes may improve the sensitivity of CNS lesion detection compared with passively diffusing contrast agents. In the future, thrombin-cleavable probes may provide a sensitive and highly specific tool to enable noninvasive imaging of CNS lesions and perhaps to predict the appearance of newly demyelinating foci in diseases, such as MS.
In addition to its utility as an imaging biomarker, fibrinogen and components of the coagulation cascade may serve as valuable blood and cerebrospinal fluid (CSF) biomarkers to diagnose and track neurological disease progression (FIG. 5b). Indeed, an elevated plasma fibrinogen level is a recognized risk factor for developing AD106,107. Several proteomic studies in AD identify plasma or CSF fibrinogen as a useful marker of disease severity and progression169–173 that correlates with neo-cortical amyloid burden174. Proteomics also revealed elevated fibrin and/or fibrinogen degradation products in the CSF of patients with traumatic brain injury175. High plasma fibrinogen levels can also be found in MS and the related inflammatory demyelinating disease neuromyelitis optica and correlate with disease severity and the presence of active lesions on MRI176. Furthermore, blood levels of the coagulation factors prothrombin and factor X are significantly higher in relapsing–remitting and secondary progressive MS patients than in healthy donors177, revealing that additional upstream components of the coagulation cascade may also serve as valuable biomarkers for MS. Indeed, FXII activity is elevated in the plasma of patients with MS during relapse178. Elevated plasma FXII is also true for AD, as shown by increased activation of the contact system, an upstream FXII-mediated cascade that drives fibrin formation through the intrinsic coagulation pathway179,180. Zamolodchikov et al. demonstrated that markers of contact system activation, including increased levels of FXIIa, high-molecular-weight kininogen cleavage and kallikrein activity, are detected in the plasma of patients with AD but not in plasma from control subjects without dementia179. Increased levels of fibrinogen in the CSF of patients with major depressive disorder were associated with white matter tract abnormalities identified by diffusion tensor imaging181. These studies highlight dysfunctional coagulation as a common thread among diverse CNS diseases with neurovascular abnormalities. As such, imaging and fluid biomarkers of coagulation may provide a valuable tool to enhance disease detection and monitoring in a number of CNS pathologies. These studies also need to be interpreted with caution because fibrinogen is an acute phase reactant.
Testing the ‘fibrin hypothesis’
Studies in neurological diseases and animal models coupled with the pleiotropic functions of fibrinogen in the CNS have set the stage for a new hypothesis for the genesis and progression of neurological diseases by proposing fibrinogen as a new molecular player that can promote and amplify neuroimmune and neurodegenerative processes. Fibrinogen is abundantly detected in the human brain in MS, AD and trauma, and animal studies support that fibrinogen is necessary and sufficient for the induction of neuroinflammatory processes. Findings in humans and animal studies coupled with the capacity of fibrinogen and fibrin to engage multiple receptors and signalling pathways in brain cells have the potential to launch a field to test the fibrin hypothesis in neurological diseases.
Four key questions would need to be addressed to further elucidate the role of fibrinogen in the CNS and its potential as a target for therapeutic intervention (FIG. 5b). First, how are the pleiotropic effects of fibrinogen integrated during the disease course to affect inflammation, degeneration and repair? To date, fibrinogen mutants have been generated only to inhibit the interaction of fibrinogen with CD11b/CD18. Expanding the ‘fibrinogen toolbox’ of genetic models and pharmacological agents and making these widely available to the neuroscience community would be required to validate the relative contributions of other cellular receptors and the in vivo contribution of its multiple cellular targets. Because cells are exposed simultaneously to multiple inflammatory and vascular-derived stimulators, studies using comparative multi-omics approaches would be essential to elucidate how CNS immune cells integrate signals at sites of BBB disruption to regulate neuroinflammation, neurodegeneration and repair. Second, what are the cellular targets and underlying molecular mechanisms of the actions of fibrinogen in the CNS? In 2002, Akassoglou and Strickland proposed that cells in the nervous tissue are equipped with receptors and intracellular signalling pathways to mediate fibrin-induced cellular responses22. Indeed, in later years the identification of microglia, OPCs, neurons, astrocytes, brain endothelial cells and Schwann cells as cellular targets for fibrinogen changed our understanding of fibrinogen from a marker of BBB disruption to an active player in nervous system pathogenesis (FIGS. 2–4). The effects of fibrinogen on other brain cells, such as mature oligodendrocytes, pericytes, different neuronal subtypes and neural progenitor cells, are now starting to be revealed (see note added in proof). Furthermore, how fibrinogen influences neuron–glia communication remains poorly understood. Studies to determine the cellular and molecular mechanisms of fibrinogen and fibrin in the CNS would be critical for the discovery of new mechanisms that link BBB dysfunction with neurological disease. Third, does fibrinogen contribute to disease pathogenesis in neurological diseases with BBB disruption other than MS and AD? If so, what is the magnitude of its pathogenic impact in these conditions or disease subtypes relative to other pathogenic factors? Does fibrinogen contribute to different conditions through the same key mechanisms or through a broad range of mechanisms that differ among diseases? Genetic and pharmacological tools have demonstrated a causal role for fibrinogen in MS and AD animal models (FIG. 3). These studies could serve as a roadmap for testing whether fibrinogen plays a role in animal models of trauma, neurodegeneration and even neuropsychiatric disorders. Indeed, emerging evidence suggests increased BBB leakage in psychiatric disorders such as schizophrenia, bipolar disorder and psychosis170,182,183. The fibrinogen toolbox (FIG. 1b) could be employed to test the role of fibrinogen across several animal models of disease with increased BBB permeability, including brain trauma, spinal cord injury, subarachnoid haemorrhage, cerebral malaria, epilepsy, pericyte-deficient mice and brain tumours (FIG. 5b; TABLE 1). Such studies have the potential to identify fibrinogen-induced neuropathology as a common thread among several neurological diseases and psychiatric conditions and help predict the potential usefulness, impact and market for individual fibrinogen-targeting treatment strategies. Finally, can we translate fibrin and/or fibrinogen therapeutics to clinical practice for neurological diseases? Potential pharmacological agents to block fibrinogen’s effects in the CNS include anticoagulants, drugs that enhance fibrin degradation, agents that protect the BBB to limit fibrin entrance in the CNS and selective inhibitors of the interactions of fibrinogen and fibrin with its CNS receptors. The benefits of anticoagulants in multiple animal models of neurological disease are well established (FIG. 3). Several US Food and Drug Administration (FDA)-approved drugs, including new oral anticoagulants, can effectively reduce fibrin formation and, thus, may be of clinical interest in the treatment of neurological diseases with chronic or repeated opening of the BBB. Similarly, thrombolytic agents such as tPA that promote fibrin degradation are widely used for the treatment of stroke. However, the potential haemorrhagic complications of fibrin depletion may limit its widespread clinical use for chronic CNS disease. In addition, these drugs do not deplete fibrinogen, which also exerts pathogenic effects in the CNS.
Drugs that protect the BBB could potentially limit fibrinogen entrance in the CNS. For example, activated protein C, which promotes endothelial barrier integrity, prevents accumulation of blood-derived products in the CNS and attenuates neurodegeneration in ALS-model mice184. Similarly, blocking the pro-inflammatory cyclophilin A pathway stabilizes the BBB in APOE transgenic mice and decreases CNS fibrin deposition and secondary neurodegenerative changes114. In EAE mice, upregulation of tight-junction molecules in endothelial cells by a protein kinase Cβ inhibitor protects the BBB and attenuates inflammation, demyelination and axonal damage. Interestingly, many disease-modifying drugs currently in clinical use for MS have BBB-stabilizing properties185. In addition to their immunomodulatory effects, interferon-β186, dimethyl fumarate187, fingolimod188 and laquinimod189 all stabilize the BBB, often through actions on endothelial tight-junction proteins. For example, laquinimod increases expression of tight-junction proteins p120 and the tight-junction protein zonula occludens 1 in human brain endothelial cells and suppresses migration of T lymphocytes across the BBB189. Statins, which are commonly used for the treatment of dyslipidaemias, also reduce BBB permeability190 and showed promise in the treatment of progressive MS patients191. A caveat of BBB-protective agents could be that if they are administered therapeutically after the initial opening of the BBB, they will not target fibrin already deposited in the CNS. Fibrin is an insoluble substrate that appears to not be easily degraded in the brain. Indeed, there is abundant fibrin deposition in the cortex in progressive MS66, which is considered to have significantly less BBB leakage than white matter MS lesions. In this case, further protection of the BBB would not be efficacious in removing or neutralizing fibrin already deposited in the CNS.
Targeting the interactions of fibrin with CD11b has proved a promising strategy in animal models to selectively inhibit the pathological effects of fibrin in the CNS while preserving its beneficial effects on blood clotting. The fibrin γ377–395 peptide inhibits the interaction of fibrin with the CD11b-I domain of CD11b/CD18 and inhibits fibrin-induced microglia activation in vitro and in vivo8,76. Therapeutic intranasal administration of the fibrin γ377–395 peptide protects mice from EAE by suppressing paralysis, relapses, demyelination and neuroinflammation8. Vaccination of EAE mice with the γ377–395 peptide also significantly protects from neurological disease8. In addition, intranasal administration of the γ377–395 peptide reduces pathology in an AD animal model124. In vivo administration of the γ377–395 peptide does not affect blood coagulation, suggesting that pharmaco-logical tools, such as monoclonal antibodies against the γ377–395 fibrin epitope, can be developed to selectively target the pro-inflammatory effects of fibrin without affecting its beneficial role in haemostasis8. Fibrin–CD11b inhibitors have not yet been evaluated in human clinical trials.
Targeting the interactions of fibrin with Aβ has shown efficacy in an AD model111. Fibrinogen and fibrin bind Aβ to form clots that are more resistant to degradation than normal clots11,120,122. These insoluble fibrin–Aβ complexes are deposited in the blood vessels and perivascular spaces in AD patients and mice11,120,122 and potentially contribute to impaired blood flow and chronic fibrin-induced inflammation. In addition, Aβ activates FXII, an upstream component of the intrinsic coagulation cascade, to further promote fibrin formation192. Activated FXII also increases inflammation through the kallikrein–kininogen–bradykinin pathway193. Therefore, the fibrin–Aβ interaction may form a feedforward loop that drives aberrant vascular and inflammatory processes in the CNS that contribute to neurodegeneration in AD. Indeed, Ahn et al. discovered that a small molecule, RU-505, which blocks the fibrin–Aβ interaction, significantly reduces vascular pathology, inhibits neuroinflammation and protects from cognitive decline in AD models111. FXII depletion also reduces inflammation and neuropathology in AD mice125. Therefore, blocking fibrin’s interaction with Aβ may break the cycle of fibrin deposition and inflammation that may potentially contribute to AD progression. Fibrin–Aβ inhibitors have not yet been evaluated in human clinical trials.
Development of novel fibrin therapeutics would pave the way for targeting blood-induced inflammation and toxicity in the CNS. A fibrin therapeutics pipeline could include selective targeting of fibrin domains with known deleterious functions in the CNS, structure-guided drug design and unbiased high-throughput chemical and genomic screens of fibrin-stimulated nervous system cells. The therapeutic effects in animal models of targeting fibrin interactions with the CD11b-I domain or with Aβ suggest that it is possible to develop pharmacological tools to target fibrin–protein interactions selectively in the CNS. An advantage of this approach is the preservation of the beneficial clotting functions of fibrinogen. The binding sites of fibrinogen to CNS targets do not overlap with its coagulation sites, giving the opportunity to develop selective CNS pharmacological inhibitors for fibrin and fibrinogen. Biochemistry studies to further identify fibrin and/or fibrinogen receptors and binding proteins in the CNS and map specific binding sites on fibrinogen would provide the basis for drug discovery. Crystallography studies of the complexes of fibrinogen with its CNS targets could also be instrumental in characterization of the interactions of fibrin with its receptors and binding proteins to enable structure-guided drug design. Finally, unbiased small-molecule, RNAi and CRISPR–Cas9 screens can be developed to identify inhibitors for intracellular pathways induced by fibrin activation of nervous system cells. Chemical screens in the presence of extrinsic regulators of nervous system pathology, such as fibrin, could enable the discovery of compounds that can overcome the toxic lesion environment in vivo. Chemical and genetic screens can also be performed in human nervous system cells isolated from patients to further enhance the translational potential of these approaches. Regardless of the therapeutic approach utilized to target fibrinogen and fibrin, patient stratification could facilitate clinical development. Fluid biomarkers of the coagulation cascade, noninvasive fibrin imaging tools to identify patients with fibrin deposits in the CNS and metagenome-wide association studies to test whether there are any potential genetic links with coagulation and/or fibrinolysis genes could be instrumental for the translation of proof-of-principle fibrin drugs into novel therapies for neurological diseases.
Acknowledgements
The authors are grateful to L. Mucke and D. Reich for critical reading of the manuscript, T. Roberts and J. Carroll for graphics and G. Howard for editorial assistance. The authors are supported by the US National Institutes of Health (NIH), National Institute of Child Health and Human Development (NICHD) K12-HD072222 grant and a Pediatric Scientist Development Program fellowship (supported by March of Dimes 4-FY10-461 and NIH/NICHD K12-HD000850) to M.A.P, a Race to Erase MS Young Investigator Award and American Heart Association Scientist Development grant to J.K.R and the National Multiple Sclerosis Society grant RG4985, NIH/NINDS grant R35 NS097976, the Conrad N. Hilton Foundation grant and US Department of Defense MS160082 grant to K.A.
Footnotes
Note added in proof
A recent study published after this review was accepted added pericyte-deficient mice as another animal model with BBB abnormalities protected from genetic or pharmacologic depletion of fibrinogen and reported effects of fibrinogen on pericytes and mature oligodendrocytes209.
Competing interests
K.A. is a co-founder of MedaRed. K.A. and J.K.R. are named inventors in patents and patent applications. Their interests are managed by the Gladstone Institutes in accordance with its conflict of interest policy.
Literature Cited
- 1.Zhang B et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 153, 707–720 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vemuri P et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 138, 761–771 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonsson T et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med 368, 107–116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerreiro R et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med 368, 117–127 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlokovic BV The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Adams RA, Schachtrup C, Davalos D, Tsigelny I & Akassoglou K Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: lessons from multiple sclerosis. Curr. Med. Chem 14, 2925–2936 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Davalos D & Akassoglou K Fibrinogen as a key regulator of inflammation in disease. Semin. Immunopathol 34, 43–62 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Adams RA et al. The fibrin-derived γ77–395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J. Exp. Med 204, 571–582 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davalos D et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat. Commun 3, 1227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul J, Strickland S & Melchor JP Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. J. Exp. Med 204, 1999–2008 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes-Canteli M et al. Fibrinogen and β-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer’s disease. Neuron 66, 695–709 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schachtrup C et al. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-β after vascular damage. J. Neurosci 30, 5843–5854 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akassoglou K, Yu WM, Akpinar P & Strickland S Fibrin inhibits peripheral nerve remyelination by regulating Schwann cell differentiation. Neuron 33, 861–875 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Liebner S, Czupalla CJ & Wolburg H Current concepts of blood-brain barrier development. Int. J. Dev. Biol 55, 467–476 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Iadecola C The Neurovascular Unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96, 17–42 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zlokovic BV Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci 12, 723–738 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tietz S & Engelhardt B Brain barriers: crosstalk between complex tight junctions and adherens junctions. J. Cell Biol 209, 493–506 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knowland D et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood–brain barrier breakdown in stroke. Neuron 82, 603–617 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baeten KM & Akassoglou K Extracellular matrix and matrix receptors in blood–brain barrier formation and stroke. Dev. Neurobiol 71, 1018–1039 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Z, Nelson AR, Betsholtz C & Zlokovic BV Establishment and dysfunction of the blood–brain barrier. Cell 163, 1064–1078 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas WS et al. Tissue factor contributes to microvascular defects after focal cerebral ischemia. Stroke 24, 847–853 (1993). [DOI] [PubMed] [Google Scholar]
- 22.Akassoglou K & Strickland S Nervous system pathology: the fibrin perspective. Biol. Chem 383, 37–45 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Abbott NJ, Ronnback L & Hansson E Astrocyte-endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci 7, 41–53 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Adams RA, Passino M, Sachs BD, Nuriel T & Akassoglou K Fibrin mechanisms and functions in nervous system pathology. Mol. Interv 4, 163–176 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Drouin-Ouellet J et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: potential implications for its pathophysiology. Ann. Neurol 78, 160–177 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Tennent GA et al. Human plasma fibrinogen is synthesized in the liver. Blood 109, 1971–1974 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Weisel JW Fibrinogen and fibrin. Adv. Protein Chem 70, 247–299 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Lord ST Molecular mechanisms affecting fibrin structure and stability. Arterioscler Thromb. Vasc. Biol 31, 494–499 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosesson MW Fibrinogen and fibrin structure and functions. J. Thromb. Haemost 3, 1894–1904 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, Mochalkin I & Doolittle RF A model of fibrin formation based on crystal structures of fibrinogen and fibrin fragments complexed with synthetic peptides. Proc. Natl Acad. Sci. USA 97, 14156–14161 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmback K, Danton M, Suh T, Daugherty C & Degen J Impaired platelet aggregation and sustained bleeding in mice lacking the fibrinogen motif bound by integrin αIIb β3. EMBO J 15, 5760–5771 (1996). [PMC free article] [PubMed] [Google Scholar]
- 32.Rooney MM, Parise LV & Lord ST Dissecting clot retraction and platelet aggregation. Clot retraction does not require an intact fibrinogen γ chain C terminus. J. Biol. Chem 271, 8553–8555 (1996). [DOI] [PubMed] [Google Scholar]
- 33.Castellino FJ & Ploplis VA Structure and function of the plasminogen/plasmin system. Thromb. Haemost 93, 647–654 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Bardehle S, Rafalski VA & Akassoglou K Breaking boundaries-coagulation and fibrinolysis at the neuro-vascular interface. Front. Cell Neurosci 9, 354 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osterwalder T et al. The axonally secreted serine proteinase inhibitor, neuroserpin, inhibits plasminogen activators and plasmin but not thrombin. J. Biol. Chem 273, 2312–2321 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Sachs BD et al. p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J. Cell Biol 177, 1119–1132 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurens N, Koolwijk P & de Maat MP Fibrin structure and wound healing. J. Thromb. Haemost 4, 932–939 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Chan JC, Duszczyszyn DA, Castellino FJ & Ploplis VA Accelerated skin wound healing in plasminogen activator inhibitor-1-deficient mice. Am. J. Pathol 159, 1681–1688 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bugge TH et al. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell 87, 709–719 (1996). [DOI] [PubMed] [Google Scholar]
- 40.Romer J et al. Impaired wound healing in mice with a disrupted plasminogen gene. Nat. Med 2, 287–292 (1996). [DOI] [PubMed] [Google Scholar]
- 41.Motley MP et al. A CCR2 macrophage endocytic pathway mediates extravascular fibrin clearance in vivo. Blood 127, 1085–1096 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doolittle RF A detailed consideration of a principal domain of vertebrate fibrinogen and its relatives. Protein Sci 1, 1563–1577 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doolittle RF, McNamara K & Lin K Correlating structure and function during the evolution of fibrinogen-related domains. Protein Sci 21, 1808–1823 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsushita M et al. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J. Biol. Chem 271, 2448–2454 (1996). [DOI] [PubMed] [Google Scholar]
- 45.Marazzi S et al. Characterization of human fibroleukin, a fibrinogen-like protein secreted by T lymphocytes. J. Immunol 161, 138–147 (1998). [PubMed] [Google Scholar]
- 46.Chiquet-Ehrismann R & Tucker RP Tenascins and the importance of adhesion modulation. Cold Spring Harb. Perspect. Biol 3, a004960 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanington PC & Zhang SM The primary role of fibrinogen-related proteins in invertebrates is defense, not coagulation. J. Innate Immun 3, 17–27 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powell PA, Wesley C, Spencer S & Cagan RL Scabrous complexes with Notch to mediate boundary formation. Nature 409, 626–630 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Lassmann H Multiple sclerosis pathology: evolution of pathogenetic concepts. Brain Pathol 15, 217–222 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lassmann H, Bruck W & Lucchinetti C Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol. Med 7, 115–121 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Lucchinetti CF, Brueck W, Rodriguez M & Lassmann H Multiple sclerosis: lessons from neuropathology. Semin. Neurol 18, 337–349 (1998). [DOI] [PubMed] [Google Scholar]
- 52.Rindficisch E Histologisches detail zu der grauen degeneration von gehirn und ruckenmark. Arch. Pathol. Anat. Physiol 26, 474–483 (1863). [Google Scholar]
- 53.Reich DS, Lucchinetti CF & Calabresi PA Multiple sclerosis. N. Engl. J. Med 378, 169–180 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grossman RI et al. Multiple sclerosis: serial study of gadolinium-enhanced MR imaging. Radiology 169, 117–122 (1988). [DOI] [PubMed] [Google Scholar]
- 55.Miller DH et al. Serial gadolinium enhanced magnetic resonance imaging in multiple sclerosis. Brain 111, 927–939 (1988). [DOI] [PubMed] [Google Scholar]
- 56.Kermode AG et al. Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenetic and clinical implications. Brain 113, 1477–1489 (1990). [DOI] [PubMed] [Google Scholar]
- 57.Cotton F, Weiner HL, Jolesz FA & Guttmann CR MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology 60, 640–646 (2003). [DOI] [PubMed] [Google Scholar]
- 58.Gaitan MI, Sati P, Inati SJ & Reich DS Initial investigation of the blood-brain barrier in MS lesions at 7 tesla. Mult. Scler 19, 1068–1073 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaitan MI et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Ann. Neurol 70, 22–29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruck W et al. Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann. Neurol 42, 783–793 (1997). [DOI] [PubMed] [Google Scholar]
- 61.Katz D et al. Correlation between magnetic resonance imaging findings and lesion development in chronic, active multiple sclerosis. Ann. Neurol 34, 661–669 (1993). [DOI] [PubMed] [Google Scholar]
- 62.Vos CM et al. Blood-brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiol. Dis 20, 953–960 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Kirk J, Plumb J, Mirakhur M & McQuaid S Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. J. Pathol 201, 319–327 (2003). [DOI] [PubMed] [Google Scholar]
- 64.Kwon EE & Prineas JW Blood-brain barrier abnormalities in longstanding multiple sclerosis lesions. An immunohistochemical study. J. Neuropathol. Exp. Neurol 53, 625–636 (1994). [DOI] [PubMed] [Google Scholar]
- 65.Han MH et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 451, 1076–1081 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Yates RL et al. Fibrin(ogen) and neurodegeneration in the progressive multiple sclerosis cortex. Ann. Neurol 82, 259–270 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Gveric D et al. Plasminogen activators in multiple sclerosis lesions: implications for the inflammatory response and axonal damage. Brain 124, 1978–1988 (2001). [DOI] [PubMed] [Google Scholar]
- 68.Gveric D, H. B, Petzold A, Lawrence DA & Cuzner ML Impaired fibrinolysis in multiple sclerosis: a role for tissue plasminogen activator inhibitors. Brain 126, 1–9 (2003). [DOI] [PubMed] [Google Scholar]
- 69.Marik C, Felts PA, Bauer J, Lassmann H & Smith KJ Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain 130, 2800–2815 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gay FW, Drye TJ, Dick GW & Esiri MM The application of multifactorial cluster analysis in the staging of plaques in early multiple sclerosis. Identification and characterization of the primary demyelinating lesion. Brain 120, 1461–1483 (1997). [DOI] [PubMed] [Google Scholar]
- 71.Wakefield AJ, More LJ, Difford J & McLaughlin JE Immunohistochemical study of vascular injury in acute multiple sclerosis. J. Clin. Pathol 47, 129–133 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adams RD & Kubik CS The morbid anatomy of the demyelinative disease. Am. J. Med 12, 510–546 (1952). [DOI] [PubMed] [Google Scholar]
- 73.Barnett MH & Prineas JW Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann. Neurol 55, 458–468 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Maggi P et al. The formation of inflammatory demyelinated lesions in cerebral white matter. Ann. Neurol 76, 594–608 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ryu JK et al. Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat. Commun 6, 8164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ugarova TP et al. Sequence γ377–395(P2), but not γ190–202(P1), is the binding site for the αMI-domain of integrin αMβ2 in the γC-domain of fibrinogen. Biochemistry 42, 9365–9373 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Lishko VK, Kudryk B, Yakubenko VP, Yee VC & Ugarova TP Regulated unmasking of the cryptic binding site for integrin αMβ2 in the γC-domain of fibrinogen. Biochemistry 41, 12942–12951 (2002). [DOI] [PubMed] [Google Scholar]
- 78.Smiley ST, King JA & Hancock WW Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J. Immunol 167, 2887–2894 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Millien VO et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science 341, 792–796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han C et al. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat. Immunol 11, 734–742 (2010). [DOI] [PubMed] [Google Scholar]
- 81.Perera PY et al. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J. Immunol 166, 574–581 (2001). [DOI] [PubMed] [Google Scholar]
- 82.Ling GS et al. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat. Commun 5, 3039 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noubir S, Hmama Z & Reiner NE Dual receptors and distinct pathways mediate interleukin-1 receptor-associated kinase degradation in response to lipopolysaccharide. Involvement of CD14/TLR4, CR3, and phosphatidylinositol 3-kinase. J. Biol. Chem 279, 25189–25195 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Hanspers K, Akassoglou K & Mendiola AS Fibrin complement receptor 3 signaling pathway (Homo sapiens) Wikipathways.org/Index.php/Pathway:WP4136 (2017).
- 85.Paterson PY Experimental allergic encephalomyelitis: role of fibrin deposition in immunopathogenesis of inflammation in rats. Fed. Proc 35, 2428–2434 (1976). [PubMed] [Google Scholar]
- 86.Akassoglou K et al. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc. Natl Acad. Sci. USA 101, 6698–6703 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang Y, Tian SJ, Wu L, Huang DH & Wu WP Fibrinogen depleting agent batroxobin has a beneficial effect on experimental autoimmune encephalomyelitis. Cell. Mol. Neurobiol 31, 437–448 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flick MJ et al. Leukocyte engagement of fibrin(ogen) via the integrin receptor αMβ2/Mac-1 is critical for host inflammatory response in vivo. J. Clin. Invest 113, 1596–1606 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flick MJ, Du X & Degen JL Fibrin(ogen)–αMβ2 interactions regulate leukocyte function and innate immunity in vivo. Exp. Biol. Med 229, 1105–1110 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Huang Y & Mucke L Alzheimer mechanisms and therapeutic strategies. Cell 148, 1204–1222 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iadecola C The pathobiology of vascular dementia. Neuron 80, 844–866 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schneider JA, Arvanitakis Z, Bang W & Bennett DA Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69, 2197–2204 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA & Schneider JA Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol 15, 934–943 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bowman GL et al. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology 68, 1809–1814 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ujiie M, Dickstein DL, Carlow DA & Jefferies WA Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation 10, 463–470 (2003). [DOI] [PubMed] [Google Scholar]
- 96.Scheltens P & Goos JD Dementia in 2011: microbleeds in dementia—singing a different ARIA. Nat. Rev. Neurol 8, 68–70 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Kirsch W et al. Serial susceptibility weighted MRI measures brain iron and microbleeds in dementia. J. Alzheimers Dis 17, 599–609 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cullen KM, Kocsi Z & Stone J Microvascular pathology in the aging human brain: evidence that senile plaques are sites of microhaemorrhages. Neurobiol. Aging 27, 1786–1796 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Merlini M & Akassoglou K Alzheimer disease makes new blood contacts. Blood 129, 2462–2463 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hultman K, Strickland S & Norris EH The APOE ε4/ε4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer’s disease patients. J. Cereb. Blood Flow Metab 33, 1251–1258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ryu JK & McLarnon JG A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J. Cell. Mol. Med 13, 2911–2925 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fiala M et al. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood-brain barrier. Eur. J. Clin. Invest 32, 360–371 (2002). [DOI] [PubMed] [Google Scholar]
- 103.Miners JS, Schulz I & Love S Differing associations between Aβ accumulation, hypoperfusion, blood-brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer’s disease. J. Cereb. Blood Flow Metab 38, 103–115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cortes-Canteli M, Mattei L, Richards AT, Norris EH & Strickland S Fibrin deposited in the Alzheimer’s disease brain promotes neuronal degeneration. Neurobiol. Aging 36, 608–617 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sengillo JD et al. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol 23, 303–310 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu G, Zhang H, Zhang S, Fan X & Liu X Plasma fibrinogen is associated with cognitive decline and risk for dementia in patients with mild cognitive impairment. Int. J. Clin. Pract 62, 1070–1075 (2008). [DOI] [PubMed] [Google Scholar]
- 107.van Oijen M, Witteman JC, Hofman A, Koudstaal PJ & Breteler MM Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke 36, 2637–2641 (2005). [DOI] [PubMed] [Google Scholar]
- 108.Montagne A, Zhao Z & Zlokovic BV Alzheimer’s disease: a matter of blood-brain barrier dysfunction? J. Exp. Med 214, 3151–3169 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Winkler EA et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci 18, 521–530 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McManus RM, Finucane OM, Wilk MM, Mills KHG & Lynch MA FTY720 attenuates infection-induced enhancement of Aβ accumulation in APP/PS1 mice by modulating astrocytic activation. J. Neuroimmune Pharmacol 12, 670–681 (2017). [DOI] [PubMed] [Google Scholar]
- 111.Ahn HJ et al. A novel Aβ-fibrinogen interaction inhibitor rescues altered thrombosis and cognitive decline in Alzheimer’s disease mice. J. Exp. Med 211, 1049–1062 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bell RD et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nikolakopoulou AM, Zhao Z, Montagne A & Zlokovic BV Regional early and progressive loss of brain pericytes but not vascular smooth muscle cells in adult mice with disrupted platelet-derived growth factor receptor-β signaling. PLOS ONE 12, e0176225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bell RD et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Soto I et al. APOE stabilization by exercise prevents aging neurovascular dysfunction and complement induction. PLOS Biol 13, e1002279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McLarnon JG & Ryu JK Relevance of Aβ1–42 intrahippocampal injection as an animal model of inflamed Alzheimer’s disease brain. Curr. Alzheimer Res 5, 475–480 (2008). [DOI] [PubMed] [Google Scholar]
- 117.Tripathy D et al. Thrombin, a mediator of cerebrovascular inflammation in AD and hypoxia. Front. Aging Neurosci 5, 19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Timmer NM et al. Enoxaparin treatment administered at both early and late stages of amyloid β deposition improves cognition of APPswe/PS1dE9 mice with differential effects on brain Aβ levels. Neurobiol. Dis 40, 340–347 (2010). [DOI] [PubMed] [Google Scholar]
- 119.Bergamaschini L et al. Peripheral treatment with enoxaparin, a low molecular weight heparin, reduces plaques and β-amyloid accumulation in a mouse model of Alzheimer’s disease. J. Neurosci 24, 4181–4186 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ahn HJ et al. Alzheimer’s disease peptide β-amyloid interacts with fibrinogen and induces its oligomerization. Proc. Natl Acad. Sci. USA 107, 21812–21817 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zamolodchikov D, Renne T & Strickland S The Alzheimer’s disease peptide β-amyloid promotes thrombin generation through activation of coagulation factor XII. J. Thromb. Haemost 14, 995–1007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zamolodchikov D & Strickland S Aβ delays fibrin clot lysis by altering fibrin structure and attenuating plasminogen binding to fibrin. Blood 119, 3342–3351 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oh SB et al. Tissue plasminogen activator arrests Alzheimer’s disease pathogenesis. Neurobiol. Aging 35, 511–519 (2014). [DOI] [PubMed] [Google Scholar]
- 124.Aso E, Serrano AL, Munoz-Canoves P & Ferrer I Fibrinogen-derived γ377–395 peptide improves cognitive performance and reduces amyloid-β deposition, without altering inflammation, in AβPP/PS1 mice. J. Alzheimers Dis 47, 403–412 (2015). [DOI] [PubMed] [Google Scholar]
- 125.Chen ZL et al. Depletion of coagulation factor XII ameliorates brain pathology and cognitive impairment in Alzheimer disease mice. Blood 129, 2547–2556 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bien-Ly N et al. Lack of widespread BBB disruption in Alzheimer’s disease models: focus on therapeutic antibodies. Neuron 88, 289–297 (2015). [DOI] [PubMed] [Google Scholar]
- 127.Sudre CH et al. White matter hyperintensities are seen only in GRN mutation carriers in the GENFI cohort. Neuroimage Clin 15, 171–180 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thal DR et al. Frontotemporal lobar degeneration FTLD-tau: preclinical lesions, vascular, and Alzheimer-related co-pathologies. J. Neural Transm. (Vienna) 122, 1007–1018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Winkler EA et al. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol 125, 111–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Evans MC, Couch Y, Sibson N & Turner MR Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol. Cell Neurosci 53, 34–41 (2013). [DOI] [PubMed] [Google Scholar]
- 131.Kortekaas R et al. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol 57, 176–179 (2005). [DOI] [PubMed] [Google Scholar]
- 132.Pisani V et al. Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson’s disease. J. Neuroinflamm 9, 188 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gray MT & Woulfe JM Striatal blood–brain barrier permeability in Parkinson’s disease. J. Cereb. Blood Flow Metab 35, 747–750 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shlosberg D, Benifla M, Kaufer D & Friedman A Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol 6, 393–403 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chodobski A, Zink BJ & Szmydynger-Chodobska J Blood–brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2, 492–516 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tomkins O et al. Blood–brain barrier disruption in post-traumatic epilepsy. J. Neurol. Neurosurg. Psychiatry 79, 774–777 (2008). [DOI] [PubMed] [Google Scholar]
- 137.Korn A, Golan H, Melamed I, Pascual-Marqui R & Friedman A Focal cortical dysfunction and blood-brain barrier disruption in patients with Postconcussion syndrome. J. Clin. Neurophysiol 22, 1–9 (2005). [DOI] [PubMed] [Google Scholar]
- 138.Hay JR, Johnson VE, Young AM, Smith DH & Stewart W Blood–brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans. J. Neuropathol. Exp. Neurol 74, 1147–1157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Armulik A et al. Pericytes regulate the blood–brain barrier. Nature 468, 557–561 (2010). [DOI] [PubMed] [Google Scholar]
- 140.Franklin RJ & Ffrench-Constant C Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci 9, 839–855 (2008). [DOI] [PubMed] [Google Scholar]
- 141.Fancy SP, Chan JR, Baranzini SE, Franklin RJ & Rowitch DH Myelin regeneration: a recapitulation of development? Annu. Rev. Neurosci 34, 21–43 (2011). [DOI] [PubMed] [Google Scholar]
- 142.Petersen MA et al. Fibrinogen activates BMP signaling in oligodendrocyte progenitor cells and inhibits remyelination after vascular damage. Neuron 96, 1003–1012.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gallo V & Deneen B Glial development: the crossroads of regeneration and repair in the CNS. Neuron 83, 283–308 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Salazar VS, Gamer LW & Rosen V BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol 12, 203–221 (2016). [DOI] [PubMed] [Google Scholar]
- 145.Wagner DO et al. BMPs: from bone to body morphogenetic proteins. Sci. Signal 3, mr1 (2010). [DOI] [PubMed] [Google Scholar]
- 146.Pera MF & Tam PP Extrinsic regulation of pluripotent stem cells. Nature 465, 713–720 (2010). [DOI] [PubMed] [Google Scholar]
- 147.See J et al. Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol. Cell Neurosci 26, 481–492 (2004). [DOI] [PubMed] [Google Scholar]
- 148.Gomes WA, Mehler MF & Kessler JA Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev. Biol 255, 164–177 (2003). [DOI] [PubMed] [Google Scholar]
- 149.Lehnardt S et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J. Neurosci 22, 2478–2486 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Back SA, Gan X, Li Y, Rosenberg PA & Volpe JJ Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J. Neurosci 18, 6241–6253 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oka A, Belliveau MJ, Rosenberg PA & Volpe JJ Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. J. Neurosci 13, 1441–1453 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Norris EH & Strickland S Fibrinogen in the nervous system: glia beware. Neuron 96, 951–953 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nave KA & Ehrenreich H A bloody brake on myelin repair. Nature 553, 31–32 (2018). [DOI] [PubMed] [Google Scholar]
- 154.Akassoglou K, Kombrinck KW, Degen JL & Strickland S Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury. J. Cell Biol 149, 1157–1166 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Previtali SC et al. The extracellular matrix affects axonal regeneration in peripheral neuropathies. Neurology 71, 322–331 (2008). [DOI] [PubMed] [Google Scholar]
- 156.Zou T et al. Exogenous tissue plasminogen activator enhances peripheral nerve regeneration and functional recovery after injury in mice. J. Neuropathol. Exp. Neurol 65, 78–86 (2006). [DOI] [PubMed] [Google Scholar]
- 157.Akassoglou K, Akpinar P, Murray S & Strickland S Fibrin is a regulator of Schwann cell migration after sciatic nerve injury in mice. Neurosci. Lett 338, 185–188 (2003). [DOI] [PubMed] [Google Scholar]
- 158.Schachtrup C et al. Fibrinogen inhibits neurite outgrowth via β3 integrin-mediated phosphorylation of the EGF receptor. Proc. Natl Acad. Sci. USA 104, 11814–11819 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lam CK, Yoo T, Hiner B, Liu Z & Grutzendler J Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature 465, 478–482 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Falati S, Gross P, Merrill-Skoloff G, Furie BC & Furie B Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat. Med 8, 1175–1181 (2002). [DOI] [PubMed] [Google Scholar]
- 161.Tsai YT et al. Optical imaging of fibrin deposition to elucidate participation of mast cells in foreign body responses. Biomaterials 35, 2089–2096 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim JY et al. Direct imaging of cerebral thromboemboli using computed tomography and fibrin-targeted gold nanoparticles. Theranostics 5, 1098–1114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Overoye-Chan K et al. EP-2104R: a fibrin-specific gadolinium-based MRI contrast agent for detection of thrombus. J. Am. Chem. Soc 130, 6025–6039 (2008). [DOI] [PubMed] [Google Scholar]
- 164.Blasi F et al. Multisite thrombus imaging and fibrin content estimation with a single whole-body PET scan in rats. Arterioscler Thromb. Vasc. Biol 35, 2114–2121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Olson ES et al. In vivo fluorescence imaging of atherosclerotic plaques with activatable cell-penetrating peptides targeting thrombin activity. Integr. Biol 4, 595–605 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen B et al. Thrombin activity associated with neuronal damage during acute focal ischemia. J. Neurosci 32, 7622–7631 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Whitney M et al. Ratiometric activatable cell-penetrating peptides provide rapid in vivo readout of thrombin activation. Angew. Chem. Int. Ed Engl 52, 325–330 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Davalos D et al. Early detection of thrombin activity in neuroinflammatory disease. Ann. Neurol 75, 303–308 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee JW et al. Fibrinogen γ-A chain precursor in CSF: a candidate biomarker for Alzheimer’s disease. BMC Neurol 7, 14 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Craig-Schapiro R et al. Multiplexed immunoassay panel identifies novel CSF biomarkers for Alzheimer’s disease diagnosis and prognosis. PLOS ONE 6, e18850 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vafadar-Isfahani B et al. Identification of SPARC-like 1 protein as part of a biomarker panel for Alzheimer’s disease in cerebrospinal fluid. J. Alzheimers Dis 28, 625–636 (2012). [DOI] [PubMed] [Google Scholar]
- 172.Thambisetty M et al. Plasma biomarkers of brain atrophy in Alzheimer’s disease. PLOS ONE 6, e28527 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang HQ et al. Prognostic polypeptide blood plasma biomarkers of alzheimer’s disease progression. J. Alzheimers Dis 40, 659–666 (2014). [DOI] [PubMed] [Google Scholar]
- 174.Ashton NJ et al. Blood protein predictors of brain amyloid for enrichment in clinical trials? Alzheimers Dement 1, 48–60 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Conti A et al. Proteome study of human cerebrospinal fluid following traumatic brain injury indicates fibrin(ogen) degradation products as trauma-associated markers. J. Neurotrauma 21, 854–863 (2004). [DOI] [PubMed] [Google Scholar]
- 176.Zhang Y et al. Elevated fibrinogen levels in neuromyelitis optica is associated with severity of disease. Neurol. Sci 37, 1823–1829 (2016). [DOI] [PubMed] [Google Scholar]
- 177.Gobel K et al. Prothrombin and factor X are elevated in multiple sclerosis patients. Ann. Neurol 80, 946–951 (2016). [DOI] [PubMed] [Google Scholar]
- 178.Gobel K et al. Blood coagulation factor XII drives adaptive immunity during neuroinflammation via CD87-mediated modulation of dendritic cells. Nat. Commun 7, 11626 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zamolodchikov D, Chen ZL, Conti BA, Renne T & Strickland S Activation of the factor XII-driven contact system in Alzheimer’s disease patient and mouse model plasma. Proc. Natl Acad. Sci. USA 112, 4068–4073 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bergamaschini L et al. Activation of the contact system in cerebrospinal fluid of patients with Alzheimer disease. Alzheimer Dis. Assoc. Disord 12, 102–108 (1998). [DOI] [PubMed] [Google Scholar]
- 181.Hattori K et al. Increased cerebrospinal fluid fibrinogen in major depressive disorder. Sci. Rep 5, 11412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pollak TA et al. The blood-brain barrier in psychosis. Lancet Psychiatry 5, 79–92 (2018). [DOI] [PubMed] [Google Scholar]
- 183.Patel JP & Frey BN Disruption in the blood–brain barrier: the missing link between brain and body inflammation in bipolar disorder? Neural Plast 2015, 708306 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Winkler EA et al. Blood–spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc. Natl Acad. Sci. USA 111, E1035–E1042 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Spencer JI, Bell JS & DeLuca GC Vascular pathology in multiple sclerosis: reframing pathogenesis around the blood–brain barrier. J. Neurol. Neurosurg. Psychiatry 89, 42–52 (2017). [DOI] [PubMed] [Google Scholar]
- 186.Kraus J & Oschmann P The impact of interferon-β treatment on the blood–brain barrier. Drug Discov. Today 11, 755–762 (2006). [DOI] [PubMed] [Google Scholar]
- 187.Kunze R et al. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood–brain barrier integrity. Exp. Neurol 266, 99–111 (2015). [DOI] [PubMed] [Google Scholar]
- 188.Nishihara H et al. Fingolimod prevents blood–brain barrier disruption induced by the sera from patients with multiple sclerosis. PLOS ONE 10, e0121488 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Luhder F et al. Laquinimod enhances central nervous system barrier functions. Neurobiol. Dis 102, 60–69 (2017). [DOI] [PubMed] [Google Scholar]
- 190.Ifergan I et al. Statins reduce human blood–brain barrier permeability and restrict leukocyte migration: relevance to multiple sclerosis. Ann. Neurol 60, 45–55 (2006). [DOI] [PubMed] [Google Scholar]
- 191.Chataway J et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet 383, 2213–2221 (2014). [DOI] [PubMed] [Google Scholar]
- 192.Zamolodchikov D & Strickland S The Alzheimer’s disease peptide β-amyloid promotes thrombin generation through activation of coagulation factor XII: reply. J. Thromb. Haemost 14, 1489 (2016). [DOI] [PubMed] [Google Scholar]
- 193.Zamolodchikov D & Strickland S A possible new role for Aβ in vascular and inflammatory dysfunction in Alzheimer’s disease. Thromb. Res 141, S59–S61 (2016). [DOI] [PubMed] [Google Scholar]
- 194.Claudio L, Raine CS & Brosnan CF Evidence of persistent blood–brain barrier abnormalities in chronic-progressive multiple sclerosis. Acta Neuropathol 90, 228–238 (1995). [DOI] [PubMed] [Google Scholar]
- 195.Inoue A, Koh CS, Shimada K, Yanagisawa N & Yoshimura K Suppression of cell-transferred experimental autoimmune encephalomyelitis in defibrinated Lewis rats. J. Neuroimmunol 71, 131–137 (1996). [DOI] [PubMed] [Google Scholar]
- 196.Inoue A et al. Fibrin deposition in the central nervous system correlates with the degree of Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J. Neuroimmunol 77, 185–194 (1997). [DOI] [PubMed] [Google Scholar]
- 197.Medved L, Tsurupa G & Yakovlev S Conformational changes upon conversion of fibrinogen into fibrin. The mechanisms of exposure of cryptic sites. Ann. NY Acad. Sci 936, 185–204 (2001). [DOI] [PubMed] [Google Scholar]
- 198.Engvall E, Ruoslahti E & Miller EJ Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J. Exp. Med 147, 1584–1595 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Suehiro K et al. Fibrinogen binds to integrin α5β1via the carboxyl-terminal RGD site of the Aα-chain. J. Biochem 128, 705–710 (2000). [DOI] [PubMed] [Google Scholar]
- 200.Smith JW, Ruggeri ZM, Kunicki TJ & Cheresh DA Interaction of integrins αvβ3 and glycoprotein IIb-IIIa with fibrinogen: differential peptide recognition accounts for distinct binding sites. J. Biol. Chem 265, 12267–12271 (1990). [PubMed] [Google Scholar]
- 201.Chernousov MA & Carey DJ αVβ8 integrin is a Schwann cell receptor for fibrin. Exp. Cell Res 291, 514–524 (2003). [DOI] [PubMed] [Google Scholar]
- 202.Gorlatov S & Medved L Interaction of fibrin(ogen) with the endothelial cell receptor VE-cadherin: mapping of the receptor-binding site in the NH2-terminal portions of the fibrin β chains. Biochemistry 41, 4107–4116 (2002). [DOI] [PubMed] [Google Scholar]
- 203.Yakovlev S et al. Identification of VLDLR as a novel endothelial cell receptor for fibrin that modulates fibrin-dependent transendothelial migration of leukocytes. Blood 119, 637–644 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Altieri DC, Duperray A, Plescia J, Thornton GB & Languino LR Structural recognition of a novel fibrinogen γ chain sequence (117–133) by intercellular adhesion molecule-1 mediates leukocyte-endothelium interaction. J. Biol. Chem 270, 696–699 (1995). [DOI] [PubMed] [Google Scholar]
- 205.Fan H et al. Protective effects of Batroxobin on spinal cord injury in rats. Neurosci. Bull 29, 501–508 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lu W, Bhasin M & Tsirka SE Involvement of tissue plasminogen activator in onset and effector phases of experimental allergic encephalomyelitis. J. Neurosci 22, 10781–10789 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Leech S, Kirk J, Plumb J & McQuaid S Persistent endothelial abnormalities and blood-brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol. Appl. Neurobiol 33, 86–98 (2007). [DOI] [PubMed] [Google Scholar]
- 208.Hochmeister S et al. Dysferlin is a new marker for leaky brain blood vessels in multiple sclerosis. J. Neuropathol. Exp. Neurol 65, 855–865 (2006). [DOI] [PubMed] [Google Scholar]
- 209.Montagne A et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat. Med 10.1038/nm.4482 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 210.Gay D & Esiri M Blood-brain barrier damage in acute multiple sclerosis plaques. An immunocytological study. Brain 114, 557–572 (1991). [DOI] [PubMed] [Google Scholar]
- 211.Lipinski B & Sajdel-Sulkowska EM New insight into Alzheimer disease: demonstration of fibrin(ogen)-serum albumin insoluble deposits in brain tissue. Alzheimer Dis. Assoc. Disord 20, 323–326 (2006). [DOI] [PubMed] [Google Scholar]
- 212.Brown H et al. Evidence of blood-brain barrier dysfunction in human cerebral malaria. Neuropathol. Appl. Neurobiol 25, 331–340 (1999). [DOI] [PubMed] [Google Scholar]
- 213.Bardos H, Molnar P, Csecsei G & Adany R Fibrin deposition in primary and metastatic human brain tumours. Blood Coagul. Fibrinolysis 7, 536–548 (1996). [PubMed] [Google Scholar]