Abstract
目的
探讨硼替佐米联用obatoclax对人急性T淋巴白血病细胞Jurkat是否具有协同抗肿瘤作用。
方法
MTT法检测单用硼替佐米和联用Bcl-2抑制剂(obatoclax,AT-101和ABT-199)对Jurkat细胞活力的影响。采用Western blot方法检测药物对Bcl-2家族蛋白、LC3B、p62、ubiquitin、BiP/Grp78、p-JNK、p-p38和CHOP蛋白表达的影响。硼替佐米和Bcl-2抑制剂对细胞凋亡的影响。实时荧光定量PCR检测未折叠蛋白反应(UPR)关键调节因子的mRNA表达水平。斑马鱼异种移植模型研究单药以及两药联用的体内抗肿瘤作用。
结果
单用硼替佐米和Bcl-2抑制剂均可抑制Jurkat细胞活力,但联用时仅obatoclax与硼替佐米具有协同细胞毒作用,引起细胞凋亡增多。obatoclax阻断自噬流,伴随LC3B-Ⅱ和p62的蓄积。此外,单用硼替佐米和obatoclax可诱导泛素化蛋白的蓄积,两药联用对泛素化蛋白蓄积具有协同作用,与药物联用的协同细胞毒作用实验结果一致。硼替佐米和obatoclax联用对蛋白酶体和自噬的双重阻断触发了未折叠蛋白反应,诱导细胞凋亡。内质网应激抑制剂牛磺熊去氧胆酸(TUDCA)可减弱硼替佐米和obatoclax联用的细胞毒作用,减少细胞凋亡。在斑马鱼体内联用硼替佐米和obatoclax显著地减少肿瘤灶形成。
结论
硼替佐米与obatoclax联用双重阻断蛋白质降解途径对人急性T淋巴白血病细胞具有协同抗肿瘤作用。
Keywords: 人急性T淋巴白血病细胞, 硼替佐米, obatoclax, 自噬, 协同作用, Bcl-2, 未折叠蛋白反应, 斑马鱼异种移植模型
Abstract
Objective
To explore whether bortezomib and a Bcl-2 inhibitor exhibit synergistic anti-tumor effect in human acute T lymphoblastic leukemia cells.
Methods
MTT assay was used to determine the cytotoxicity of bortezomib in the absence or presence of Bcl-2 inhibitors (obatoclax, AT-101 and ABT-199) in Jurkat cells. The effects of drug treatment on the expression of Bcl-2 family proteins, LC3B, p62, ubiquitin, BiP/Grp78, p-JNK, p-p38 and CHOP proteins were examined by Western blotting. Flow cytometry was used to determine the effects of bortezomib and Bcl-2 inhibitors (obatoclax, AT-101 and ABT-199) on cell apoptosis. Quantitative real-time PCR was used to measure the mRNA expression levels of the key regulatory factors of unfolded protein reaction (UPR). A zebrafish xenograft model was used to study the anti-tumor effect of bortezomib, obatoclax and their combination in vivo.
Results
Bortezomib or Bcl-2 inhibitors alone inhibited the cell viability of Jurkat cells, but only obatoclax and bortezomib showed synergistic cytotoxicity and pro-apoptotic effect. Obatoclax, rather than AT-101 and ABT- 199, blocked autophagic flux in the cells evidenced by concomitant accumulation of LC3B-Ⅱ and p62. Both bortezomib and obatoclax alone caused accumulation of polyubiquinated proteins, and their combination showed a synergistic effect, which was consistent with their synergistic cytotoxicity. The dual blockade of proteasome and autophagy by the combination of bortezomib and obatoclax triggered unfolded protein response followed by cell apoptosis. Preventing UPS dysfunction by tauroursodeoxycholic acid (TUDCA) significantly attenuated the cytotoxicity and pro-apoptotic effect of bortezomib in combination with obatoclax. In zebrafish xenograft models, bortezomib combined with obatoclax significantly decreased tumor foci formation.
Conclusion
Bortezomib and obatoclax for dual blockade of protein degradation pathways show synergistic anti-tumor effect in human acute T lymphoblastic leukemia cells.
Keywords: human acute T lymphoblastic leukemia cells, bortezomib, obatoclax, autophagy, synergism, Bcl-2, unfolded protein response, zebrafish xenograft model
急性淋巴细胞白血病(ALL)是一种由B淋巴细胞或T淋巴细胞前体细胞分化和增殖的遗传改变而导致的恶性疾病。其发病率遵循双峰分布,第1个发病高峰在2~5岁之间,第2个发病高峰在50岁左右[1-2]。目前尽管ALL患者的生存率,特别是儿童ALL患者的生存率有了显著改善,但成人的长期治愈率仍低于40%[3]。而且,难治性/复发性ALL的预后很差[4-5]。鉴于目前ALL治疗方法的局限性,迫切需要开发新的治疗方案。
硼替佐米是美国FDA批准的第一个蛋白酶体抑制剂,用于治疗多发性骨髓瘤(MM)、难治性/复发性MM和套细胞淋巴瘤[6]。最近,有研究建议将硼替佐米作为一种新的治疗方案用于ALL患者的临床治疗[6]。硼替佐米的抗ALL作用,最早的临床证据来自一份病例报告,记录了硼替佐米联合地塞米松给药后对一个患有多发性复发性ALL儿童显示了一定的临床疗效[8]。虽然硼替佐米在Ⅰ期临床研究中没有令人满意的单药活性[9],但已有研究报道硼替佐米与常规化疗或靶向药物的联合应用能够显著的改善单药治疗的临床疗效[10-13]。
泛素-蛋白酶体途径(UPP)是细胞内蛋白质降解的主要机制,调控着调节细胞周期、细胞凋亡和基因转录的蛋白质的降解,是维持细胞稳态和存活的基础,而蛋白酶体抑制剂主要通过阻断UPP来发挥抗肿瘤活性[7, 14]。虽然硼替佐米抗肿瘤活性的具体机制尚未完全阐明,但越来越多的证据表明,硼替佐米引起Bcl-2家族促凋亡蛋白成员的蓄积是其抗肿瘤的重要机制,如BH3-only蛋白BIK,NOXA,PUMA和BIM的积累[15-16]。然而,也有文献报道Bcl-2家族的抗细胞凋亡成员如Mcl-1,在细胞中蓄积减弱了硼替佐米疗效[15, 17]。此外,还有文献报道了Bcl-2和Mcl-1的过表达可能是硼替佐米耐药的潜在机制[18-19]。在难治性/复发性MM,Bcl-2抑制剂ABT-199(venetoclax)与硼替佐米联用后,在不良反应可接受的情况下,表现出了有前景的治疗效果[20]。越来越多的研究显示,与其他化疗或靶向药物联合能够增强硼替佐米的抗肿瘤作用[10-13]。但是,硼替佐米与Bcl-2抑制剂在急性T淋巴细胞白血病细胞是否具有协同抗肿瘤作用,目前还未见文献报道。
因此,本研究目的是探索硼替佐米联合Bcl-2抑制剂是否具有协同抗肿瘤作用。为此我们检测了硼替佐米联用不同的Bcl-2抑制剂(obatoclax,AT-101和ABT-199)是否可以对Jurkat细胞表现出协同抗肿瘤活性。研究结果显示硼替佐米只有联用obatoclax才具有协同抗肿瘤活性,其机制与双重阻断UPP和自噬相关。
1. 材料和方法
1.1. 化合物
Obatoclax,AT-101,ABT-199,牛磺熊去氧胆酸盐(TUDCA)和地塞米松(Selleck Chemicals)。所有其他化学品(Sigma-Aldrich)。
1.2. 细胞培养
急性T淋巴细胞白血病细胞株Jurkat从中国典型培养物保藏中心(武汉)购买。用含10%胎牛血清(invitrogen)、100 U/mL青霉素和100 μg/mL链霉素(invitrogen)的RPMI 1640培养基(invitrogen)在37 ℃、5% CO2的孵箱中培养。
1.3. 细胞活力检测
MTT分析用于评估药物处理后细胞的存活率。将细胞接种于96孔板,24 h后单独或联合使用不同浓度的药物培养48 h,然后加入MTT溶液,终浓度为0.5 mg/mL,培养4 h。用570 nm的酶联免疫检测仪(Bio-Rad实验室)以分光光度法测定还原产物蓝紫色甲瓒产量。应用Compusyn软件计算硼替佐米与Bcl-2抑制剂联合用药对细胞活力的影响。计算联合指数(CI)值。CI < 1,CI=1,CI>1分别表示协同作用、相加作用和拮抗作用[21]。为了研究硼替佐米和obatoclax联合是否通过内质网应激通路发挥细胞毒作用,内质网应激抑制剂牛磺熊去氧胆酸(TUDCA)[22-23]与以上两药物共孵育48 h,然后检测细胞存活。
1.4. Annexin Ⅴ染色
为了研究药物对Jurkat细胞的细胞毒性,通过Annexin Ⅴ(DOJINDO)染色检测细胞凋亡。采用单药和两药联用分别处理细胞48 h,然后收集细胞,用冰冷的PBS洗涤2次,避光用AnnexinⅤ标记细胞,室温静置15 min。使用BD FACSCanto Ⅱ流式细胞仪(BD Biosciences)分析细胞凋亡,地塞米松为阳性对照。由于obatoclax为红色的化合物在PI通道下会自发红色荧光,所以细胞没有做PI染色。
1.5. 蛋白印迹法
Jurkat细胞经硼替佐米(5nmol/L),obatoclax(1μmol/L),AT-101(2 μmol/L)和ABT-199(5 μmol/L)单用或联用处理后,用含有蛋白酶抑制剂(Merck)和磷酸酶抑制剂(Merck)的RIPA缓冲液裂解细胞。通过SDS-PAGE分离蛋白质并转移至PVDF膜(Roche Diagnostics Corp),一抗4 ℃孵育过夜。然后室温孵育二抗1 h(Cell Signaling Technology)。除检测GAPDH(SC-25778,Santa Cruz Biotechnology)和vinculin(036M4797Ⅴ,Sigma-Aldrich)的一抗外,其余所有一抗和二抗均购自Cell Signaling Technology。抗体目录编号:Mcl-1(5453),Bcl-2(2870),Bcl-xl(2764),p62(8025),LC3B(2775),β-actin(4970),ubiquitin(3936),BiP/Grp78(3177S),p-JNK(9251)、p-p38(9211)和CHOP(5554)。利用化学发光在X射线胶片显影(Fuji Phot Film)。
1.6. 逆转录和实时荧光定量PCR
实验采用硼替佐米(5nmol/L)联用obatoclax(1μmol/L)处理Jurkat细胞3、6及12 h,使用TRIZOL(Invitrogen)提取总RNA。根据PrimeScript RT试剂盒(TAKARA)说明书操作,逆转录2 μg总RNA为cDNA。使用SYBR Select Master Mix(Applied Biosystems),运用标准的两步法(变性:95 ℃/15 s,退火/延伸:60 ℃/1 min,40个循环),于7500实时荧光定量PCR系统(Applied Biosystems)进行PCR反应。采用比较阈值循环(CT)进行数据分析。引物序列从qPrimerDepot(National Center for Biotechnology Information)获得。
1.7. 建立斑马鱼异种移植模型
在显微注射前,将受精后48 h(hpf)的野生型AB斑马鱼胚胎进行破膜处理,然后将约300个用CM-DiI(Invitrogen)标记的Jurkat细胞显微注射接种于斑马鱼胚胎的卵黄囊中。再将胚胎经DMSO(溶剂对照)、obatoclax(0.1 μmol/L)、bortezomib(5 nmol/L)或两药联用处理,于34 ℃的培养箱中培养4 d。每天更换含有上述药物的培养液,通过荧光显微镜观察肿瘤的生长情况。所有涉及斑马鱼的实验都依据南医科大学动物保护和使用委员会制定的指南进行。
1.8. 统计分析
实验数据均以至少3次独立重复实验的均数±标准差表示。采用单因素方差分析和Tukey's t检验对数据进行分析。P < 0.05时认为差异具有统计学意义。
2. 结果
2.1. 蛋白酶体抑制剂硼替佐米抑制了Jurkat细胞的细胞活力
将Jurkat细胞用硼替佐米处理48 h后,采用MTT法检测药物对Jurkat细胞活力的影响。硼替佐米在Jurkat细胞中以剂量依赖性方式降低细胞活力(图 1)。
1.
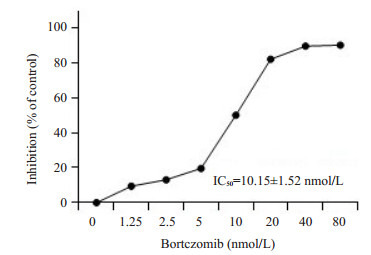
硼替佐米对Jurkat细胞的毒性作用
Cytotoxicity of bortezomib in Jurkat cells. The value of IC50 was calculated by Prism 5.0 software (n=3)
2.2. 硼替佐米增加了Jurkat细胞Bcl-2家族抗凋亡蛋白的水平
在Jurkat细胞中,硼替佐米显著增加Mcl-1蛋白表达,并轻微增加了Bcl-2和Bcl-xl蛋白水平(图 2)。
2.
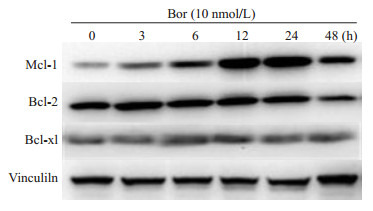
硼替佐米诱导对Jurkat细胞中Bcl-2家族抗凋亡蛋白表达增加
Effects of bortezomib on protein levels of anti-apoptotic Bcl-2 family members in Jurkat cells (n=3)
2.3. 硼替佐米联用obatoclax在Jurkat细胞中的协同细胞毒作用
Bcl-2抑制剂(obatoclax,AT-101和ABT-199)处理Jurkat细胞48 h后,通过MTT法检测细胞活力。结果显示药物以剂量依赖性的方式降低Jurkat细胞的活力(图 3A)。此外,硼替佐米联合obatoclax在Jurkat细胞中显示出协同的细胞毒作用,而另外两种Bcl-2抑制剂(AT-101和ABT-199)联用硼替佐米没有显示这种协同效应(图 3B)。硼替佐米和obatoclax单用均能诱导细胞凋亡,但两药联用后进一步促进了细胞凋亡(图 3C)。
3.
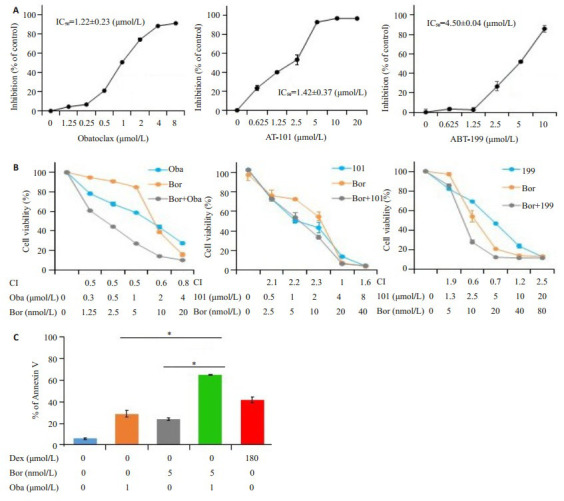
硼替佐米联用obatoclax在Jurkat细胞中的协同细胞毒性作用
Bortezomib combined with obatoclax show synergistic cytotoxicity in Jurkat cells. A: Bcl-2 inhibitors decrease the viability of Jurkat cells (n=3); B: Cytotoxicity of bortezomib in combination with obatoclax, AT-101, or ABT-199 determined by MTT assay (n=3); C: Cell apoptosis determined by phosphatidylserine (PS) externalization and binding of Annexin Ⅴ (n=3). *P < 0.05
2.4. Obatoclax抑制了Jurkat细胞的自噬活性
Bcl-2抑制剂(obatoclax,AT-101,和ABT-199)分别处理Jurkat细胞24 h,只有obatoclax显著增加Jurkat细胞中的LC3B-Ⅱ和p62蛋白水平,而ABT-199和AT-101没有显示相似的作用(图 4A)。Obatoclax或硼替佐米处理48 h,可单独诱导泛素化蛋白质在Jurkat细胞蓄积,而两药联合能够进一步增加泛素化蛋白的蓄积(图 4B)。
4.
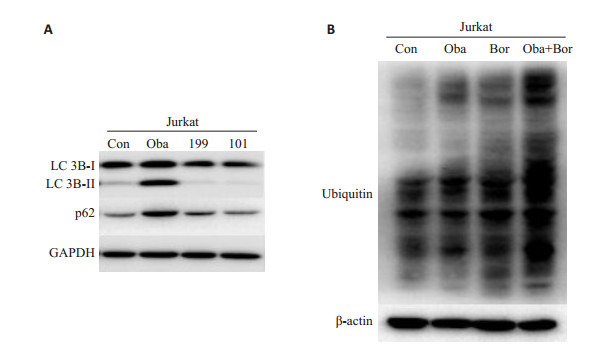
Bcl-2抑制剂对Jurkat细胞自噬活性的影响
Effects of Bcl-2 inhibitors on autophagic activity of Jurkat cells. A: Conversion of LC3B-Ⅰ to LC3B-Ⅱ and protein expression of p62 determined by Western blotting (n=3); B: Amount of polyubiquitinated proteins determined by Western blotting (n=3)
2.5. 硼替佐米联用obatoclax激活未折叠蛋白反应(UPR)
Bip是未折叠蛋白反应(UPR)的主要调节因子,药物处理24 h后检测发现,单用硼替佐米没有改变Bip的蛋白水平,单用obatoclax略微增加Bip蛋白表达,单用AT-101或ABT-199也没有改变Bip的蛋白水平。当硼替佐米联用obatoclax后显著增强了Bip蛋白表达,而硼替佐米联用AT-101或ABT-199不影响Bip蛋白水平。此外,硼替佐米联合obatoclax明显增加了Jurkat细胞中的p-JNK表达,而联用AT-101或ABT-199不影响p-JNK表达。相反,单用硼替佐米或与不同的Bcl-2抑制剂联合处理细胞后,p-p38表达水平都保持相对稳定或仅略微增加(图 5A)。
5.
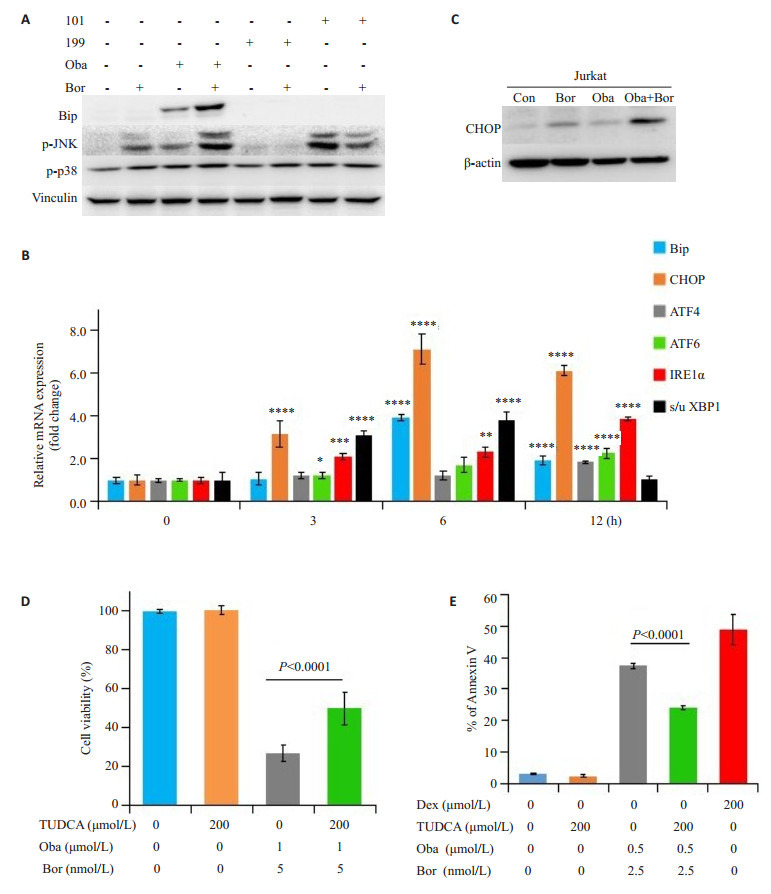
硼替佐米联用obatoclax在Jurkat细胞中诱导了未折叠蛋白反应
Bortezomib combined with obatoclax induces unfolded protein response (UPR) in Jurkat cells. A: Protein expressions of Bip, p-JNK, and p-p38 determined by Western blotting (n=3); B: mRNA levels of key regulators of the UPR determined by realtime PCR (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 vs control); C: Protein expression of CHOP determined by Western blotting (n=3); D: Cell viability determined by MTT assay; E: Cellular apoptosis determined by PS externalization and binding of Annexin Ⅴ (n=3)
硼替佐米与obatoclax联用上调了Jurkat细胞中UPR关键调节因子的mRNA水平,包括Bip,CHOP,ATF4,ATF6,IRE1α和剪接/未剪接-XBP1(图 5B)。此外,硼替佐米与obatoclax联用处理Jurkat细胞48 h导致CHOP的蛋白质表达显著增加(图 5C)。
牛磺熊去氧胆酸(TUDCA)本身不影响细胞活力,但它显著降低了硼替佐米与obatoclax联合诱导的细胞毒性(图 5D)。相同的是,TUDCA削弱了联合用药的促细胞凋亡作用(图 5E)。
2.6. 硼替佐米联合obatoclax抑制斑马鱼移植瘤模型中肿瘤的生长
本研究利用斑马鱼早期胚胎(48 hpf)免疫缺陷的特点,将CM-DiI标记的Jurkat细胞显微注射接种于斑马鱼胚胎的卵黄囊中,然后观察肿瘤的生长。图 6A展示了4 dpi时药物治疗后肿瘤病灶形成的代表性图像。单用硼替佐米或obatoclax可略微减少肿瘤病灶的形成,但联合用药能比单用更显著的减少肿瘤病灶的形成(图 6B),且上述处理对斑马鱼的生存没有影响。
6.
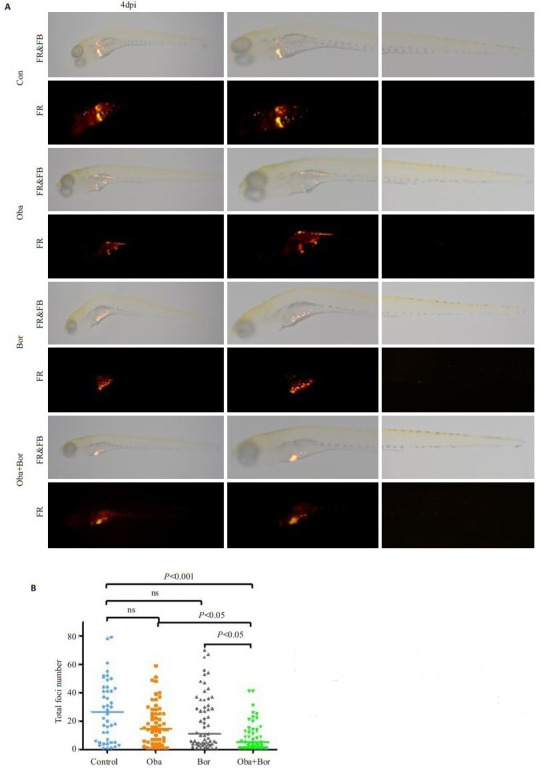
硼替佐米联合obatoclax抑制斑马鱼移植瘤模型中肿瘤的生长
Bortezomib combined with obatoclax more strongly suppresses tumor foci formation than either drug alone in zebrafish xenograft models. A: Representative fluorescence microscopic images at 4 dpi (day post injection); B: Quantification of tumor foci formation in zebrafish (n>40 in each group)
3. 讨论
在本研究中,我们发现硼替佐米抑制了Jurkat细胞的细胞活力,其IC50值在nmol/L级别。Bcl-2抑制剂(obatoclax,AT-101和ABT-199)也以剂量依赖性方式抑制Jurkat细胞的细胞活力。值得注意的是,3个Bcl-2抑制剂中,只有obatoclax联用硼替佐米显示出协同的细胞毒作用。
Bcl-2家族抗凋亡蛋白Mcl-1是通过UPP被降解,已有文献报道蛋白酶体抑制剂可以在几个小时内使Mcl-1蛋白水平快速增加[15]。与此相似的是,我们用硼替佐米处理细胞3 h,检测发现Mcl-1蛋白水平显著增加。虽然硼替佐米也增加了Bcl-2和Bcl-xL的蛋白水平,但效果并不明显。当硼替佐米联用obatoclax时,两药显示出协同细胞毒性作用,而联用其他两种Bcl-2抑制剂均未显示协同细胞毒性作用。Obatoclax和AT-101是抗凋亡Bcl-2家族成员的泛抑制剂,抑制Bcl-2,Bcl-xL,Mcl-1和Bcl-w[24-26]。而ABT-199是高选择性的Bcl-2抑制剂[27]。考虑到本实验中Bcl-2抑制剂的选择性,尽管硼替佐米增加Mcl-1蛋白水平,但obatoclax和硼替佐米之间的协同作用看起来并不依赖于抑制Mcl-1或Bcl-2家族的其他抗凋亡成员。
我们之前的研究表明,obatoclax在溶酶体中蓄积,导致人食道癌细胞中溶酶体功能和自噬降解能力受损,泛素化蛋白蓄积[28]。在我们的研究之后,其他研究小组也陆续报道在其他类型肿瘤细胞obatoclax也对溶酶体功能有类似影响[29-30]。这些发现表明obatoclax的自噬抑制活性可能在不同类型的肿瘤细胞中普遍存在。obatoclax是一个含有吲哚基团的碱性化合物,进入细胞后在溶酶体内富集,破坏了溶酶体内的酸性环境和组织蛋白酶的水解活性,最终导致细胞自噬活性的降低[28]。与这些发现一致的是,我们在Jurkat细胞中发现obatoclax处理后,LC3B-Ⅱ和p62蛋白水平的增加,提示obatoclax阻断了自噬流。相反,AT-101和ABT-199没有改变Jurkat细胞的自噬活性。UPP和自噬-溶酶体途径是真核细胞中蛋白质降解的两种主要途径,这两个系统在细胞中相互协调,发挥维持蛋白质稳态的作用[31]。研究显示抑制UPP通路导致细胞自噬活性增加,也就是说在一条蛋白降解通路受阻时,细胞通过增强另外一条降解通路来缓解压力,这是细胞应对UPP降解通路缺陷的一种促生存的机制。因此,抑制自噬能够增强UPP抑制剂的抗肿瘤作用[32-33]。与预期结果相似,硼替佐米和obatoclax对蛋白酶体和自噬的双重阻断导致了泛素化蛋白质的进一步积累。
错误折叠的蛋白质通常靶向UPP进行降解,而蛋白酶体抑制剂就是通过增加错误折叠蛋白质的积累来触发内质网(ER)应激[34]。考虑到硼替佐米联用obatoclax增加了泛素化蛋白质的蓄积,我们研究了联合用药是否能通过双重阻断UPP和自噬从而诱发内质网应激。内质网功能紊乱导致细胞产生应激反应,即UPR。UPR最初的功能是恢复蛋白质稳态,但如果稳态无法重建,最终可能触发细胞凋亡[35]。硼替佐米和obatoclax单用几乎不影响Bip和CHOP蛋白表达,而联合应用显著增加了Bip和CHOP蛋白水平。目前已经有文献报道内质网应激能够激活JNK信号传导途径[36]。我们的研究也表明,硼替佐米联用obatoclax可显著增加p-JNK的表达。值得注意的是,内质网应激抑制剂TUDCA减弱了联合用药的细胞毒作用。这些研究结果表明硼替佐米联用obatoclax是通过双重抑制蛋白酶体和自噬活性,引起内质网应激,从而诱导细胞毒性作用。
因此,本研究显示硼替佐米和obatoclax在Jurkat细胞中通过双重阻断蛋白质降解途径,表现出协同的细胞毒性。虽然在体外实验中该协同作用不依赖于obtatoclax对Bcl-2的抗凋亡成员的抑制,但这种抑制作用可为联合用药提供益处。此外,单用硼替佐米或obatoclax仅略微减少斑马鱼异种移植模型中的肿瘤灶形成,而联合用药比单用更显著地减少肿瘤病灶的形成,显示了联合用药在体内的优势。本研究结果为急性T淋巴细胞白血病的治疗提供了新的联合给药方案。
Biography
周丹,在读硕士,E-mail: 121585564@qq.com
Funding Statement
国家自然科学基金(81773773);广州市科技计划项目(201607010350)
Supported by National Natural Science Foundation of China (81773773)
Contributor Information
周 丹 (Dan ZHOU), Email: 121585564@qq.com.
余 乐 (Le YU), Email: yulezy@smu.edu.cn.
References
- 1.Paul S, Kantarjian H, Jabbour EJ. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2016;91(11):1645–66. doi: 10.1016/j.mayocp.2016.09.010. [Paul S, Kantarjian H, Jabbour EJ. Adult acute lymphoblastic leukemia[J]. Mayo Clin Proc, 2016, 91(11): 1645-66.] [DOI] [PubMed] [Google Scholar]
- 2.Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia:a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6):e577. doi: 10.1038/bcj.2017.53. [Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia:a comprehensive review and 2017 update[J]. Blood Cancer J, 2017, 7 (6): e577.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabbour E, O'brien S, Konopleva M, et al. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer. 2015;121(15):2517–28. doi: 10.1002/cncr.v121.15. [Jabbour E, O'brien S, Konopleva M, et al. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia [J]. Cancer, 2015, 121(15): 2517-28.] [DOI] [PubMed] [Google Scholar]
- 4.Pui CH, Carroll WL, Meshinchi S, et al. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29(5):551–65. doi: 10.1200/JCO.2010.30.7405. [Pui CH, Carroll WL, Meshinchi S, et al. Biology, risk stratification, and therapy of pediatric acute leukemias: an update[J]. J Clin Oncol, 2011, 29(5): 551-65.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. http://cn.bing.com/academic/profile?id=a6660d65dfffaa30d896b1b3e994af62&amp;amp;encoded=0&amp;amp;v=paper_preview&amp;amp;mkt=zh-cn. Blood. 2007;109(3):944–50. doi: 10.1182/blood-2006-05-018192. [Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study[J]. Blood, 2007, 109(3): 944-50.] [DOI] [PubMed] [Google Scholar]
- 6.Du XL, Chen Q. Recent advancements of bortezomib in acute lymphocytic leukemia treatment. Acta Haematol. 2013;129(4):207–14. doi: 10.1159/000345260. [Du XL, Chen Q. Recent advancements of bortezomib in acute lymphocytic leukemia treatment[J]. Acta Haematol, 2013, 129(4): 207-14.] [DOI] [PubMed] [Google Scholar]
- 7.Zahid MF. The role of bortezomib in the treatment of acute lymphoblastic leukemia. Future Oncol. 2016;12(16):1861–4. doi: 10.2217/fon-2016-0126. [Zahid MF. The role of bortezomib in the treatment of acute lymphoblastic leukemia[J]. Future Oncol, 2016, 12(16): 1861-4.] [DOI] [PubMed] [Google Scholar]
- 8.Brown RE, Bostrom B, Zhang PL. Brief communication: Morphoproteomics and bortezomib/dexamethasone-induced response in relapsed acute lymphoblastic leukemia. http://cn.bing.com/academic/profile?id=874e666993d164d9d0fafeb08cbf4543&amp;amp;encoded=0&amp;amp;v=paper_preview&amp;amp;mkt=zh-cn. Ann Clin Lab Sci. 2004;34(2):203–5. [Brown RE, Bostrom B, Zhang PL. Brief communication: Morphoproteomics and bortezomib/dexamethasone-induced response in relapsed acute lymphoblastic leukemia[J]. Ann Clin Lab Sci, 2004, 34(2): 203-5.] [PubMed] [Google Scholar]
- 9.Houghton PJ, Morton CL, Kolb E, et al. Initial testing (stage 1) of the proteasome inhibitor bortezomib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(1):37–45. doi: 10.1002/pbc.v50:1. [Houghton PJ, Morton CL, Kolb E, et al. Initial testing (stage 1) of the proteasome inhibitor bortezomib by the pediatric preclinical testing program[J]. Pediatr Blood Cancer, 2008, 50(1): 37-45.] [DOI] [PubMed] [Google Scholar]
- 10.Messinger Y, Gaynon P, Raetz E, et al. Phase Ⅰ study of bortezomib combined with chemotherapy in children with relapsed childhood acute lymphoblastic leukemia (ALL): A report from the therapeutic advances in childhood leukemia (TACL) consortium. Pediatr Blood Cancer. 2010;55(2):254–9. doi: 10.1002/pbc.v55:2. [Messinger Y, Gaynon P, Raetz E, et al. Phase Ⅰ study of bortezomib combined with chemotherapy in children with relapsed childhood acute lymphoblastic leukemia (ALL): A report from the therapeutic advances in childhood leukemia (TACL) consortium[J]. Pediatr Blood Cancer, 2010, 55(2): 254-9.] [DOI] [PubMed] [Google Scholar]
- 11.Hu XH, Xu JJ, Sun AN, et al. Successful T-cell acute lymphoblastic leukemia treatment with proteasome inhibitor bortezomib based on evaluation of nuclear factor-kappa B activity. Leuk Lymphoma. 2011;52(12):2393–5. doi: 10.3109/10428194.2011.593271. [Hu XH, Xu JJ, Sun AN, et al. Successful T-cell acute lymphoblastic leukemia treatment with proteasome inhibitor bortezomib based on evaluation of nuclear factor-kappa B activity[J]. Leuk Lymphoma, 2011, 52(12): 2393-5.] [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Wang C, Song Y, et al. Treatment of refractory/relapsed adult acute lymphoblastic leukemia with bortezomib-based chemotherapy. http://cn.bing.com/academic/profile?id=2f5b8aa441de6a5936c1ce35a9739b57&amp;amp;encoded=0&amp;amp;v=paper_preview&amp;amp;mkt=zh-cn. Int J Gen Med. 2015;8:211–4. doi: 10.2147/IJGM.S59537. [Zhao J, Wang C, Song Y, et al. Treatment of refractory/relapsed adult acute lymphoblastic leukemia with bortezomib-based chemotherapy [J]. Int J Gen Med, 2015, 8: 211-4.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messinger YH, Gaynon PS, Sposto R, et al. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: therapeutic advances in childhood leukemia& lymphoma (TACL) study. Blood. 2012;120(2):285–90. doi: 10.1182/blood-2012-04-418640. [Messinger YH, Gaynon PS, Sposto R, et al. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: therapeutic advances in childhood leukemia& lymphoma (TACL) study[J]. Blood, 2012, 120(2): 285-90.] [DOI] [PubMed] [Google Scholar]
- 14.Citrin R, Foster JB, Teachey DT. The role of proteasome inhibition in the treatment of malignant and non-malignant hematologic disorders. Expert Rev Hematol. 2016;9(9):873–89. doi: 10.1080/17474086.2016.1216311. [Citrin R, Foster JB, Teachey DT. The role of proteasome inhibition in the treatment of malignant and non-malignant hematologic disorders[J]. Expert Rev Hematol, 2016, 9(9): 873-89.] [DOI] [PubMed] [Google Scholar]
- 15.Fennell DA, Chacko A, Mutti L. BCL-2 family regulation by the 20S proteasome inhibitor bortezomib. Oncogene. 2008;27(9):1189–97. doi: 10.1038/sj.onc.1210744. [Fennell DA, Chacko A, Mutti L. BCL-2 family regulation by the 20S proteasome inhibitor bortezomib[J]. Oncogene, 2008, 27(9): 1189-97.] [DOI] [PubMed] [Google Scholar]
- 16.Poulaki V, Mitsiades CS, Kotoula V, et al. The proteasome inhibitor bortezomib induces apoptosis in human retinoblastoma cell lines in vitro. Invest Ophthalmol Vis Sci. 2007;48(10):4706–19. doi: 10.1167/iovs.06-1147. [Poulaki V, Mitsiades CS, Kotoula V, et al. The proteasome inhibitor bortezomib induces apoptosis in human retinoblastoma cell lines in vitro[J]. Invest Ophthalmol Vis Sci, 2007, 48(10): 4706-19.] [DOI] [PubMed] [Google Scholar]
- 17.Shore GC, Viallet J. Modulating the bcl-2 family of apoptosis suppressors for potential therapeutic benefit in cancer. http://cn.bing.com/academic/profile?id=9f730a2e17662dcc9b2618d6a9e15e32&amp;amp;encoded=0&amp;amp;v=paper_preview&amp;amp;mkt=zh-cn. Hematology Am Soc Hematol Educ Program. 2005;2015:226–30. doi: 10.1182/asheducation-2005.1.226. [Shore GC, Viallet J. Modulating the bcl-2 family of apoptosis suppressors for potential therapeutic benefit in cancer[J]. Hematology Am Soc Hematol Educ Program, 2005, 2015: 226-30.] [DOI] [PubMed] [Google Scholar]
- 18.Ailawadhi S, Miecznikowski J, Gaile DP, et al. Bortezomib mitigates adverse prognosis conferred by Bcl-2 overexpression in patients with relapsed/refractory multiple myeloma. Leuk Lymphoma. 2012;53(6):1174–82. doi: 10.3109/10428194.2011.637212. [Ailawadhi S, Miecznikowski J, Gaile DP, et al. Bortezomib mitigates adverse prognosis conferred by Bcl-2 overexpression in patients with relapsed/refractory multiple myeloma[J]. Leuk Lymphoma, 2012, 53(6): 1174-82.] [DOI] [PubMed] [Google Scholar]
- 19.Smith AJ, Dai HM, Correia C, et al. Noxa/Bcl-2 protein interactions contribute to bortezomib resistance in human lymphoid cells. J Biol Chem. 2011;286(20):17682–92. doi: 10.1074/jbc.M110.189092. [Smith AJ, Dai HM, Correia C, et al. Noxa/Bcl-2 protein interactions contribute to bortezomib resistance in human lymphoid cells[J]. J Biol Chem, 2011, 286(20): 17682-92.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touzeau C, Maciag P, Amiot M, et al. Targeting Bcl-2 for the treatment of multiple myeloma. Leukemia. 2018;32(9):1899–907. doi: 10.1038/s41375-018-0223-9. [Touzeau C, Maciag P, Amiot M, et al. Targeting Bcl-2 for the treatment of multiple myeloma[J]. Leukemia, 2018, 32(9): 1899- 907.] [DOI] [PubMed] [Google Scholar]
- 21.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81. doi: 10.1124/pr.58.3.10. [Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies [J]. Pharmacol Rev, 2006, 58(3): 621-81.] [DOI] [PubMed] [Google Scholar]
- 22.Yoon YM, Lee JH, Yun SP, et al. Tauroursodeoxycholic acid reduces ER stress by regulating of Akt-dependent cellular prion protein. Sci Rep. 2016;6:39838. doi: 10.1038/srep39838. [Yoon YM, Lee JH, Yun SP, et al. Tauroursodeoxycholic acid reduces ER stress by regulating of Akt-dependent cellular prion protein[J]. Sci Rep, 2016, 6: 39838.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Q, Khaoustov VI, Chung CC, et al. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36(3):592–601. doi: 10.1053/jhep.2002.35441. [Xie Q, Khaoustov VI, Chung CC, et al. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation [J]. Hepatology, 2002, 36(3): 592-601.] [DOI] [PubMed] [Google Scholar]
- 24.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7(12):989–1000. doi: 10.1038/nrd2658. [Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy[J]. Nat Rev Drug Discov, 2008, 7(12): 989-1000.] [DOI] [PubMed] [Google Scholar]
- 25.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. http://cn.bing.com/academic/profile?id=81951159e1d2ca6e0e31120312f8d002&amp;amp;encoded=0&amp;amp;v=paper_preview&amp;amp;mkt=zh-cn. Clin Cancer Res. 2009;15(4):1126–32. doi: 10.1158/1078-0432.CCR-08-0144. [Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy[J]. Clin Cancer Res, 2009, 15 (4): 1126-32.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhai D, Jin C, Satterthwait AC, et al. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13(8):1419–21. doi: 10.1038/sj.cdd.4401937. [Zhai D, Jin C, Satterthwait AC, et al. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins[J]. Cell Death Differ, 2006, 13(8): 1419-21.] [DOI] [PubMed] [Google Scholar]
- 27.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. http://cn.bing.com/academic/profile?id=8110cfe48bb367dd82988ff704909f89&amp;amp;encoded=0&amp;amp;v=paper_preview&amp;amp;mkt=zh-cn. Nat Med. 2013;19(2):202–8. doi: 10.1038/nm.3048. [Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets[J]. Nat Med, 2013, 19(2): 202-8.] [DOI] [PubMed] [Google Scholar]
- 28.Zhao XY, Lin QH, Que FC, et al. Synergistic anti-tumor effect of obatoclax and MG-132 in esophageal cancer cell line CaES-17. http://cn.bing.com/academic/profile?id=64879316a15791957f23a0f0c3ca3dc9&amp;amp;encoded=0&amp;amp;v=paper_preview&amp;amp;mkt=zh-cn. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36(4):506–13. [Zhao XY, Lin QH, Que FC, et al. Synergistic anti-tumor effect of obatoclax and MG-132 in esophageal cancer cell line CaES-17[J]. Nan Fang Yi Ke Da Xue Xue Bao, 2016, 36(4): 506-13.] [PubMed] [Google Scholar]
- 29.Stamelos VA, Fisher N, Bamrah HA, et al. The BH3 mimetic obatoclax accumulates in lysosomes and causes their alkalinization. http://cn.bing.com/academic/profile?id=45c2977d6eb5576c58772bf6a7a5791d&amp;amp;encoded=0&amp;amp;v=paper_preview&amp;amp;mkt=zh-cn. PLoS One. 2016;11(3):e150696. doi: 10.1371/journal.pone.0150696. [Stamelos VA, Fisher N, Bamrah HA, et al. The BH3 mimetic obatoclax accumulates in lysosomes and causes their alkalinization [J]. PLoS One, 2016, 11(3): e150696.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Champa D, Orlacchio A, Patel B, et al. Obatoclax kills anaplastic thyroid cancer cells by inducing lysosome neutralization and necrosis. http://cn.bing.com/academic/profile?id=dc243afb9fe626d724103c110566a6d2&amp;amp;encoded=0&amp;amp;v=paper_preview&amp;amp;mkt=zh-cn. Oncotarget. 2016;7(23):34453–71. doi: 10.18632/oncotarget.9121. [Champa D, Orlacchio A, Patel B, et al. Obatoclax kills anaplastic thyroid cancer cells by inducing lysosome neutralization and necrosis[J]. Oncotarget, 2016, 7(23): 34453-71.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Wang X. The interplay between autophagy and the ubiquitin-proteasome system in cardiac proteotoxicity. http://cn.bing.com/academic/profile?id=133688357c929f24a386eca2719b7ee0&amp;amp;encoded=0&amp;amp;v=paper_preview&amp;amp;mkt=zh-cn. Biochim Biophys Acta. 2015;1852(2, SI):188–94. doi: 10.1016/j.bbadis.2014.07.028. [Wang C, Wang X. The interplay between autophagy and the ubiquitin-proteasome system in cardiac proteotoxicity[J]. Biochim Biophys Acta, 2015, 1852(2, SI): 188-94.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu WK, Cho CH, Lee CW, et al. Macroautophagy and ERK phosphorylation counteract the antiproliferative effect of proteasome inhibitor in gastric cancer cells. http://cn.bing.com/academic/profile?id=2ccad75c57e59a786580ec95918d78f7&amp;amp;encoded=0&amp;amp;v=paper_preview&amp;amp;mkt=zh-cn. Autophagy. 2010;6(2):228–38. doi: 10.4161/auto.6.2.11042. [Wu WK, Cho CH, Lee CW, et al. Macroautophagy and ERK phosphorylation counteract the antiproliferative effect of proteasome inhibitor in gastric cancer cells[J]. Autophagy, 2010, 6(2): 228-38.] [DOI] [PubMed] [Google Scholar]
- 33.Wang ZH, Zhu SC, Zhang GS, et al. Inhibition of autophagy enhances the anticancer activity of bortezomib in B-cell acute lymphoblastic leukemia cells. http://cn.bing.com/academic/profile?id=bcf1b1436f4beedabded4987327cd11f&amp;amp;encoded=0&amp;amp;v=paper_preview&amp;amp;mkt=zh-cn. Am J Cancer Res. 2015;5(2):639–50. [Wang ZH, Zhu SC, Zhang GS, et al. Inhibition of autophagy enhances the anticancer activity of bortezomib in B-cell acute lymphoblastic leukemia cells[J]. Am J Cancer Res, 2015, 5(2): 639- 50.] [PMC free article] [PubMed] [Google Scholar]
- 34.Fels DR, Ye J, Segan AT, et al. Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways. http://cn.bing.com/academic/profile?id=b009f4dfabe1c922d71e78ce3c86d01a&amp;amp;encoded=0&amp;amp;v=paper_preview&amp;amp;mkt=zh-cn. Cancer Res. 2008;68(22):9323–30. doi: 10.1158/0008-5472.CAN-08-2873. [Fels DR, Ye J, Segan AT, et al. Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways[J]. Cancer Res, 2008, 68 (22): 9323-30.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell Life and death decisions. J Clin Invest. 2005;115(10):2656–64. doi: 10.1172/JCI26373. [Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell Life and death decisions[J]. J Clin Invest, 2005, 115(10): 2656-64.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–6. doi: 10.1126/science.287.5453.664. [Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1[J]. Science, 2000, 287(5453): 664-6.] [DOI] [PubMed] [Google Scholar]


