Abstract
目的
探讨大鼠骨髓间充质干细胞(BMSCs)来源的外泌体对睾丸缺血再灌注损伤的保护作用。
方法
采用原代培养方法分离、培养、并鉴定大鼠BMSCs。超高速离心法提取BMSCs来源的外泌体,并采用纳米颗粒跟踪分析仪(NTA)分析其粒径大小,透射电子显微镜观察其形态,蛋白免疫印迹法检测其典型标志蛋白质鉴定提取的外泌体。构建雄性SD大鼠睾丸缺血再灌注损伤(IRI)模型,24只健康雄性SD大鼠随机分为3组;A组:假手术组(Sham组),B组:生理盐水处理组(I/R+NS),C组:BMSCs来源外泌体(100 μg/mL)处理组(I/R+BMSCs-exo)。最后取各组大鼠扭转侧(左侧)睾丸并用光学显微镜检测睾丸组织病理学结构改变以及对生精结构进行组织评分,采用生物化学的方法检测各组睾丸组织中超氧化物歧化酶的活性,丙二醛的含量,蛋白免疫印迹法检测高迁移率族蛋B1(HMGB1),含半胱氨酸的天冬氨酸蛋白水解酶3(caspases-3)和剪切型caspase-3的表达。
结果
成功从大鼠骨髓中原代分离并培养BMSCs,并成功提取了BMSCs分泌的外泌体。动物模型中,与睾丸生精结构正常的A组(Sham)相比,B组(I/R+NS)的睾丸生精结构明显有不同程度受损,而C处理组(I/R+BMSCs-exo)中有一定的改善(P < 0.05)。睾丸组织生化指标检测中,B组(I/R+NS)组织中的MDA的含量较A组(Sham)明显升高,而SOD的活性与A组(Sham)相比则降低(P < 0.01),而在经外泌体处理之后的C组(I/R+BMSCs-exo)中,与生理盐水处理组B组相比,组织中MDA的含量有一定程度降低而SOD的水平则上调(P < 0.05)。睾丸组织蛋白免疫印迹上,与A组(Sham)相比,B组(I/R+NS)的HMGB1,caspase-3以及剪切型caspase-3明显升高,而C组(IR+BMSCs-exo)的HMGB1,caspase-3和剪切型caspase-3一定程度的降低(P < 0.05)。
结论
BMSCs来源的外泌体对睾丸缺血再灌注损伤有抗氧化,抗炎症和抗凋亡的保护作用。
Keywords: 外泌体, 间充质干细胞, 睾丸缺血再灌注, 抗氧化, 炎症, 凋亡
Abstract
Objective
To investigate the protective effect of bone marrow mesenchymal stem cells (BMSCs)-derived exosomes against testicular ischemia-reperfusion injury (IRI) in rats.
Methods
Rat BMSCs were isolated, cultured and identified in the primary culture. The exosomes were extracted from the BMSCs and characterized using nanoparticle tracking analysis, transmission electron microscopy, and Western blotting. Twenty-four healthy male SD rats were randomly divided into sham operation group, testicular IRI with saline treatment group and IRI with exosome treatment group. The contralateral testes of the rats were collected for pathological observation, aseessment of superoxide dismutase (SOD) and malondialdehyde (MDA), and detection of HMGB1, caspases-3 and cleaved caspase-3 expressions using Western blotting.
Results
We successfully obtained exosomes from rat BMSCs. Testicular IRI significantly impaired testicular spermatogenesis, which was markedly improved by treatment with the exosomes (P < 0.05). Testicular IRI also caused significant increase in the protein expression of HMGB1, caspase-3 and cleaved caspase-3 in the testicular tissue, and treatment with the exosomes obviously ameliorated these changes (P < 0.05).
Conclusion
BMSCs-derived exosomes protects against testicular IRI due to the anti-oxidant, antiinflammatory and anti-apoptosis activities of the exosomes.
Keywords: exosomes, bone marrow mesenchymal stem cells, testis, ischemia-reperfusion injury, antioxidant, inflammation, apoptosis
睾丸扭转又称精索扭转,是泌尿外科常见的急症,常见于新生儿和青少年时期[1]。睾丸缺血再灌注损伤(IRI)是睾丸扭转后手术复位的主要病理生理过程,其损害男性生精功能,甚至致使睾丸坏死萎缩从而导致不育[2-3]。睾丸IRI机理复杂,由多种因素共同参与,目前认为其主要与氧自由基大量产生、中性粒细胞聚集、炎性细胞因子过表达等因素密切相关[3-5]。及时针对灌注损伤早期产生大量的氧自由基及炎性细胞因子进行预处理可以减轻睾丸IRI。研究发现通过外源性的抗氧化药物、自由基清除剂[6-8]、抗炎症药物[9-10]等可以减轻睾丸IRI,改善睾丸的生精功能。因此,及时的外科干预联合有效的抗氧化、抗炎药物等干预措施是治疗睾丸扭转的重要手段,能有效减少睾丸损害改善睾丸生精。
间充质干细胞(MSCs)是一种具有多种分化潜能的细胞,其在治疗组织器官缺血再灌注损伤中发挥抗炎、抗氧化、抗凋亡等[11-13]多种功能作用,但目前对异基因MSCs输注后在体内的增殖、分化、寿命以及以此带来的免疫调节功能的变化的认识尚不明确,这使得MSCs的临床应用受到限制[14]。有研究表明MSCs所分泌的外泌体在心肌损伤、急性肝、肾损伤[15-19]、神经再生[20]、肺损伤[21]等方面发挥着主要作用,而且因其低免疫原性使MSCs外泌体的应用较MSCs直接应用更为安全,可开启非细胞治疗的方法。目前骨髓间充质干细胞(BMSCs)源性外泌体在睾丸IRI的作用及其相关机制尚不清楚。因此,本研究主要通过原代培养及提取骨髓间充质干细胞来源的外泌体,在进一步建立大鼠睾丸缺血再灌注损伤模型上,探讨骨髓间充质干细胞来源的外泌体对睾丸缺血再灌注损伤的保护作用,为探索BMSCs来源的外泌体治疗睾丸IRI提供新的思路和理论依据。
1. 材料和方法
1.1. 材料
1.1.1. 实验动物及分组
7~8周龄健康雄性SD大鼠(SPF级)24只,体质量为240~300 g,购自南方医科大学实验动物中心,采用完全随机方法分成3组。A:假手术组(Sham);B:生理盐水组(I/R+NS);C:外泌体处理组(I/R+BMSCs-exo),100 μg/mL;每组8只。
1.1.2. 主要仪器及试剂
低温超高速智能超速离心机(Beckman);纳米颗粒跟踪分析仪(NanoSight);透射电子显微镜(Hitachi);0.45 μm和0.22 μm滤器(Millipore);电泳仪和电转仪(Bio-Rad);PVDF膜(BioTrace);总蛋白提取试剂盒(Beyotime);蛋白浓度测定试剂盒(Beyotime);CD63,TSG101,ALIX多克隆抗体(abconal);Calnexin多克隆抗体(Bioworld);HMGB1,caspase-3,剪切型caspase-3单克隆抗体(abconal);CD90、CD44、CD34抗体及同型对照(BD);HRP标记羊抗兔IgG(Ray Antibody);超氧化物歧化酶(SOD)、丙二醛(MDA)(比色法)检测试剂盒(建成生物)。
1.2. 方法
1.2.1. BMSCs的原代分离,培养及鉴定
根据文献[22]所述,雄性SPF级SD大鼠颈椎脱臼处死,无菌条件下取出股骨与胫骨。L-DEME液冲洗骨髓腔,获得的骨髓冲洗液,1000 r/min离心5 min,重悬后继续培养。24 h后,全量换液,之后每隔2~3 d换液1次,去除未贴壁的细胞。重复上述操作,当细胞传代扩增至P4代时,用于后续实验。制备P4代BMSCs细胞悬液,采用流式细胞仪检测BMSCs表面标志物表达。取P4代BMSCs,分别加入成骨细胞诱导液、成脂细胞诱导液,21 d后进行细胞爬片并固定,分别对诱导分化的成骨细胞、脂肪细胞进行茜素红染色以及油红O染色,显微镜下观察诱导分化结果。
1.2.2. BMSCs来源外泌体的提取及鉴定
超速离心去除FBS中的外泌体待用,P3-6代BMSCs融合至70%左右时将培养基更换为含10%去外泌体FBS的低糖DMEM培养基,48 h后收集上清。利用超速离心方法从BMSCs培养上清中获取BMSCs-exo,分别采用透射电镜观察外泌体形态特征,纳米颗粒跟踪分析其粒径大小及蛋白免疫印迹术检测其表面标志物CD63,TSG101,ALIX,利用BCA蛋白定量法对获取的外泌体进行定量。
1.2.3. 睾丸IRI动物模型的建立
手术全程在无菌术室进行,睾丸缺血再灌注模型采用Turner法[23]建立:予2%戊巴比妥钠(50 mg/kg)腹腔注射麻醉后,左下腹小切口,游离左侧睾丸,切断睾丸引带,分离周围组织至附睾头。假手术组仅将左侧睾丸游离后还纳睾丸,不予扭转,缝合切口后术毕。其他两组待左侧睾丸完全游离后, 睾丸顺时针扭转720°,固定扭转侧睾丸防止自然复位,维持4 h后原切口打开复位扭转睾丸,关闭切口,全程用湿润的无菌纱布覆盖切口。复位前30 min,手术组分别尾静脉注射等量的外泌体(100 μg/mL)和生理盐水,复位维持4 h。术后取各组大鼠睾丸,将所有睾丸标本沿中轴切开分两部分,用于组织的石蜡包埋和置-80 ℃冰箱中保存待测。
1.2.4. HE染色观察睾丸组织病理变化
采集各组扭转侧睾丸组织,置于10%中性甲醛溶液中浸泡固定24 h,梯度酒精脱水,二甲苯透明石蜡包埋,制备厚度为5 μm的连续切片,常规HE染色。光镜下进行各组睾丸生精结构组织形态学观察。用改良的Johnson评分法[24]评估组织病理学评分,评分标准如下:10分生精功能正常;9分许多晚期精子细胞,排列紊乱,管腔内有脱落生精细胞;8分每小管小于5条精子,后期精子细胞较少;7分无精子和后期精子细胞,早期精子细胞较多;6分无精子和后期精子细胞,早期精子细胞较少;5分无精子和精子细胞,精母细胞较多;4分无精子和精子细胞,精母细胞较少;3分仅有精原细胞;2分无生精细胞,仅有支持细胞;1分曲细精管无生精上皮。
1.2.5. 睾丸组织SOD,MDA的检测
组织匀浆的制备:准确称取各组睾丸组织质量并记录,以无菌生理盐水作为匀浆介质,按质量体积比为1:9加匀浆介质制备成10%的组织匀浆,BCA试剂盒检测各自匀浆的浓度,严格根据SOD与MDA各自说明书不同要求进行操作并计算。
1.2.6. Western blot检测睾丸组织
caspase-3,剪切型caspase-3和HMGB1提取睾丸组织蛋白和外泌体悬液添加上样缓冲液,95 ℃,变性10 min,每个上样孔添加40 μg样品,10% SDS-PAGE凝胶电泳(80 V,30 min;100 V,60 min)分离蛋白,湿法(200 mA,120 min)转印至PVDF膜,用含5%牛血清白蛋白的TBS液室温封闭1 h,对应的抗体严格根据说明书参考配置相应比例抗体浓度,分别置于4 ℃过夜,TBST液洗膜5次,5 min/次,二抗兔抗羊IgG 1:10 000室温孵育1 h,TBST液洗膜5次,5 min/次,Bio-Rad凝胶成像系统获取图像。
1.3. 统计学分析
实验数据用Graph Pad Prism 7进行统计分析,正态分布的计量资料用均数±标准差表示,多组比较用单因素方差分析,组间两两比较方差齐时用LSD-t检验,方差不齐时用Dunnett-t检验,检验水准为α=0.05;组织病理学评分用Tukey's Multiple Comparison Test,检验分析用GraphPad Prism 7统计软件进行。
2. 结果
2.1. BMSCs的鉴定
通过贴壁法纯化的BMSCs以集落方式迅速增殖,呈梭形或多角形贴壁生长。细胞增殖明显,高度融合,呈漩涡状或辐射状排列。经成骨细胞诱导液培养后,可见细胞形态由长梭形变为多角形,胞核变大,呈圆形或卵圆形,细胞可呈多层重叠排列生长。茜素红S染色可见间质内沉积的钙化结节,经成脂细胞诱导液培养后,细胞形态变为短梭形,细胞中可见充满脂滴的脂肪细胞形成,油红O染色可见脂滴为红色(图 1A~C)。BMSCs特异性表达如CD29,CD44,CD90和CDl05等表面标志,而缺乏CD45,CD34。本实验流式检测表明所培养细胞为CD90+CD44+CD34-MSC(图 1D)。
1.
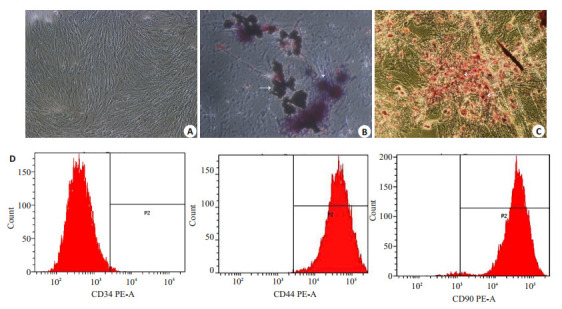
骨髓间充质干细胞(BMSCs)的鉴定
Identification of bone marrow mesenchymal stem cells (BMSCs). A: Normal BMSCs; B: Osteogenic induction of the cells stained by alizarin red S (arrow); C: Adipogenic induction of the cells stained by oil red O (arrow); D: Detection of BMSCs markers CD34(-), CD90(+), and CD44(+) using flow cytometry
2.2. BMSCs来源外泌体的鉴定
透射电子显微镜下观察提取的外泌体呈圆形、椭圆形的囊泡,有完整双层膜包绕,内部含有低电子密度物质。纳米颗粒跟踪分析仪(NTA)分析其直径约在30~ 150 nm之间,峰值约为109 nm。Western blot检测外泌体蛋白标志物均阳性表达CD63,ALIX,TSG101蛋白,且不含细胞内质网Calnexin蛋白(图 2)。
2.
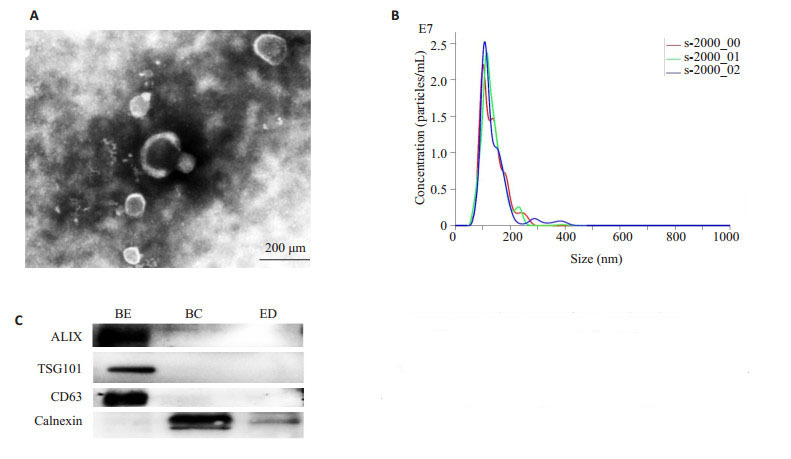
骨髓间充质干细胞(BMSCs)来源外泌体的鉴定
Identification of exosomes derived from rat BMSCs. A: Transmission electron microscopy; B: Nanoparticle tracking analysis; C: Western blotting for the markers of the exosomes. BE: BMSCsderived exosomes; BC: BMSCs; ED: Exosome depleted
2.3. HE染色观察睾丸组织病理改变
假手术组睾丸形态正常,结构层次分明,生殖细胞排列整齐有序,有正常的生精小管和生精细胞。与假手术(Sham)组比,I/R+NS组睾丸组织损伤最显著,表现为管腔内可见大量渗出,空泡形成。生精细胞排列紊乱,细胞层次不清,生精上皮或一定程度缺失或脱落。而I/ R+BMSCs-exo治疗组睾丸结构虽紊乱但有一定层次,生殖细胞排列紧密,生精细胞空泡形成和杂乱无章的上皮细胞减少;睾丸生精结构的HE评分,I/R+BMSCsexo组较I/R+NS组有一定修复作用(图 3)。
3.
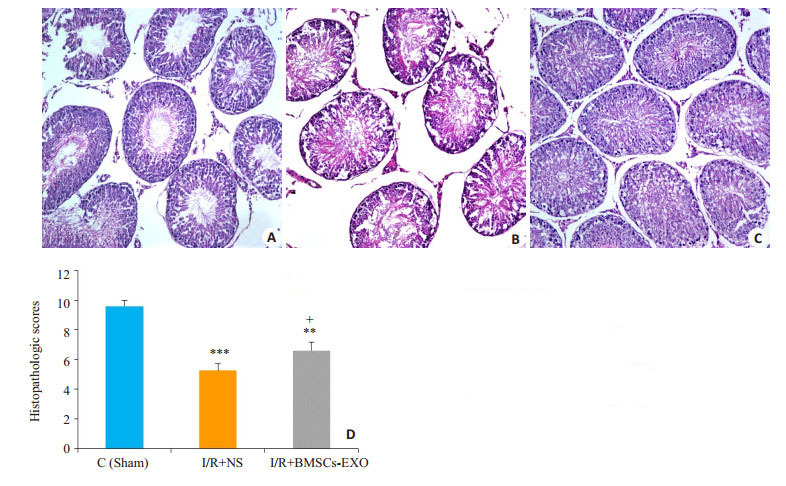
大鼠睾丸组织病理改变
Pathological changes of rat testis (HE staining, × 20). A: Sham group; B: I/R + NS group; C: I/R + BMSCs-exo group; D: Histopathologic scores of testicular tissue in the 3 groups (Mean±SD, n=4 or 5). ***P < 0.001, **P < 0.01 vs sham group; +P < 0.05 vs I/R + NS group
2.4. 检测睾丸组织SOD活性与MDA含量
睾丸组织生化指标检测中,B组(I/R+NS)组织中的MDA的含量较A组(Sham)明显升高,而SOD的活性与A组(Sham)相比则降低(P < 0.01),而在经外泌体处理之后的C组(I/R+BMSCs-exo)中,与生理盐水处理组B组相比,组织中MDA的含量有一定程度降低而SOD的水平则上调,差异均具有统计学意义(P < 0.05,图 4)。
4.
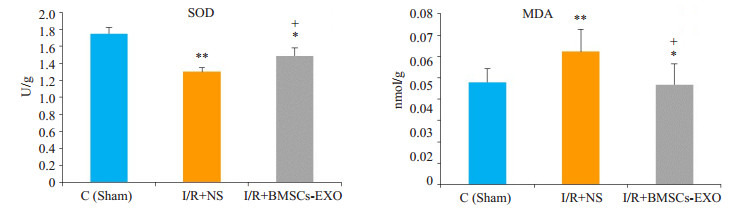
各组睾丸组织SOD活性和MDA含量变化
Changes of SOD activity and MDA content in rat testis (Mean ± SD, n=4 or 5). **P < 0.01, *P < 0.05 vs sham group; +P < 0.05 vs I/R+NS group
2.5. Western blot法检测睾丸组织剪切型caspase-3, caspase-3和HMGB1水平
与假手术组(Sham)相比,I/R+NS组睾丸组织中caspase-3,剪切型caspase-3和HMGB1表达水平升高。经BMSCs-exo处理后的组织中caspase-3,剪切型caspase-3以及HMGB1表达水平下调,两组表达量差异有统计学意义(P < 0.05,图 5、6)。
5.
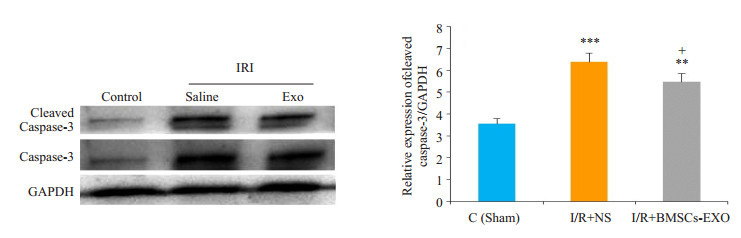
剪切型caspase-3蛋白水平检测
Protein level of cleaved caspase-3 in the testicular tissue in different groups determined by Western blotting (Mean±SD, n=4). ***P < 0.001, **P < 0.01 vs control group; +P < 0.05 vs I/R+NS group
6.

HMGB1蛋白水平检测
Protein level of HMGB1 in testicular tissue in different groups determined by Western blotting (Mean±SD, n= 4). ***P < 0.001, **P < 0.01 vs control group; +P < 0.05 vs I/R+NS group
3. 讨论
睾丸扭转后复位是一种典型的缺血再灌注损伤(IRI)病理生理过程[3],其产生大量氧自由基、激活炎症反应,造成睾丸生精功能损害,是导致男性不育原因之一。既往研究报道通过抗氧化、抗炎症等药物治疗可减轻睾丸IRI,改善睾丸生精功能,然而目前临床上尚无特异的治疗药物,因此有效的药物治疗对此具有重大的临床意义。既往研究报道间充质干细胞来源的外泌体可通过抗氧化作用、抗炎症反应、抗细胞凋亡等效应能有效改善心脏,肾脏,肝脏等[15-19]组织器官IRI,而骨髓间充质干细胞来源的外泌体在睾丸IRI中的作用及其机制目前尚不清楚。
本研究在建立大鼠睾丸IRI模型的基础上,初步探索了大鼠BMSCs来源的外泌体对睾丸IRI的作用及其相关机制。首先,从3~4周SD大鼠骨髓中原代分离培养至第三代骨髓间充质干细胞,分别对其成功进行成骨、成脂诱导,并应用流式细胞仪方法鉴定该细胞阳性表达CD90、CD44,而缺乏CD34抗原,这些结果也与目前文献报道骨髓间充质干细胞特点一致。外泌体是一种直径大小约30~150 nm的微囊泡,其表面常特异性表达CD63、TSG101、ALIX等[25]蛋白,而缺乏Calnexin蛋白表达,这与我们鉴定的BMSCs来源的外泌体特点一致。
在正常生理状态下,机体氧化与抗氧化处于动态平衡状态,而睾丸IRI过程产生大量活性氧自由基及大量炎性介质,这严重损害睾丸的生精功能。曾有研究表明睾丸IRI中睾丸组织SOD活性降低,其清除超氧阴离子自由基能力下降,同时MDA含量升高,睾丸组织受氧自由基破坏加重[26-27]。在本研究动物模型中,睾丸IRI组与对照组相比,其睾丸SOD活性显著降低,而MDA含量也显著增高,同时睾丸IRI组睾丸组织HE评分显著低于对照组,这提示睾丸IRI中抗氧化与氧化动态平衡被打破,过多的活性氧可损害睾丸生精细胞,从而导致不育的可能。我们发现在接受BMSCs来源的外泌体处理后,较对照组相比,睾丸组织的SOD活性增加,MDA含量下降,同样睾丸组织HE评分也增高,这初步表明BMSCs来源的外泌体可减轻睾丸IRI发挥保护睾丸生精的作用,但其具体机制尚不清楚。
睾丸IRI产生大量活性氧自由基及大量炎症因子,可致使生精细胞过度凋亡从而导致不育[28]。细胞的凋亡受半胱氨酸蛋白酶(caspase)家族的调节,而caspase-3是不同凋亡途径的关键汇集点,剪切型caspase-3的活化则提示凋亡已发生,可间接反应细胞凋亡情况[29-30]。既往研究表明睾丸IRI致使caspase-3升高[31-33],影响睾丸的生精功能。为了进一步探索BMSCs来源的外泌体对睾丸IRI的分子机制,我们研究表明经过BMSCs来源的外泌体处理实验组中睾丸组织的剪切型caspase-3蛋白表达降低,提示BMSCs来源的外泌体可能对睾丸IRI有抗凋亡的保护作用。另一方面,高迁移率族蛋白B1(HMGB1)被认为是机体炎症反应的重要介质,有研究报道它可通过刺激免疫应答和炎症加重肾小管缺血-再灌注损伤[34],又能通过增强TNF-a/JNK的作用而促进缺血再灌注诱导的心肌细胞凋亡[35],此外HMGB1拮抗剂还可显著抑制炎症,从而减轻脑、肝、肾IRI[36-39]。本实验中,睾丸IRI组中炎症蛋白HMGB1表达量明显升高, 而经BMSCs外泌体处理后,睾丸组织中HMGB1显著下调,这也提示着BMSCs来源的外泌体对睾丸IRI具有抗炎症的保护作用。
综上所述,BMSCs来源的外泌体可通过抗氧化、抗炎症、抗凋亡等作用减轻睾丸IRI,从而改善睾丸生精功能,但其具体作用机制还不清楚,仍需我们对其进行更深入的探讨。我们相信随着研究的不断深入,外泌体内容物及其功能、作用机制也将更为明了,这将为间充质干细胞外泌体在临床上治疗睾丸IRI提供一个新的思路和理论依据。
Biographies
张万松,在读硕士研究生,E-mail: 497510495@qq.com
杨诚,医师,E-mali: yaking91@163.com
Funding Statement
国家自然科学基金(81772257)
Supported by National Natural Science Foundation of China (81772257)
Contributor Information
张 万松 (Wansong ZHANG), Email: 497510495@qq.com.
杨 诚 (Cheng YANG), Email: yaking91@163.com.
刘 存东 (Cundong LIU), Email: cundongliu@163.com.
References
- 1.Tuglu D, Yuvanc E, Yilmaz E, et al. The antioxidant effect of dexmedetomidine on testicular ischemia-reperfusion injury. Acta Cir Bras. 2015;30(6):414–21. doi: 10.1590/S0102-865020150060000007. [Tuglu D, Yuvanc E, Yilmaz E, et al. The antioxidant effect of dexmedetomidine on testicular ischemia-reperfusion injury[J]. Acta Cir Bras, 2015, 30(6): 414-21.] [DOI] [PubMed] [Google Scholar]
- 2.Barada JH, Weingarten JL, Cromie WJ. Testicular salvage and age-related delay in the presentation of testicular torsion. J Urol. 1989;142(3):746–8. doi: 10.1016/S0022-5347(17)38875-4. [Barada JH, Weingarten JL, Cromie WJ. Testicular salvage and age-related delay in the presentation of testicular torsion[J]. J Urol, 1989, 142(3): 746-8.] [DOI] [PubMed] [Google Scholar]
- 3.Turner TT, Tung KS, Tomomasa H, et al. Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol Reprod. 1997;57(6):1267–74. doi: 10.1095/biolreprod57.6.1267. [Turner TT, Tung KS, Tomomasa H, et al. Acute testicular ischemia results in germ cell-specific apoptosis in the rat[J]. Biol Reprod, 1997, 57(6): 1267-74.] [DOI] [PubMed] [Google Scholar]
- 4.Pagliaro P. Differential biological effects of products of nitric oxide (NO)synthase: it is not enough to say NO. Life Sci. 2003;73(17):2137–49. doi: 10.1016/S0024-3205(03)00593-9. [Pagliaro P. Differential biological effects of products of nitric oxide (NO)synthase: it is not enough to say NO[J]. Life Sci, 2003, 73 (17): 2137-49.] [DOI] [PubMed] [Google Scholar]
- 5.Zini A, Abitbol J, Girardi SK, et al. Germ cell apoptosis and endothelial nitric oxide synthase (eNOS) expression following ischemia-reperfusion injury to testis. Arch Androl. 1998;41(1):57–65. doi: 10.3109/01485019808988547. [Zini A, Abitbol J, Girardi SK, et al. Germ cell apoptosis and endothelial nitric oxide synthase (eNOS) expression following ischemia-reperfusion injury to testis[J]. Arch Androl, 1998, 41(1): 57-65.] [DOI] [PubMed] [Google Scholar]
- 6.Ozbek O, Altintas R, Polat A, et al. The protective effect of apocynin on testicular ischemia-reperfusion injury. J Urol. 2015;193(4):1417–22. doi: 10.1016/j.juro.2014.11.086. [Ozbek O, Altintas R, Polat A, et al. The protective effect of apocynin on testicular ischemia-reperfusion injury[J]. J Urol, 2015, 193(4): 1417-22.] [DOI] [PubMed] [Google Scholar]
- 7.Dilber Y, Inan S, Ercan GA, et al. The role of CAPE in PI3K/AKT/ mTOR activation and oxidative stress on testis torsion. Acta Histochem. 2016;118(1):31–7. doi: 10.1016/j.acthis.2015.11.004. [Dilber Y, Inan S, Ercan GA, et al. The role of CAPE in PI3K/AKT/ mTOR activation and oxidative stress on testis torsion[J]. Acta Histochem, 2016, 118(1): 31-7.] [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Xia ZY, Meng QT, et al. Shen-Fu injection preconditioning inhibits myocardial ischemia-reperfusion injury in diabetic rats: activation of eNOS via the PI3K/Akt pathway. https://www.hindawi.com/journals/bmri/2011/384627/fig2/ J Biomed Biotechnol. 2011;2011:384627. doi: 10.1155/2011/384627. [Wu Y, Xia ZY, Meng QT, et al. Shen-Fu injection preconditioning inhibits myocardial ischemia-reperfusion injury in diabetic rats: activation of eNOS via the PI3K/Akt pathway[J]. J Biomed Biotechnol, 2011, 2011: 384627.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fouad AA, Qutub HO, Jresat I. Dose-dependent protective effect of baicalin against testicular torsion-detorsion in rats. https://onlinelibrary.wiley.com/doi/full/10.1111/and.12580#references-section. Andrologia. 2017;49(1):10. doi: 10.1111/and.12580. [Fouad AA, Qutub HO, Jresat I. Dose-dependent protective effect of baicalin against testicular torsion-detorsion in rats[J]. Andrologia, 2017, 49(1): 10.] [DOI] [PubMed] [Google Scholar]
- 10.Un H, Bayir Y, Halici Z, et al. The effects of RAAS inhibition in rate limiting step by aliskiren on testicular torsion injury in rats. J Urol. 2015;194(3):828–33. doi: 10.1016/j.juro.2015.03.117. [Un H, Bayir Y, Halici Z, et al. The effects of RAAS inhibition in rate limiting step by aliskiren on testicular torsion injury in rats[J]. J Urol, 2015, 194(3): 828-33.] [DOI] [PubMed] [Google Scholar]
- 11.Timmers L, Lim SK, Arslan F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. http://cardiovascres.oxfordjournals.org/external-ref?access_num=19383393&link_type=MED. Stem Cell Res. 2007;1(2):129–37. doi: 10.1016/j.scr.2008.02.002. [Timmers L, Lim SK, Arslan F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium[J]. Stem Cell Res, 2007, 1(2): 129-37.] [DOI] [PubMed] [Google Scholar]
- 12.Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180(11):1122–30. doi: 10.1164/rccm.200902-0242OC. [Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease[J]. Am J Respir Crit Care Med, 2009, 180(11): 1122-30.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Koppen A, Joles JA, Van Balkom BW, et al. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS One. 2012;7(6):e38746. doi: 10.1371/journal.pone.0038746. [Van Koppen A, Joles JA, Van Balkom BW, et al. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease[J]. PLoS One, 2012, 7(6): e38746.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Si YL, Zhao YL, Hao HJ, et al. MSCs: biological characteristics, clinical applications and their outstanding concerns. Ageing Res Rev. 2011;10(1, SI):93–103. doi: 10.1016/j.arr.2010.08.005. [Si YL, Zhao YL, Hao HJ, et al. MSCs: biological characteristics, clinical applications and their outstanding concerns[J]. Ageing Res Rev, 2011, 10(1, SI): 93-103.] [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Jin X, Hu CF, et al. Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy via AMPK and Akt pathways. Cell Physiol Biochem. 2017;43(1):52–68. doi: 10.1159/000480317. [Liu L, Jin X, Hu CF, et al. Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy via AMPK and Akt pathways [J]. Cell Physiol Biochem, 2017, 43(1): 52-68.] [DOI] [PubMed] [Google Scholar]
- 16.Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cellderived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301–12. doi: 10.1016/j.scr.2013.01.002. [Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cellderived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury[J]. Stem Cell Res, 2013, 10(3): 301-12.] [DOI] [PubMed] [Google Scholar]
- 17.Zhu F, Chong Lee Shin OLS, Pei G, et al. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKICKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget. 2017;8(41):70707–26. doi: 10.18632/oncotarget.19979. [Zhu F, Chong Lee Shin OLS, Pei G, et al. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKICKD transition through tubular epithelial cell dependent Sox9 activation[J]. Oncotarget, 2017, 8(41): 70707-26.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nong KT, Wang WW, Niu X, et al. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy. 2016;18(12):1548–59. doi: 10.1016/j.jcyt.2016.08.002. [Nong KT, Wang WW, Niu X, et al. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats[J]. Cytotherapy, 2016, 18(12): 1548-59.] [DOI] [PubMed] [Google Scholar]
- 19.Du Y, Li D, Han C, et al. Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (hiPSCMSCs) Protect Liver against Hepatic Ischemia/Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway. Cell Physiol Biochem. 2017;43(2):611–25. doi: 10.1159/000480533. [Du Y, Li D, Han C, et al. Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (hiPSCMSCs) Protect Liver against Hepatic Ischemia/Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway[J]. Cell Physiol Biochem, 2017, 43(2): 611-25.] [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Han S. Modified exosomes reduce apoptosis and ameliorate neural deficits induced by traumatic brain injury. http://europepmc.org/abstract/MED/29762232. ASAIO J. 2018 doi: 10.1097/MAT.0000000000000810. [Wang B, Han S. Modified exosomes reduce apoptosis and ameliorate neural deficits induced by traumatic brain injury[J]. ASAIO J, 2018, [Epub ahead of print].] [DOI] [PubMed] [Google Scholar]
- 21.Tan JL, Lau SN, Leaw B, et al. Amnion epithelial Cell-Derived exosomes restrict lung injury and enhance endogenous lung repair. Stem Cells Transl Med. 2018;7(2):180–96. doi: 10.1002/sct3.2018.7.issue-2. [Tan JL, Lau SN, Leaw B, et al. Amnion epithelial Cell-Derived exosomes restrict lung injury and enhance endogenous lung repair [J]. Stem Cells Transl Med, 2018, 7(2): 180-96.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phinney DG. Isolation of mesenchymal stem cells from murine bone marrow by immunodepletion. https://link.springer.com/protocol/10.1007/978-1-60327-169-1_12. Methods Mol Biol. 2008;449:171–86. doi: 10.1007/978-1-60327-169-1_12. [Phinney DG. Isolation of mesenchymal stem cells from murine bone marrow by immunodepletion[J]. Methods Mol Biol, 2008, 449: 171-86.] [DOI] [PubMed] [Google Scholar]
- 23.Turner TT, Bang HJ, Lysiak JL. The molecular pathology of experimental testicular torsion suggests adjunct therapy to surgical repair. http://d.old.wanfangdata.com.cn/NSTLQK/10.1097-01.ju.0000144203.30718.19/ J Urol. 2004;172(6 Pt 2):2574–8. doi: 10.1097/01.ju.0000144203.30718.19. [Turner TT, Bang HJ, Lysiak JL. The molecular pathology of experimental testicular torsion suggests adjunct therapy to surgical repair[J]. J Urol, 2004, 172(6 Pt 2): 2574-8.] [DOI] [PubMed] [Google Scholar]
- 24.Gozen A, Demiryurek S, Taskin A, et al. Protective activity of ischemic preconditioning on rat testicular ischemia: effects of Y- 27632 and 5-hydroxydecanoic acid. J Pediatr Surg. 2013;48(7):1565–72. doi: 10.1016/j.jpedsurg.2012.10.074. [Gozen A, Demiryurek S, Taskin A, et al. Protective activity of ischemic preconditioning on rat testicular ischemia: effects of Y- 27632 and 5-hydroxydecanoic acid[J]. J Pediatr Surg, 2013, 48(7): 1565-72.] [DOI] [PubMed] [Google Scholar]
- 25.Shabbir A, Cox A, Rodriguez-Menocal LA, et al. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24(14):1635–47. doi: 10.1089/scd.2014.0316. [Shabbir A, Cox A, Rodriguez-Menocal LA, et al. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro [J]. Stem Cells Dev, 2015, 24(14): 1635-47.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal A, Said TM: Oxidative stress. DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005;95(4):503–7. doi: 10.1111/bju.2005.95.issue-4. [Agarwal A, Said TM: Oxidative stress. DNA damage and apoptosis in male infertility: a clinical approach[J]. BJU Int, 2005, 95(4): 503- 7.] [DOI] [PubMed] [Google Scholar]
- 27.Fakouri A, Asghari A, Akbari G, et al. Effects of folic acid administration on testicular ischemia/reperfusion injury in rats. Acta Cir Bras. 2017;32(9):755–66. doi: 10.1590/s0102-865020170090000008. [Fakouri A, Asghari A, Akbari G, et al. Effects of folic acid administration on testicular ischemia/reperfusion injury in rats[J]. Acta Cir Bras, 2017, 32(9): 755-66.] [DOI] [PubMed] [Google Scholar]
- 28.Filho DW, Torres MA, Bordin AL, et al. Spermatic cod torsion, reactive Oxygen and Nitrogen species and ischemia-reperfusion injury. http://www.sciencedirect.com/science/article/pii/S0098299704000214. Mol Aspects Med. 2004;25(1/2):199–210. doi: 10.1016/j.mam.2004.02.020. [Filho DW, Torres MA, Bordin AL, et al. Spermatic cod torsion, reactive Oxygen and Nitrogen species and ischemia-reperfusion injury[J]. Mol Aspects Med, 2004, 25(1/2): 199-210.] [DOI] [PubMed] [Google Scholar]
- 29.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6(2):99–104. doi: 10.1038/sj.cdd.4400476. [Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis [J]. Cell Death Differ, 1999, 6(2): 99-104.] [DOI] [PubMed] [Google Scholar]
- 30.Fan TJ, Han LH. Cong RS et al:caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005;37(11):719–27. doi: 10.1111/abbs.2005.37.issue-11. [Fan TJ, Han LH. Cong RS et al:caspase family proteases and apoptosis[J]. Acta Biochim Biophys Sin (Shanghai), 2005, 37(11): 719-27.] [DOI] [PubMed] [Google Scholar]
- 31.Gultekin A, Tanriverdi HI, Inan S, et al. The effect of tunica albuginea incision on testicular tissue after detorsion in the experimental model of testicular torsion. http://journals.sbmu.ac.ir/urolj/index.php/uj/article/view/3804/1372. Urol J. 2018;15(1):32–9. doi: 10.22037/uj.v0i0.3804. [Gultekin A, Tanriverdi HI, Inan S, et al. The effect of tunica albuginea incision on testicular tissue after detorsion in the experimental model of testicular torsion[J]. Urol J, 2018, 15(1): 32-9.] [DOI] [PubMed] [Google Scholar]
- 32.Boettcher M, Meier D, Jiménez-Alcázar M, et al. Degradation of extracellular DNA by DNase1 significantly reduces testicular damage after testicular torsion in rats. http://www.ncbi.nlm.nih.gov/pubmed/28774773. Urology. 2017;109(223):223. doi: 10.1016/j.urology.2017.07.031. [Boettcher M, Meier D, Jiménez-Alcázar M, et al. Degradation of extracellular DNA by DNase1 significantly reduces testicular damage after testicular torsion in rats[J]. Urology, 2017, 109(223): 223.e1-7.] [DOI] [PubMed] [Google Scholar]
- 33.Kostakis ID, Zavras N, Damaskos C, et al. Erythropoietin and sildenafil protect against ischemia/reperfusion injury following testicular torsion in adult rats. Exp Ther Med. 2017;13(6):3341–7. doi: 10.3892/etm.2017.4441. [Kostakis ID, Zavras N, Damaskos C, et al. Erythropoietin and sildenafil protect against ischemia/reperfusion injury following testicular torsion in adult rats[J]. Exp Ther Med, 2017, 13(6): 3341-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CB, Liu LS, Zhou J, et al. Up-Regulation of HMGB1 exacerbates renal Ischemia-Reperfusion injury by stimulating inflammatory and immune responses through the TLR4 signaling pathway in mice. Cell Physiol Biochem. 2017;41(6):2447–60. doi: 10.1159/000475914. [Chen CB, Liu LS, Zhou J, et al. Up-Regulation of HMGB1 exacerbates renal Ischemia-Reperfusion injury by stimulating inflammatory and immune responses through the TLR4 signaling pathway in mice[J]. Cell Physiol Biochem, 2017, 41(6): 2447-60.] [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Yao YW, Su ZL, et al. Endogenous HMGB1 contributes to ischemia-reperfusion-induced myocardial apoptosis by potentiating the effect of TNF-alpha/JNK. Am J Physiol Heart Circ Physiol. 2011;300(3):H913–21. doi: 10.1152/ajpheart.00703.2010. [Xu H, Yao YW, Su ZL, et al. Endogenous HMGB1 contributes to ischemia-reperfusion-induced myocardial apoptosis by potentiating the effect of TNF-alpha/JNK[J]. Am J Physiol Heart Circ Physiol, 2011, 300(3): H913-21.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong G, Xiang L, Yuan LB, et al. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/ReperfusionInduced inflammation, oxidative stress, and apoptosis in rats. PLoS One. 2014;9(3):e89450. doi: 10.1371/journal.pone.0089450. [Gong G, Xiang L, Yuan LB, et al. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/ReperfusionInduced inflammation, oxidative stress, and apoptosis in rats[J]. PLoS One, 2014, 9(3): e89450.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen M, Lu J, Dai W, et al. Ethyl pyruvate ameliorates hepatic ischemia-reperfusion injury by inhibiting intrinsic pathway of apoptosis and autophagy. https://www.hindawi.com/journals/mi/2013/461536/ Mediators Inflamm. 2013;2013:461536. doi: 10.1155/2013/461536. [Shen M, Lu J, Dai W, et al. Ethyl pyruvate ameliorates hepatic ischemia-reperfusion injury by inhibiting intrinsic pathway of apoptosis and autophagy[J]. Mediators Inflamm, 2013, 2013: 461536.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao G, Fu C, Wang L, et al. Down-regulation of nuclear HMGB1 reduces ischemia-induced HMGB1 translocation and release and protects against liver ischemia-reperfusion injury. Sci Rep. 2017;7:46272. doi: 10.1038/srep46272. [Zhao G, Fu C, Wang L, et al. Down-regulation of nuclear HMGB1 reduces ischemia-induced HMGB1 translocation and release and protects against liver ischemia-reperfusion injury[J]. Sci Rep, 2017, 7: 46272.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Gong Q, Zhong S, et al. Neutralization of the extracellular HMGB1 released by ischaemic damaged renal cells protects against renal ischaemia-reperfusion injury. Nephrol Dial Transplant. 2011;26(2):469–78. doi: 10.1093/ndt/gfq466. [Li J, Gong Q, Zhong S, et al. Neutralization of the extracellular HMGB1 released by ischaemic damaged renal cells protects against renal ischaemia-reperfusion injury[J]. Nephrol Dial Transplant, 2011, 26(2): 469-78.] [DOI] [PubMed] [Google Scholar]


