Abstract
目的
探究蜂胶对Triton-WR1339所致高脂血症的降脂作用及调控脂质代谢机制。
方法
将C57BL/6小鼠随机分为7组,10只/组,雌雄各半,分别为正常组、模型组、非诺贝特组(30 mg/kg)、蜂胶HB01高剂量组(60 mg/kg)、蜂胶HB01低剂量组(30 mg/kg)、蜂胶HB02高剂量组(60 mg/kg)和蜂胶HB02低剂量组(30 mg/kg)。采用肌肉注射Triton WR-1339试剂造成高脂血症模型,灌胃1周后眼眶取血,离心后测定血清中总胆固醇(TC)、甘油三酯(TG)、高密度脂蛋白胆固醇(HDL)、低密度脂蛋白胆固醇(LDL)、丙二醛(MDA)、总超氧化物歧化酶(SOD)、谷丙转氨酶(GPT)、谷草转氨酶(GOT)等指标;取肝脏组织,置-80 ℃保存,Western blot法测定脂质转运蛋白的表达水平。
结果
与正常组相比,模型组血清中TC、TG、LDL、MDA、GPT、GOT的含量升高,HDL含量降低、SOD活力下降(P < 0.05)。与模型组比较,阳性药(非诺贝特:30 mg/kg)和两种蜂胶(高剂量组:60 mg/kg;低剂量组:30 mg/kg)都能明显降低TC、TG、LDL、MDA、GPT、GOT含量而升高HDL含量,促进SOD活力,差异有统计学意义(P < 0.05),且蜂胶的效果略优于阳性药组。与正常组相比,Triton-WR1339诱导的模型组肝脏中的ABCA1、ABCG8、低密度脂蛋白和SR-B1等脂质转运蛋白显著下降,差异有统计学意义(P < 0.05),但阳性药(非诺贝特:30 mg/kg)和蜂胶(高剂量组:60 mg/kg;低剂量组:30 mg/kg)干预后均能逆转这一下降趋势,蜂胶组的干预效果略优于阳性药组,差异有统计学意义(P < 0.05)。
结论
蜂胶具有显著的降血脂作用,其作用机制与改善肝脏脂质代谢紊乱、调控脂质代谢转运蛋白相关。
Keywords: 蜂胶, 降血脂, Triton-WR1339
Abstract
Objective
To evaluate the therapeutic effect of propolis against Triton-WR1339-induced hyperlipidemia in mice and explore the underlying mechanism.
Methods
C57BL/6 mice were randomly divided into 7 groups (n=10), including the control group, hyperlipidemia model group, fenofibrate (30 mg/kg) treatment group, and 4 treatment groups treated with low- (30 mg/kg) or high-dose (60 mg/kg) propolis HB01 or HB02. In all but the control group, acute hyperlipidemia models were established by intramuscular injection of Triton WR-1339, and corresponding treatments were administered via gastric lavage for 7 days. After the treatments, blood samples were collected for testing the levels of total cholesterol (TC), triglycerides (TG), highdensity lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), malondialdehyde (MDA), superoxide dismutase (SOD), alanine aminotransferase (GPT), and aspartate aminotransferase (GOT); Western blotting was used to detect the expressions of the proteins involved in lipid metabolism in the liver tissues including ABCA1, ABCG8, LDLR, and SR-B1.
Results
Compared with the normal control group, the mice with Triton-WR1339-induced hyperlipidemia showed significantly increased levels of TC, TG, LDL, MDA, GPT, and GOT and lowered HDL-C levels and SOD activity (P < 0.05). Treatments with fenofibrate and the 2 propolis at either low or high dose significantly reversed Triton-WR1339-induced changes in blood lipids (P < 0.05), and the effects of propolis were more potent. Triton-WR1339 injection also significantly decreased the expressions levels of ABCA1, ABCG8, LDLR, and SR-B1 in the liver (P < 0.05), and these changes were obviously reversed by treatments with fenofibrate and propolis (P < 0.05), especially by the latter.
Conclusion
The lipid-lowering effects of propolis are mediated by improving lipid metabolism and regulating the expressions of lipid transport proteins in the liver tissue.
Keywords: propolis, hypolipidemia, Triton-WR1339, lipid metabolism
高脂血症是诱发动脉粥样硬化发生及发展的危险因素,也是诱发代谢性疾病(糖尿病、高血压、脂肪肝)的病因之一。高脂血症主要危害是诱发包括动脉粥样硬化在内的心脑血管疾病[1]。因此,防治高脂血症的发生具有重要的治疗意义。
蜂胶收载于《中国药典》2015年版,功能补虚弱,化浊脂,止消渴,外用解毒消肿,收敛生肌,用于体虚早衰、高脂血症、消渴,外治皮肤皲裂,烧烫伤[3]。蜂胶具有抑菌[4],抗肿瘤[5-7],降血糖[8-10],抗氧化[11-13],抗炎[14-15],保护心脏[16-17]等功效。现有研究发现蜂胶对不同类型的高血脂症动物模型均具有降血脂的作用,其机制也都与抗氧化有关[18-20]。近年来,研究报道高血脂症与脂质蛋白的转运有密切的关系[21-23],然而,蜂胶对高血脂症中肝脏脂质转运代谢的影响尚未见文献报道。因此,本文拟对蜂胶降血脂及调控血脂转运代谢的分子机制进行研究。
1. 材料和方法
1.1. 实验动物
4周龄C57BL/6小鼠,SPF级,70只,体质量18~20 g,雌雄各半。由广东省动物实验中心提供,合格证号:[SCXK(粤)2013-0002]。
1.2. 药物、试剂与仪器
非诺贝特胶囊(法利博福尼制药,批号:23504),蜂胶(批号:HB01产地:云南省昆明邹康蜂业有限公司,HB02产地:四川省资阳市安岳县铁峰山养蜂场。制备工艺:取蜂胶粉碎,用95%乙醇浸泡溶解,滤过,滤液回收乙醇,晾干。用PEG-400混合溶剂(PEG 400-吐温80-纯水,体积比5: 1: 4)溶液溶解)全波长多功能酶标仪(Thermo scientific multiskan FC),低温离心机(Thermo scientific legend micro 17R),高压灭菌锅(ZEALWAY GI65DWS),玻璃匀浆机(宁波新芝生物科技股份有限公司DY89-Ⅱ),高速离心机(eppendorf 5810R),垂直板电泳转移装置(Bio-rad 1658004);甘油三酯(TG)试剂盒(A110- 1)、总胆固醇(TC)试剂盒(A111-1)、高密度脂蛋白胆固醇(HDL)试剂盒(A112-1)、低密度脂蛋白胆固醇(LDL)试剂盒(A113-1)、丙二醛(MDA)试剂盒(A003-2)、总超氧化物歧化酶(SOD)试剂盒(A001-3)、谷丙转氨酶(GPT)试剂盒(C009-2)、谷草转氨酶(GOT)试剂盒(C010-2),购于南京建成生物工程研究所;低密度脂蛋白受体(LDLR)抗体(ab52818),B类Ⅰ型清道夫受体(SR-B1)抗体(ab52818),腺苷三磷酸结合转运体A1(ABCA1)抗体(ab7360),腺苷三磷酸结合转运蛋白G超家族成员8(ABCG8)抗体(ab223056)购于Abcam,甘油醛- 3-磷酸脱氢酶(GAPDH)抗体(ab8425);二抗购于Cell Signaling Technology;试剂盒和抗体置于-20 ℃保存。
1.3. 动物分组
将小鼠随机分为7组,10只/组,分为正常对照组(Con)、模型组(Tr1339W)、非诺贝特组(FF)、蜂胶HB01高剂量组(PHD01)、蜂胶HB01低剂量组(PLD01)、蜂胶HB02高剂量组(PHD02)、蜂胶HB02低剂量组(PLD02)。各组每天喂食普通饲料和蒸馏水,非诺贝特及蜂胶各剂量组灌胃给药1周。给药剂量,非诺贝特组:30 mg/kg,蜂胶HB01高剂量组:60 mg/kg,蜂胶HB01低剂量组:30 mg/kg,蜂胶HB02高剂量组:60 mg/kg,蜂胶HB02低剂量组:30 mg/kg。给药第3天,模型组及给药组采用Triton WR-1339进行造模,肌注0.6 g/kg,在第5天取小鼠眼球血,10 000 r/min离心10 min后进行血脂指标测试。在进行混合型高脂血症造模的研究中,取血样后应当对新鲜血样标本即时或当天测定。按照试剂盒说明书加入试剂和酶,置于37 ℃温箱保温一定时间让酶与试剂充分混合反应,最后拿出来使用酶标仪测定D值。
1.4. Western blotting检测
用Western blotting法检测脂质代谢信号通路中ABCA1(ATP-binding cassette, sub-family A, member 1), ABCG1(ATP-binding cassette, sub-family G, member 1), LDLR(Low density lipoprotein receptor), SRB1(Scavenger receptor class B type Ⅰ)的蛋白表达情况。具体操作如下:取各组肝脏提取蛋白,BCA法蛋白定量并调平。进行聚丙烯酰胺凝胶电泳,转印至硝酸纤维素膜(PVDF膜)上。将PVDF膜上裁剪出的目的蛋白条带全部浸没在封闭液中,4 ℃过夜。取出封闭后的膜,一抗4 ℃孵育过夜,漂洗后二抗室温振荡孵育2 h。最后制备化学发光检测底物工作液检测蛋白。Western blot结果用凝胶图像分析软件Quantity one进行分析。
1.5. 统计学分析
采用SPSS 20.0和Graph-pad Prism 6.0统计软件,正态分布计量资料均采用均数±标准差表示,采用方差分析进行组间比较,采用SNK法进行两两比较。P < 0.05为差异有统计学意义。
2. 结果
2.1. 蜂胶对血脂的影响
与对照组比较,模型组小鼠血清TG、TC及LDL水平显著升高(P < 0.05),HDL水平显著降低(P < 0.05);与模型组比较,蜂胶干预后TG、TC及LDL水平均显著降低(P < 0.05),蜂胶干预后HDL水平显著升高(P < 0.05,表 1、图 1~2)。
1.
蜂胶对小鼠血脂的调控作用
Regulatory effects of propolis on blood lipid levels in mice (Mean±SD)
| Group | TG (mmol/L) | TC (mmol/L) | HDL (mmol/L) | LDL (mmol/L) |
| #P < 0.05 vs control group. *P < 0.05 vs Tr1339W group. Con: Control group; Tr1339W: Triton WR-1339 model group; FF: Triton WR-1339 + fenofibrate group; PHD01: Triton WR-1339 + Propolis HB01 high dosage group; PLD01: Triton WR-1339 + Propolis HB01 low dosage group; PHD02: Triton WR-1339 + Propolis HB02 high dosage group; PLD02: Triton WR-1339+Propolis HB02 low dosage group. | ||||
| Control | 0.92±0.85 | 4.16±0.55 | 2.53±2.56 | 1.53±0.36 |
| Tr1339W | 5.33±2.50# | 13.6±5.66# | 0.07±0.03# | 5.92±2.13# |
| FF | 0.37±0.09* | 10.19±5.32* | 4.96±2.50* | 3.19±1.35* |
| PHD01 | 3.59±1.36* | 8.35±4.13* | 4.12±2.24* | 6.33±3.77 |
| PLD01 | 2.26±1.84* | 9.90±3.69* | 4.29±1.59* | 2.57±1.57* |
| PHD02 | 4.21±2.39 | 8.16±3.59* | 0.64±0.10* | 4.22±1.47* |
| PLD02 | 4.88±2.43 | 7.96±4.54* | 2.79±1.27* | 3.48±1.72* |
1.
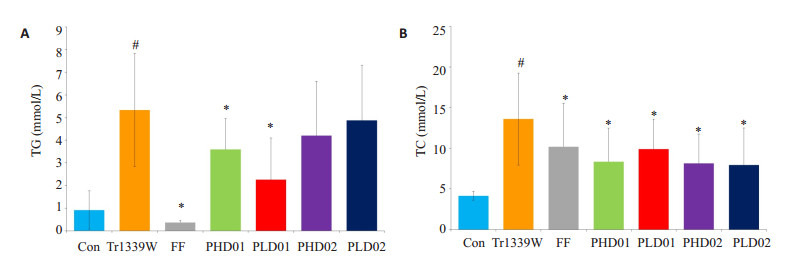
蜂胶对Triton小鼠血清中TG、TC的影响
Effect of propolis on TG (A) and TC (B) levels in Triton-1339W-induced hyperlipidemic mice. #P < 0.05 vs control group. *P < 0.05 vs Tr1339W group.
2.
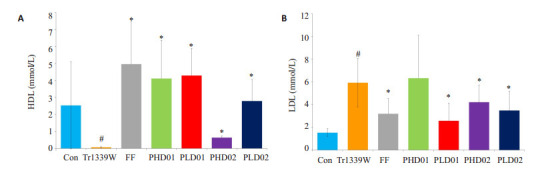
蜂胶对Triton小鼠HDL、LDL的影响
Effects of propolis on HDL (A) and LDL (B) in Triton-1339W-induced hyperlipidemic mice. #P < 0.05 vs control group. *P < 0.05 vs Tr1339W group.
2.2. 蜂胶对肝酶活性的影响
与对照组比较,模型组小鼠血清GOP及GPT水平显著升高(P < 0.05);与模型组比较,蜂胶干预后GOP及GPT水平均显著降低(P < 0.05,表 2、图 4)。
2.
蜂胶对小鼠过氧化及肝酶活性的影响
Effects of propolis on peroxidation and liver enzyme actovoty in the mice (Mean±SD)
| Group | MDA (mmol/L) | SOD (U/mgprot) | GPT (mmol/L) | GOT (mmol/L) |
| #P < 0.05 vs control group. *P < 0.05 vs Tr1339W group. | ||||
| Control | 74.80±45.12 | 20.55±3.57 | 13.44±6.36 | 45.08±21.33 |
| Tr1339W | 86.53±67.33# | 1.98±0.24# | 230.61±156.36# | 679.84±348.67# |
| FF | 34.29±19.67* | 24.24±2.47* | 33.80±20.93* | 85.59±46.48* |
| PHD01 | 23.54±12.32* | 3.05±1.01* | 95.25±67.08* | 273.06±220.25* |
| PLD01 | 19.55±10.78* | 7.85±3.30* | 65.84±83.43* | 210.59±268.17* |
| PHD02 | 33.77±22.57* | 2.27±0.83 | 115.69±76.63* | 275.30±215.59* |
| PLD02 | 13.04±5.64* | 1.37±0.40 | 103.53±91.60* | 224.45±285.75 |
4.
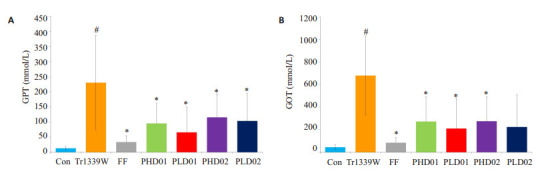
蜂胶对Triton小鼠GPT和GOT的影响
Effects of propolis on GPT (A) and GOT (B) in Triton-1339W-induced hyperlipidemic mice. #P < 0.05 vs control group. *P < 0.05 vs Tr1339W group.
2.3. 蜂胶对氧化应激的影响
与对照组比较,模型组小鼠血清SOD水平显著降低(P < 0.05),MDA水平显著降低(P < 0.05);与模型组比较,蜂胶干预后SOD水平均显著升高(P < 0.05),蜂胶干预后MDA水平显著降低(P < 0.05,表 2、图 3)。
3.
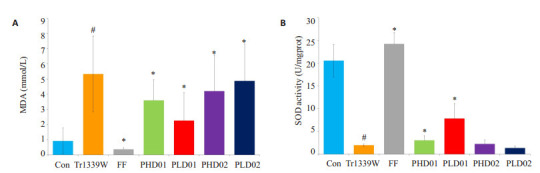
蜂胶对Triton小鼠MDA和SOD活性的影响
Effects of propolis on MDA (A) and SOD (B) activity in Triton-1339W-induced hyperlipidemic mice. #P < 0.05 vs control group. *P < 0.05 vs Tr1339W group.
2.4. 蜂胶对脂质转运蛋白(ABCA1,ABCG8,LDLR和SR-B1)的影响
采用Western blotting对脂质转运蛋白(ABCA1,ABCG8,LDLR和SR-B1)的表达水平进行检测,将各蛋白条带的灰度值与对应的内参进行比较,得到的比值制成柱状图(n=6)。与正常组比较,模型组肝脏中的ABCA1,ABCG8,LDLR和SR-B1表达量显著下降,在给予蜂胶及阳性药干预后,ABCA1,ABCG8,LDLR和SR-B1显著上升,提示蜂胶可以通过上调脂质转运蛋白的表达量(图 6)。
6.
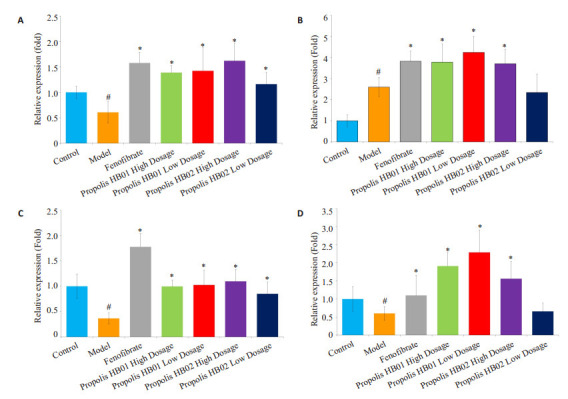
蜂胶对小鼠ABCA1,ABCG8,LDLR和SR-B1表达水平的影响
Effects of propolis on expression of ABCA1, ABCG8, LDLR and SR-B1 in mice. A: ABCA1; B: ABCG8; C: LDLR; D: SRB1. #P < 0.05 vs control group. *P < 0.05 vs Tr1339W group.
3. 讨论
蜂胶作为重要的蜂产品之一,临床上已广泛报道其对各型高脂血症患者均有效[1, 9, 12, 17]。研究发现,蜂胶可通过抗氧化来发挥降血脂的作用[18-20],但是蜂胶降血脂的其他作用机制仍未见报道。本研究采用TritonWR-1339注射小鼠造成高血脂症,观察蜂胶对小鼠血清中TC、TG、LDL和HDL含量的影响,实验结果表明,与对照组比较,模型组小鼠血清中的TC、TG、LDL水平显著升高,HDL含量显著下降(P < 0.05)。而蜂胶能明显降低高脂血症小鼠血清TC、TG、LDL含量和升高HDL的含量(P < 0.05),证实了蜂胶具有显著降血脂的作用。
通过检测肝酶活性,本研究发现Triton WR-1339注射能显著升高GOP和GPT的活性(P < 0.05),说明高血脂影响了肝酶活性,对肝脏造成损伤;但蜂胶能显著降低GOP和GPT的活性(P < 0.05),说明蜂胶在降血脂的同时还能保护肝脏。此外,本实验还证实了蜂胶能显著提高SOD的活性和降低MDA的水平(P < 0.05),验证了蜂胶是通过抗氧化来发挥降脂保肝作用[18-20]。其他降血脂的植物提取物同样也具有抗氧化的作用,如苹果多酚、北五味子多糖及胡柚皮黄酮等[27-29]。由此说明,蜂胶和其他植物提取物的相同点是可以通过提高机体的SOD活力,减轻脂质过氧化,加强自由基的消除,从而起到降血脂的作用。但是本研究中蜂胶对Triton-WR1339所致高脂血症小鼠的给药剂量较低,仅30 mg/kg,即可达到显著的抗氧化降血脂效果,而苹果多酚的给药剂量则需要高达200 mg/kg和400 mg/kg方能达到显著效果[27]。由此看出,蜂胶的抗氧化降血脂的作用较强。
5.
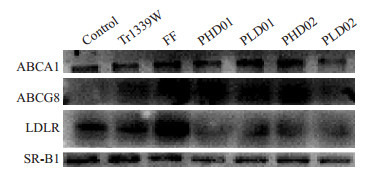
ABCA1, ABCG8, LDLR和SR-B1的蛋白表达水平
Western blotting for detecting the expressions of ABCA1, ABCG8, LDLR and SR-B1 in the liver of the mice.
本研究还探讨了蜂胶通过调控脂质代谢降血脂的作用机制。胆固醇在血管壁的过度积聚是造成动脉粥样硬化的根本原因,而胆固醇逆转运是机体排出过多胆固醇的唯一途径[30]。因此,胆固醇逆转运功能及分子机制的研究在动脉样硬化的防治过程中起到重要的作用。ABCA1和ABCG8介导的单向胆固醇流出是外周组织胆固醇流出的主要途径,可有效减少外周组织脂质的蓄积[31-32]。本研究发现,Triton WR-1339注射诱导的高血脂模型能显著降低ABCA1和ABCG8的水平,干扰了胆固醇的外排,而蜂胶可显著升高ABCA1和ABCG8的水平,说明蜂胶可通过促进胆固醇的外排起到降脂的作用。SR-BI可以改变细胞膜上的胆固醇分布,介导胆固醇的摄取和逆转运,可促进胆固醇的外排。而LDLR则可结合LDL,促进胆固醇的外排[31-32]。本研究发现,Triton WR-1339注射诱导的高脂血症模型能显著降低LDLR和SR-BI的水平,进一步阻碍胆固醇的外排,加剧血脂的升高。而蜂胶可显著升高LDLR和SR-BI的水平。鉴于肝脏中过量的胆固醇聚集是高血脂症的病因。ABCA1,ABCG8,LDLR和SR-B1都是胆固醇转运蛋白,可以促进胆固醇的外排[23-26]。许多植物提取物可通过提高ABCA1,ABCG8,LDLR和SRB1的水平,从而有效地降低血脂的水平[33-34]。本研究也证实蜂胶的干预可显著升高ABCA1,ABCG8,LDLR和SR-B1的水平。由此可以推测,蜂胶是通过提高胆固醇转运蛋白的表达水平,进而起到降低血脂的作用。
综上所述,蜂胶具有显著的降血脂作用,其作用机制不仅通过提高机体的SOD活力,减轻脂质过氧化,加强自由基的消除,还可以调控脂质代谢,提高体内胆固醇转运蛋白的水平,促进胆固醇的外排。本研究为蜂胶应用于高脂血症的治疗提供了进一步的理论基础。
Biography
黄晓其, 硕士, 讲师, E-mail: huangxiaoqi@gzucm.edu.cn
Funding Statement
广东省产学研合作项目(2014B090902002);广东高校优秀青年新人才培养计划项目(育苗项目2012LYM_0081)
Contributor Information
黄 晓其 (Xiaoqi HUANG), Email: huangxiaoqi@gzucm.edu.cn.
兰 天 (Tian LAN), Email: lantian@gdpu.edu.cn.
References
- 1.邝 枣园, 宋 梦微, 马 嫚, et al. 黄连解毒汤对高脂血症小鼠血脂及炎症因子的影响. http://d.old.wanfangdata.com.cn/Periodical/zgwsbzgl201602138. 广州中医药大学学报. 2014;31(2):268–71. [邝枣园, 宋梦微, 马嫚, 等.黄连解毒汤对高脂血症小鼠血脂及炎症因子的影响[J].广州中医药大学学报, 2014, 31(2): 268-71.] [Google Scholar]
- 2.罗 广波, 伍 家鸣, 朴 胜华, et al. 以血脂异常为主的代谢紊乱人群中医证候规律研究. http://www.cnki.com.cn/Article/CJFDTOTAL-REST201404033.htm. 广州中医药大学学报. 2014;31(4):637–9. [罗广波, 伍家鸣, 朴胜华, 等.以血脂异常为主的代谢紊乱人群中医证候规律研究[J].广州中医药大学学报, 2014, 31(4): 637-9.] [Google Scholar]
- 3.国家药典委员会 . 中华人民共和国药典(2015年版) 北京: 中国医药科技出版社; 2015. pp. 352–8. [国家药典委员会.中华人民共和国药典(2015年版) [M].北京:中国医药科技出版社, 2015: 352-8.] [Google Scholar]
- 4.周 先汉, 张 秀喜, 朱 稀檩, et al. 蜂胶提取物抑菌活性及其抑菌机理的研究. http://lib.cqvip.com/qk/81668X/200001/30349204.html. 食品科技. 2009;34(5):233–6. [周先汉, 张秀喜, 朱稀檩, 等.蜂胶提取物抑菌活性及其抑菌机理的研究[J].食品科技, 2009, 34(5): 233-6.] [Google Scholar]
- 5.郑 宇斐, 王 凯, 胡 福良. 蜂胶抗肿瘤活性及其机制的研究进展. http://www.cqvip.com/QK/96327X/201604/668673194.html. 天然产物研究与开发. 2016;28(4):627–36. [郑宇斐, 王凯, 胡福良.蜂胶抗肿瘤活性及其机制的研究进展[J].天然产物研究与开发, 2016, 28(4): 627-36.] [Google Scholar]
- 6.Ippolito E, Floreno B, Rinaldi CG, et al. Efficacy of a propolisbased syrup (FARINGEL) in preventing radiation-induced esophagitis in locally advanced lung cancer. Chemotherapy. 2018;63(2):76–82. doi: 10.1159/000487897. [Ippolito E, Floreno B, Rinaldi CG, et al. Efficacy of a propolisbased syrup (FARINGEL) in preventing radiation-induced esophagitis in locally advanced lung cancer[J]. Chemotherapy, 2018, 63(2): 76-82.] [DOI] [PubMed] [Google Scholar]
- 7.Vukovic NL, Obradovic AD, Vukic MD, et al. Cytotoxic, proapoptotic and antioxidative potential of flavonoids isolated from propolis against colon (HCT-116) and breast (MDA-MB-231) cancer cell lines. http://linkinghub.elsevier.com/retrieve/pii/S0963996917309109. Food Res Int. 2018;106(4):71–80. doi: 10.1016/j.foodres.2017.12.056. [Vukovic NL, Obradovic AD, Vukic MD, et al. Cytotoxic, proapoptotic and antioxidative potential of flavonoids isolated from propolis against colon (HCT-116) and breast (MDA-MB-231) cancer cell lines [J]. Food Res Int, 2018, 106(4): 71-80.] [DOI] [PubMed] [Google Scholar]
- 8.杨 明, 隋 殿军, 陈 文学, et al. 蜂胶总黄酮对STZ诱导糖尿病大鼠降血糖机制研究. http://d.old.wanfangdata.com.cn/Periodical/zyc201409030. 中药材. 2014;37(9):1623–6. [杨明, 隋殿军, 陈文学, 等.蜂胶总黄酮对STZ诱导糖尿病大鼠降血糖机制研究[J].中药材, 2014, 37(9): 1623-6.] [PubMed] [Google Scholar]
- 9.Aral CA, Kesim S, Greenwell H, et al. Alveolar bone protective and hypoglycemic effects of systemic propolis treatment in experimental periodontitis and diabetes mellitus. J Med Food. 2015;18(2):195–201. doi: 10.1089/jmf.2013.3137. [Aral CA, Kesim S, Greenwell H, et al. Alveolar bone protective and hypoglycemic effects of systemic propolis treatment in experimental periodontitis and diabetes mellitus[J]. J Med Food, 2015, 18(2): 195-201.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang LJ, Lee HB, Bae HJ, et al. Antidiabetic effect of propolis: reduction of expression of glucose-6-phosphatase through inhibition of Y279 and Y216 autophosphorylation of GSK-3 alpha/beta in HepG2 cells. PhytoTther Res. 2010;24(10):1554–61. doi: 10.1002/ptr.v24:10. [Kang LJ, Lee HB, Bae HJ, et al. Antidiabetic effect of propolis: reduction of expression of glucose-6-phosphatase through inhibition of Y279 and Y216 autophosphorylation of GSK-3 alpha/beta in HepG2 cells [J]. PhytoTther Res, 2010, 24(10): 1554-61.] [DOI] [PubMed] [Google Scholar]
- 11.张 江临, 王 凯, 胡 福良. 蜂胶的抗氧化活性及其分子机制研究进展. http://d.old.wanfangdata.com.cn/Periodical/zgzyzz201316021. 中国中药杂志. 2013;38(16):2645–52. [张江临, 王凯, 胡福良.蜂胶的抗氧化活性及其分子机制研究进展[J].中国中药杂志, 2013, 38(16): 2645-52.] [PubMed] [Google Scholar]
- 12.李荣玮. 金银花和蜂胶的抗氧化活性及其色谱分析研究[D]. 西安: 西北大学, 2008.
- 13.Gao WN, Pu LL, Wei JY, et al. Serum antioxidant parameters are significantly increased in patients with type 2 diabetes mellitus after consumption of Chinese propolis: a randomized controled trial based on fasting serum glucose level. Diabetes Ther. 2018;9(1):101–11. doi: 10.1007/s13300-017-0341-9. [Gao WN, Pu LL, Wei JY, et al. Serum antioxidant parameters are significantly increased in patients with type 2 diabetes mellitus after consumption of Chinese propolis: a randomized controled trial based on fasting serum glucose level [J]. Diabetes Ther, 2018, 9(1): 101-11.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.王 凯, 张 江临, 胡 福良. 蜂胶抗炎活性及其分子机制研究进展. http://d.old.wanfangdata.com.cn/Periodical/zcy201316025. 中草药. 2013;44(16):2321–9. [王凯, 张江临, 胡福良.蜂胶抗炎活性及其分子机制研究进展[J].中草药, 2013, 44(16): 2321-9.] [Google Scholar]
- 15.Bueno-Silva B, Kawamoto D, Ando-Suguimoto ES, et al. Brazilian red propolis attenuates inflammatory signaling cascade in LPSActivated macrophages. http://dx.plos.org/10.1371/journal.pone.0144954. PLoS One. 2015;10(12):e144954. doi: 10.1371/journal.pone.0144954. [Bueno-Silva B, Kawamoto D, Ando-Suguimoto ES, et al. Brazilian red propolis attenuates inflammatory signaling cascade in LPSActivated macrophages [J]. PLoS One, 2015, 10(12): e144954.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.杨 明, 隋 殿军, 朱 姝, et al. 蜂胶总黄酮对大鼠心肌缺血再灌注损伤Fas、Bax和Bcl-2基因蛋白表达的影响. 中国药理学通报. 2005;21(7):799–803. doi: 10.3321/j.issn:1001-1978.2005.07.008. [杨明, 隋殿军, 朱姝, 等.蜂胶总黄酮对大鼠心肌缺血再灌注损伤Fas、Bax和Bcl-2基因蛋白表达的影响[J].中国药理学通报, 2005, 21 (7): 799-803.] [DOI] [Google Scholar]
- 17.Alyane M, Benguedouar L, Kebsa W, et al. Cardioprotective effects and mechanism of action of polyphenols extracted from propolis against doxorubicin toxicity. http://www.ncbi.nlm.nih.gov/pubmed/18614413. Pak J Pharm Sci. 2008;21(3):201–9. [Alyane M, Benguedouar L, Kebsa W, et al. Cardioprotective effects and mechanism of action of polyphenols extracted from propolis against doxorubicin toxicity[J]. Pak J Pharm Sci, 2008, 21(3): 201- 9.] [PubMed] [Google Scholar]
- 18.El-Sayed E, Abo-Salem OM, Aly HA, et al. Potential antidiabetic and hypolipidemic effects of propolis extract in streptozotocininduced diabetic rats. http://www.ncbi.nlm.nih.gov/pubmed/19339227/ Pak J Pharm Sci. 2009;22(2):167–8. [El-Sayed E, Abo-Salem OM, Aly HA, et al. Potential antidiabetic and hypolipidemic effects of propolis extract in streptozotocininduced diabetic rats [J]. Pak J Pharm Sci, 2009, 22(2): 167-8.] [PubMed] [Google Scholar]
- 19.Oliveira Lopes RH, Benitez Macorini LF, de Toledo Espindola PP, et al. Antioxidant and hypolipidemic activity of the hydroethanolic extract of curatella americana L. leaves. http://jglobal.jst.go.jp/en/public/20090422/201702276382797310. Oxid Med Cell Longev. 2016;26(9):9681425. doi: 10.1155/2016/9681425. [Oliveira Lopes RH, Benitez Macorini LF, de Toledo Espindola PP, et al. Antioxidant and hypolipidemic activity of the hydroethanolic extract of curatella americana L. leaves[J]. Oxid Med Cell Longev, 2016, 26(9): 9681425.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun YZ, Chen JF, Shen LM, et al. Anti-atherosclerotic effect of hesperidin in LDLr-/- mice and its possible mechanism. http://linkinghub.elsevier.com/retrieve/pii/S0014299917305952. Eur J Pharmacol. 2017;815(3):109–17. doi: 10.1016/j.ejphar.2017.09.010. [Sun YZ, Chen JF, Shen LM, et al. Anti-atherosclerotic effect of hesperidin in LDLr-/- mice and its possible mechanism[J]. Eur J Pharmacol, 2017, 815(3): 109-17.] [DOI] [PubMed] [Google Scholar]
- 21.Singh V, Jain M, Misra A, et al. Curcuma oil ameliorates hyperlipidaemia and associated deleterious effects in golden Syrian hamsters. Br J Nutr. 2013;110(3):437–46. doi: 10.1017/S0007114512005363. [Singh V, Jain M, Misra A, et al. Curcuma oil ameliorates hyperlipidaemia and associated deleterious effects in golden Syrian hamsters [J]. Br J Nutr, 2013, 110(3): 437-46.] [DOI] [PubMed] [Google Scholar]
- 22.de Boer JF, Schonewille M, Boesjes MA, et al. Intestinal farnesoid X receptor controls transintestinal cholesterol excretion in mice. Gastroenterology. 2017;152(5):1126. doi: 10.1053/j.gastro.2016.12.037. [de Boer JF, Schonewille M, Boesjes MA, et al. Intestinal farnesoid X receptor controls transintestinal cholesterol excretion in mice[J]. Gastroenterology, 2017, 152(5): 1126.] [DOI] [PubMed] [Google Scholar]
- 23.Li YB, Jiang BM, Liang PF, et al. Nucleolin protects macrophages from oxLDL-induced foam cell formation through up-regulating ABCA1 expression. Biochem Biophys Res Commun. 2017;486(2):364–71. doi: 10.1016/j.bbrc.2017.03.047. [Li YB, Jiang BM, Liang PF, et al. Nucleolin protects macrophages from oxLDL-induced foam cell formation through up-regulating ABCA1 expression[J]. Biochem Biophys Res Commun, 2017, 486 (2): 364-71.] [DOI] [PubMed] [Google Scholar]
- 24.Xu LQ, Shi YF, Zhuang SG, et al. Recent advances on uric acid transporters. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3226109. Oncotarget. 2017;8(59):100852–62. doi: 10.18632/oncotarget.20135. [Xu LQ, Shi YF, Zhuang SG, et al. Recent advances on uric acid transporters [J]. Oncotarget, 2017, 8(59): 100852-62.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarzecki MS, Araujo SM, Bortolotto VC, et al. Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=Doaj000004742479. Toxicol Rep. 2014;1(2):200–9. doi: 10.1016/j.toxrep.2014.02.003. [Zarzecki MS, Araujo SM, Bortolotto VC, et al. Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice[J]. Toxicol Rep, 2014, 1(2): 200-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwak YS, Kyung JS, Kim JS, et al. Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from korean red ginseng. Biol Pharm Bull. 2010;33(3):468–72. doi: 10.1248/bpb.33.468. [Kwak YS, Kyung JS, Kim JS, et al. Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from korean red ginseng[J]. Biol Pharm Bull, 2010, 33(3): 468-72.] [DOI] [PubMed] [Google Scholar]
- 27.姚 楠, 何 蓉蓉, 曾 晓会, et al. Hypotriglyceridemic effects of Apple polyphenols extract via Up-Regulation of lipoprotein lipase in triton WR-1339-Induced mice. Chin J Integr Med. 2014;20(1):31–5. doi: 10.1007/s11655-012-1243-3. [姚楠, 何蓉蓉, 曾晓会, 等. Hypotriglyceridemic effects of Apple polyphenols extract via Up-Regulation of lipoprotein lipase in triton WR-1339-Induced mice[J]. Chin J Integr Med, 2014, 20(1): 31-5.] [DOI] [PubMed] [Google Scholar]
- 28.苑 荣爽, 李 贺, 孙 靖辉, et al. 北五味子多糖对高脂诱导非酒精性脂肪性肝病大鼠的降血脂作用及其抗氧化活性. http://d.old.wanfangdata.com.cn/Periodical/bqeykdxxb201706007. 吉林大学学报:医学版. 2017;43(6):1103–8, 13. [苑荣爽, 李贺, 孙靖辉, 等.北五味子多糖对高脂诱导非酒精性脂肪性肝病大鼠的降血脂作用及其抗氧化活性[J].吉林大学学报:医学版, 2017, 43(6): 1103-8, 13.] [Google Scholar]
- 29.杨 佩磊, 蒋 剑平, 李 全清, et al. 胡柚皮黄酮对高脂血症大鼠的降血脂作用研究. http://d.old.wanfangdata.com.cn/Periodical/zgzyzz201705021. 中国中药杂志. 2017;42(5):936–43. doi: 10.19540/j.cnki.cjcmm.20170121.008. [杨佩磊, 蒋剑平, 李全清, 等.胡柚皮黄酮对高脂血症大鼠的降血脂作用研究[J].中国中药杂志, 2017, 42(5): 936-43.] [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Ding WX, Li T. Cholesterol and bile acid-mediated regulation of autophagy in fatty liver diseases and atherosclerosis. Biochim Biophys Acta. 2018;1863(7):726–8. doi: 10.1016/j.bbalip.2018.04.005. [Wang Y, Ding WX, Li T. Cholesterol and bile acid-mediated regulation of autophagy in fatty liver diseases and atherosclerosis [J]. Biochim Biophys Acta, 2018, 1863(7): 726-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.姚雨宏. 阿托伐他汀对脂多糖诱导的细胞及小鼠炎症模型中脂质代谢和胆固醇流出的影响[D]. 北京: 中国医学科学院, 2017.
- 32.Hao S, Xiao Y, Lin Y, et al. Cholrogenic acid-enriched extract from Eucommia ulmoides leaves inhibits hepatic lipid accumulation through regulation of cholesterol metabolism in HepG2 cells. Pharm Biol. 2016;54(2):251–9. doi: 10.3109/13880209.2015.1029054. [Hao S, Xiao Y, Lin Y, et al. Cholrogenic acid-enriched extract from Eucommia ulmoides leaves inhibits hepatic lipid accumulation through regulation of cholesterol metabolism in HepG2 cells[J]. Pharm Biol, 2016, 54(2): 251-9.] [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Si YH, Zhai L, et al. Celastrus orbiculatus Thumb. reduces lipid accumulation by promoting reverse cholesterol transport in hyperlipidemic mice. Lipids. 2016;51(6):677–92. doi: 10.1007/s11745-016-4145-x. [Zhang Y, Si YH, Zhai L, et al. Celastrus orbiculatus Thumb. reduces lipid accumulation by promoting reverse cholesterol transport in hyperlipidemic mice[J]. Lipids, 2016, 51(6): 677-92.] [DOI] [PubMed] [Google Scholar]
- 34.Yang JH, Cho SS, Kim KM, et al. Neoagarooligosacchrides enhance the level and efficiency of LDL receptor and improve cholesterol homeostasis. J Funct Foods. 2017;38:529–39. doi: 10.1016/j.jff.2017.09.053. [Yang JH, Cho SS, Kim KM, et al. Neoagarooligosacchrides enhance the level and efficiency of LDL receptor and improve cholesterol homeostasis [J]. J Funct Foods, 2017, 38: 529-39.] [DOI] [Google Scholar]


