Abstract
目的
探讨预测胰腺导管腺癌(PDAC)鉴别于胰腺其他实性肿瘤病变的因素,评估内镜超声引导下细针抽吸术(EUS-FNA)对PDAC的诊断价值。
方法
回顾性分析2009年1月~2016年5月南方医科大学南方医院消化内窥镜中心因胰腺占位行EUS-FNA检查的患者的临床资料,排除诊断不明、资料缺失、重复穿刺、囊性占位病变以及良性病变病例,将患者分为PDAC组与non-PDAC组,比较两组的EUS-FNA穿刺阳性率,统计EUS-FNA对PDAC诊断的敏感度、特异度、阳性预测值、阴性预测值和准确率,纳入PDAC组和non-PDAC组患者的人口特征、临床特征、实验室检查和超声内镜成像特征等相关因素进行单因素和多因素非条件logistic回归分析。
结果
纳入75例胰腺实体肿瘤病变中,PDAC占72.0%,non-PDAC占28.0%。EUS-FNA诊断PDAC的敏感度、特异度、阳性预测值、阴性预测值和准确率分别是77.8%、100.0%、100.0%、63.6%、84.0%。PDAC组与non-PDAC组的EUS-FNA穿刺阳性率差异无统计学意义(77.8% vs 76.2%,P>0.05)。多因素logistic分析显示腹痛(OR=5.163,95% CI:1.093~24.389,P=0.038)、病灶性状(OR=7.105,95%CI:1.440~35.043,P=0.016)、病灶大小(OR=0.926,95%CI:0.877~0.978,P=0.006)、病灶转移(OR=6.165,95%CI:1.332~28.533,P=0.020)是预测PDAC的独立影响因子。
结论
腹痛、病灶转移、病灶大小和病灶性状的超声内镜成像特征可以可靠地预测PDAC,并且EUS-FNA对PDAC的诊断是具有较高的敏感度和特异度。
Keywords: 胰腺导管腺癌, 胰腺实体肿瘤病变, 内镜超声检查术, 细针抽吸术, 预测诊断
Abstract
Objective
To identify the predictive factors for differentiating pancreatic ductal adenocarcinoma (PDAC) from other neoplastic solid pancreatic lesions and assess the accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of PDAC.
Methods
We retrospectively analyzed the clinical data of patients referred for EUS-FNA evaluation of pancreatic lesions in the Digestive Endoscopic Center of Nanfang Hospital between January, 2009 and May, 2016. The cases with unknown diagnosis, missing data, repeated punctures, cystic lesions and benign lesions were excluded from the analysis. The positivity rates of EUS-FNA were compared between patients with PDAC and those with non-PDAC lesions, and the sensitivity, specificity, positive predictive value, negative predictive value and accuracy of EUS-FNA were assessed in the diagnosis of PDAC. Univariate and multivariate logistic regression analyses were used to identify the factors for differentiating PDAC from non-PDAC lesions based on the demographic characteristics, clinical presentations, laboratory data, and endoscopic ultrasonography imaging features of the patients.
Results
Among the 75 patients with solid neoplastic pancreatic lesions, 54 (72.0%) were found to have PDAC and 21 (28.0%) had non-PDAC lesions. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of EUS-FNA for the diagnosis of PDAC were 77.8%, 100.0%, 100.0%, 63.6% and 84.0%, respectively. No significant difference was found in the positivity rate of EUS-FNA between patients with PDAC and those with non-PDAC lesions (77.8% vs 76.2%, P > 0.05). Multivariate regression analysis identified abdominal pain (OR=5.163, 95%CI: 1.093-24.389, P=0.038), lesion size (OR=0.926, 95%CI: 0.877-0.978, P=0.006), characteristics of the solid lesions (OR=7.105, 95%CI: 1.440-35.043, P=0.016), and evidence of metastases (OR=6.165, 95%CI: 1.332-28.533, P=0.020) as the independent factors for predicting PDAC.
Conclusion
The pretest characteristics including abdominal pain, evidence of metastases, and lesion size and lesion characteristics defined by endoscopic ultrasonography findings can reliably predict a diagnosis of PDAC. EUS-FNA has a high sensitivity and a high specificity for the diagnosis of PDAC.
Keywords: pancreatic ductal adenocarcinoma, neoplastic solid pancreatic lesions, endoscopic ultrasonography, fine needle aspiration, predictive diagnosis
在胰腺实体肿瘤病变中,胰腺导管腺癌(PDAC)约占90%,而其他实体肿瘤例如神经内分泌肿瘤、转移癌等胰腺非导管腺癌(non-PDAC)约占10%~15%[1],因此“胰腺癌”泛指PDAC。PDAC是一种恶性程度极高的消化系统肿瘤,其发病隐匿,进展迅速,治疗效果及预后极差,5年生存率仅为5%,死亡率与发病率几乎相等[2]。PDAC高死亡率的原因一方面是超过80%的患者诊断时已出现局部晚期或转移性疾病,另一方面是早期或局部病变的患者手术切除后5年生存率仅为25%[3],并且,在手术探查时,26%的患者被发现是不可切除的[4]。而大多数non-PDAC进展缓慢,其中胰腺神经内分泌瘤患者的10年生存率为45%[5],甚至不能用手术治疗的患者靶向药物治疗后生存预后能够获益[6]。这表明早期诊断和明确病理学性质可提高胰腺实体肿瘤病变患者的生存率。因此,鉴别诊断PDAC和non-PDAC对指导早期发现和诊断胰腺实体肿瘤病变尤显重要。
内镜超声引导下细针抽吸术(EUS-FNA)获取胰腺实性占位病变的细胞组织学的敏感度和特异度较高,对于明确其病理学性质尤为重要。近两篇超过30例研究荟萃分析[7-8]均证明EUS-FNA诊断胰腺实性占位总的敏感度和特异度可分别达91%和94%,并且内镜超声检查术(EUS)在发现胰腺实性占位和对胰腺肿瘤分期方面优于CT、MRI影像学。一旦EUS-FNA诊断胰腺实体肿瘤,并能够根据EUS特征加以鉴别PDAC和non-PDAC,将进一步证实肿瘤的特异性诊断及最小化诊断的不确定性。
国内目前尚无关于EUS-FNA对胰腺实体肿瘤病变鉴别诊断的评价报道,本研究将对我院行EUS-FNA检查发现胰腺实体肿瘤病变的患者资料进行回顾性分析,旨在探讨人口特征、临床表现、EUS影像学特征及危险因素对PDAC与non-PDAC的鉴别诊断,同时评估EUS-FNA对PDAC的诊断价值,分析EUS在不同类型胰腺实体肿瘤的特异性特征。
1. 资料和方法
1.1. 研究对象
选取2009年1月~2016年5月因B超、CT、磁共振、经内镜逆行胰胆管造影术等检查明确发现或怀疑胰腺占位性病变,就诊南方医科大学南方医院消化内窥镜中心且行EUS-FNA检查的患者。收集的指标:人口特征(年龄、性别)、临床表现(腹痛、体质量下降)、既往史(抽烟饮酒史、慢性胰腺炎病史、糖尿病病史)、实验室检查[总胆红素、糖链抗原199(CA199)]、EUS影像学特征(病变部位、病灶大小、病灶性状、胰主管是否扩张、血管是否侵犯、病灶是否转移、胰腺周围淋巴结是否肿大)、术后并发症。
纳入标准:住院初诊患者,临床资料记录齐全,因胰腺实性占位(包括囊实性)而行EUS-FNA检查,且最终诊断明确为PDAC或non-PDAC。
排除标准:(1)年龄小于18岁;(2)诊断不确定、资料缺失、重复穿刺、囊性占位病变、良性病变(如胰腺浆液性腺瘤、急慢性胰腺炎、胰腺假性囊肿、胰腺囊肿、胰腺脓肿、胰腺结核、自身免疫性胰腺炎等);(3)已行手术治疗明确诊断或已行放化疗治疗。
1.2. 病理分型及诊断标准
病理分型:PDAC为研究组,其表现为由分化不同程度的导管样结构的腺体构成,伴有丰富的纤维间质;non-PDAC为对照组,为不包括PDAC的其他病理分型,本研究包括胰腺神经内分泌瘤、胰腺实性-假乳头瘤、胰腺黏液性肿瘤伴不典型增生、胰腺转移癌等。
诊断标准:EUS-FNA细胞学或组织条病理检查之一发现PDAC细胞即定义为EUS-FNA阳性,仅发现异型细胞或正常细胞或炎性细胞或non-PDAC病理分型均定义为EUS-FNA阴性。EUS-FNA检查追加手术治疗者以手术病理为最终诊断,未行手术治疗者随访6月,根据临床表现、实验室检查及影像学结果确定(包括CT、MRI和EUS)。
1.3. 专有名词的定义
比值比(OR)又称风险比,指比较PDAC组和non-PDAC组的相关影响因素暴露的情况差异时的定量描述,OR值大于1且有意义时(P>0.05),说明该因素是预测PDAC的危险因子。
以计算EUS-FNA在PDAC的诊断价值指标为例,敏感度指最终诊断PDAC患病中被EUS-FNA诊断出来的百分比;特异度指最终诊断non-PDAC患病中被EUS-FNA诊断出来的百分比;阳性预测值指在所有EUS-FNA诊断PDAC阳性者中,真正为最终诊断PDAC的比例;阴性预测值指EUS-FNA诊断non-PDAC中,真正为最终诊断non-PDAC的比例;准确率指EUS-FNA诊断PDAC和non-PDAC与最终诊断的符合率。
1.4. EUS-FNA检查使用器材及方法
使用器材:EU-ME2超声内镜主机(OLYMPUS);GF-UCT260型和GF-UCT2000-OL5型超声电子内镜(OLYMPUS);UM-2R和UM-DP12-25R微型超声探头,频率12.0 MHz(OLYMPUS);19G EUS穿刺针(COOK ECHO-19、COOK ECHO-HD-19-C、Boston ExpectTM),22G EUS穿刺针(COOK ECHO-1-22、COOK ECHO-3-22、COOK ECHO-HD-22-C、Boston ExpectTM)。
方法:术前完善相关评估,确定无内镜检查和穿刺的禁忌证。术者向患者告知操作相关事项,患者均签署知情同意书。患者术前常规禁食4~6 h,检查前予咽喉部利多卡因局部麻醉,检查时给予持续生命体征监测。术中实时超声监控,由超声内镜医师使用EUS探查病变及其周围邻近脏器,确定病灶的部位、大小、内部回声特点及其与周围脏器、血管的关系等,对病变长、短径进行测量,避开血管进行穿刺,视情况给予抽吸负压吸引。穿刺物立即置于玻片上涂片数张,自然干燥,送病理科行细胞学检查。穿刺组织条用10%中性甲醛溶液固定后,送病理科检查。穿刺后嘱患者卧床休息4~6 h,术后禁食8~24 h,静脉给药以预防感染、出血、抑酸治疗,密切观察并发症发生情况(如发热、消化道大出血、局部血肿、消化道穿孔、急性胰腺炎等)。
1.5. 统计学分析
采用IBM SPSS 22.0软件处理数据,根据最终诊断结果统计EUS-FNA诊断PDAC与non-PDAC的敏感度、特异度、阳性预测值、阴性预测值和准确率。不同类型间率的比较行χ2检验,两组均数比较采用t检验,对PDAC与non-PDAC诊断的影响因素进行单因素和多因素logistic回归分析。本研究中胰腺病灶大小记录缺失值,采用组内序列平均数替代,总胆红素和CA199水平的记录缺失均纳入正常值范围,不予具体值替代,若缺失值比例超过25%,不予纳入多因素分析。P值的检验水准设为0.05。
2. 结果
2.1. 患者的基本资料情况
本研究中,因胰腺占位性病变行EUS-FNA患者193例,逐步排除了诊断不明43例,资料缺失7例,重复穿刺6例,囊性占位15例,良性病变47例,最后纳入研究75例,PDAC与non-PDAC患者的基本资料情况如表 1所示。21例non-PDAC中,包括黏液性囊性肿瘤伴不典型增生5例,神经内分泌瘤和实性-假乳头瘤各3例,黏液性囊性腺癌和黏液性非囊性腺癌各2例,印戒细胞癌、低分化内分泌癌、导管内乳头状肿瘤伴不典型增生各1例,以及继发性胰腺转移癌3例,其原发病灶分别是肝细胞癌、胆管细胞癌、结肠腺癌。75例EUS-FNA中,1例出现急性胰腺炎,并发症发生率为1.3%(1/75),经保守对症处理后好转。
1.
胰腺导管腺癌与胰腺非导管腺癌患者的基本信息、临床特征、实验室检查和影像学特征
Demographic factors, clinical features, laboratory data, and imaging characteristics of patients with PDAC and non-PDAC lesions
| Characteristics | All patients* | |
| PDAC(n=54) | non-PDAC (n=21) | |
| *Values are expressed as Mean ± SD or the number of patients (%) or median; PDAC: Pancreatic ductal adenocarcinoma; SD: Standard deviation; CA199, carbohydrate antigens 199; IQR: Interquartile range; EUS: Endoscopic ultrasonography; LAP: Lymphadenopathy; **Cystic component within solid lesion was not included. | ||
| Demographic | ||
| Age (year) | 57.7±11.6 | 51.4±12.1 |
| Male | 40/54 (74.1%) | 11/21 (52.4%) |
| Habits | ||
| Smoker | 15/54 (27.8%) | 5/21 (23.8%) |
| Alcohol use | 5/54 (9.3%) | 1/21 (4.8%) |
| Clinical features | ||
| Abdominal pain | 48/54 (88.9%) | 13/21 (61.9%) |
| Weight loss | 37/54 (68.5%) | 8/21 (38.1%) |
| History of diabetes mellitus | 13/54 (24.1%) | 4/21 (19.0%) |
| History of chronic pancreatitis | 11/54 (20.4%) | 2/21 (9.5%) |
| Laboratory data | ||
| Serum CA199 [U/mL, median (IQR)] | 336.6 (58.8-1379.5), n=50 | 20.4 (7.4-207.6), n=20 |
| Total bilirubin [jimol/L, median (IQR)] | 11.2(7.9-17.4), n=46 | 10.9 (6.9-13.2), n=20 |
| Location of pancreatic lesion | ||
| Head/neck | 36/54 (66.7%) | 12/21 (57.1%) |
| Body | 20/54 (37.0%) | 8/21 (38.1%) |
| Tail | 17/54 (31.5%) | 7/21 (33.3%) |
| EUS imaging features | ||
| Lesion size (mm) | 35.8±14.5, n=52 | 46.7±16.8, n=21 |
| Presence of ≥2 lesions | 17/54 (31.5%) | 6/21 (28.6%) |
| Pancreatic main duct dilation | 28/54 (51.9%) | 5/21 (23.8%) |
| Evidence of arterial invasion | 26/54 (48.1%) | 6/21 (28.6%) |
| Evidence of metastases | 32/54 (59.3%) | 7/21 (33.3%) |
| Evidence of peripancreatic LAP | 35/54 (64.8%) | 7/21 (33.3%) |
| Solid lesion** | 50/54 (92.6%) | 14/21 (66.7%) |
2.2. PDAC与non-PDAC的诊断结果比较
PDAC与non-PDAC的诊断结果比较情况如表 2所示。54例PDAC中,由EUS-FNA诊断者为42例,穿刺阳性率为77.8%,另外12例穿刺阴性者中,10例进一步行手术治疗明确病理诊断,2例经超声引导下穿刺活检术明确病理诊断。EUS-FNA诊断PDAC的敏感度、特异度、阳性预测值、阴性预测值和准确率分别是77.8%、100.0%、100.0%、63.6%、84.0%。21例non-PDAC中,由EUS-FNA诊断者为16例,穿刺阳性率为76.2%,另外5例穿刺阴性者进一步行手术治疗明确病理诊断。EUS-FNA诊断non-PDAC的敏感度、特异度、阳性预测值、阴性预测值和准确率分别是76.2%、100.0%、100.0%、91.5%、93.3%。PDAC与non-PDAC的穿刺阳性率、敏感度、特异度、阳性预测值、准确率差异均无统计学意义(P>0.05),而non-PDAC的阴性预测值明显高于PDAC,差异有统计学意义(χ2=10.927,P=0.001)。
2.
75例胰腺实体肿瘤病变患者的EUS-FNA结果与最终诊断结果(例)
EUS-FNA findings and the final diagnosis in 75 patients with PDAC and non-PDAC solid neoplastic pancreatic lesions
| EUS-FNA diagnosis | Final diagnosis | Total | |
| PDAC | non-PDAC | ||
| EUS-FNA: Endoscopic ultrasound-guided fine needle aspiration; PDAC: Pancreatic ductal adenocarcinoma. | |||
| PDAC | 54 | - | - |
| Positive | 42 | 0 | 42 |
| Negative | 12 | 21 | 33 |
| non-PDAC | - | 21 | - |
| Positive | 0 | 16 | 16 |
| Negative | 54 | 5 | 59 |
| Total | 54 | 21 | 75 |
2.3. PDAC与non-PDAC的单因素与多因素分析
PDAC与non-PDAC比较的单因素分析表明年龄、腹痛、体重下降、病灶大小、病灶性状(实性/囊实性)、病灶转移、胰主管扩张、胰腺周围淋巴结肿大、CA199水平等多个因子能预测PDAC(表 3),而抽烟、嗜酒、糖尿病、慢性胰腺炎等既往史以及性别、病灶部位(头颈部/体部/尾部)、血管侵犯、总胆红素水平等因子均不是预测PDAC的影响因子。其中,单因素分析显示PDAC的CA199水平高于non-PDAC,在中度升高水平即101~1000 IU/mL范围趋势更明显;PDAC的病灶平均值小于non-PDAC组,且PDAC中病灶<30 mm比例为42.3%(22/52),当病灶>30 mm时,偏向于non-PDAC诊断。多因素分析表明,与non-PDAC比较,腹痛、实性病灶、病灶转移、病灶大小偏小是预测PDAC的独立影响因子(表 4)。
3.
胰腺导管腺癌鉴别于胰腺非导管腺癌的影响因素的单因素分析
Univariate logistic regression of the predictive factors for differentiating PDAC from non-PDAC
| Variable | P* value | OR | 95%CI | Missing values (n) |
| PDAC: Pancreatic ductal adenocarcinoma; CI: Confidence interval; OR: Odds ratio; CA199: Carbohydrate antigens 199; LAP: Lymphadenopathy; *Univariable logistic regression analysis was used for continuous variables and categorical factors. | ||||
| Age (year) | 0.048 | 1.046 | 1.001-1.091 | 0 |
| Gender (male vs female) | 0.075 | 2.597 | 0.908-7.427 | 0 |
| Smoking | 0.727 | 1.231 | 0.383-3.955 | 0 |
| Abdominal pain | 0.011 | 4.923 | 1.449-16.727 | 0 |
| Weight loss | 0.019 | 3.537 | 1.236-10.121 | 0 |
| History of diabetes mellitus | 0.641 | 1.348 | 0.384-4.728 | 0 |
| History of chronic pancreatitis | 0.277 | 2.430 | 0.490-12.043 | 0 |
| Lesion size (mm) | 0.010 | 0.955 | 0.922-0.989 | 2 |
| Lesion size (>30 mm vs ≤30 mm) | 0.015 | 0.144 | 0.030-0.681 | 2 |
| Total bilirubin (>17.1 μmol/L vs ≤17.1 μmol/L) | 0.157 | 3.176 | 0.640-15.769 | 9 |
| Serum CA199 (≤37 IU/mL) | 0.020 | 5 | ||
| Elevated (38-100) vs normal | 0.624 | 1.636 | 0.229-11.703 | |
| High (101-1000) vs normal | 0.004 | 8.364 | 1.952-35.832 | |
| Markedly high (>1000) vs normal | 0.042 | 4.727 | 1.056-21.153 | |
| Head/neck | 0.442 | 1.500 | 0.534-4.214 | 0 |
| Body | 0.932 | 0.956 | 0.338-2.703 | 0 |
| Tail | 0.877 | 0.919 | 0.314-2.689 | 0 |
| lesion characteristics (solid vs cystic-solid) | 0.008 | 6.250 | 1.598-24.448 | 0 |
| Pancreatic main duct dilation | 0.033 | 3.446 | 1.105-10.746 | 0 |
| Evidence of arterial invasion | 0.129 | 2.321 | 0.783-6.883 | 0 |
| Evidence of metastases | 0.048 | 2.909 | 1.011-8.374 | 0 |
| Evidence of peripancreatic LAP | 0.016 | 3.684 | 1.270-10.692 | 0 |
4.
鉴别胰腺导管腺癌与胰腺非导管腺癌的预测相关因素的多因素分析
Multivariate logistic regression of the predictive factors for differentiating PDAC from non-PDAC
| Variable | P* value | OR | 95%CI |
| PDAC: Pancreatic ductal adenocarcinoma; CI: Confidence interval; OR: Odds ratio; *Univariable logistic regression analysis was used for continuous variables and categorical factors. | |||
| Abdominal pain | 0.038 | 5.163 | 1.093-24.389 |
| lesion characteristics (solid vs cystic-solid) |
0.016 | 7.105 | 1.440-35.043 |
| Evidence of metastases | 0.020 | 6.165 | 1.332-28.533 |
| Lesion size (mean), mm | 0.006 | 0.926 | 0.877-0.978 |
2.4. PDAC与non-PDAC的EUS影像学表现
1例PDAC的EUS影像学表现为胰头部见一不均质低回声光团,肿物边界不规则,与肠系膜上静脉门静脉汇合部界限模糊(图 1),1例non-PDAC黏液性囊性肿瘤的EUS影像学表现为胰尾部可见一囊实性病变,内部可见一低回声结构突向囊腔,囊壁厚而不规则(图 2),1例non-PDAC神经内分泌瘤的EUS影像学表现为胰腺钩突部可见一不均质低回声团,形状不规则,血供丰富(图 3)。
1.

胰腺导管腺癌的超声内镜及病理表现
Endosonographic and pathological features of pancreatic ductal adenocarcinoma. A: A 29.7 mm × 22.5 mm hypoechoic, heterogeneous mass in the head of the pancreas (white arrow), blurred with the confluence of superior mesenteric vein (#) and portal vein (*). B: Pathological examination of the surgical specimen confirmed diagnosis of adenocarcinoma (HE staining, original magnification × 200). C: Endoscopic ultrasound-guided fine needle aspiration with both cytology and microhistology. Cytology showed a cluster of malignant cells (HE staining, × 400). D: Microhistology confirmed the diagnosis of adenocarcinoma (HE staining, ×200).
2.
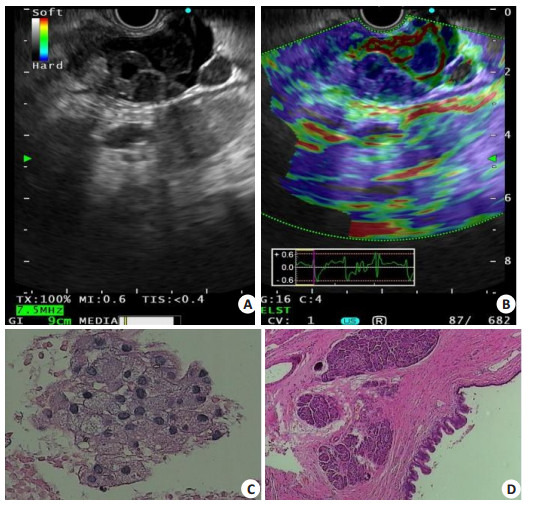
胰腺黏液性囊性肿瘤的超声内镜和病理表现
Endosonographic and pathological features of mucinous cystic neoplasm of the pancreas. A: The tail of the pancreas showed a cystic component within the solid lesion, with a hypoechoic structure protruding into the lumen; B: ELST elastography showing a pancreatic lesion area dominated by blue and green with increased hardness; C: Fine needle aspiration performed with cytology showing a mucinous epithelial cell cluster (HE staining, ×400); D: Surgical pathology supporting the diagnosis of pancreatic mucinous cystadenoma with moderate to severe atypical hyperplasia (HE staining, ×200).
3.
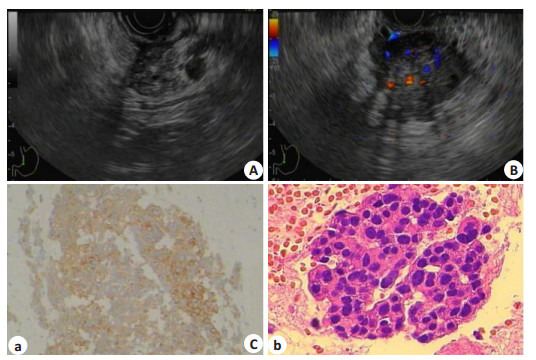
胰腺神经内分泌瘤的超声内镜及病理表现
Endosonographic and pathological features of pancreatic neuroendocrine tumor. A: A 29 mm × 34 mm hypoechoic, heterogeneous mass in the uncinate region of the pancreas (white arrow); B: EUS findings showing a hypervascular mass with distinct margins; C: Fine needle aspiration performed with microhistology, which confirmed the diagnosis of grade 2 neuroendocrine tumor (immunohistochemical staining: (a) for CgA (+), original magnification ×100; (b) for Ki-67 (+), original magnification ×400).
3. 讨论
本研究是华南地区的一项单中心回顾性研究,EUS-FNA在诊断PDAC和non-PDAC上均获得较高的敏感度,但与国外的水平仍有一定的差距[9],可能与研究数据来源不同有关,如研究对象选择、定义标准和研究目的的不同。虽然EUS-FNA在诊断PDAC与non-PDAC的敏感度、特异度、阳性预测值、准确率方面均无统计学差异,但其能获取胰腺实体肿瘤病变的细胞和组织学的病理诊断[10-11],对临床上鉴别诊断PDAC和non-PDAC尤其重要。同时,本研究在可疑的胰腺实体肿瘤病变中联合利用EUS影像学表现和临床特征探讨鉴别诊断PDAC和non-PDAC的意义。本研究单因素分析显示,年龄、腹痛、体质量下降、病灶大小、病灶性状、病灶转移、胰主管扩张、胰腺周围淋巴结肿大、CA199水平等多个因子能预测PDAC,且多因素分析表明腹痛、病灶性状、病灶转移、病灶大小是预测PDAC的独立影响因子。因此,患者的临床特征和EUS成像特征可以指导临床医生对PDAC与non-PDAC的鉴别诊断。
大多数胰腺肿瘤早期无明显临床症状,一项病例对照研究[12]显示胰腺癌早期的临床症状主要表现为腹痛、体重下降、恶心呕吐、新发糖尿病、腰背放射痛、嗜睡等,其中腰背放射痛、嗜睡、新发糖尿病是胰腺癌特征性表现。另一项研究[13]显示腹痛、新发糖尿病多为胰腺癌晚期的表现。流行病学研究[14]发现吸烟、嗜酒、慢性胰腺炎史以及年龄的增长为胰腺癌的危险因素,男性高发于女性。本研究中,以non-PDAC为对照组,PDAC的诊断与腹痛、体质量下降、年龄的增长有关,而与吸烟、糖尿病、性别无关,与国外的一项胰腺实性肿瘤病变的研究结果具有相似性[15]。
CA199水平的显著增加与PDAC的诊断有关,本研究显示当CA199水平在101~1000 IU/mL范围时,诊断PDAC的风险是non-PDAC的8倍,并且,CA199>1000 IU/mL时,诊断风险仍高于non-PDAC。一项系统评价研究[16]显示CA199作为肿瘤标志物在2283例人群中诊断胰腺癌的敏感度和特异度的中位数分别是79%和82%,研究没有单独探讨其对PDAC的敏感度和特异度,但考虑PDAC占胰腺癌的90%,仍显示CA199显著升高在PDAC中的诊断价值。本研究中总胆红素水平不是预测PDAC的影响因子,总胆红素水平升高往往提示病灶在胰头,引起胆道梗阻所致,本研究PDAC的胰头占位比例尽管高于non-PDAC,但单因素分析显示病灶部位不是PDAC的预测因子,而国外大部分研究发现胰头病变多见于PDAC[1, 15, 17],这可能与样本量有关。
本研究中,病灶平均大小是PDAC与non-PDAC的鉴别诊断的影响因子。Gagovic等[1]的研究显示病灶大小平均值在比较PDAC与non-PDAC的差异无统计意义,但当>50 mm,偏向于non-PDAC诊断。一项1000例回顾性研究[18]显示EUS在发现病灶<30 mm的准确性优于B超和CT,且另一项胰腺肿瘤预后分析[19]显示病灶<30 mm时其生存预后改善。本研究中,病灶<30 mm占PDAC为42.3%,且病灶>30 mm时,偏向non-PDAC诊断。因此,在一定程度上,早期检测发现肿瘤能够指导患者的临床管理且有助于改善生存预后。
另外,本研究还发现PDAC合并病灶转移较non-PDAC多。PDAC恶性程度极高,发病隐匿,进展迅速,80%可出现病灶转移,5年生存率低[20],而大多数non-PDAC尽管存在潜在恶性倾向,但发展相对缓慢,生存预期较PDAC高。通常地,PDAC病灶转移首先扩散到附近的淋巴结,然后扩散到肝脏或腹膜腔,结直肠或肺,而骨骼或大脑不常见。本研究没有探讨PDAC病灶转移分布情况,但伴有病灶转移也能支持其侵袭性这一特点。研究中没有纳入穿刺阴性且无病理明确诊断的胰腺占位伴侵袭性转移的病例,这些病例影像学表现为胰腺恶性肿瘤占位伴有其他器官病灶转移,考虑PDAC可能性大,临床上对患者生存预后管理仍有一定的提示作用。
最后,本研究还发现PDAC实性病灶比例较non-PDAC高,而non-PDAC的囊实性比例居多。EUS对胰腺病变的性状及形态学可提供实时高分辨率图像,有时部分不典型囊性病变可表现为囊实性,研究中non-PDAC囊实性病灶占1/3,其中黏液性囊性肿瘤均为囊实性。黏液性囊性肿瘤的EUS影像学常表现为单房或小分隔样囊性病变,边界清,低密度包绕胰腺实质,囊壁上伴周边钙化,而呈恶性倾向时表现为厚而不规则囊壁,壁内有结节,囊液内混有固体成分,表现为囊实性。神经内分泌瘤的EUS影像学常表现为边界尚清楚的低回声团块,伴内部高回声或等回声团,血供丰富。而PDAC的EUS影像学多表现为不均质低回声且边缘不规则的实性病灶,伴有胰主管扩张和邻近低回声斑块形成,但有时难与局灶性胰腺炎鉴别[21]。但总体上,EUS对于PDAC和non-PDAC的病灶性状的图像鉴别起到重大的作用,将进一步证实胰腺实体肿瘤的特异性诊断及最小化诊断的不确定性。
德州大学安德森癌症中心[15]的一项临床回顾性研究对胰腺实体肿瘤病变的生存分析显示神经内分泌瘤2年的生存率为80%,而PDAC与胰腺转移癌仅分别为21%和48%,该中心的另一项研究[22]也表明PDAC可手术切除病灶患者接受辅助化疗的5年生存率仅有21%。可见,PDAC患者病情进展迅速,预后欠佳。
目前,PDAC患者的生存预后欠佳,病情大多处于进展期阶段,这可能与其早期诊断困难有关。最近由日本胰腺协会分析日本胰腺癌登记处数据显示[23-24],早期诊断对改善胰腺癌的预后发挥重要作用,且胰腺癌小于10 mm具有良好的预后。Hanada等[25]对胰腺癌早期筛查分析结果显示,提高生存率应在于通过有效筛查,其中包括EUS检查。对于胰腺癌小于10 mm的病例诊断,EUS检查的肿瘤检出率高于CT或其他方式,EUS-FNA有助于确认组织学诊断。对于原位胰腺癌的诊断,EUS和磁共振胰胆管造影可能在检测胰管的局部不规则狭窄中起重要作用。
本研究的不足之处,首先是一项单中心临床回顾性研究,样本量有限,临床上部分患者影像学发现胰腺恶性肿瘤伴病灶转移后往往放弃进一步明确诊治,抑或EUS-FNA穿刺阴性者未再进一步重复穿刺或手术病理明确诊断。本研究6例重复EUS-FNA穿刺中,尽管只有1例穿刺结果为阳性,对其明确诊断仍显重要。本研究中纳入对象为病理诊断明确的胰腺实体恶性或恶性前病变的肿瘤病变,排除临床上无明确病理而影像学支持胰腺恶性肿瘤伴转移的病例,这部分患者考虑为PDAC的可能性大,但不能确诊,这也许可以部分解释本研究中PDAC占胰腺实体肿瘤病变比例偏低的原因。另外,本研究多因素分析中缺失值的处理方法可能对数据分析存在一定的偏倚,2例病灶大小缺失值采取的是组内序列平均值法,5例CA199和9例总胆红素水平缺失值均视为正常值组,原因考虑临床病历中没有记录具体数值,只描述为正常。本研究另外不足之处是没有对胰腺肿瘤进行分期和预后生存分析,主要原因是患者无良好的随访,资料缺失。
综上所述,本研究胰腺实体肿瘤病变中,大多数是PDAC,non-PDAC占28.0%。腹痛、病灶转移、病灶大小和病灶性状的EUS成像特征是预测PDAC的独立影响因子。PDAC进展快且生存率低,及早发现和诊断,尤其是对于小于10 mm的病灶,可提高患者生存和预后。EUS在胰腺肿瘤诊断中起重要作用,尤其是对于小胰腺肿瘤诊断较敏感,EUS-FNA对PDAC的诊断是具有较高的敏感度和特异度,能获取细胞和组织学的病理诊断,指导临床诊断和治疗。
Biography
吴丽权,博士,E-mail: liquanwu@126.com
Funding Statement
广东省自然科学基金(2017A030313472)
Contributor Information
吴 丽权 (Liquan WU), Email: liquanwu@126.com.
郭 文 (Wen GUO), Email: gwdoc@163.com.
朱 薇 (Wei ZHU), Email: chnz_w@126.com.
References
- 1.Gagovic V, Spier BJ, Delee RJ, et al. Endoscopic ultrasound fineneedle aspiration characteristics of primary adenocarcinoma versus other malignant neoplasms of the pancreas. Can J Gastroenterol. 2012;26(10):691–6. doi: 10.1155/2012/761721. [Gagovic V, Spier BJ, Delee RJ, et al. Endoscopic ultrasound fineneedle aspiration characteristics of primary adenocarcinoma versus other malignant neoplasms of the pancreas[J]. Can J Gastroenterol, 2012, 26(10): 691-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ma JM, Zou ZH, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [Siegel R, Ma JM, Zou ZH, et al. Cancer statistics, 2014[J]. CA Cancer J Clin, 2014, 64(1): 9-29.] [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet. 2016;388(10039):73–85. doi: 10.1016/S0140-6736(16)00141-0. [Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer[J]. Lancet, 2016, 388(10039): 73-85.] [DOI] [PubMed] [Google Scholar]
- 4.Toomey P, Childs C, Luberice K, et al. Nontherapeutic celiotomy incidence is not affected by volume of pancreaticoduodenectomy for pancreatic adenocarcinoma. http://europepmc.org/abstract/med/23896244. Am Surg. 2013;79(8):781–5. [Toomey P, Childs C, Luberice K, et al. Nontherapeutic celiotomy incidence is not affected by volume of pancreaticoduodenectomy for pancreatic adenocarcinoma[J]. Am Surg, 2013, 79(8): 781-5.] [PubMed] [Google Scholar]
- 5.Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63(5):318–48. doi: 10.3322/caac.v63.5. [Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer[J]. CA Cancer J Clin, 2013, 63(5): 318-48.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raymond E, Hobday T, Castellano DA, et al. Therapy innovations: tyrosine kinase inhibitors for the treatment of pancreatic neuroendocrine tumors. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_f67e6eead5dafde7395073a0f3b620ad. Cancer Metastasis Rev. 2011;30(1, 1):19–26. doi: 10.1007/s10555-011-9291-2. [Raymond E, Hobday T, Castellano DA, et al. Therapy innovations: tyrosine kinase inhibitors for the treatment of pancreatic neuroendocrine tumors[J]. Cancer Metastasis Rev, 2011, 30(1, 1): 19-26.] [DOI] [PubMed] [Google Scholar]
- 7.Hewitt MJ, Mcphail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75(2):319–31. doi: 10.1016/j.gie.2011.08.049. [Hewitt MJ, Mcphail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis[J]. Gastrointest Endosc, 2012, 75(2): 319-31.] [DOI] [PubMed] [Google Scholar]
- 8.Chen G, Liu S, Zhao Y, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer:a meta-analysis. Pancreatology. 2013;13(3):298–304. doi: 10.1016/j.pan.2013.01.013. [Chen G, Liu S, Zhao Y, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer:a meta-analysis[J]. Pancreatology, 2013, 13(3): 298-304.] [DOI] [PubMed] [Google Scholar]
- 9.Kamata K, Takenaka M, Omoto S, et al. Impact of avascular areas, as measured by contrast-enhanced harmonic EUS, on the accuracy of FNA for pancreatic adenocarcinoma. Gastrointest Endosc. 2018;87(1):158–63. doi: 10.1016/j.gie.2017.05.052. [Kamata K, Takenaka M, Omoto S, et al. Impact of avascular areas, as measured by contrast-enhanced harmonic EUS, on the accuracy of FNA for pancreatic adenocarcinoma[J]. Gastrointest Endosc, 2018, 87(1): 158-63.] [DOI] [PubMed] [Google Scholar]
- 10.Cheng B, Zhang YE, Chen Q, et al. Analysis of Fine-Needle biopsy vs Fine-Needle aspiration in diagnosis of pancreatic and abdominal masses: a prospective, multicenter, randomized controlled trial. Clin Gastroenterol Hepatol. 2018;16(8):1314–21. doi: 10.1016/j.cgh.2017.07.010. [Cheng B, Zhang YE, Chen Q, et al. Analysis of Fine-Needle biopsy vs Fine-Needle aspiration in diagnosis of pancreatic and abdominal masses: a prospective, multicenter, randomized controlled trial[J]. Clin Gastroenterol Hepatol, 2018, 16(8): 1314-21.] [DOI] [PubMed] [Google Scholar]
- 11.葛 楠, 孙 思予, 金 震东. 中国内镜超声引导下细针穿刺临床应用指南. 中华消化内镜杂志. 2017;34(1):3–13. doi: 10.3760/cma.j.issn.1007-5232.2017.01.002. [葛楠, 孙思予, 金震东.中国内镜超声引导下细针穿刺临床应用指南[J].中华消化内镜杂志, 2017, 34(1): 3-13.] [DOI] [Google Scholar]
- 12.Keane MG, Horsfall L, Rait G, et al. A case-control study comparing the incidence of early symptoms in pancreatic and biliary tract cancer. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc4244441/ BMJ Open. 2014;4(11):e5720. doi: 10.1136/bmjopen-2014-005720. [Keane MG, Horsfall L, Rait G, et al. A case-control study comparing the incidence of early symptoms in pancreatic and biliary tract cancer[J]. BMJ Open, 2014, 4(11): e5720.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma C, Eltawil KM, Renfrew PD, et al. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990-2010. World J Gastroenterol. 2011;17(7):867–97. doi: 10.3748/wjg.v17.i7.867. [Sharma C, Eltawil KM, Renfrew PD, et al. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990-2010[J]. World J Gastroenterol, 2011, 17(7): 867-97.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barone E, Corrado A, Gemignani F, et al. Environmental risk factors for pancreatic cancer: an update. Arch Toxicol. 2016;90(11):2617–42. doi: 10.1007/s00204-016-1821-9. [Barone E, Corrado A, Gemignani F, et al. Environmental risk factors for pancreatic cancer: an update[J]. Arch Toxicol, 2016, 90 (11): 2617-42.] [DOI] [PubMed] [Google Scholar]
- 15.Krishna SG, Li F, Bhattacharya A, et al. Differentiation of pancreatic ductal adenocarcinoma from other neoplastic solid pancreatic lesions: a tertiary oncology center experience. Gastrointest Endosc. 2015;81(2):370–9. doi: 10.1016/j.gie.2014.08.023. [Krishna SG, Li F, Bhattacharya A, et al. Differentiation of pancreatic ductal adenocarcinoma from other neoplastic solid pancreatic lesions: a tertiary oncology center experience[J]. Gastrointest Endosc, 2015, 81(2): 370-9.] [DOI] [PubMed] [Google Scholar]
- 16.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266–70. doi: 10.1016/j.ejso.2006.10.004. [Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer[J]. Eur J Surg Oncol, 2007, 33(3): 266-70.] [DOI] [PubMed] [Google Scholar]
- 17.Tox U, Hackenberg R, Stelzer A, et al. Endosonographic diagnosis of solid pancreatic tumors:a retrospective analysis from a tertiary referral center. http://www.ncbi.nlm.nih.gov/pubmed/17427113. J Gastroenterol. 2007;45(4):307–12. doi: 10.1055/s-2007-962824. [Tox U, Hackenberg R, Stelzer A, et al. Endosonographic diagnosis of solid pancreatic tumors:a retrospective analysis from a tertiary referral center[J]. J Gastroenterol, 2007, 45(4): 307-12.] [DOI] [PubMed] [Google Scholar]
- 18.Volmar KE, Vollmer RT, Jowell PS, et al. Pancreatic FNA in 1000 cases: a comparison of imaging modalities. Gastrointest Endosc. 2005;61(7):854–61. doi: 10.1016/S0016-5107(05)00364-0. [Volmar KE, Vollmer RT, Jowell PS, et al. Pancreatic FNA in 1000 cases: a comparison of imaging modalities[J]. Gastrointest Endosc, 2005, 61(7): 854-61.] [DOI] [PubMed] [Google Scholar]
- 19.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas - 616 patients: Results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4(6):567–79. doi: 10.1016/S1091-255X(00)80105-5. [Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas - 616 patients: Results, outcomes, and prognostic indicators[J]. J Gastrointest Surg, 2000, 4(6): 567-79.] [DOI] [PubMed] [Google Scholar]
- 20.Barhli A, Cros J, Bartholin L, et al. Prognostic stratification of resected pancreatic ductal adenocarcinoma: Past, present, and future[J]. Dig Liver Dis, 2018, [Epub ahead of print].https://www.sciencedirect.com/science/article/pii/S1590865818308892
- 21.Krishna SG, Lee JH. Endosonography in solid and cystic pancreatic tumors. J Interv Gastroenterol. 2011;1(4):193–201. doi: 10.4161/jig. [Krishna SG, Lee JH. Endosonography in solid and cystic pancreatic tumors[J]. J Interv Gastroenterol, 2011, 1(4): 193-201.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz MH, Fleming JB, Lee JE. Current status of adjuvant therapy for pancreatic cancer. Oncologist. 2010;15(11):1205–13. doi: 10.1634/theoncologist.2010-0121. [Katz MH, Fleming JB, Lee JE. Current status of adjuvant therapy for pancreatic cancer[J]. Oncologist, 2010, 15(11): 1205-13.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egawa S, Toma H, Ohigashi H, et al. Japan pancreatic cancer registry; 30th year anniversary Japan pancreas society. Pancreas. 2012;41(7):985–92. doi: 10.1097/MPA.0b013e318258055c. [Egawa S, Toma H, Ohigashi H, et al. Japan pancreatic cancer registry; 30th year anniversary Japan pancreas society[J]. Pancreas, 2012, 41(7): 985-92.] [DOI] [PubMed] [Google Scholar]
- 24.Egawa S, Takeda K, Fukuyama S, et al. Clinicopathological aspects of small pancreatic cancer. Pancreas. 2004;28(3):235–40. doi: 10.1097/00006676-200404000-00004. [Egawa S, Takeda K, Fukuyama S, et al. Clinicopathological aspects of small pancreatic cancer[J]. Pancreas, 2004, 28(3): 235-40.] [DOI] [PubMed] [Google Scholar]
- 25.Hanada K, Okazaki A, Hirano N, et al. Effective screening for early diagnosis of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2015;29(6):929–39. doi: 10.1016/j.bpg.2015.09.017. [Hanada K, Okazaki A, Hirano N, et al. Effective screening for early diagnosis of pancreatic cancer[J]. Best Pract Res Clin Gastroenterol, 2015, 29(6): 929-39.] [DOI] [PubMed] [Google Scholar]


