Abstract
目的
探讨姜黄素对香烟烟雾提取物(CSE)诱导的人气道上皮16HBE细胞氧化应激和炎症反应的抑制作用及相关的分子机制。
方法
使用shRNA-PPARγ(shPPARγ)转染人气道上皮16HBE细胞下调PPARγ表达。将16HBE细胞分为5组即对照组、姜黄素组、CSE组、CSE+姜黄素组和CSE+姜黄素+shRNA-PPARγ组。在0 h和24 h时使用MTT法对细胞活性进行检测;干预24 h后使用qPCR对细胞TNF-α、iNOS和PPARγ mRNA表达进行检测,采用western blot检测16HBE细胞中iNOS、PPARγ蛋白表达以及NF-κB p65磷酸化水平。
结果
16HBE细胞在干预24 h后各组之间细胞活性未有明显统计学差异(P>0.05)。与对照组相比较,CSE干预24 h后PPARγ表达水平明显降低,TNF-α、iNOS表达及NF-κB p65磷酸化水平明显升高,差异均有统计学意义(P < 0.05)。但CSE组较CSE+姜黄素组和CSE+姜黄素+shPPARγ组PPARγ表达水平下降以及TNF-α、iNOS表达和NF-κB p65磷酸化水平升高更显著,差异均有统计学意义(P < 0.05);且CSE+姜黄素+shPPARγ组较CSE+姜黄素组PPARγ表达水平下降以及TNF-α、iNOS表达和NF-κB p65磷酸化水平升高更明显,差异均有统计学意义(P < 0.05)。
结论
姜黄素可以通过抑制PPARγ/ NF-κB信号通路减轻CSE诱导的人气道上皮16HBE细胞的炎症反应及氧化应激。为姜黄素应用于慢性阻塞性肺疾病等疾病的临床治疗奠定了理论基础。
Keywords: CSE, 16HBE细胞, 氧化应激, PPARγ, NF-κB,
Abstract
Objective
To investigate the effect of curcumin against cigarette smoke extract (CSE)- induced oxidative stress in human bronchial epithelial cells and explore the underlying mechanism.
Methods
Human bronchial epithelial cell line 16HBE was treated for 24 h with curcumin, CSE, CSE + curcumin, and CSE + curcumin with transfection by a short hairpin RNA targeting PPARγ (shPPARγ). MTT assay was used to observe the changes in the cell viability after the treatments. Quantitative real-time PCR was performed to detect the mRNA expressions of tumor necrosis factor-α (TNF-α), iNOS and PPARγ in the cells, and the protein expressions of iNOS, PPARγ and the phosphorylation of NF-κB p65 were detected using Western blotting.
Results
The treatments did not cause significant changes in the cell viability. Exposure to CSE for 24 h significantly lowered PPARγ expression and increased TNF-α and iNOS expressions and phosphorylation of NF-κB p65 in the cells. The effects of CSE were significantly suppressed by curcumin, but transfection of the cells with shRNA-PPARγ obviously abrogated the suppressive effects of curcumin.
Conclusions
Curcumin suppresses CSE-induced oxidative stress and inflammation via the PPARγ/NF-κB signaling pathway in 16HBE cells, suggesting the potential of curcumin in the treatment of chronic obstructive pulmonary disease.
Keywords: cigarette smoke extract, bronchial epithelial cells, 16HBE cells, oxidative stress, PPARγ, nuclear factor-κB
慢性阻塞性肺疾病(COPD)作为最常见的慢性气道炎症性疾病,已成为导致人类死亡的第4大疾病,研究发现烟雾及颗粒诱导的持续性的气道氧化应激和炎症反应是导致COPD发生和发展的核心因素[1-2]。因此抑制过度的氧化应激和炎症反应已成为延缓疾病进展和治疗的关键。过氧化物酶体增殖物激活受体γ(PPARγ)是配体激活的核转录因子,在机体脂质平衡、糖代谢、炎症反应和免疫反应等方面具有重要的调控作用。研究发现PPARγ具有调节肺免疫反应的作用[3],其还可能作为治疗支气管哮喘和COPD等慢性肺疾病的潜在分子靶点[4]。姜黄素是从姜黄类植物的根茎中提取的一种具有抑制氧化应激、炎症反应、器官纤维化、调节血糖及调控细胞凋亡等多种生物学活性的酚类化合物[5-7]。大量研究发现,姜黄素的效应与上调PPARγ的表达有关[8-11]。另外目前研究证实姜黄素调控香烟烟雾介导的炎症反应和氧化应激作用主要包括:直接清除自由基、增强抗氧化防御系统、抑制NF-κB信号通路、激活核因子E2相关因子2(Nrf2)等,但具体分子机制仍不明确。鉴于PPARγ是调控炎症和应激的上游关键因子,那么姜黄素以上的药理作用是否与诱导PPARγ表达密切相关呢?本实验将使用姜黄素对香烟烟雾提取物(CSE)诱导的人气道上皮16HBE细胞进行干预,观察其对PPARγ和NF-κB表达和活化的影响,进一步探讨姜黄素对COPD的治疗作用及与PPARγ/NF-κB信号通路的关系。
1. 材料和方法
1.1. 试剂和材料
人气道上皮细胞16HBE细胞株(中国科学院上海细胞库提供);姜黄素(纯度>98%)(西安崇信);总RNA抽提试剂盒(北京百泰克);第一链cDNA合成试剂盒和PCR试剂盒(Takara公司);iQTM5 Multicolor Real-time PCR Detection System(百乐);shRNA-PPARγ(sc-44220- V)和shRNA-scrambled(sc-108060,阴性对照shRNA)(Santa Cruz);鼠抗人iNOS抗体、鼠抗人PPARγ抗体、鼠抗人NF-κB p65抗体、鼠抗人phospho-NF-κB p65抗体和鼠抗人β-actin抗体(Santa Cruz)。DMEM培养基(Gibco),小牛血清(四季清),甲醇(色谱纯),乙腈(色谱纯,Tedia),DMSO(二甲基亚砜,国药集团),MTT试剂盒(Promega)。其余试剂均为分析纯。
1.2. 细胞培养及shRNA-PPARγ转染效果鉴定
16HBE细胞常规接种在含10% FBS、100 g/L青霉素、100 g/L链霉素的DMEM培养液中,置于37 ℃、95%空气、5% CO2孵箱内培养。每48 h换液、传代1次,取对数生长期细胞用于实验。根据前期研究方法,首先使用shRNA-PPARγ和shRNA-scrambled对16HBE细胞干预24 h,观察shRNA-PPARγ对于16HBE细胞PPARγ表达的抑制作用,在干预24 h后使用qPCR和western blot法对PPARγ表达水平进行检测,了解shRNA-PPARγ转染效率[12]。
1.3. 细胞分组及干预方法
1.3.1. 细胞分组方法
16HBE细胞分为对照组、姜黄素组、CSE组、CSE+姜黄素组和CSE+姜黄素+shRNAPPARγ组。根据文献介绍方法制备CSE[13-14]。其中对照组细胞加入PBS干预24 h;姜黄素组加入终浓度为25 μmol/L的姜黄素溶液进行干预24 h;CSE组加入CSE(终浓度为2%)干预24 h;CSE+姜黄素组加入终浓度为25 μmol/L的姜黄素溶液进行干预4 h然后再给予CSE(终浓度为2%)干预24 h;CSE+姜黄素+shPPARγ组在给予shRNA-PPARγ干预24 h后加入终浓度为25 μmol/L的姜黄素溶液干预4 h,然后再加入CSE(终浓度为2%)干预24 h。在干预结束后提取细胞mRNA和蛋白保存于-80 ℃备用。
1.3.2. 细胞活性分析(MTT比色试验)
取对数生长期细胞消化制成单细胞悬液,接种于96孔培养板中,每孔接种100 μL约含5×103个细胞。贴壁后无血清培养细胞16~24 h,使细胞同步化。使用MTT比色试验对0 h和24 h时的细胞生长状态进行测定,重复实验5次。细胞活性(%)=对应时间点(OD干预组/OD对照组)×100(%)[12, 15-16]。
1.3.3. QPCR检测16HBE细胞TNF-α、iNOS和PPARγ mRNA表达检测
干预24 h后,按照qPCR试剂盒说明书提取细胞总RNA。引物:TNF-α正义5'-CTCTTCTC CTTCCTGATCGTGG-3',反义5'-CTTGTCACTCGG GGTTCGAG -3';PPARγ正义5'-ACTGTCGGTTTCA GAAGTGC-3',反义5'-ATGGACACCATACTTGAG C-3';iNOS正义:5'-CACAGAACTGAGGGTACA-3',反义:5'-AGAGAGATCGGGTTCACA-3';β-actin正义:5'-CTTAGTTGCGTTACACCCTTTCTTG-3',反义:5'- CTGTCACCTTCACCGTTCCAGTTT-3'。使用β-actin作为内参基因,使用2-△△CT公式计算目的基因相对表达量。ΔΔCt=(Ct, 目标−Ct, β-actin)干预组(- Ct, 目标−Ct, β-actin)对照组[17-18]。
1.3.4. Western blot法检测16HBE细胞中iNOS、PPARγ蛋白表达以及NF-κB p65活化
干预24 h后,按照试剂盒操作要求,首先提取细胞总蛋白,并进行聚丙烯酰胺凝胶电泳(SDS-PAGE),然后转移至硝酸纤维素滤膜上,用脱脂奶粉封闭1 h,分别加入鼠抗人单克隆抗体PPARγ(1:1000)、phospho-NF-κB p65(1:800)、NF-κB p65抗体(1:800)和β-actin(1:1200),4 ℃孵育过夜,洗膜后加入相应的辣根过氧化物酶标记的二抗(1:2000),用ECL进行显色,用凝胶成像分析系统进行扫描。
1.3.5. 统计学方法
计量资料以均数±标准差表示。采用SPSS17.0进行单因素方差分析,多重比较采用LSD法,P < 0.05为差异有统计学意义。
2. 结果
2.1. shRNA-PPARγ转染后16HBE PPARγ的表达水平及细胞活性
16HBE细胞转染shRNA-PPARγ和shRNAscrambled 24 h后,使用qPCR和western blot法对PPARγ mRNA和蛋白表达进行分析,发现转染shRNA-PPARγ后细胞PPARγ表达水平较对照组明显下降(P < 0.05,图 1,表 1),而shRNA-scrambled组和对照组未见显著差异(P>0.05,图 1,表 1)。
1.

16HBE细胞PPARγ western blot结果
The western blot results of PPARγ in 16HBE cells.
1.
16HBE细胞PPARγ mRNA和蛋白的表达水平
Changes in mRNA and protein expressions of PPARγ in 16HBE cells transfected with shRNA-PPARγ (n=5, Mean±SD)
| Group | PPARγ mRNA | PPARγ protein |
| #P < 0.05 compared with Control. | ||
| Control | 1 | 0.98±0.05 |
| shRNA-scrambled | 0.98±0.04 | 0.96±0.03 |
| shRNA-PPARγ | 0.18±0.07# | 0.21±0.11# |
2.2. 姜黄素对于16HBE细胞活性的影响
16HBE细胞在干预24 h后使用MTT法对各组细胞活性进行检查,发现对照组、姜黄素组、CSE组、CSE+姜黄素组和CSE+姜黄素+shPPARγ组之间细胞活性未有明显统计学差异(P>0.05,表 2)。
2.
MTT比色法测定16HBE细胞活性(0 h and 24 h)
Changes in 16HBE cell viability at different time points of treatments (n=5, Mean±SD)
| Intervention time | 0 h | 24 h |
| Control | 100% | 100% |
| Curcumin | (99.7±1.5)% | (103.7±1.9)% |
| CSE | (101.3±2.7)% | (98.6±2.1)% |
| CSE+Curcumin | (101.4±2.6)% | (99.5±2.8)% |
| CSE+Curcumin+shPPARγ | (104.2±1.9)% | (101.3±1.3)% |
2.3. 姜黄素对于CSE诱导的16HBE细胞TNF-α和iNOS表达的影响
与对照组相比较,16HBE细胞在CSE干预24 h后iNOS mRNA和蛋白的表达水平及TNF-α mRNA的表达水平明显升高,差异均有统计学意义(P < 0.05,图 2,表 3)。但CSE组较CSE+姜黄素组和CSE+姜黄素+shPPARγ组iNOS和TNF-α表达水平升高更加显著,差异均有统计学意义(P < 0.05,图 2,表 3);且CSE +姜黄素+ shPPARγ组较CSE+姜黄素组升高更加明显,差异均有统计学意义(P < 0.05,图 2,表 3)。
2.
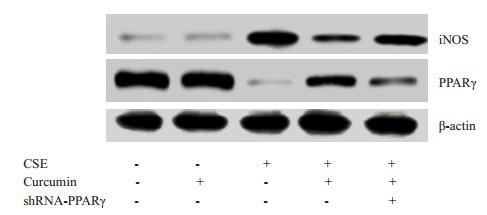
16HBE细胞iNOS和PPARγ western blot结果
Western blotting of iNOS and PPARγ in 16HBE cells with different treatments.
3.
16HBE细胞TNF-α和iNOS mRNA的表达水平
The mRNA expression of TNF-α and iNOS in 16HBE cells with different treatments (n=5, Mean±SD)
| Group | TNF-α mRNA | iNOS mRNA |
| *P < 0.05 compared with Control; #P < 0.05 compared with CSE; †P < 0.05 compared with CSE+Curcumin+shPPARγ. | ||
| Control | 1 | 1 |
| Curcumin | 1.05±0.04 | 1.07±0.06 |
| CSE | 12.15±4.18* | 13.27±2.17* |
| CSE+Curcumin | 4.52±1.22*#† | 3.58±1.06*#† |
| CSE+Curcumin+shPPARγ | 8.97±2.41*# | 6.77±1.62*# |
2.4. 姜黄素对于CSE诱导的16HBE细胞PPARγ表达的影响
与对照组相比较,16HBE细胞在CSE干预24 h后PPARγ mRNA和蛋白的表达水平明显降低,差异均有统计学意义(P < 0.05,图 2,表 4)。但CSE组较CSE+姜黄素组和CSE+姜黄素+shPPARγ组PPARγ表达水平下降更加显著,差异均有统计学意义(P < 0.05,图 2,表 4);且CSE+姜黄素+shPPARγ组较CSE+姜黄素组PPARγ表达水平下降更加明显,差异均有统计学意义(P < 0.05,图 2,表 4)。
4.
16HBE细胞PPARγ mRNA和蛋白的表达水平及NF-κB p65活化水平
The mRNA and protein expression of PPARγ and activation of NF- κB p65 in 16HBE cells with different treatments (n=5, Mean±SD)
| Group | PPARγ mRNA | PPARγ protein | Phospho-NF-κB p65/NF-κB p65 |
| *P < 0.05 compared with Control; #P < 0.05 compared with CSE; †P < 0.05 compared with CSE+Curcumin+shPPARγ. | |||
| Control | 1 | 0.98±0.05 | 0.17±0.04 |
| Curcumin | 0.98±0.04 | 0.96±0.06 | 0.15±0.03 |
| CSE | 0.18±0.07* | 0.18±0.08* | 0.93±0.04* |
| CSE+Curcumin | 0.69±0.14*#† | 0.81±0.12*#† | 0.35±0.07*#† |
| CSE+Curcumin+shPPARγ | 0.37±0.12*# | 0.41±0.11*# | 0.59±0.09*# |
2.5. 姜黄素对于CSE诱导的16HBE细胞NF-κB p65磷酸化水平的影响
与对照组相比较,16HBE细胞在CSE干预24 h后NF-κB p65的磷酸化水平明显升高,差异均有统计学意义(P < 0.05,图 3,表 4)。但CSE组较CSE+姜黄素组和CSE+姜黄素+shPPARγ组NF-κB p65的磷酸化水平升高更加显著,差异均有统计学意义(P < 0.05,图 3,表 4);且CSE+姜黄素+shPPARγ组较CSE+姜黄素组NF-κB p65的磷酸化水平升高更加明显,差异均有统计学意义(P < 0.05,图 3,表 4)。
3.

16HBE细胞NF-κB p65磷酸化水平的Western blot结果
Western blotting for assessing activation of NF-κB p65 in 16HBE cells with different treatments.
3. 讨论
慢性阻塞性肺疾病是以持续呼吸道症状和持续气流受限为特征的一种慢性肺病,与气道和肺对有害微粒或气体的异常反应有关。流行病学调查发现近年来我国的COPD发病率呈上升趋势,目前发病率已高达8.6%~13.6%[1-2]。大量研究证示吸入有害颗粒和烟雾如香烟、生物燃料及空气污染等诱导的中小气道慢性持续性氧化应激及炎症反应是COPD发生和发展的根本原因[1-2, 19-20]。抑制氧化应激及炎症反应已成为COPD治疗的关键靶点。气道上皮细胞(AECs)作为人体与外界接触的第一道物理性和生物性屏障,时刻暴露于炎症性和感染性的刺激和损伤,AECs损伤引起的急、慢性炎症浸润是改变和破坏气道局部和整体免疫调节水平的重要因素[21-22]。本研究通过香烟烟雾提取物(CSE)干预人气道上皮16HBE细胞模拟吸烟状态下气道上皮细胞的炎症模型,通过姜黄素干预,观察气道上皮细胞的活性和炎症反应程度,探讨了姜黄素对COPD的治疗作用及机制。
本研究结果显示,与对照组比较,CSE干预16HBE细胞24 h后iNOS mRNA和蛋白质及TNF-α mRNA的表达水平明显升高。诱导型一氧化氮合酶(iNOS)属于NOS家族,过度的或病理性氧化应激和炎症反应状态下多种组织细胞均出现高表达[23]。iNOS的高表达将导致组织细胞内NO(重要的氧自由基分子)的大量合成诱导过度的氧化应激反应,造成组织损伤和炎症反应的持续[24-25]。Seimetz等[25]的研究显示在香烟诱导的小鼠肺气肿模型中抑制iNOS表达可有效改善气道炎症反应、肺气肿的发生及肺血管的重塑。而TNF-α作为重要的炎症介质,在COPD气道炎症反应中扮演者重要的角色[26]。COPD相关的炎症状态下TNF-α可由多种组织细胞合成和分泌,TNF-α既可直接导致气道上皮细胞损伤,也可间接通过趋化中性粒细胞和巨噬细胞等炎症细胞的聚集及活化导致气道炎症和氧化应激反应[16, 27]。有研究显示气道和血中TNF-α水平与COPD气道及全身炎症反应程度呈正相关,可作为检查COPD严重程度的标记物[26]。
研究中在CSE的基础上加用姜黄素干预后,我们发现iNOS和TNF-α mRNA的表达水平明显下降。该结果说明16HBE细胞中姜黄素对CSE诱导的TNF-α和iNOS表达有显著的抑制作用。表明姜黄素可有效减轻CSE诱导的气道上皮炎症反应和氧化应激反应。NF- κB在生理状态下与IκB形成复合物存在于包浆中,当细胞受刺激后,IκB发生磷酸化并与NF-κB解离,激活的NF-κB在核内与特定的κB位点结合,引起靶基因表达增强[16-17]。Yuan等[28]的研究发现姜黄素可以通过抑制NF-κB信号通路的活化下调LPS诱导的BEAS-2B细胞COX-2过度表达。在本研究中,与对照组相比较,CES组和CSE+姜黄素组NF-κB p65的磷酸化水平均升高,但CES组较CSE+姜黄素组升高更为显著。NF-κB被证实在炎症的调控中起到关键性作用[15, 18],且研究发现TNF-α和iNOS基因启动子部位存在NF-κB结合位点。结合本研究可发现,TNF-α、iNOS的表达水平与NF-κB p65的磷酸化呈正相关,提示姜黄素可通过抑制NF-κB信号通路下调炎症相关基因的转录,从而发挥其抗炎、抗氧化作用。
Meng等[29]的研究发现姜黄素可通过上调PPARγ信号通路减轻自发性高血压大鼠模型(SHRs)的心肌纤维化。Liu等[30]的实验显示姜黄素可通过促进PPARγ信号通路抑制TGF-β2诱导的肺成纤维细胞向肌成纤维细胞的分化。杨雪梅等[11]的研究显示姜黄素既可结合PPARγ,又可激活PPARγ,是PPARγ的天然配体。目前大量研究证实PPARγ是NF-κB活化重要的调控因子,在多种病理状态下和不同组织中上调PPARγ可导致NF- κB活化水平下降[14]。本研究中首先我们发现在CSE诱导的16HBE细胞状态下姜黄素可以明显上调PPARγ的表达而下调NF-κB p65的磷酸化水平。进一步我们在使用shRNA-PPARγ转染细胞特异性抑制PPARγ表达后发现姜黄素促进PPARγ合成及抑制NF-κB p65磷酸化的作用明显减弱。同时发现姜黄素对TNF-α和iNOS的抑制作用也被shRNA-PPARγ明显阻断。该结果表明姜黄素可通过PPARγ/NF-κB信号通路减轻CSE诱导的16HBE细胞TNF-α和iNOS表达。
综上所述,本研究发现姜黄素可以通过PPARγ/NF- κB信号通路减轻CSE诱导的人气道上皮16HBE细胞的炎症反应及氧化应激,为姜黄素应用于COPD和支气管哮喘等慢性气道炎症性疾病的临床治疗奠定了理论基础。
Biographies
朱涛,博士,博士后,副主任医师,E-mail: zhutao063020@163. com
施婵妹,硕士研究生,E-mail: 775710485@qq.com
Funding Statement
广东省科技计划(2016A020215099,2014A020212627);广东省自然科学基金(博士科研启动纵向协同管理试点项目)(2017A030310286);广州市科学研究专项(一般项目专题)(201707010282)
Contributor Information
施 婵妹 (Chanmei SHI), Email: 775710485@qq.com.
邓 火金 (Huojin DENG), Email: denghj51889@126.com.
References
- 1.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health[CPH]study): a National cross-sectional study. Lancet. 2018;391(1131):1706–17. doi: 10.1016/S0140-6736(18)30841-9. [Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health[CPH]study): a National cross-sectional study[J]. Lancet, 2018, 391(1131): 1706-17.] [DOI] [PubMed] [Google Scholar]
- 2.Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_d1d927d88df3d77a5078096b2c630013. Lancet Respir Med. 2018;6(6):421–30. doi: 10.1016/S2213-2600(18)30103-6. [Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study[J]. Lancet Respir Med, 2018, 6(6): 421-30.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nobs SP, Kopf M. PPAR-gamma in innate and adaptive lung immunity. J Leukoc Biol. 2018;104(4):737–41. doi: 10.1002/jlb.2018.104.issue-4. [Nobs SP, Kopf M. PPAR-gamma in innate and adaptive lung immunity[J]. J Leukoc Biol, 2018, 104(4): 737-41.] [DOI] [PubMed] [Google Scholar]
- 4.Hart CM, Roman J, Reddy R, et al. PPARγ: A novel molecular target in lung disease. J Investig Med. 2008;56(2):515–7. doi: 10.2310/JIM.0b013e318165e89d. [Hart CM, Roman J, Reddy R, et al. PPARγ: A novel molecular target in lung disease[J]. J Investig Med, 2008, 56(2): 515-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li HY, Yang M, Li Z, et al. Curcumin inhibits angiotensin Ⅱinduced inflammation and proliferation of rat vascular smooth muscle cells by elevating PPAR-gamma activity and reducing oxidative stress. Int J Mol Med. 2017;39(5):1307–16. doi: 10.3892/ijmm.2017.2924. [Li HY, Yang M, Li Z, et al. Curcumin inhibits angiotensin Ⅱinduced inflammation and proliferation of rat vascular smooth muscle cells by elevating PPAR-gamma activity and reducing oxidative stress[J]. Int J Mol Med, 2017, 39(5): 1307-16.] [DOI] [PubMed] [Google Scholar]
- 6.尹 海燕, 邱 敏珊, 何 丹, et al. 姜黄素对脓毒症大鼠肝细胞的保护作用. 中华危重病急救医学. 2017;29(2):162–6. doi: 10.3760/cma.j.issn.2095-4352.2017.02.013. [尹海燕, 邱敏珊, 何丹, 等.姜黄素对脓毒症大鼠肝细胞的保护作用[J].中华危重病急救医学, 2017, 29(2): 162-6.] [DOI] [PubMed] [Google Scholar]
- 7.王 翠娟, 尚 明, 邹 微, et al. 姜黄素诱导NSCLC细胞凋亡机制探讨. http://d.old.wanfangdata.com.cn/Periodical/qlzlzz201710002. 中华肿瘤防治杂志. 2017;24(10):663–9. [王翠娟, 尚明, 邹微, 等.姜黄素诱导NSCLC细胞凋亡机制探讨[J].中华肿瘤防治杂志, 2017, 24(10): 663-9.] [Google Scholar]
- 8.Mohsen M, Ehsan K, et al. Potential effects of curcumin on peroxisome proliferatoractivated receptor-γ in vitro and in vivo. World J of Methodol. 2016;6(1):112–7. doi: 10.5662/wjm.v6.i1.112. [Mohsen M, Ehsan K, et al. Potential effects of curcumin on peroxisome proliferatoractivated receptor-γ in vitro and in vivo[J]. World J of Methodol, 2016, 6(1): 112-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu S, Jiang B, Wang H, et al. Curcumin suppresses intestinal fibrosis by inhibition of PPARγ-mediated epithelial-mesenchymal transition. Evid Based Complement Alternat Med. 2017:7876064. doi: 10.1155/2017/7876064. [Xu S, Jiang B, Wang H, et al. Curcumin suppresses intestinal fibrosis by inhibition of PPARγ-mediated epithelial-mesenchymal transition[J]. Evid Based Complement Alternat Med, 2017: 7876064.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng KP, Yang AJ, Hu XH, et al. Curcumin attenuates pulmonary inflammation in lipopolysaccharide induced acute lung injury in neonatal rat model by activating peroxisome Proliferator-Activated receptor gamma (PPAR gamma) pathway. Med Sci Monit. 2018;24:1178–84. doi: 10.12659/MSM.908714. [Cheng KP, Yang AJ, Hu XH, et al. Curcumin attenuates pulmonary inflammation in lipopolysaccharide induced acute lung injury in neonatal rat model by activating peroxisome Proliferator-Activated receptor gamma (PPAR gamma) pathway[J]. Med Sci Monit, 2018, 24: 1178-84.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.杨 雪梅, 邱 红梅, 田 蜜, et al. 姜黄素对人过氧化物酶体增殖物激活受体γ_1激活作用的研究. http://d.old.wanfangdata.com.cn/Periodical/zcy201715017. 中草药. 2017;48(15):3122–6. [杨雪梅, 邱红梅, 田蜜, 等.姜黄素对人过氧化物酶体增殖物激活受体γ_1激活作用的研究[J].中草药, 2017, 48(15): 3122-6.] [Google Scholar]
- 12.Zhu T, Li C, Zhang X, et al. GLP-1 enhances SP-A expression in LPS-induced acute lung injury through the TTF-1 signaling pathway[J], 2018: 3601454.
- 13.Chen ZH, Kim HP, Sciurba FC, et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One. 2008;3(10):e3316. doi: 10.1371/journal.pone.0003316. [Chen ZH, Kim HP, Sciurba FC, et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease[J]. PLoS One, 2008, 3(10): e3316.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Hu J, Wang T, et al. Silymarin attenuates cigarette smoke extract-induced inflammation via simultaneous inhibition of autophagy and ERK/p38 MAPK pathway in human bronchial epithelial cells. Sci Rep. 2016;6:37751. doi: 10.1038/srep37751. [Li D, Hu J, Wang T, et al. Silymarin attenuates cigarette smoke extract-induced inflammation via simultaneous inhibition of autophagy and ERK/p38 MAPK pathway in human bronchial epithelial cells[J]. Sci Rep, 2016, 6: 37751.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu T, Zhang W, Feng SJ, et al. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPAR gammadependent pathway. Int Immunopharmacol. 2016;34:16–24. doi: 10.1016/j.intimp.2016.02.014. [Zhu T, Zhang W, Feng SJ, et al. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPAR gammadependent pathway[J]. Int Immunopharmacol, 2016, 34: 16-24.] [DOI] [PubMed] [Google Scholar]
- 16.Zhu T, Wang DX, Zhang W, et al. Andrographolide protects against LPS-Induced acute lung injury by inactivation of NF-kappa B. PLoS One. 2013;8(2):e56407. doi: 10.1371/journal.pone.0056407. [Zhu T, Wang DX, Zhang W, et al. Andrographolide protects against LPS-Induced acute lung injury by inactivation of NF-kappa B[J]. PLoS One, 2013, 8(2): e56407.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu T, Wu XL, Zhang W, et al. Glucagon like peptide-1 (GLP-1) modulates OVA-Induced airway inflammation and mucus secretion involving a protein kinase a (PKA)-Dependent nuclear factor-kappa B (NF-kappa B) signaling pathway in mice. Int J Mol Sci. 2015;16(9):20195–211. doi: 10.3390/ijms160920195. [Zhu T, Wu XL, Zhang W, et al. Glucagon like peptide-1 (GLP-1) modulates OVA-Induced airway inflammation and mucus secretion involving a protein kinase a (PKA)-Dependent nuclear factor-kappa B (NF-kappa B) signaling pathway in mice[J]. Int J Mol Sci, 2015, 16(9): 20195-211.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu T, Li CY, Zhang XE, et al. GLP-1 analogue liraglutide enhances SP-A expression in LPS-Induced acute lung injury through the TTF-1 signaling pathway. Mediators Inflamm. 2018:3601454. doi: 10.1155/2018/3601454. [Zhu T, Li CY, Zhang XE, et al. GLP-1 analogue liraglutide enhances SP-A expression in LPS-Induced acute lung injury through the TTF-1 signaling pathway[J]. Mediators Inflamm, 2018: 3601454.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.张 毅, 朱 涛, 于 化鹏. 全身性和吸入性糖皮质激素治疗对AECOPD系统性炎症反应水平的影响. 西部医学. 2018;30(1):52–5. doi: 10.3969/j.issn.1672-3511.2018.01.013. [张毅, 朱涛, 于化鹏.全身性和吸入性糖皮质激素治疗对AECOPD系统性炎症反应水平的影响[J].西部医学, 2018, 30 (1): 52-5.] [DOI] [Google Scholar]
- 20.刘 杰, 陈 荣昌, 钟 南山. 呼出气二氧化碳和体表氧饱和度监测在慢性阻塞性肺疾病呼吸衰竭患者中的应用. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=2010071565. 南方医科大学学报. 2010;30(7):1565–8. [刘杰, 陈荣昌, 钟南山.呼出气二氧化碳和体表氧饱和度监测在慢性阻塞性肺疾病呼吸衰竭患者中的应用[J].南方医科大学学报, 2010, 30(7): 1565-8.] [PubMed] [Google Scholar]
- 21.Holt PG, Strickland DH, Wikström ME, et al. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8(2):142–52. doi: 10.1038/nri2236. [Holt PG, Strickland DH, Wikström ME, et al. Regulation of immunological homeostasis in the respiratory tract[J]. Nat Rev Immunol, 2008, 8(2): 142-52.] [DOI] [PubMed] [Google Scholar]
- 22.Hallstrand TS, Hackett TL, Altemeier WA, et al. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol. 2014;151(1):1–15. doi: 10.1016/j.clim.2013.12.003. [Hallstrand TS, Hackett TL, Altemeier WA, et al. Airway epithelial regulation of pulmonary immune homeostasis and inflammation[J]. Clin Immunol, 2014, 151(1): 1-15.] [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharyya A, Chattopadhyay R, Mitra S, et al. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94(2):329–54. doi: 10.1152/physrev.00040.2012. [Bhattacharyya A, Chattopadhyay R, Mitra S, et al. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases[J]. Physiol Rev, 2014, 94(2): 329-54.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malerba M, Radaeli A, Olivini A, et al. Exhaled nitric oxide as a biomarker in COPD and related comorbidities. Biomed Res Int. 2014:271918. doi: 10.1155/2014/271918. [Malerba M, Radaeli A, Olivini A, et al. Exhaled nitric oxide as a biomarker in COPD and related comorbidities[J]. Biomed Res Int, 2014: 271918.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seimetz M, Parajuli N, Pichl A, et al. Inducible NOS inhibition reverses Tobacco-Smoke-Induced emphysema and pulmonary hypertension in mice. Cell. 2011;147(2):293–305. doi: 10.1016/j.cell.2011.08.035. [Seimetz M, Parajuli N, Pichl A, et al. Inducible NOS inhibition reverses Tobacco-Smoke-Induced emphysema and pulmonary hypertension in mice[J]. Cell, 2011, 147(2): 293-305.] [DOI] [PubMed] [Google Scholar]
- 26.陈 博, 戴 爱国. TNF-α对老年COPD患者调节性T细胞CTLA-4表达的影响. 国际呼吸杂志. 2017;37(21):1601–9. doi: 10.3760/cma.j.issn.1673-436X.2017.21.001. [陈博, 戴爱国. TNF-α对老年COPD患者调节性T细胞CTLA-4表达的影响[J].国际呼吸杂志, 2017, 37(21): 1601-9.] [DOI] [Google Scholar]
- 27.王 林梅, 齐 景宪, 冯 青青. 慢性阻塞性肺疾病急性加重期和稳定期患者血清IL-8、TNF-α及免疫因子的检测. http://d.old.wanfangdata.com.cn/Periodical/henanykdx201802028. 郑州大学学报:医学版. 2018;(2):255–8. [王林梅, 齐景宪, 冯青青.慢性阻塞性肺疾病急性加重期和稳定期患者血清IL-8、TNF-α及免疫因子的检测[J].郑州大学学报:医学版, 2018(2): 255-8.] [Google Scholar]
- 28.Yuan J, Liu R, Ma Y, et al. Curcumin attenuates airway inflammation and airway remolding by inhibiting NF-κB signaling and COX-2 in cigarette Smoke-Induced COPD mice. http://cn.bing.com/academic/profile?id=e32132fc5dd1529f09a23b6a7a204ff7&encoded=0&v=paper_preview&mkt=zh-cn. Inflammation. 2018;41(5):1804–14. doi: 10.1007/s10753-018-0823-6. [Yuan J, Liu R, Ma Y, et al. Curcumin attenuates airway inflammation and airway remolding by inhibiting NF-κB signaling and COX-2 in cigarette Smoke-Induced COPD mice[J]. Inflammation, 2018, 41 (5): 1804-14.] [DOI] [PubMed] [Google Scholar]
- 29.Meng Z, Yu XH, Chen J, et al. Curcumin attenuates cardiac fibrosis in spontaneously hypertensive rats through PPAR-gamma activation. http://cn.bing.com/academic/profile?id=95730054122ae267d6f44106a61dbebf&encoded=0&v=paper_preview&mkt=zh-cn. Acta Pharmacol Sin. 2014;35(10):1247–56. doi: 10.1038/aps.2014.63. [Meng Z, Yu XH, Chen J, et al. Curcumin attenuates cardiac fibrosis in spontaneously hypertensive rats through PPAR-gamma activation[J]. Acta Pharmacol Sin, 2014, 35(10): 1247-56.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D, Gong L, Zhu H, et al. Curcumin inhibits transforming growth factor β induced differentiation of mouse lung fibroblasts to myofibroblasts. Front Pharmacol. 2016;7:419. doi: 10.3389/fphar.2016.00419. [Liu D, Gong L, Zhu H, et al. Curcumin inhibits transforming growth factor β induced differentiation of mouse lung fibroblasts to myofibroblasts[J]. Front Pharmacol, 2016, 7: 419.] [DOI] [PMC free article] [PubMed] [Google Scholar]


