Abstract
Objective
To identify whether musculoskeletal ultrasound (MSUS) abnormalities are associated with specific phases of rheumatoid arthritis (RA) development in individuals at risk of RA.
Methods
This is a prospective cohort study of individuals at risk of developing RA, namely first-degree relatives of patients with RA (RA-FDRs) without evidence of established rheumatic disease at inclusion. The inflammatory activity on MSUS was assessed according to a validated score (SONAR). Active MSUS was defined as a total B-mode score greater than 8, including at least one joint with significant synovitis (grade 2 or 3) or significant synovial hyperaemia (Doppler score greater than 1). We used logistic regression to analyse associations between MSUS findings and recognised preclinical phases of RA development, adjusting for other demographic and biological characteristics.
Results
A total of 273 RA-FDRs were analysed, of whom 23 (8%) were anticitrullinated protein autoantibodies-positive, 58 (21%) had unclassified arthritis and 96 (35%) had an active MSUS, which was only associated with unclassified arthritis (OR: 1.8, 95% CI 1.0 to 3.3).
Conclusion
In individuals at risk of RA, active MSUS was associated with the presence of unclassified arthritis, but not with any of the earlier described phases of RA development. These findings do not support an indiscriminate use of ultrasound in a screening strategy for preclinical RA.
Keywords: rheumatoid arthritis, ultrasonography, early rheumatoid arthritis
Key messages.
What is already known about this subject?
Identifying preclinical rheumatoid arthritis (RA) has become a high-stakes undertaking.
Initiation of appropriate treatment at the preclinical stage may change the course of the disease.
The added value of musculoskeletal ultrasound (MSUS) in RA management is well recognised, especially for early diagnosis.
What does the study add?
In this cohort study of individuals genetically at risk of developing RA, with no established rheumatic disease, MSUS inflammatory activity was associated with the presence of unclassified arthritis, the latest phase of preclinical RA.
However, MSUS was not associated with earlier preclinical phases of RA development.
How might this impact on clinical practice?
Although MSUS may be useful in detecting imminent RA, our study does not support the systematic use of MSUS in a screening algorithm for preclinical RA.
Introduction
Rheumatoid arthritis (RA) remains a frequent and debilitating disease even with the development of highly effective new treatments. It is well established that starting aggressive antirheumatic therapy early may change the course of the disease.1 It is thought that the initiation of appropriate treatment at the preclinical stage of RA may enable the disease to be prevented.2 3 Several randomised controlled trials in preclinical disease are currently under way. Thus, identifying preclinical RA has become a high-stakes undertaking.4 5
Specific preclinical phases of RA development have been proposed,6 namely genetic risk factors for RA, environmental risk factors, systemic autoimmunity associated with RA, symptoms without clinical arthritis and unclassified arthritis. Systemic autoimmunity associated with RA is considered the immunological onset of the disease and is characterised by the presence of autoantibodies, such as rheumatoid factor (RF) and anticitrullinated protein autoantibodies (ACPAs), and both autoantibodies precede the onset of RA by several years.3 Preclinical phases have been studied among first-degree relatives with RA (RA-FDRs), a population with an increased risk of developing RA compared with the general population.7
Several predictive factors for RA development have been identified, which can schematically be grouped as clinical predictors (ie, environmental exposures, family history), biomarkers (ie, genetic factors, autoantibodies) and imaging modalities. Among the various imaging techniques, subclinical MRI inflammation was shown to precede clinical arthritis by a few months.8 Musculoskeletal ultrasound (MSUS) has gained a prominent role in RA management, given technical improvements and accessibility in daily clinical practice.9 In established RA, MSUS is more sensitive than clinical assessment for the detection of synovitis.10 Moreover, MSUS plays an important role in two critical stages of the disease: early RA diagnosis and prediction of relapse in patients clinically in remission.11 12 At an earlier stage of the disease, namely in autoantibody-positive patients with arthralgias but without clinical evidence of synovitis, MSUS abnormalities have been associated with subsequent development of arthritis.13 14
The aim of this study was to assess the value of MSUS in a screening strategy of healthy individuals at increased risk of RA. We analysed the association of MSUS with the recognised preclinical phases of RA development.
Methods
Patient population and study design
The SCREEN-RA study is an ongoing cohort study of individuals genetically at risk of developing RA, namely first-degree relatives (FDR) of patients with RA who had no established rheumatic disease and no antirheumatic treatment at enrolment, described in detail elsewhere.7 15 Briefly at enrolment, RA-FDRs answer a questionnaire about potential environmental risk factors and are examined by a rheumatologist or specialised study nurse to rule out the presence of RA, other autoimmune conditions, and tender or swollen joints. Serum samples are collected for genetic testing and autoantibodies (RF and ACPA) assessment. Participants are followed annually to assess for the development of signs and symptoms of arthritis.
We performed a cross-sectional study nested within the SCREEN-RA cohort to analyse MSUS in individuals in preclinical phases of the disease. The analysis included consecutive participants with complete MSUS and ACPA status included in the registry after December 2011. For this analysis, we considered the first visit with an MSUS assessment, which may have occurred a few years after enrolment into the SCREEN-RA cohort. Prospective follow-up is ongoing, and at this time only two patients have developed classifiable RA, which is insufficient to allow a longitudinal analysis.
MSUS assessment
MSUS was performed using an Esaote MyLab 60 machine with a linear transducer at 18 MHz by senior rheumatologists with expertise in MSUS, blinded to clinical and biological data. Power Doppler settings, depth and gain were optimised to the lowest achievable pulse repetition frequency (PRF) and maximum gain without perceptual noise artefact as per published guidelines.16 A standardised MSUS examination was performed according to the validated, semiquantitative Swiss Sonography in Arthritis and Rheumatism (SONAR) score.9 17–19 Bilateral wrists, metacarpophalangeal and interphalangeal joints 2–5, olecranon fossa, and suprapatellar recess were assessed. Synovitis in B-mode and hyperaemia with power Doppler were graded in each joint in line with the Outcome Measures in Rheumatology clinical trials definitions.16 20 Significant level of inflammatory activity on articular MSUS or ‘active MSUS’ was defined as a total B-mode score of greater than 8, including at least one joint with significant synovitis (defined as grade 2 or 3) or significant synovial hyperaemia (defined as Doppler score greater than 1), as previously published.9 21
Primary outcome
Operationally we classified the participants into the following four groups:
FDRs without risk factors, specifically individuals without ‘shared epitope’, without ACPA, without RF, and without symptoms or signs associated with possible RA.
FDRs with genetic risk factors, defined as the presence of one or two copies of the shared epitope or with ‘systemic autoimmunity associated with RA’, defined by the presence of ACPA positivity, but without symptoms or signs associated with possible RA. ACPA positivity was operationally characterised by a positive result to any of the anticyclic citrullinated peptide antibodies tests (commercial ELISA tests anti-CCP 2.0, 3.0 or 3.1, according to the manufacturer’s cut-off values: anti-CCP2 ≥25 U/mL, and anti-CCP3.1 and anti-CCP3 ≥20 U/mL).7
FDRs with inflammatory arthralgias or self-reported symptoms associated with possible RA, according to the Connective Tissue Disease Screening Questionnaire (CSQ), but without clinical arthritis.22 The CSQ includes six RA-relevant items: morning stiffness, arthritis in the hand joints or wrists, arthritis in three or more joint areas, symmetric arthritis, subcutaneous nodules, and RF test results. The presence of three positive responses or more was considered to represent symptoms associated with possible RA.23
Unclassified arthritis, namely FDRs with at least one swollen joint on physical examination.
Statistical analysis
We performed logistic regression to analyse univariable and multivariable associations between MSUS findings and the preclinical phases of RA development,6 adjusting for other patient characteristics, including age, gender and tobacco smoking.
Sporadically missing covariates were managed using multiple imputations. All analyses were performed with STATA V.14.0. P values less than 0.05 were considered statistically significant.
Results
Among 1233 RA-FDR participants in the SCREEN-RA cohort, MSUS was performed in 273, of whom 96 (35%) had an active MSUS. Individuals with active MSUS tended to be older (years median (IQR): 52 (44–61) vs 50 (38–59), p=0.03), but without differences in gender, body mass index or tobacco smoking (table 1). ACPAs were present in 8% of RA-FDRs, which is a prevalence commonly found in unaffected FDR populations.24 25 MSUS was performed at enrolment in 153 (56%) RA-FDRs; the rest had a median of 2.7 (IQR: 2.0–4.3) years of follow-up. There was no significant difference in the ultrasound results comparing ACPA-positive and ACPA-negative RA-FDRs (mean B-mode score (SD): 6.7 (3.6) vs 6.8 (3.6), OR (95% CI): 1.0 (0.9 to 1.1); mean Doppler score (SD): 0.8 (1.3) vs 1.2 (1.9), OR (95% CI): 1.2 (0.9 to 1.6)).
Table 1.
Characteristics of FDRs with ultrasound (MSUS)
| Non-active MSUS (n=177) |
Active MSUS (n=96) |
Univariable† OR (95% CI) |
|
| Age, years, median (IQR) | 50 (38–59) | 52 (44–61) | 1.0 (1.0 to 1.0)* |
| Gender (female), n (%) | 135 (76) | 70 (73) | 0.8 (0.5 to 1.5) |
| Tobacco smoking current, n (%)‡ | 36 (22) | 17 (21) | 0.9 (0.5 to 1.8) |
| Tobacco smoking ever, n (%) | 80 (45) | 46 (48) | 1.1 (0.7 to 1.8) |
| Alcohol regular consumption, n (%) | 51 (29) | 33 (34) | 1.3 (0.8 to 2.2) |
| Body mass index, median (IQR) | 24 (21–27) | 23 (22–25) | 0.9 (0.9 to 1.0) |
| Activity (frequent manual activity) | 20 (11) | 17 (18) | 1.7 (0.8 to 3.4) |
| ACPA positivity, n (%) | 15 (8) | 8 (8) | 0.9 (0.4 to 2.4) |
| SE (one or two alleles), n (%) | 90 (51) | 40 (42) | 0.71 (0.5 to 1.1) |
| Time since first enrolment in SCREEN-RA | 0 (0–2.5) | 0 (0–2.4) | 1.1 (0.9 to 1.2) |
Active MSUS defined as a total B-mode score >8, including at least one joint with synovitis of grade 2 or 3, or Doppler score greater than 1.
*P<0.05.
†Univariable logistic regression analysis.
‡A total of 10% of observations were missing.
ACPA, anticitrullinated protein autoantibodies; FDRs, first-degree relatives; MSUS, musculoskeletal ultrasound; SE, shared epitope.
In univariable analyses, there was a significant correlation between active MSUS and the presence of unclassified arthritis on clinical examination. Twenty-eight per cent of RA-FDRs with active MSUS had at least one swollen joint on physical examination compared with 18% in the group without MSUS signs (OR 1.8, 95% CI 1.0 to 3.3) (table 2). In multivariable analyses, adjusted for age, gender and tobacco smoking, the relationship between MSUS and unclassified arthritis was only borderline significant (OR: 1.6, 95% CI 0.9 to 3.0). We found no association with genetic risk factors (presence of shared epitope), systemic autoimmunity (ACPA) or self-reported symptoms in the absence of swollen joints. We found a significant trend for more active MSUS in the later phases of preclinical disease development (p=0.03; figure 1).
Table 2.
MSUS status in relation to the stages of RA development
| Non-active MSUS (n=177) |
Active MSUS (n=96) |
Univariable* OR (95% CI) |
|
| 1. FDR without risk factors | 28 | 17 | 1.1 (0.6 to 2.2) |
| 2. Genetic risk factors (1 or 2 copies of SE) or systemic autoimmunity associated with RA (ACPA positivity), but without arthralgias or arthritis | 28 | 10 | 0.6 (0.3 to 1.3) |
| 3. Inflammatory arthralgias or self-reported symptoms associated with possible RA, but without clinical arthritis (some ACPA-positive)† | 90 | 42 | 0.7 (0.5 to 1.2) |
| 4. Unclassified arthritis (at least one swollen joint) (some ACPA-positive) | 31 | 27 | 1.8 (1.0 to 3.3)‡ |
The stages of RA development were defined as mutually exclusive for the purpose of this analysis. Active MSUS defined as a total B-mode score >8, including at least one joint with synovitis of grade 2 or 3, or Doppler score greater than 1.
*Univariable logistic regression analysis.
†Inflammatory arthralgias or self-reported symptoms associated with possible RA (Connective Tissue Disease Screening Questionnaire, CSQ >3).
‡P<0.05.
ACPA, anticitrullinated protein antibodies; FDR, first-degree relative; MSUS, musculoskeletal ultrasound; RA, rheumatoid arthritis; SE, shared epitope.
Figure 1.
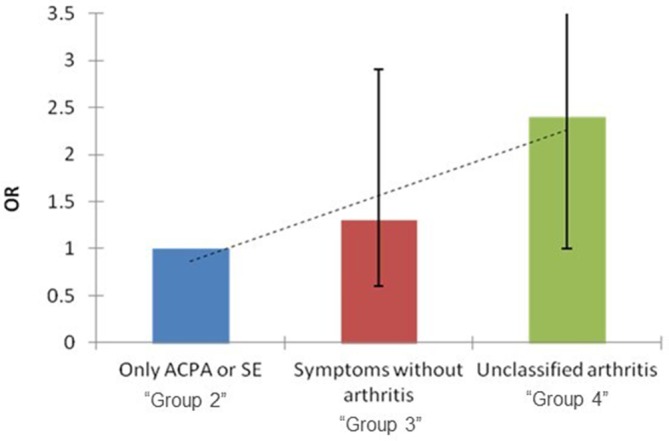
Active MSUS in preclinical phases of RA. In individuals with at least one risk factor for RA, the prevalence of an active ultrasound is increased in the first-degree relatives classified in the later phases of preclinical RA development (table 2) (p value for trend: 0.03). Unclassified arthritis defined as the presence of at least one swollen joint. ACPA, anticitrullinated protein antibodies; MSUS, musculoskeletal ultrasound; RA, rheumatoid arthritis; SE, shared epitope.
Discussion
This is the first study to assess MSUS in a population with the whole spectrum of preclinical phases of RA development. Active MSUS was associated with the presence of unclassified arthritis, but not with earlier preclinical phases of RA development. In particular, we did not find more MSUS inflammatory activity in participants with inflammatory arthralgias or systemic autoimmunity of RA. Furthermore, active MSUS was not significantly associated with any demographic or clinical risk factors, except for older age (table 1).
As preventive trials are currently being conducted in preclinical stages of RA, it has become more relevant to accurately identify high-risk individuals. New imaging methods have demonstrated high sensitivity for the detection of synovitis and to identify patients’ subclinical inflammation. van Steenbergen et al8 demonstrated that MRI could detect subclinical inflammation in patients with ‘clinically suspect arthralgia’, 4–5 months prior to the diagnosis of clinically apparent arthritis. In a prospective study of patients with arthralgias and a positive ACPA, in a later phase of RA development, patients with ultrasound features of inflammation were more likely to subsequently develop clinical synovitis.14 In a population of patients with inflammatory joint complaints without clinical synovitis, van der Ven et al26 found that Doppler signal at baseline was associated with the development of arthritis at 1 year. However, the positive predictive value of MSUS was low (26%), while the negative predictive value was 89%.
In contrast to previous MSUS studies, we included a broader population including the whole spectrum of preclinical phases of RA development. Only a limited number of subjects were ACPA-positive or reported symptoms or signs of arthritis. We found no association between MSUS and the early phases of RA development, such as autoimmunity associated with RA or arthralgias without arthritis. Active MSUS was only significantly associated with unclassified arthritis. This is in accordance with the current notion that autoimmunity in RA is initiated outside of the joints, such as mucosal surfaces, and that synovitis develops only late in the disease course.
We found a high prevalence of active MSUS in our population, with 35% of participants having MSUS signs of arthritis. A recent study conducted in healthy subjects described MSUS abnormalities in 88% of the population, 13% with synovial hypertrophy with or without Doppler signal,27 which underscores the limited specificity of MSUS joint abnormalities. The SONAR score used in this study has been validated in patients with RA,17 18 but has also been shown to be prognostic for the development of RA in patients with inflammatory arthralgias, with an OR of 7.4, independently of other prognostic factors.28 Currently several validated MSUS scoring systems exist, and it is not established which MSUS scoring system is most discriminant in preclinical stages of the disease. It is possible that MSUS scoring systems including the feet may be more sensitive, however potentially with even less specificity. In accordance with these data, 18% of our control group of FDRs without risk factors or symptoms of arthritis displayed an active MSUS. Previous studies have demonstrated that osteoarthritis is associated with signs of synovitis on MSUS,29 which may be in line with our findings that active MSUS signs were more frequent in older individuals, although we have no radiographic data to formally support this hypothesis.
Our study is limited by its cross-sectional nature, which does not allow for definite conclusions about the prognostic value of active MSUS for the future development of RA. However, the lack of association with the earlier phases of disease development6 does not suggest a strong clinical value of MSUS in a broad-range screening strategy for RA. As described in other studies,11 12 MSUS was only correlated with later phase of RA development, suggesting that MSUS may be of value only in patients with a high probability of subsequent RA development. Finally, we were not able to analyse any further subgroups, such as ACPA-positive patients with arthralgia, given the limited sample size.
In conclusion, our study does not support the systematic use of MSUS in a screening algorithm for preclinical RA. However, MSUS may be useful to detect imminent RA, for example in selected patients considering preventive antirheumatic therapy.
Footnotes
Contributors: LB, DA-R, AF and CG conceived the presented idea. DA-R and AF performed most of the statistics. All authors contributed to patient inclusion, discussed the results and contributed to the final manuscript. All authors have contributed to researching data for the article, discussion of its content, and writing, reviewing and editing the manuscript before submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Ethics Committee of University Hospital of Geneva, Switzerland (CER 08-102), and all participants signed an informed consent before enrolment.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Finckh A, Liang MH, van Herckenrode CM, et al. Long-Term impact of early treatment on radiographic progression in rheumatoid arthritis: a meta-analysis. Arthritis Rheum 2006;55:864–72. 10.1002/art.22353 [DOI] [PubMed] [Google Scholar]
- 2.Deane KD. Can rheumatoid arthritis be prevented? Best Pract Res Clin Rheumatol 2013;27:467–85. 10.1016/j.berh.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mankia K, Emery P. Review: preclinical rheumatoid arthritis: progress toward prevention: preclinical rheumatoid arthritis. Arthritis Rheumatol 2016;68:779–88. [DOI] [PubMed] [Google Scholar]
- 4.van de Stadt LA, Witte BI, Bos WH, et al. A prediction rule for the development of arthritis in seropositive arthralgia patients. Ann Rheum Dis 2013;72:1920–6. 10.1136/annrheumdis-2012-202127 [DOI] [PubMed] [Google Scholar]
- 5.Karlson EW, van Schaardenburg D, van der Helm-van Mil AH. Strategies to predict rheumatoid arthritis development in at-risk populations. Rheumatology 2016;55:6–15. 10.1093/rheumatology/keu287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlag DM, Raza K, van Baarsen LGM, et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the study Group for risk factors for rheumatoid arthritis. Ann Rheum Dis 2012;71:638–41. 10.1136/annrheumdis-2011-200990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alpizar-Rodriguez D, Brulhart L, Mueller RB, et al. The prevalence of anticitrullinated protein antibodies increases with age in healthy individuals at risk for rheumatoid arthritis. Clin Rheumatol 2017;36:677–82. 10.1007/s10067-017-3547-3 [DOI] [PubMed] [Google Scholar]
- 8.van Steenbergen HW, Mangnus L, Reijnierse M, et al. Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis. Ann Rheum Dis 2016;75:1824–30. 10.1136/annrheumdis-2015-208138 [DOI] [PubMed] [Google Scholar]
- 9.Zufferey P, Tamborrini G, Gabay C, et al. Recommendations for the use of ultrasound in rheumatoid arthritis: literature review and sonar score experience. Swiss Med Wkly 2013. 10.4414/smw.2013.13861 [DOI] [PubMed] [Google Scholar]
- 10.Naredo E. Assessment of inflammatory activity in rheumatoid arthritis: a comparative study of clinical evaluation with grey scale and power Doppler ultrasonography. Ann Rheum Dis 2004;64:375–81. 10.1136/ard.2004.023929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen H, Ruyssen-Witrand A, Gandjbakhch F, et al. Prevalence of ultrasound-detected residual synovitis and risk of relapse and structural progression in rheumatoid arthritis patients in clinical remission: a systematic review and meta-analysis. Rheumatology 2014;53:2110–8. 10.1093/rheumatology/keu217 [DOI] [PubMed] [Google Scholar]
- 12.Colebatch AN, Edwards CJ, Østergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis 2013;72:804–14. 10.1136/annrheumdis-2012-203158 [DOI] [PubMed] [Google Scholar]
- 13.van de Stadt LA, Bos WH, Meursinge Reynders M, et al. The value of ultrasonography in predicting arthritis in auto-antibody positive arthralgia patients: a prospective cohort study. Arthritis Res Ther 2010;12 10.1186/ar3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam JL, Hensor EMA, Hunt L, et al. Ultrasound findings predict progression to inflammatory arthritis in anti-CCP antibody-positive patients without clinical synovitis. Ann Rheum Dis 2016;75:2060–7. 10.1136/annrheumdis-2015-208235 [DOI] [PubMed] [Google Scholar]
- 15.Alpizar-Rodriguez D, Mueller RB, Möller B, et al. Female hormonal factors and the development of anti-citrullinated protein antibodies in women at risk of rheumatoid arthritis. Rheumatology 2017;56:1579–85. 10.1093/rheumatology/kex239 [DOI] [PubMed] [Google Scholar]
- 16.Mandl P, Naredo E, Wakefield RJ, et al. A systematic literature review analysis of ultrasound joint count and scoring systems to assess synovitis in rheumatoid arthritis according to the OMERACT filter. J Rheumatol 2011;38:2055–62. 10.3899/jrheum.110424 [DOI] [PubMed] [Google Scholar]
- 17.Brulhart L, Ziswiler H-R, Tamborrini G, et al. The importance of sonographer experience and machine quality with REGARDS to the role of musculoskeletal ultrasound in routine care of rheumatoid arthritis patients. Clin Exp Rheumatol 2015;33:98–101. [PubMed] [Google Scholar]
- 18.Zufferey P, Brulhart L, Tamborrini G, et al. Ultrasound evaluation of synovitis in RA: correlation with clinical disease activity and sensitivity to change in an observational cohort study. Joint Bone Spine 2014;81:222–7. 10.1016/j.jbspin.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 19.Tamborrini G, Ziswiler HR, Stärkle-Bär A, et al. Books on demand GmbH (Norderstedt). ultrasound scoring in rheumatoid arthritis and spondyloarthritis Sonar-scoring. 2016.
- 20.Wakefield RJ, Balint PV, Szkudlarek M, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol 2005;32:2485–7. [PubMed] [Google Scholar]
- 21.Zufferey P, Möller B, Brulhart L, et al. Persistence of ultrasound synovitis in patients with rheumatoid arthritis fulfilling the DAS28 and/or the new ACR/EULAR RA remission definitions: results of an observational cohort study. Joint Bone Spine 2014;81:426–32. 10.1016/j.jbspin.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 22.Karlson EW, Sanchez-Guerrero J, Wright EA, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol 1995;5:297–302. 10.1016/1047-2797(94)00096-C [DOI] [PubMed] [Google Scholar]
- 23.Potter J, Odutola J, Gonzales CA, et al. Validation of English and Spanish-language versions of a screening questionnaire for rheumatoid arthritis in an underserved community. J Rheumatol 2008;35:1545–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Unriza-Puin S, Bautista-Molano W, Lafaurie GI, et al. Are obesity, ACPAs and periodontitis conditions that influence the risk of developing rheumatoid arthritis in first-degree relatives? Clin Rheumatol 2017;36:799–806. 10.1007/s10067-016-3519-z [DOI] [PubMed] [Google Scholar]
- 25.Smolik I, Robinson DB, Bernstein CN, et al. First-Degree relatives of patients with rheumatoid arthritis exhibit high prevalence of joint symptoms. J Rheumatol 2013;40:818–24. 10.3899/jrheum.121016 [DOI] [PubMed] [Google Scholar]
- 26.van der Ven M, van der Veer-Meerkerk M, Ten Cate DF, et al. Absence of ultrasound inflammation in patients presenting with arthralgia rules out the development of arthritis. Arthritis Res Ther 2017;19 10.1186/s13075-017-1405-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padovano I, Costantino F, Breban M, et al. Prevalence of ultrasound synovial inflammatory findings in healthy subjects. Ann Rheum Dis 2016;75:1819–23. 10.1136/annrheumdis-2015-208103 [DOI] [PubMed] [Google Scholar]
- 28.Zufferey P, Rebell C, Benaim C, et al. Ultrasound can be useful to predict an evolution towards rheumatoid arthritis in patients with inflammatory polyarthralgia without anticitrullinated antibodies. Joint Bone Spine 2017;84:299–303. 10.1016/j.jbspin.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 29.Wittoek R, Carron P, Verbruggen G. Structural and inflammatory sonographic findings in erosive and non-erosive osteoarthritis of the interphalangeal finger joints. Ann Rheum Dis 2010;69:2173–6. 10.1136/ard.2010.128504 [DOI] [PubMed] [Google Scholar]


