Abstract
目的
探讨针刺对气道重塑小鼠肺组织TGF-β1/Smads信号通路的调控作用。
方法
将30只SPF级小鼠随机分为空白组、模型组、针刺组,10只/组。模型组、针刺组制作小鼠哮喘模型。针刺组自造模之日起第10天行针刺操作,穴取肺俞、大椎、足三里(两侧),每次留针20 min,隔日针刺1次,共治疗7次。并于最后1次针刺结束后24 h行1%卵蛋白雾化吸入3 d,最后1次激发后24 h于不同浓度乙酰甲基氯化胆碱(Mch)吸入下通过无创体积描计器检测小鼠肺阻力,然后采用HE染色法观察小鼠肺组织的病理变化,ELISA检测小鼠支气管肺泡灌洗液(BALF)及血清中TGF-β1的表达,Western blot对各组气道平滑肌中差异蛋白进行检测。将针刺组分离提取气道平滑肌细胞,空白组加入PBS液,抑制组加入TGF-β1抑制剂(LY2157299)分别进行培养,然后Western blot检测两组细胞type-I及Smads蛋白表达相对量。
结果
模型组小鼠气管痉挛明显,管腔狭窄,支气管黏膜增厚,部分上皮细胞脱落,肺泡隔增厚,气道平滑肌明显增厚;针刺组较模型组有明显改善;不同浓度Mch激发后模型组小鼠肺阻力最高,较其它两组均差异有统计学意义(P < 0.05)。且3组小鼠的肺阻力都随着Mch的浓度加大而增加。模型组血清中TGF-β1阳性表达OD值较空白组增加(P < 0.05);针刺组阳性表达低于模型组(P < 0.05)。针刺组BALF中TGF-β1含量低于模型组(P < 0.05)高于空白组(P < 0.05)。模型组气道平滑肌中α-SMA、TGF-β1及Smads蛋白表达相对量均高于针刺组及空白组(均P < 0.05),抑制组细胞中type-I及Smads蛋白表达相对量较空白组高(P>0.05)。
结论
针刺能通过抑制气道TGF-β1的表达,下调Smads及α-SMA的表达来抑制气道重塑,减轻哮喘气道炎性反应,且抑制TGF-β1后,type-I及Smads蛋白表达相对量较高,针刺可以通过TGF-β1/Smads通路来控制哮喘进展,为临床上哮喘的治疗提供理论依据。
Keywords: 针刺, 气道重塑, TGF-β1/Smads, 哮喘
Abstract
Objective
To investigate the effect of acupuncture on TGF-β1/Smads signaling pathway in the lung tissue of mice with airway remodeling.
Methods
Thirty specific pathogen-free mice were randomly divided into blank group, model group and acupuncture group (n=10). Mouse models of asthma were established in the model group and the acupuncture group, and the mice in the latter group received 7 acupuncture therapies (at bilateral Fei Shu, Da Zhui and Zu Sanli, 20 min each time) every other day, starting on the 10th day after the modeling. At 24 h after the last acupuncture, the mice were subjected to inhalation of 1% OVA for 3 days, and 24 h after the last challenge, the mice were given methacholine chloride (Mch) inhalation at different concentrations for measurement of lung resistance using a noninvasive stroke volume meter. HE staining was used to observe the pathological changes in the lung tissues, and TGF-β1 levels in the the bronchoalveolar lavage fluid (BALF) and serum were detected using ELISA; Western blotting was used to detect the differential protein expressions in the airway smooth muscles between the two groups. The airway smooth muscle cells were isolated from the mice in the acupuncture group and treated with a TGF- β1 inhibitor (LY2157299), and the relative expressions of type-Ⅰ and Smads proteins were detected using Western blotting.
Results
The mice in the model showed obvious tracheal fistula with airway pathologies including lumen narrowing, bronchial mucosa thickening, dissociation of the epithelial cells, and thickening of the alveolar septum and airway smooth muscles. These pathological changes were obviously milder in the acupuncture group. The asthmatic mice exhibited significantly increased lung resistance in positive correlation with Mch concentration. Serum TGF-β1 level was significantly elevated in asthmatic mice (P < 0.05); TGF-β1 levels in the serum and BALF were significantly lower in the acupuncture group than in the model group (P < 0.05). In the model group, the expressions of α-SMA, TGF-β1 and Smads in the airway smooth muscles were significantly higher than those in the other two groups (both P < 0.05). In cultured airway smooth muscle cells, the expressions of type-Ⅰ and Smads were significantly higher in cells treated with LY2157299 than in the control cells (P>0.05).
Conclusion
Acupuncture can inhibit airway remodeling by inhibiting the expression of airway TGF-β1 and down-regulating the expression of Smads and α-SMA to reduce airway inflammatory response. Airway expressions of type-Ⅰ and Smads proteins remain high after inhibiting TGF-β1. Acupuncture may control asthma progression through the TGF-β1/Smads pathway.
Keywords: acupuncture, airway remodeling, transforming growth factor-β1/Smads, asthma
支气管哮喘是我国常见的慢性疾病之一,近年来发病呈上升趋势[1]。其病因多种多样,主要与气道炎症、气道高反应性和气道重塑有关[2-4]。已有研究结果表明气道重塑的结构可独立于炎症或肺功能变化而出现[5-6]。气道重塑主要由气道平滑肌(ASM)肥大增生引起。临床表现为ASM持续收缩反应,气道过度缩窄,长期收缩引发不可逆的气道阻塞,并发展成为顽固难治性哮喘[7]。
Grainge等[8]认为气道重塑的主要病理改变是胶原沉积。有学者认为TGF-β1是气道重塑的主要中介[9-10],并且具有强烈的致纤维化作用,哮喘急性发作期,在某些细胞因子作用下,能促使气道平滑肌细胞向上皮层移动,形成新的平滑肌束。Smads蛋白是TGF-β家族的特异性细胞内信号转导分子,TGF-β1/Smads信号转导通路可能通过调控α-SMA(平滑肌肌动蛋白)的上调诱导成纤维细胞分化成肌成纤维细胞,产生type-I胶原蛋白,控制大多数与上皮间充质转化有关的基因等方式致哮喘气道重塑。本次实验我们选取与气道重塑密切相关的TGF-β1、Smads、α-SMA及type-I胶原蛋白,检查其在大鼠肺组织的表达情况及针刺对其的干预作用。为进一步验证针刺对TGF-β1/Smads信号通路的影响,我们将针刺组气道平滑肌细胞加入TGF-β1抑制剂(LY2157299)进行培养,然后检测其下游蛋白Smads及type-I胶原蛋白含量。
针刺做为祖国医学的瑰宝,不仅经济实惠、操作简单,而且无副作用。研究认为针刺具有抗炎、调节免疫、缓解气道高反应、防治气道重塑等效果[11-16],但具体机制仍不明确。本研究中针刺治疗选取肺俞、大椎、足三里,以探讨针刺对哮喘的治疗作用及TGF-β1/Smads信号通路在气道炎性反应及重塑中的作用。
1. 材料和方法
1.1. 实验动物与分组
SPF级雌性BALB/C小鼠,6~8周,体质量20±2 g,购自广州中医药大学动物实验中心。饲养在广州中医药大学第一附属医院实验中心,自由进食去卵蛋白饲料和三蒸水。小鼠饲育环境符合SPF级环境设施标准:温度20~26 ℃,日温差≤3 ℃;相对湿度40%~60%,昼夜明暗交替时间12/12 h。购买的30只小鼠放置检疫室3 d检测并适应新环境,检测合格后根据计算机随机数字随机分为空白对照组(Control)、模型组(OVA)、针刺组(Acupuncture),每组10只开始造模。实验过程中对动物的处置符合中华人民共和国科学技术部2006年颁布的《关于善待实验动物的指导性意见》标准。
1.2. 实验试剂及仪器
卵蛋白(A5503-1G, Sigma);Mch(A2251-25G,Sigma);α-SMA抗体(Ab124964, Abcam);兔抗大鼠TGF-β1抗体(ab92486, Abcam);Smads抗体(8685T, CST);小鼠TGF-β1- ELISA试剂盒(Ab119557, R & D);TGF-β1抑制剂(LY2157299, MCE);Hybond-P PVDF膜(Amersham);β-Actin(D110001-0010, Proteintech);type-I胶原蛋白(812017, Abcam);无创体积描计器(RC, Buxco);SuperRX医用X光胶片(Fujifilm);光学显微镜(BX51型,日本奥林巴斯公司);低速离心机SL01/SLO2(上海知信);酶标仪(M200PRO,TECAN帝肯(上海)贸易有限公司);雾化吸入器(085G3005, PARI);一次性无菌针灸针(0.35 mm×25 mm)。
1.3. 模型制备
模型组及针刺组BALB/C小鼠均于实验的第1天、第8天和第14天共3次在腹腔分2点注射含50 μg OVA和2 mg Al(OH)3凝胶的生理盐水0.2 mL,且于第10天开始捆绑固定小鼠,隔天1次,20 min/次(针刺组见1.4干预方法);第23天小鼠放进自制30 cm×25 cm×15 cm琼树脂透明雾化器(左侧钻一4 cm×1 cm孔以便置入雾化喷头,对侧钻6个直径1 cm小孔便换气),予以1% OVA雾化,雾化时间调为30 min,出雾量调为最小以便观察小鼠雾化反应(雾化每次每箱不超过8只),1次/d,连续3 d[17-18](图 1)。
1.
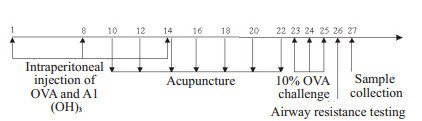
造模及针刺日程图
Schedule of modeling and acupuncture
1.4. 干预方法
空白组、模型组不予处理。针刺组于10 d后,隔日针刺肺俞、足三里、大椎(均双侧)1次,共7次。针刺定位[19]:(1)肺俞:第3胸椎下两旁肋间,直刺3 mm;(2)足三里:膝关节下方,腓骨小头下0.3 cm处的肌沟中,直刺3 mm;(3)大椎:第7颈椎与第1胸椎棘突间的凹瞻中,直刺3 mm。针刺方法:平补平泻,每次留针20 min,每隔5 min行针1次(以200次/min捻转速度,捻针20次为行针1次),隔日针刺1次,共针7次(由针灸专业研究生完成)。
1.5. 标本采集
1.5.1. 动物标本采集
末次激发24 h后,每只小鼠雾化PBS(磷酸盐缓冲液)3 min建立肺阻力(Lung Resistance, RL)基线后,用无创体积描计器通过雾化小鼠吸入300 μL倍増浓度的Mch检测小鼠RL。激发浓度从低到髙,依次为3.21、6.25、12.5、25、50 mg/mL,每次雾化20 s,记录3 min时各个浓度级的平均RL值。将每个浓度级的RL值与生理盐水时的RL值相比(各浓度级RL/生理盐水RL×100%),所得的RL%值作为评价气道高反应的指标[20]。
RL值检测后24 h腹腔注射10%水合氯醛(0.3 mL/ 100 g)麻醉,固定,暴露气管,在环状软骨上方行V型切口,插管(连接三通管),连接小动物呼吸机,潮气量10 mL/kg,频率60 counts/min。
暴露肺及气管,结扎右侧肺门后,在气管上剪一个小口,将钝头插管插入气管内并用线固定,用注射器抽取3 mL、37 ℃无菌氯化钠溶液,缓慢经气管注入左肺,间隔30 s后抽出支气管肺泡灌洗液,反复灌洗3次收集BALF,离心(4 ℃,3500 r/min,15 min)。取上清液于-20 ℃保存备用。
然后无菌条件下小鼠心脏取血,静置1 h,离心(4 ℃,3500 r/min,15 min),取上清液于-20 ℃保存备用。取出右上肺组织,0.9% Nacl溶液漂洗干净,吸水纸拭干。于4%多聚甲醛固定,48 h后进行石蜡包埋。
各组无菌条件下取气道组织,然后分离出气道平滑肌碾碎,加入laemml sample buffer用超声破碎仪助溶处理,最后进行高速离心(13 000 r/min,10 min,4 ℃),取上清,用于Western blot检测差异蛋白。
1.5.2. 细胞培养
针刺组气道平滑肌进行分离提取气道平滑肌细胞进行原代培养,空白组加入PBS液,抑制组加入TGF-β1抑制剂(LY2157299)分别培养,每2~3 d换全液1次,细胞贴壁融合到80%左右进行传代培养。将第3代细胞收集后,加入300 μL laemml sample buffer用超声破碎仪处理10 s后,进行高速离心(13 000 r/min,10 min,4 ℃),取上清,进行Western blot检测蛋白表达。
1.6. 肺组织病理学观察
将取得的肺组织置于4%多聚甲醛中浸泡固定,常规梯度乙醇脱水、透明、石蜡包埋后连续切片(5 μm),HE染色,光镜观察气道、肺组织病理改变并拍照。
1.7. 用ELISA法分别测定血清和BALF细胞因子TGF-β1
采用心脏取血法,同时收集肺泡灌洗液,根据ELISA试剂盒(Ab119557, R & D)要求操作。
1.8. 统计学分析
采用SPSS16.0统计软件包进行分析,各组数据符合正态分布,所有数据均以均数±标准差表示。采用单因素方差分析检验其差异性,多组间两两比较采用LSD法,以P < 0.05表示差异有统计学意义。
2. 结果
2.1. 各组小鼠造模情况
模型组及针刺组激发时小鼠就出现不同程度的呼吸喘促、烦躁、口唇发青、抓耳、喷嚏、哮鸣音等症状,针刺组较模型组轻,空白组无任何表现。结合小鼠的症状及激发后肺阻力的变化表明哮喘模型制备成功。在实验过程中,模型组及针刺组各有1只小鼠雾化激发时抢救无效死亡。
2.2. 不同浓度Mch激发后3组小鼠肺阻力的变化
不同浓度乙酰胆碱激发后模型组小鼠肺阻力最高,较其它两组差异均有统计学意义(P < 0.05,表 1,图 2)。且3组小鼠的RL都随着Mch的浓度加大而增加。
1.
小鼠肺阻力RL%的变化
Changes of lung resistance in the mice (%, Mean±SD)
| Mch (mg/mL) | Control (n=10) | OVA(n=9) | Acupuncture (n=9) |
| 3.21 | 1.88±0.35* | 6.46±2.21# | 2.56±0.87#* |
| 6.25 | 2.12±0.32* | 8.80±0.46# | 3.50±1.38#* |
| 12.5 | 2.60±0.56* | 8.47±2.55# | 4.79±1.16#* |
| 25 | 3.05±1.01* | 10.83±2.90# | 5.22±0.71#* |
| 50 | 3.78±1.28* | 13.98±3.18# | 6.080±0.64#* |
| #P < 0.05 vs control; *P < 0.05 vs OVA group. | |||
2.
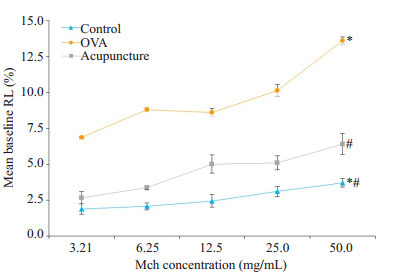
3组小鼠不同浓度Mch吸入时气道阻力RL的变化
Changes of lung resistance (%) after methacholine (Mch) challenge in mice (#P < 0.05 vs control; *P < 0.05 vs OVA group)
2.3. 各组小鼠肺组织病理变化
光镜下可见空白组小鼠肺组织结构清晰,肺泡壁薄且光滑,肺泡腔内无渗出物,无炎性细胞浸润;模型组小鼠气管痉挛明显,管腔狭窄,支气管黏膜增厚,皱襞增多,部分上皮细胞脱落,肺泡隔增厚,气道平滑肌明显增厚。针刺组整体较模型组改善(图 3)。
3.
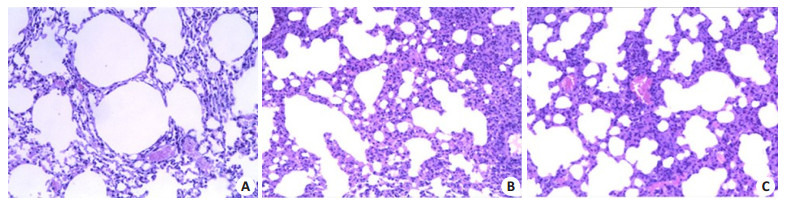
小鼠肺组织病理HE染色
Histological analysis of the lung tissues of the mice. A: It can be seen that the tissue structure was clear, there was no exudation in the alveolar; B: It can be seen that trachea stenosis was obvious, lumen was narrow, bronchial mucosa was thickened, folds were increased; C: The acupuncture group showed some improvement over the acupuncture group. HE staining, original magnification, ×100)
2.4. 各组小鼠BALF及血清中TGF-β1含量
BALF中TGF-β1含量模型组及针刺组均高于空白组(P < 0.05),且针刺组较模型组低(P < 0.05)。血清中TGF-β1含量模型组高于空白组(P < 0.05),而针刺组与空白组无统计学差异(P > 0.05),且针刺组较模型组低(P < 0.05)。表明气道高反应时小鼠的TGF-β1含量增加,并且针刺处理有一定的治疗效果(表 2、图 4、5)。
2.
小鼠BALF及血清中TGF-β1A值
TGF-β1 expression (absorbance) in BALF and serum of the mice (Mean±SD)
| Group | Mice (n) | BALF | Serum |
| #P < 0.05 vs control; *P < 0.05 vs OVA group. | |||
| Control | 10 | 0.0035±0.004* | 0.0093±0.003* |
| OVA | 9 | 0.0365±0.012# | 0.0222±0.006# |
| Acupuncture | 9 | 0.0272±0.011#* | 0.0112±0.009* |
4.
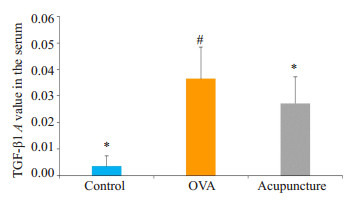
各组小鼠血清中TGF-β1A值95%CI
TGF-β1 level 95% CI in the serum of mice in each group (#P < 0.05 vs control; *P < 0.05 vs OVAgroup)
5.
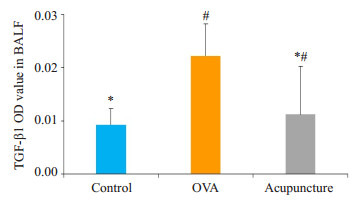
各组小鼠BALF中TGF-β1A值95%CI
TGF-β1 level 95% CI in the BALF of mice in each group (#P < 0.05 vs control; *P < 0.05 vs OVAgroup)
2.5. Western blot检测气道平滑肌差异蛋白
各组气道平滑肌TGF-β1及其下游蛋白Smads的变化和ASMC中α-SMA的表达(图 6)。模型组TGF-β1蛋白的灰度测定值均较空白组差异有统计学意义(P < 0.05),模型组与针刺组比较(P < 0.05),针刺组与空白组比较差异无统计学意义(P > 0.05)。气道平滑肌中Smads蛋白模型组的灰度测定值与空白组比较差异有统计学意义(P < 0.05),模型组与针刺组比较差异有统计学意义(P < 0.05),针刺组与空白组比较差异无统计学意义(P > 0.05)。气道平滑肌中α-SMA蛋白模型组的灰度测定值与空白组比较有统计学差异(P < 0.05),模型组与针刺组,针刺组与空白组比较有统计学差异(P < 0.05)。
6.
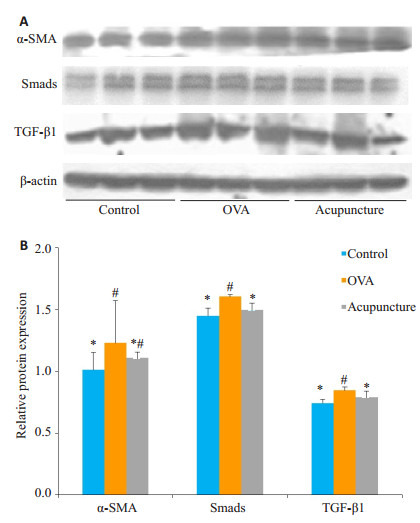
气道平滑肌中α-SMA、Smads、TGF-β1蛋白表达相对量
Relative expression levels of α-SMA, Smads and TGF-β 1protein in airway smooth muscle (#P < 0.05 vs control; *P < 0.05 vs OVAgroup)
2.6. 抑制TGF-β1后,检测下游蛋白表达量
抑制剂组Smads及type-I表达量较对照组高,但差异无统计学意义(P > 0.05,图 7)。
7.
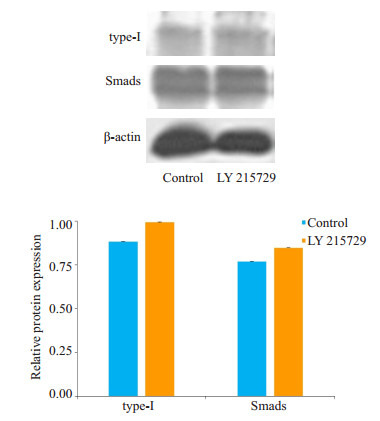
两组ASM中type-Ⅰ和Smads蛋白表达相对量
Acupuncture regulated TGF-β1 associated factors type-Ⅰ collagen and Smads expression in ASMCs of experimental asthma after added the inhibitor LY215729
3. 讨论
世界范围内有超过3亿的哮喘患者,虽然近年来防治哮喘的工作不断深入,但是其发病率及死亡率仍呈上升趋势[1]。针刺被认为具有抗炎、调节免疫,缓解气道高反应、防治气道重塑等效果[11],有学者研究[12]认为轻/中持续性哮喘患者针灸治疗后,患者报告的咳嗽较少、喘息、呼吸困难和夜间觉醒发作均减少,也有学者认为针刺还通过增加交感神经兴奋性来改善鼻通气[13]。针刺改善通气的临床效果是肯定的,但是针刺防治气道重塑的具体机制仍不明确。
气道重塑是哮喘难治的关键因素,气道平滑肌肥大增生是其主要表现,TGF-β1被认为是气道重塑的主要中介[15-16]。本研究中针刺治疗选取肺俞、大椎、足三里。针刺肺俞能影响血液和组织细胞的环核苷酸,从而减少炎性介质的释放,减轻炎症反应。大椎为足云阳交会穴和督脉强身要穴,有振阳补虚之功效[17]。足三里为足阳明胃经主要穴位,有调节免疫力、补中益气、通经活络之效[21]。研究结果表明,卵蛋白连续致敏后,哮喘大鼠症状明显;在Mch激发后,3组小鼠RL均增加,而模型组在Mch 25 mg/mL和50 mg/mL激发下明显增加(图 2),说明存在气道高反应。模型组与空白组HE染色比较,支气管痉挛明显,管腔狭窄,支气管黏膜增厚,皱襞增多,肺泡隔增厚。而针刺组较模型组气道炎性反应减轻(图 3),说明针刺能减轻气道的炎症反应,具有一定抑制气道重塑的作用。
小鼠BALF及血清中TGF-β1模型组及针刺组均较空白组高,而针刺组较模型TGF-β1低差异有统计学意义(图 4、5),结合病理我们推测气道上皮的病理改变与TGF-β1的表达量呈正相关,说明TGF-β1会刺激气道上皮细胞发生重塑。这也与韩君萍等的研究结果一致[22-23],卵蛋白可以致小鼠哮喘模型,而针刺在一定程度上可以减轻炎症反应,防治气道重塑。但具体的机制是什么?
有研究运用蛋白组学对哮喘模型小鼠气道平滑肌中差异蛋白进行检测,发现针刺治疗后某些蛋白的表达差异消失或得到部分逆转[24]。Rosendahl等[25]发现卵白蛋白致敏的Balb/c小鼠支气管上皮细胞、成纤维细胞以及血管内皮细胞中的磷酸化Smad2表达水平在吸入卵白蛋白后迅速升高,而未进行吸入激发的致敏Balb/c小鼠的磷酸化Smad2呈低表达。也有研究认为哮喘的整个过程中均有TGF-β1的分泌,TGF-β1具有强烈的致纤维化作用,哮喘急性发作期,在某些细胞因子作用下,能促使气道平滑肌细胞向上皮层移动,形成新的平滑肌束[26-27]。本次实验我们选取与气道重塑密切相关的TGF-β1、Smads及α-SMA蛋白,检测其在哮喘大鼠肺组织中的蛋白表达情况及针刺对其的干预作用。
本研究肺组织Western blot检测结果表明(图 6),针刺组Smads蛋白及TGF-β1蛋白含量均较模型组降低,而较空白组无明显差异,这也同时验证了我们的推测,针刺可能通过TGF-β1/Smads信号转导通路来防治气道重塑的进展。针刺组较模型组α-SMA(平滑肌肌动蛋白)低,较空白组高。α-SMA可诱导成纤维细胞分化成肌成纤维细胞,产生type-I胶原蛋白,增加ECM的含量,ECM不仅是气道重塑的病理产物,同时也是气道重塑的致病因素,ECM作为细胞黏附分化迁移和基因表达的场所,可以促进平滑肌细胞(ASMC)增殖分化,进一步导致ASMC增殖肥大[28],形成恶性循环。针刺组α- SMA较低,也说明针刺可一定程度上防治肺纤维化。但是否经TGF-β1/Smads信号通路,我们提取针刺组ASMC加入抑制剂LY2157299进行培养,发现抑制剂组Smads及type-I胶原蛋白相对量增加,但两组无统计学差异。这也一定程度上说明针刺有经TGF-β1/Smads信号转导通路调控α-SMA(平滑肌肌动蛋白)的上调诱导成纤维细胞分化成肌成纤维细胞,产生type-I胶原蛋白的方式控制气道重塑的发展,但并非通过这单一通路来作用。有学者认为外源性S100A8蛋白可抑制了血小板源生长因子诱导的ASMC的迁移,这也给我们进一步研究哮喘进展提供新思路[29-30]。
综上所述,TGF-β1蛋白参与哮喘气道炎性反应,针刺能通过抑制气道TGF-β1的表达,下调Smads及α-SMA的表达来抑制气道重塑,减轻哮喘气道炎性反应。抑制TGF-β1后,type-I胶原蛋白及Smads蛋白表达相对量较高,认为针刺有通过TGF-β1/Smads通路来控制哮喘进展,这也为临床上针刺治疗哮喘提供理论支持。
Biography
刘慧慧,主治医师,E-mail: gzylhh@163.com
Funding Statement
广东省中医药管理局项目(20172054);广州中医药大学高水平项目青年基金(A1-AFD018171Z11075);广州中医药大学第一附属医院“创新强院”优秀青年基金(10900088);广东省科技计划项目(2016A020226016)
Contributor Information
刘 慧慧 (Huihui LIU), Email: gzylhh@163.com.
李 春桥 (Chunqiao LI), Email: lichunqiao18154920@163.com.
References
- 1.Lambrecht BN, Hammad H. The immunology of asthma. http://d.old.wanfangdata.com.cn/NSTLQK/10.1016-S1081-1206(10)63545-4/ Nat Immunol. 2015;16(1):45–56. doi: 10.1038/ni.3049. [Lambrecht BN, Hammad H. The immunology of asthma[J]. Nat Immunol, 2015, 16(1): 45-56.] [DOI] [PubMed] [Google Scholar]
- 2.Khan MA. In fl ammation signals airway smooth muscle cell proliferation in asthma pathogenesis. Multidiscip Respir Med. 2013;8(1):11. doi: 10.1186/2049-6958-8-11. [Khan MA. In fl ammation signals airway smooth muscle cell proliferation in asthma pathogenesis[J]. Multidiscip Respir Med, 2013, 8(1): 11.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shifren A, Witt C, Christie C, et al. Mechanisms of remodelingin asthmatic airways. http://www.ncbi.nlm.nih.gov/pubmed/22315625. JAllergy (Cairo) 2012;25(7):316049. doi: 10.1155/2012/316049. [Shifren A, Witt C, Christie C, et al. Mechanisms of remodelingin asthmatic airways[J]. JAllergy (Cairo), 2012, 25(7): 316049.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamid Q, Tulic M. Immunobiology of asthma. http://d.old.wanfangdata.com.cn/NSTLQK/10.1146-annurev.physiol.010908.163200/ Annu Rev Physiol. 2009;71(11):489–507. doi: 10.1146/annurev.physiol.010908.163200. [Hamid Q, Tulic M. Immunobiology of asthma[J]. Annu Rev Physiol, 2009, 71(11): 489-507.] [DOI] [PubMed] [Google Scholar]
- 5.Jenkins HA, Cherniack R, Szefler SJ, et al. A comparison of the clinical charateristics of children and adults with severe asthma. Chest. 2003;124(4):1318–24. doi: 10.1378/chest.124.4.1318. [Jenkins HA, Cherniack R, Szefler SJ, et al. A comparison of the clinical charateristics of children and adults with severe asthma[J]. Chest, 2003, 124(4): 1318-24.] [DOI] [PubMed] [Google Scholar]
- 6.Jenkins HA, Cool C, Szefler SJ, et al. Histopathology of severe childhood asthma-A case series. Chest. 2003;124(1):32–41. doi: 10.1378/chest.124.1.32. [Jenkins HA, Cool C, Szefler SJ, et al. Histopathology of severe childhood asthma-A case series[J]. Chest, 2003, 124(1): 32-41.] [DOI] [PubMed] [Google Scholar]
- 7.Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc. 2009;6(3):301–5. doi: 10.1513/pats.200808-089RM. [Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma[J]. Proc Am Thorac Soc, 2009, 6(3): 301-5.] [DOI] [PubMed] [Google Scholar]
- 8.Grainge CL, Lau LC, Ward JA, et al. Effect of bronchoconstriction on airway remodeling inasthma. http://www.ncbi.nlm.nih.gov/pubmed/21612469. N Engl J Med. 2011;364(3):2006–15. doi: 10.1056/NEJMoa1014350. [Grainge CL, Lau LC, Ward JA, et al. Effect of bronchoconstriction on airway remodeling inasthma[J]. N Engl J Med, 2011, 364(3): 2006-15.] [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Mirza S, Xu T, et al. Aryl hydrocarbon receptor (AhR) modulates cockroach allergen- induced immune responses through active TGF beta 1 release. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3b0823b82b376a984f3c6f21a4c9fa40. Mediators Inflamm. 2014;5(1):1–13. doi: 10.1155/2014/591479. [Zhou Y, Mirza S, Xu T, et al. Aryl hydrocarbon receptor (AhR) modulates cockroach allergen- induced immune responses through active TGF beta 1 release[J]. Mediators Inflamm, 2014, 5(1): 1-13.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zc Y, Yi MJ, Ran N, et al. Transforming growth factor-β1 induces bronchial epithelial cells to mesenchymal transition by activating the snail pathway and promotes airway remodeling inasthma. Mol Med Rep. 2013;8(6):1663–8. doi: 10.3892/mmr.2013.1728. [Zc Y, Yi MJ, Ran N, et al. Transforming growth factor-β1 induces bronchial epithelial cells to mesenchymal transition by activating the snail pathway and promotes airway remodeling inasthma[J]. Mol Med Rep, 2013, 8(6): 1663-8.] [DOI] [PubMed] [Google Scholar]
- 11.李双.针刺对支气管哮喘大鼠肺组织CollagenⅠ, Fibronection, VEGF表达的影响[D].[出版地不详]: 华北理工大学, 2016.http://cdmd.cnki.com.cn/Article/CDMD-10081-1017741353.htm
- 12.谭 程, 赵 吉平, 张 昶. 近十年针刺治疗支气管哮喘的临床及实验研究现状. http://d.old.wanfangdata.com.cn/Periodical/zcyj201104013. 针刺研究. 2011;36(4):302–6, 312. [谭程, 赵吉平, 张昶.近十年针刺治疗支气管哮喘的临床及实验研究现状[J].针刺研究, 2011, 36(4): 302-6, 312.] [PubMed] [Google Scholar]
- 13.杨金华.针刺对哮喘大鼠肺组织PI3K/AKT信号通路的影响[D].华北理工大学, 2017.
- 14.Li J, Wu S, Tang HT, et al. Long-term effects of acupuncture treatment on airway smooth muscle in a rat model of smoke-induced chronic obstructive pulmonary disease. Acupunct Med. 2016;34(2):107–13. doi: 10.1136/acupmed-2014-010674. [Li J, Wu S, Tang HT, et al. Long-term effects of acupuncture treatment on airway smooth muscle in a rat model of smoke-induced chronic obstructive pulmonary disease[J]. Acupunct Med, 2016, 34 (2): 107-13.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pai HJ, Azevedo RS, Ferreira Braga AL, et al. A randomized, controlled, crossover study in patients with mild and moderate asthma undergoing treatment with traditional Chinese acupuncture. Clinics (Sao Paulo) 2015;70(10):663–9. doi: 10.6061/clinics. [Pai HJ, Azevedo RS, Ferreira Braga AL, et al. A randomized, controlled, crossover study in patients with mild and moderate asthma undergoing treatment with traditional Chinese acupuncture [J]. Clinics (Sao Paulo), 2015, 70(10): 663-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang KJ, Chen LQ, Wang Y, et al. Sphenopalatine ganglion acupuncture improves nasal ventilation and modulates autonomic nervous activity in healthy volunteers: a randomized controlled study. http://www.nature.com/articles/srep29947. Sci Rep. 2016;6(3):29947. doi: 10.1038/srep29947. [Wang KJ, Chen LQ, Wang Y, et al. Sphenopalatine ganglion acupuncture improves nasal ventilation and modulates autonomic nervous activity in healthy volunteers: a randomized controlled study [J]. Sci Rep, 2016, 6(3): 29947.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.庄月容.不同频次针刺预处理对OVA致敏气道高反应性小鼠的炎症调节作用[D].广州: 广州中医药大学, 2015.http://cdmd.cnki.com.cn/Article/CDMD-10572-1015363799.htm
- 18.Debeuf N, Haspeslagh E, van Helden M, et al. Mouse models of asthma. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3459343. Curr Protoc Mouse Biol. 2016;6(3):169–84. doi: 10.1002/cpmo.4. [Debeuf N, Haspeslagh E, van Helden M, et al. Mouse models of asthma[J]. Curr Protoc Mouse Biol, 2016, 6(3): 169-84.] [DOI] [PubMed] [Google Scholar]
- 19.李 忠仁. 实验针灸学. 北京: 中国中医药出版社; 2003. p. 255. [李忠仁.实验针灸学[M].北京:中国中医药出版社, 2003: 255.] [Google Scholar]
- 20.孙 晓春, 胡 晓燕, 王 莉佳, et al. 氯喹对哮喘小鼠气道高反应性的抑制作用. http://www.j-smu.com/oa/darticle.aspx?type=view&id=20150112. 南方医科大学学报. 2015;35(1):12–6. doi: 10.3969/j.issn.1673-4254.2015.01.03. [孙晓春, 胡晓燕, 王莉佳, 等.氯喹对哮喘小鼠气道高反应性的抑制作用[J].南方医科大学学报, 2015, 35(1): 12-6.] [DOI] [PubMed] [Google Scholar]
- 21.Stockert K, Schneider B, Porenta G, et al. Laser acupuncture and probiotics in school age children with asthma: a randomized, placebo-controlled pilot study of therapy guided by principles of Traditional Chinese Medicine. Pediatr Allergy Immunol. 2007;18(2):160–6. doi: 10.1111/pai.2007.18.issue-2. [Stockert K, Schneider B, Porenta G, et al. Laser acupuncture and probiotics in school age children with asthma: a randomized, placebo-controlled pilot study of therapy guided by principles of Traditional Chinese Medicine[J]. Pediatr Allergy Immunol, 2007, 18 (2): 160-6.] [DOI] [PubMed] [Google Scholar]
- 22.韩 君萍, 李 双, 赵 叶, et al. 针刺对哮喘模型大鼠肺组织中TGF-β1表达的调控. http://d.old.wanfangdata.com.cn/Periodical/zgzj201707017. 中国针灸. 2017;37(7):741–6. doi: 10.13703/j.0255-2930.2017.07.016. [韩君萍, 李双, 赵叶, 等.针刺对哮喘模型大鼠肺组织中TGF-β1表达的调控[J].中国针灸, 2017, 37(7): 741-6.] [DOI] [PubMed] [Google Scholar]
- 23.Li HZ, Qin XY, Shao SJ, et al. Effect of acupuncture intervention on airway remodeling and transforming growth factor-beta1 expression in asthma rats. http://europepmc.org/abstract/med/25219122. Zhen Ci Yan Jiu. 2014;39(4):278–81. [Li HZ, Qin XY, Shao SJ, et al. Effect of acupuncture intervention on airway remodeling and transforming growth factor-beta1 expression in asthma rats[J]. Zhen Ci Yan Jiu, 2014, 39(4): 278-81.] [PubMed] [Google Scholar]
- 24.Wang Y, Cui JM, Ma SL, et al. Proteomics analysis of component in serum with anti-asthma activity derived from rats treated by acupuncture. JAcupunct Tuina Sci. 2009;7(6):326–31. doi: 10.1007/s11726-009-0326-y. [Wang Y, Cui JM, Ma SL, et al. Proteomics analysis of component in serum with anti-asthma activity derived from rats treated by acupuncture[J]. JAcupunct Tuina Sci, 2009, 7(6): 326-31.] [DOI] [Google Scholar]
- 25.Rosendahl A, Checchin D, Fehniger TE, et al. Activation of the TGFbeta/activin-Smad2 pathway during allergic airway inflammation. Am J Respir Cell Mol Biol. 2001;25(1):60–8. doi: 10.1165/ajrcmb.25.1.4396. [Rosendahl A, Checchin D, Fehniger TE, et al. Activation of the TGFbeta/activin-Smad2 pathway during allergic airway inflammation [J].Am J Respir Cell Mol Biol, 2001, 25(1): 60-8.] [DOI] [PubMed] [Google Scholar]
- 26.Todorova L, Bjermer L, Westergren-Thorsson G, et al. TGF-β1- induced matrix productionby bronchial fibroblasts in asthma: budesonide and formoterol effects. Respir Med. 2011;105(9):1296–307. doi: 10.1016/j.rmed.2011.03.020. [Todorova L, Bjermer L, Westergren-Thorsson G, et al. TGF-β1- induced matrix productionby bronchial fibroblasts in asthma: budesonide and formoterol effects[J]. Respir Med, 2011, 105(9): 1296-307.] [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto S, Gon Y, Takeshita I, et al. Transforming growth factor-β induces phenotypic modulation of human lung fibrolasts to myofibroblast through a c-jun-NH2 terminal kinase-dependent pathway. Am J Respir Crit Care Med. 2001;163(1):152–7. doi: 10.1164/ajrccm.163.1.2005069. [Hashimoto S, Gon Y, Takeshita I, et al. Transforming growth factor-β induces phenotypic modulation of human lung fibrolasts to myofibroblast through a c-jun-NH2 terminal kinase-dependent pathway[J].Am J Respir Crit Care Med, 2001, 163(1): 152-7.] [DOI] [PubMed] [Google Scholar]
- 28.O'reilly R, Ullmann N, Irving S, et al. Increased airway smooth muscle in preschool wheezers who have asthma at school age. J Allergy Clin Immunol. 2013;131(4):1024. doi: 10.1016/j.jaci.2012.08.044. [O'reilly R, Ullmann N, Irving S, et al. Increased airway smooth muscle in preschool wheezers who have asthma at school age[J]. J Allergy Clin Immunol, 2013, 131(4): 1024.] [DOI] [PubMed] [Google Scholar]
- 29.Xu YD, Wei Y, Wang Y, et al. Exogenous S100A8 protein inhibits PDGF-induced migration of airway smooth muscle cells in a RAGEdependent manner. Biochem Biophys Res Commun. 2016;472(1):243–9. doi: 10.1016/j.bbrc.2016.02.098. [Xu YD, Wei Y, Wang Y, et al. Exogenous S100A8 protein inhibits PDGF-induced migration of airway smooth muscle cells in a RAGEdependent manner[J]. Biochem Biophys Res Commun, 2016, 472 (1): 243-9.] [DOI] [PubMed] [Google Scholar]
- 30.Xu YD, Wang Y, Yin LM, et al. S100A8 inhibits PDGF-induced proliferation of airway smooth muscle cells dependent on the receptor for advanced glycation end-products. Biol Res. 2017;50(1):23–30. doi: 10.1186/s40659-017-0128-5. [Xu YD, Wang Y, Yin LM, et al. S100A8 inhibits PDGF-induced proliferation of airway smooth muscle cells dependent on the receptor for advanced glycation end-products[J]. Biol Res, 2017, 50 (1): 23-30.] [DOI] [PMC free article] [PubMed] [Google Scholar]


