Abstract
目的
研究肌醇(MI)和木樨草素(LUT)对人肺腺癌A549细胞的作用,并探讨其机制。
方法
不同浓度的肌醇和木樨草素处理A549细胞24 h或48 h,MTT法检测细胞活性。10 mmol/L肌醇和20 μmol/L木樨草素单独或共同处理A549细胞和永生化肺支气管上皮细胞Beas-2B 48 h,MTT法、平板克隆形成实验和划痕愈合实验分别检测肌醇和木樨草素对两种细胞活性、细胞克隆形成和迁移的影响,Western blot法检测肌醇和木樨草素对A549细胞中相关蛋白质表达水平的影响。
结果
肌醇和木樨草素均可抑制A549细胞的活性,且呈浓度依赖性。10 mmol/L肌醇和20 μmol/L木樨草素作用A549细胞48 h后,肌醇处理组、木樨草素处理组和联合处理组A549细胞的活性分别是对照组的92%、83%和70%,平板克隆形成数分别是对照组的79%、73%和43%,划痕愈合度分别为24.61%、13.08%和8.65%,对照组划痕愈合度为29.99%;同等剂量下的肌醇和木樨草素单独或共同处理对Beas-2B细胞的活性、克隆形成和迁移没有明显的抑制作用。与对照组相比,肌醇处理组细胞中p-PDK1和p-Akt以及木樨草素处理组细胞中p-PDK1的蛋白质表达明显减少(P < 0.05),联合处理组下调的幅度更为明显(P < 0.05)。
结论
肌醇和木樨草素可选择性的抑制A549细胞的活性、克隆形成和迁移,合用时抑制作用更为明显,可能是通过下调p-PDK1和p-Akt的表达发挥的作用。
Keywords: 肌醇, 木樨草素, A549细胞, 联合应用, 化学预防
Abstract
Objective
To study the effects of myo-inositol and luteolin on human lung cancer A549 cells and explore the possible mechanisms.
Methods
A549 cells were treated with different concentrations of myo-inositol and luteolin, either alone or in combination, and the cell viability was examined using MTT assay. A549 cells and human bronchial epithelial Beas-2B cells were treated for 48 h with 10 mmol/L myo-inositol and 20 μmol/L luteolin, alone or in combination, and the cell proliferation was detected using MTT assay; the colony formation and migration of the cells were examined with colony formation assay and wound healing assay, respectively. The protein expression levels in A549 cells were detected using Western blotting.
Results
Both myo-inositol and luteolin could dose-dependently inhibit the viability of A549 cells. Treatments with 10 mmol/L myo-inositol, 20 μmol/L luteolin, and both for 48 h caused significant reduction in the cell viability (92%, 83% and 70% of the control level, respectively) and colony number (79%, 73% and 43%, respectively), and significantly lowered the wound closure rate (24.61%, 13.08% and 8.65%, respectively, as compared with 29.99% in the control group). Similar treatments with myoinositol and luteolin alone or in combination produced no significant inhibitory effect on the growth, colony formation or migration of Beas-2B cells. The expressions of p-PDK1 and p-Akt in myo-inositol-treated A549 cells and the expression of pPDK1 in luteolin-treated cells were significantly decreased (P < 0.05), and the decrements were more obvious in the combined treatment group (P < 0.05).
Conclusion
Luteolin combined with myo-inositol can selectively inhibit the proliferation and migration of A549 cells, and these effects are probably mediated, at least in part, by suppressing the activation of PDK1 and Akt.
Keywords: myo-inositol, luteolin, A549 cells, combined treatment, chemoprevention
肺癌的发病率和死亡率都比较高,严重威胁人类健康,患者5年存活率只有18%。探索与研究肺癌预防和治疗的新方法具有重要的意义。基于食物及其简单提取物的干预措施在癌症预防中获得了越来越多的关注[1-3]。肌醇和木樨草素在许多可食用植物中广泛存在,属于膳食营养成分[4-5]。一些研究显示肌醇和木樨草素均具有较好的抗癌活性[6-9]。不同具有抗癌活性的膳食营养成分合用时可能会产生相加或协同的联合效用,在同等剂量下取得更好的预防效果,增加通过膳食干预进行癌症的化学预防的可行性[10-12]。肌醇和木樨草素的联合应用尚未见报道。本研究试图探索肌醇和木樨草素的联合应用效果,对肌醇和木樨草素对A549细胞增殖和迁移的影响及其作用机制进行了初步的探讨。
1. 材料和方法
1.1. 试剂和材料
人肺腺癌细胞A549细胞株(中国科学院上海细胞库提供);永生化人支气管上皮细胞Beas-2B细胞株(ATCC细胞库提供);肌醇和木樨草素(sigma);RPMI 1640细胞培养基、DMEM细胞培养基和胎牛血清(Gibco);单克隆抗体PDK1、p-PDK1、Akt、p-Akt、GAPDH和辣根过氧化物酶标记的山羊抗兔二抗(CST);ECL免疫印迹分析试剂盒(Thermo);BCA蛋白浓度测定试剂盒和蛋白标准、Western及IP细胞裂解液和苯甲基磺酰氟化物(PMSF)(碧云天)。
1.2. 细胞培养
A549细胞株接种于含10%胎牛血清的RPMI 1640培养基中,Beas-2B细胞株接种于含5%胎牛血清的DMEM培养基中,培养基中青霉素和链霉素的浓度均为100 U/mL,置于37 ℃、CO2体积分数5%的细胞培养箱中培养。
1.3. 细胞增殖实验
取对数生长期细胞接种于96孔培养板中(3×103/孔),培养过夜,待细胞贴壁后,用不同浓度的肌醇、木樨草素或二者的混合溶液处理细胞,每个浓度设平行孔6个,对照孔加入不含药物的含有0.1% DMSO的培养液,于37 ℃、5% CO2条件下继续培养24 h或48 h,终止培养前每孔加入噻唑蓝溶液(MTT,5 mg/mL)10 μL,继续培养4 h,吸去上清液,每孔加入DMSO 100 μL,水平振摇10 min,在490 nm波长处测量吸光度(A)值,计算细胞存活率。细胞存活率=实验组A值/对照组A值× 100%。该实验重复了3次。
1.4. 划痕实验
用含0.1% DMSO的培养液作为溶剂对照,用肌醇(10 mmol/L)和/或木樨草素(20 μmol/L)处理细胞,48 h后,将各处理组细胞分别重新接种到6孔培养板中(1 × 105/孔)。待细胞貼壁并长至九成满时,血清饥饿12 h。用小枪头制造等宽划痕,用PBS冲洗掉悬浮细胞,继续无血清培养。每组细胞选取5个观察点,之后分别在0 h和24 h时间点对所选择的观察点进行拍照。划痕弥合度计算公式为:[Δarea/area (day0)]×100%。
1.5. 平板克隆形成实验
用含0.1% DMSO的培养液作为溶剂对照,用肌醇(10 mmol/L)和/或木樨草素(20 μmol/L)处理细胞,48 h后,将各处理组细胞消化制成单细胞混悬液,稀释后接种到六孔细胞培养板(1×103/孔)。继续培养7~10 d,终止细胞培养。取适量4%多聚甲醛固定液固定30 min,随后用0.5%结晶紫染色液缓慢振摇染色1 h。晾干,拍照。计数每个培养孔内形成的克隆。该实验重复了3次。
1.6. 蛋白质免疫印迹实验
用含0.1% DMSO的培养液作为溶剂对照,用肌醇(10 mmol/L)和/或木樨草素(20 μmol/L)处理细胞,48 h后,终止培养。将收集起来的各处理组细胞按1:10的比例加入适量冰上溶解的Western及IP细胞裂解液(碧云天),置于冰上裂解30 min,4 ℃,12 000 r/min离心,30 min,取上清液,用BCA蛋白定量法测定上清液蛋白浓度。每泳道取50 μg总蛋白上样,经10% SDS-PAGE凝胶垂直电泳分离蛋白,随后将之转移到PVDF膜上,5%脱脂奶粉室温封闭1 h,与抗3-磷酸肌醇依赖性蛋白激酶1(PDK1)、p-PDK1、Akt、p-Akt和GAPDH抗体(1: 1000)4 ℃孵育过夜,Tris-HCl缓冲盐溶液(TBST)充分洗膜后,用辣根过氧化酶标记的羊抗兔二抗(1:2000)室温孵育1 h,TBST充分洗膜后用ECL免疫印迹分析试剂盒曝光并拍照,实验重复3次。条带灰度用Gel-pro analyzer4图像分析软件(Media Cybernetics)分析,计算目的条带同相对应的GAPDH条带间的相对灰度值。
1.7. 统计学方法
实验所得数据用均数±标准差表示,然后经SPSS18.0软件采用Student-Newman-Keuls test法分析组间的统计学差异。当P < 0.05时,认为组间的差异具有统计学意义。
2. 结果
2.1. 肌醇和/或木樨草素对A549细胞或Beas-2B细胞活性的影响
如图 1A,B所示,同对照组相比,10 mmol/L以上浓度的肌醇和20 μmol/L以上浓度的木樨草素作用48 h后才对A549细胞具有一定的毒性作用。与对照组相比,A549细胞的增殖活性随着肌醇和木樨草素浓度的增加和作用时间的延长而下降。在A549细胞,肌醇作用48 h的半数致死浓度(LC50)为65.31 mmol/L,木樨草素作用24 h和48 h的半数致死浓度(LC50)分别为80.50 μmol/L和42.95 μmol/L。如图 1C所示,同对照组相比,单独或联合应用肌醇(10 mmol/L)和木樨草素(20 μmol/L)48 h后对Beas-2B细胞的活性没有明显的抑制作用,各组间没有明显的差别,但是同样浓度的肌醇或木樨草素单独应用或联合应用48 h后,肌醇处理组、木樨草素处理组和联合处理组A549细胞的活性分别为对照组的92%、83%和70%,组间差别显著,联合处理组A549细胞的活性下降最为明显。
1.
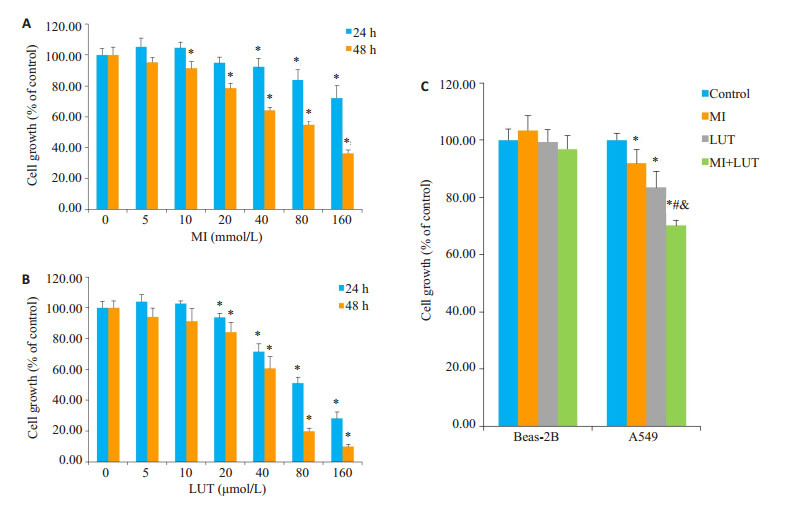
细胞活性检测
Effect of myo-inositol (MI) and luteolin (LUT) on proliferation of A549 cells (A, B) and Beas-2B cells (C). *P < 0.05 vs control group; #P < 0.05 vs MI group; & P < 0.05 vs LUT group
2.2. 肌醇和/或木樨草素对A549细胞或Beas-2B细胞平板克隆形成的影响
用10 mmol/L肌醇和20 μmol/L木樨草素单独或联合作用于A549细胞和Beas-2B细胞48 h后,用平板克隆形成实验检测肌醇和木樨草素对这两种细胞克隆形成的影响,结果如图 2所示,Beas-2B细胞形成的克隆团数目比较少和松散,处理组同对照组间没有显著性差别;对照组A549细胞能在培养平面形成许多大而紧密的细胞克隆团;肌醇处理组和木樨草素处理组A549细胞形成的克隆团数目与对照组相比有所减少,联合处理组A549细胞形成的克隆团数目远少于对照组细胞和另外2个处理组的细胞,形成的克隆团比较小。这些结果表明该剂量的肌醇和木樨草素可选择性的抑制A549细胞的克隆形成,合用时抑制作用要强于肌醇或木樨草素单独应用时。
2.
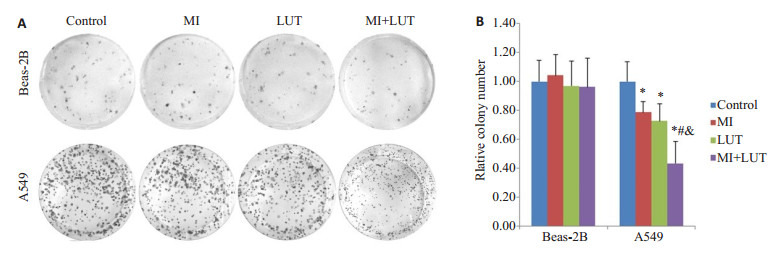
平板克隆形成实验结果
Colony formation by A549 cells and Beas-2B cells treated for 48 h with MI and LUT alone or in combination. A: Representative images of cell colonies of A549 and Beas-2B cells; B: Relative colony number of A549 and Beas-2B cells. *P < 0.05 vs control group; #P < 0.05 vs MI group; & P < 0.05 vs LUT group
2.3. 肌醇和/或木樨草素对A549细胞或Beas-2B细胞划痕愈合能力的影响
用10 mmol/L肌醇和20 μmol/L木樨草素单独或联合作用于A549细胞和Beas-2B细胞48 h,用划痕实验检测肌醇和木樨草素对细胞侧向迁移能力的影响,结果如图 3所示。在划痕后24 h,对照组、肌醇处理组、木樨草素处理组和联合处理组A549细胞的划痕愈合度分别是29.99%、24.61%、13.08%和8.65%,联合处理组A549细胞的划痕愈合程度显著低于其它3组细胞。然而,经过同样的处理,Beas-2B细胞各个处理组间划痕愈合度的差别不具有统计学意义。
3.
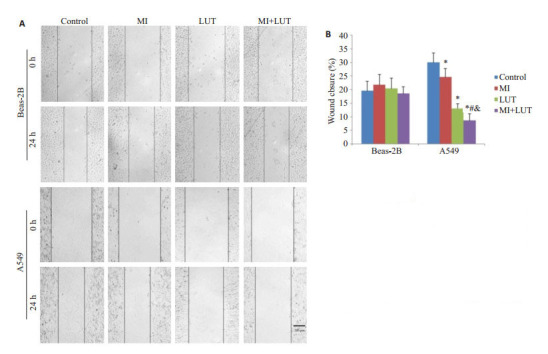
划痕实验结果
Wound closure in A549 cells and Beas-2B cells treated for 48 h with myo-inositol (MI) and luteolin (LUT)alone or in combination. A: Representative images of wound closure of A549 and Beas- 2B cells. Scale bar: 200 μm; B: Quantitative analysis of wound closure rates of A549 and Beas-2B cells. *P < 0.05 vs control group; #P < 0.05 vs MI group; & P < 0.05 vs LUT group
2.4. 肌醇和/或木樨草素对A549细胞中p-PDK1、p-Akt蛋白表达水平的调节
为了检测肌醇和/或木樨草素对A549细胞的抑制作用同PDK1和Akt活化的抑制是否平行,我们检测了A549细胞中p-PDK1和p-Akt的表达水平,如图 4所示。同对照组相比,肌醇、木樨草素可引起A549细胞pPDK1和p-Akt蛋白质表达的减少,3个处理组A549细胞中蛋白质表达水平的改变趋势一致,联合处理组变化幅度较大。结果表明用肌醇、木樨草素单独或联合处理A549细胞时,可能是通过这两个关键蛋白调控相应的通路发挥的抑制作用。
4.
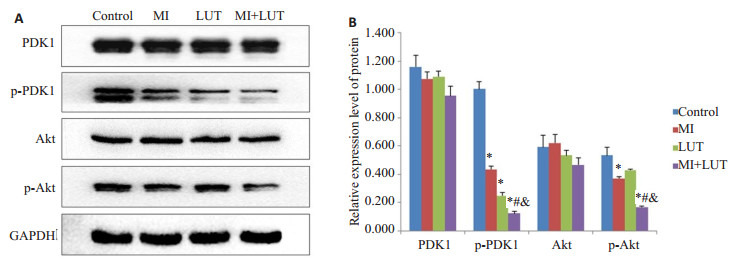
Western blotting检测结果
Protein levels in A549 cells treated for 48 h with myo-inositol (MI) and luteolin (LUT) alone or in combination detected by Western blotting. *P < 0.05 vs control group; #P < 0.05 vs MI group; & P < 0.05 vs LUT group
3. 讨论
肺癌发病率高,在男性和女性癌症发病率中肺癌发病率均位居第2位[13]。PI3K/PDK1/Akt通路是一个致癌的信号通路,其失调参与了多种癌症的发展[14-15]。PDK1和Akt都是PI3K/PDK1/Akt信号通路的重要组成成分。PDK1是一个丝氨酸/苏氨酸蛋白激酶,是PI3K/PDK1/Akt通路的第一个节点,能激活多个下游的效应因子,在多种肿瘤细胞中高表达,同晚期肺癌较低的分化程度相关[16-19]。Akt是PDK1下游的一个关键的效应因子,Akt的高表达在肺癌组织和细胞系中常见,在肺癌的发生发展过程中发挥重要作用[20-22]。抑制PDK1和Akt的表达可明显抑制肿瘤细胞的增殖、克隆形成、迁移和侵袭,提示抑制PDK1或Akt可减少癌症的进展,调控二者表达水平的改变可能是预防癌症进展和治疗的合理的治疗策略[23-26]。
癌症发病率在不同国家和地区相差悬殊,主要归因于环境因素,特别是膳食因素[27-29]。用具有抗癌活性的天然化学物质进行癌症化学预防或抑制其进一步发展是一种可行的方法[28, 30]。联合应用不同的天然活性化学物质对癌细胞共处理时可能会在减少毒副作用的同时取得更好的处理效果[11]。如HJ Kim等人的研究显示肌醇(10 mmol/L)同六磷酸肌醇酯(IP6)共处理前列腺癌PC3细胞时对细胞生长的抑制程度就明显高于单独应用IP6[31]。
肌醇和木樨草素是从可食用植物中提取得到的膳食营养成分,具有一定的抗癌活性[6-9]。本实验研究首次探寻了肌醇和木樨草素的联合应用效果。本实验观察了肌醇和木樨草素对A549细胞活性、增殖和迁移的影响,结果显示肌醇和木樨草素都能降低A549细胞的活性,且呈浓度依赖性。用低浓度水平的肌醇(10 mmol/L)和木樨草素(20 μmol/L)处理A549细胞和Beas-2B细胞后,联合处理组A549细胞的活性、平板克隆形成和划痕愈合受到的抑制较肌醇或木樨草素单独应用时更为明显。与此同时,同样的处理对Beas-2B细胞的活性、克隆形成和划痕愈合程度没有明显的影响,安全性比较好。这些结果表明肌醇和木樨草素的合用能特异性的抑制A549细胞的增殖和迁移,较单用时效果更好,二者的合用可能产生了相加或协同的作用。
肌醇可调节不同的信号通路,有研究显示肌醇的添加能降低PI3K的表达,抑制Akt的活化,调节癌细胞的上皮间质转化[32-33]。木樨草素是一种膳食黄酮类化合物,可抑制过氧化氢诱导的血管平滑肌细胞Akt的活化[34]。为探讨肌醇和木樨草素合用时对A549细胞发挥抑制作用的可能的作用机制,本研究检测了A549细胞中PDK1和Akt蛋白表达水平的改变,结果可见:同对照组相比,联合处理组细胞中p-PDK1和p-Akt的表达较肌醇或木樨草素单用时出现了更为明显的下调,提示肌醇和木樨草素的合用可能是通过抑制PDK1和Akt的活性发挥的作用。
Biography
王允,副教授,博士,E-mail: wy_sunnyday@126.com
Funding Statement
安徽省高校自然科学项目(KJ2016A476);蚌埠医学院自然科学项目(BYKY1706ZD)
Contributor Information
王 允 (Yun WANG), Email: wy_sunnyday@126.com.
张 玉媛 (Yuyuan ZHANG), Email: zhyy8727@126.com.
References
- 1.黎 钧耀. 癌症的营养干预研究. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ201309007.htm. 化学进展. 2013;25(9):1462–79. [黎钧耀.癌症的营养干预研究[J].化学进展, 2013, 25(9): 1462-79.] [Google Scholar]
- 2.Rahman MA, Amin AR, Shin DM. Chemopreventive potential of natural compounds in head and neck cancer. Nutr Cancer. 2010;62(7):973–87. doi: 10.1080/01635581.2010.509538. [Rahman MA, Amin AR, Shin DM. Chemopreventive potential of natural compounds in head and neck cancer[J]. Nutr Cancer, 2010, 62(7): 973-87.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakao K, Hahm ER, Singh SV. In vitro and in vivo effects of phenethyl isothiocyanate treatment on vimentin protein expression in cancer cells. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3671484. Nutr Cancer. 2013;65(Suppl 1):61–7. doi: 10.1080/01635581.2013.785002. [Sakao K, Hahm ER, Singh SV. In vitro and in vivo effects of phenethyl isothiocyanate treatment on vimentin protein expression in cancer cells[J]. Nutr Cancer, 2013, 65(Suppl 1): 61-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam S, McWilliams A, LeRiche J, et al. A phase Ⅰ study of myoinositol for lung cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1526–31. doi: 10.1158/1055-9965.EPI-06-0128. [Lam S, McWilliams A, LeRiche J, et al. A phase Ⅰ study of myoinositol for lung cancer chemoprevention[J]. Cancer Epidemiol Biomarkers Prev, 2006, 15(8): 1526-31.] [DOI] [PubMed] [Google Scholar]
- 5.Lin Y, Shi R, Wang X, et al. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8(7):634–46. doi: 10.2174/156800908786241050. [Lin Y, Shi R, Wang X, et al. Luteolin, a flavonoid with potential for cancer prevention and therapy[J]. Curr Cancer Drug Targets, 2008, 8 (7): 634-46.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kassie F, Melkamu T, Endalew A, et al. Inhibition of lung carcinogenesis and critical cancer-related signaling pathways by N-acetylS-(N-2-phenethylthiocarbamoyl)-L-cysteine, indole-3-carbinol and myo-inositol, alone and in combination. Carcinogenesis. 2010;31(9):1634–41. doi: 10.1093/carcin/bgq139. [Kassie F, Melkamu T, Endalew A, et al. Inhibition of lung carcinogenesis and critical cancer-related signaling pathways by N-acetylS-(N-2-phenethylthiocarbamoyl)-L-cysteine, indole-3-carbinol and myo-inositol, alone and in combination[J]. Carcinogenesis, 2010, 31 (9): 1634-41.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kassie F, Matise I, Negia M, et al. Combinations of N-Acetyl-S-(N-2- PhenethylthiocarbamoyI)-L-Cysteine and myo-inositol inhibit tobacco carcinogen-induced lung adenocarcinoma in mice. Cancer Prev Res. 2008;1(4):285–97. doi: 10.1158/1940-6207.CAPR-08-0012. [Kassie F, Matise I, Negia M, et al. Combinations of N-Acetyl-S-(N-2- PhenethylthiocarbamoyI)-L-Cysteine and myo-inositol inhibit tobacco carcinogen-induced lung adenocarcinoma in mice[J]. Cancer Prev Res, 2008, 1(4): 285-97.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu X, Li Y, Li X, et al. Luteolin induces apoptosis in vitro through suppressing the MAPK and PI3K signaling pathways in gastric cancer. Oncol Lett. 2017;14(2):1993–2000. doi: 10.3892/ol.2017.6380. [Lu X, Li Y, Li X, et al. Luteolin induces apoptosis in vitro through suppressing the MAPK and PI3K signaling pathways in gastric cancer[J]. Oncol Lett, 2017, 14(2): 1993-2000.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han K, Meng W, Zhang JJ, et al. luteolin inhibited proliferation and induced apoptosis of prostate cancer cells through miR-301. http://europepmc.org/articles/PMC4888721/ Onco Targets Ther. 2016;9(1):3085–94. doi: 10.2147/OTT.S102862. [Han K, Meng W, Zhang JJ, et al. luteolin inhibited proliferation and induced apoptosis of prostate cancer cells through miR-301[J]. Onco Targets Ther, 2016, 9(1): 3085-94.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagne A, Melkamu T, Schutten MM, et al. Enhanced inhibition of lung adenocarcinoma by combinatorial treatment with indole-3- carbinol and silibinin in A/J mice. Carcinogenesis. 2011;32(4):561–7. doi: 10.1093/carcin/bgr010. [Dagne A, Melkamu T, Schutten MM, et al. Enhanced inhibition of lung adenocarcinoma by combinatorial treatment with indole-3- carbinol and silibinin in A/J mice[J]. Carcinogenesis, 2011, 32(4): 561-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang P, Wang B, Chung S, et al. Increased chemopreventive effect by combining arctigenin, green tea polyphenol and curcumin in prostate and breast cancer cells. http://www.ncbi.nlm.nih.gov/pubmed/25243063. RSCAdv. 2014;4(66):35242–50. doi: 10.1039/C4RA06616B. [Wang P, Wang B, Chung S, et al. Increased chemopreventive effect by combining arctigenin, green tea polyphenol and curcumin in prostate and breast cancer cells[J]. RSCAdv, 2014, 4(66): 35242-50.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark R, Lee J, Lee SH. Synergistic anticancer activity of capsaicin and 3, 3'-diindolylmethane in human colorectal cancer. J Agric Food Chem. 2015;63(17):4297–304. doi: 10.1021/jf506098s. [Clark R, Lee J, Lee SH. Synergistic anticancer activity of capsaicin and 3, 3'-diindolylmethane in human colorectal cancer[J]. J Agric Food Chem, 2015, 63(17): 4297-304.] [DOI] [PubMed] [Google Scholar]
- 13.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017[J]. CA Cancer J Clin, 2017, 67(1): 7-30.] [DOI] [PubMed] [Google Scholar]
- 14.Bunney TD, Katan M. Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nat Rev Cancer. 2010;10(5):342–52. doi: 10.1038/nrc2842. [Bunney TD, Katan M. Phosphoinositide signalling in cancer: beyond PI3K and PTEN[J]. Nat Rev Cancer, 2010, 10(5): 342-52.] [DOI] [PubMed] [Google Scholar]
- 15.So L, Fruman DA. PI3K signalling in B-and T-lymphocytes: new developments and therapeutic advances. http://europepmc.org/articles/PMC3539736. Biochem J. 2012;442(3):465–81. doi: 10.1042/BJ20112092. [So L, Fruman DA. PI3K signalling in B-and T-lymphocytes: new developments and therapeutic advances[J]. Biochem J, 2012, 442(3): 465-81.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vosset E, Oeztuerk-Winder F, Ruiz EJ, et al. p38α negatively regulates survival and malignant selection of transformed bronchioalveolar stem cells. PLoS One. 2013;8(11):e78911. doi: 10.1371/journal.pone.0078911. [Vosset E, Oeztuerk-Winder F, Ruiz EJ, et al. p38α negatively regulates survival and malignant selection of transformed bronchioalveolar stem cells[J]. PLoS One, 2013, 8(11): e78911.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen H, Zhu Y, Wu YJ, et al. Genomic alterations in lung adenocarcinomas detected by multicolor fluorescence in situ hybridization and comparative genomic hybridization. Cancer Ggenet Cytogenet. 2008;181(2):100–7. doi: 10.1016/j.cancergencyto.2007.11.012. [Shen H, Zhu Y, Wu YJ, et al. Genomic alterations in lung adenocarcinomas detected by multicolor fluorescence in situ hybridization and comparative genomic hybridization[J]. Cancer Ggenet Cytogenet, 2008, 181(2): 100-7.] [DOI] [PubMed] [Google Scholar]
- 18.Maurer M, Su T, Saal LH, et al. 3-Phosphoinositide-dependent kinase 1 potentiates upstream lesions on the phosphatidylinositol 3- kinase pathway in breast carcinoma. Cancer Res. 2009;69(15):6299–306. doi: 10.1158/0008-5472.CAN-09-0820. [Maurer M, Su T, Saal LH, et al. 3-Phosphoinositide-dependent kinase 1 potentiates upstream lesions on the phosphatidylinositol 3- kinase pathway in breast carcinoma[J]. Cancer Res, 2009, 69(15): 6299-306.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlay DK, Sinclair LV, Feijoo C, et al. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in PTEN-null lymphocytes. J Exp Med. 2009;206(11):2441–54. doi: 10.1084/jem.20090219. [Finlay DK, Sinclair LV, Feijoo C, et al. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in PTEN-null lymphocytes[J]. J Exp Med, 2009, 206(11): 2441-54.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balsara BR, Pei J, Mitsuuchi Y, et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25(11):2053–9. doi: 10.1093/carcin/bgh226. [Balsara BR, Pei J, Mitsuuchi Y, et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions [J]. Carcinogenesis, 2004, 25(11): 2053-9.] [DOI] [PubMed] [Google Scholar]
- 21.Brognard J, Clark AS, Ni Y, et al. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. http://jcp.bmj.com/cgi/ijlink?linkType=ABST&journalCode=canres&resid=61/10/3986. Cancer Res. 2001;61(10):3986–97. [Brognard J, Clark AS, Ni Y, et al. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation[J]. Cancer Res, 2001, 61(10): 3986-97.] [PubMed] [Google Scholar]
- 22.Brognard J, Dennis PA. Variable apoptotic response of NSCLC cells to inhibition of the MEK/ERK pathway by small molecules or dominant negative mutants. Cell Death Differ. 2002;9(9):893–904. doi: 10.1038/sj.cdd.4401054. [Brognard J and Dennis PA. Variable apoptotic response of NSCLC cells to inhibition of the MEK/ERK pathway by small molecules or dominant negative mutants[J]. Cell Death Differ, 2002, 9(9): 893- 904.] [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Zhang Z, Wang H, et al. miR-138-1* regulates aflatoxin B1- induced malignant transformation of BEAS-2B cells by targeting PDK1. Arch Toxicol. 2016;90(5):1239–49. doi: 10.1007/s00204-015-1551-4. [Wang Y, Zhang Z, Wang H, et al. miR-138-1* regulates aflatoxin B1- induced malignant transformation of BEAS-2B cells by targeting PDK1[J].Arch Toxicol, 2016, 90(5): 1239-49.] [DOI] [PubMed] [Google Scholar]
- 24.Peng Y, Qiu L, Xu D, et al. M4IDP, a zoledronic acid derivative, induces G1 arrest, apoptosis and autophagy in HCT116 colon carcinoma cells via blocking PI3K/Akt/mTOR pathway. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=08a91d9099af800fa97160822c3c2f31. Life Sci. 2017;185(1):63–72. doi: 10.1016/j.lfs.2017.07.024. [Peng Y, Qiu L, Xu D, et al. M4IDP, a zoledronic acid derivative, induces G1 arrest, apoptosis and autophagy in HCT116 colon carcinoma cells via blocking PI3K/Akt/mTOR pathway[J]. Life Sci, 2017, 185(1): 63-72.] [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Sun Z, Qi M, et al. INPP4B restrains cell proliferation and metastasis via regulation of the PI3K/AKT/SGK pathway. J Cell Mol Med. 2018;22(5):2935–43. doi: 10.1111/jcmm.2018.22.issue-5. [Chen Y, Sun Z, Qi M, et al. INPP4B restrains cell proliferation and metastasis via regulation of the PI3K/AKT/SGK pathway[J]. J Cell Mol Med, 2018, 22(5): 2935-43.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimobaba S, Taqa S, Akizuki R, et al. Claudin-18 inhibits cell proliferation and motility mediated by inhibition of phosphorylation of pdk1 and akt in human lung adenocarcinoma a549 cells. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b37e0c99aeace166908a33e83ea04853. Biochim BiophysActa. 2016;1863(6):1170–8. doi: 10.1016/j.bbamcr.2016.02.015. [Shimobaba S, Taqa S, Akizuki R, et al. Claudin-18 inhibits cell proliferation and motility mediated by inhibition of phosphorylation of pdk1 and akt in human lung adenocarcinoma a549 cells[J]. Biochim BiophysActa, 2016, 1863(6): 1170-8.] [DOI] [PubMed] [Google Scholar]
- 27.Willett W. C. Diet and cancer. Oncologist. 2000;5(5):393–404. doi: 10.1634/theoncologist.5-5-393. [W. C. Willett. Diet and cancer[J]. Oncologist, 2000, 5(5): 393-404.] [DOI] [PubMed] [Google Scholar]
- 28.Koumbi L. Dietary factors can protect against liver cancer development. World J Hepatol. 2017;9(3):119–25. doi: 10.4254/wjh.v9.i3.119. [Koumbi L. Dietary factors can protect against liver cancer development[J]. World J Hepatol, 2017, 9(3): 119-25.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui Y, Morgenstern H, Greenland S, et al. Dietary flavonoid intake and lung cancer-A population-based case-control study. Cancer. 2008;112(10):2241–8. doi: 10.1002/cncr.23398. [Cui Y, Morgenstern H, Greenland S, et al. Dietary flavonoid intake and lung cancer-A population-based case-control study[J]. Cancer, 2008, 112(10): 2241-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo Y, Ryu K, Park J, et al. Inhibition of ANO1 by luteolin and its cytotoxicity in human prostate cancer PC-3 cells. Plos One. 2017;12(3):e0174935. doi: 10.1371/journal.pone.0174935. [Seo Y, Ryu K, Park J, et al. Inhibition of ANO1 by luteolin and its cytotoxicity in human prostate cancer PC-3 cells[J]. Plos One, 2017, 12(3): e0174935.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HJ, Jang Y, Kim H, et al. Apoptotic effect of IP6 was not enhanced by co-treatment with myo- inositol in prostate carcinoma PC3 cells. Nutr Res Pract. 2007;1(3):195–9. doi: 10.4162/nrp.2007.1.3.195. [Kim HJ, Jang Y, Kim H, et al. Apoptotic effect of IP6 was not enhanced by co-treatment with myo- inositol in prostate carcinoma PC3 cells[J]. Nutr Res Pract, 2007, 1(3): 195-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bizzarri M, Dinicola S, Bevilacqua A, et al. Broad spectrum anticancer activity of myo-inositol and inositol hexakisphosphate. http://europepmc.org/articles/PMC5067332/ Int J Endocrinol. 2016;2016(1):14. doi: 10.1155/2016/5616807. [Bizzarri M, Dinicola S, Bevilacqua A, et al. Broad spectrum anticancer activity of myo-inositol and inositol hexakisphosphate [J]. Int J Endocrinol, 2016, 2016(1): 14.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bizzarri M, Cucina A, Dinicola S, et al. Does myo-inositol effect on PCOS follicles involve cytoskeleton regulation? http://www.ncbi.nlm.nih.gov/pubmed/27142131. Med Hypotheses. 2016;91(1):1–5. doi: 10.1016/j.mehy.2016.03.014. [Bizzarri M, Cucina A, Dinicola S, et al. Does myo-inositol effect on PCOS follicles involve cytoskeleton regulation[J]? Med Hypotheses, 2016, 91(1): 1-5.] [DOI] [PubMed] [Google Scholar]
- 34.Lang Y, Chen D, Li D, et al. Luteolin inhibited hydrogen peroxideinduced vascular smooth muscle cells proliferation and migration by suppressing the Src and Akt signalling pathways. J Pharm Pharmacol. 2012;64(4):597–603. doi: 10.1111/j.2042-7158.2011.01438.x. [Lang Y, Chen D, Li D, et al. Luteolin inhibited hydrogen peroxideinduced vascular smooth muscle cells proliferation and migration by suppressing the Src and Akt signalling pathways[J]. J Pharm Pharmacol, 2012, 64(4): 597-603.] [DOI] [PubMed] [Google Scholar]


