Abstract
目的
研究大肠杆菌胞外囊泡(E.coli-OMVs)对人神经母细胞瘤SK-N-SH细胞增殖、凋亡和迁移的影响。
方法
采用差速离心法提取大肠杆菌胞外囊泡,观察囊泡形态并测定囊泡粒径范围。随机分为4组:正常对照组、胞外囊泡低剂量组(100 μg/mL E.coli-OMVs)、胞外囊泡中剂量组(500 μg/mL E.coli-OMVs)和胞外囊泡高剂量组(1000 μg/mL E.coli-OMVs)。MTT检测各组细胞增殖能力;TUNEL和Western blot分别检测各组细胞凋亡情况及凋亡相关蛋白caspase3的表达;Transwell检测各组细胞迁移能力。
结果
透射电镜下E.coli-OMVs呈双层膜、中空、圆形或椭圆形,其内含低密度或高密度物质,具有明显的异质性,粒径分析结果表明其直径分布在30~450 nm。与对照组相比,经E.coli-OMVs干预后,SK-N-SH细胞增殖能力显著降低;细胞凋亡增加,caspase3蛋白表达水平明显上调;而迁移能力显著降低(P<0.05)。
结论
E.coli-OMVs对SK-N-SH细胞具有直接杀伤作用,有望成为难治性神经母细胞瘤的治疗药物。
Keywords: SK-N-SH细胞, 大肠杆菌胞外囊泡, 增殖, 凋亡, 迁移, ,
Abstract
Objective
To investigate the effect of outer membrane vesicles derived from Escherichia coli on proliferation, apoptosis and migration of human neuroblastoma SK-N-SH cells in vitro.
Methods
The outer membrane vesicles (OMVs) were obtained from wild-type Escherichia coli with ultracentrifugation method, and the morphology of the OMVs was observed by transmission electron microscopy and the vesicle diameter was determined using MALVERN ZEN3690. Human neuroblastoma SK-N-SH cells were treated with the OMVs at low (100 μg/mL), moderate (500 μg/mL) and high (1000 μg/mL) doses, and 24, 48 and 72 h later, the cell proliferation activity was detected with MTT assay. The expressions of apoptosisrelated marker caspase-3 was detected using Western blotting, and TUNEL assay was performed to detect the cell apoptosis. The migration capacity of SK-N-SH cells was evaluated using Transwell migration assay.
Results
The isolated OMVs showed a circular or elliptical hollow structure with double-layer membrane and a diameter range of 30-450 nm. Compared with the control cells, SK-N-SH cells treated with the OMVs showed significantly lowered cell proliferation capacity with enhanced expression of caspase-3. Treatment of the cells with the OMVs resulted in increased cell apoptosis and significantly lowered migration capacity (P < 0.05).
Conclusion
The OMVs derived from Escherichia coli can produce cytotoxicity against SK-N-SH cells and might serve as a therapeutic agent for refractory neuroblastoma.
Keywords: neuroblastoma, SK-N-SH cells, outer membrane vesicles, Escherichia coli, cell proliferation, apoptosis, migration
神经母细胞瘤(NB)是婴幼儿最常见的恶性肿瘤之一,约占儿童实体肿瘤的7%,其中难治性神经母细胞瘤恶性程度高,临床治疗效果不佳,预后不良[1]。细菌胞外囊泡(OMVs)最初是在霍乱弧菌中被发现[2],后有研究证实G-菌均可释放类似的囊泡[3-5],直径分布在20~500 nm [6]。G-菌释放的OMVs中含有磷脂[7]、磷酸脂多糖(LPS) [8]、蛋白质以及少量的核酸物质[9],与宿主细胞结合后,可将其中的生物活性物质运输到宿主细胞中,从而改变受体细胞的生物活性[10-12]。有研究表明,G-菌释放的OMVs在小鼠结肠癌模型体内可以显著抑制肿瘤的生长[13],但其是否直接对肿瘤组织进行杀伤,目前尚未可知。因此,本实验拟研究E.coli-OMVs对SK-N-SH细胞增殖、凋亡以及迁移能力的影响,明确G-菌OMVs是否对SKN-SH细胞具有杀伤作用,以期为难治性神经母细胞瘤及其他肿瘤的治疗提供新的思路及研究基础。
1. 材料和方法
1.1. 实验材料与准备
SK-N-SH细胞(中国科学院典型培养物保藏委员会细胞库)、DMEM/F12培养基(Hyclone)、0.25%胰酶(Gibco)、胎牛血清(Gibco)、LB (生工)、琼脂粉(生工)、MTT (索莱宝)、小鼠抗caspase3抗体(abcam)、TUNEL凋亡试剂(罗氏)、大肠杆菌野生株(重庆医科大学附属儿童医院检验科,上行尿路感染患者)、圆形细胞爬片:直径14 mm (中国生物器材网)。
1.2. 实验分组
将SK-N-SH细胞随机分为4组:正常对照组;胞外囊泡低剂量组(100 μg/mL E.coli-OMVs);胞外囊泡中剂量组(500 μg/mL E.coli-OMVs);胞外囊泡高剂量组(1000 μg/mL E.coli-OMVs)。
1.3. SK-N-SH细胞培养
用含10% FBS的DMEM/F12培养基重悬SK-NSH细胞后,接种于25 cm2的培养瓶中,置于37 ℃,5% CO2孵箱中继续培养,细胞密度生长至70%~80%时进行传代或下一步干预。
1.4. 大肠杆菌野生株培养
大肠杆菌野生株于LB培养基(100 mL ddH2O+ 2.5 g LB)中培养,5 mL LB培养基中加入20 µL大肠杆菌野生株原液,37 ℃,200 r/min摇床上培养12~16 h,然后将细菌悬液转移至250 mL LB培养基中,继续培养12~16 h至吸光度(A600):0.9-1.0。
1.5. E.coli-OMVs提取与鉴定
1.5.1. E.coli-OMVs提取方法
采用差速离心法提取E.coli-OMVs,将细菌菌液置于250 mL超离管(美国Beckman coulter公司),10 000 g,4 ℃离心10 min,留取上清液。0.45 μm PVDF过滤器过滤上清液,38 400 g超速离心1 h,弃去上清液,50 mmoL Hepes缓冲液重悬沉淀,再次38 000 g超速离心1~2 h,DMEM/F12培养基重悬沉淀,0.45 μm PVDF膜过滤重悬液,取样用琼脂板检测样本是否无菌,剩余样本-80 ℃保存[14-15]。
1.5.2. 透射电镜鉴定E.coli-OMVs形态
取E.coli-OMVs悬液20 μL,2%多聚甲醛稀释并固定后,吸取20 μL滴加于2 nm电镜铜网状栅中,室温干燥后醋酸双氧铀负染30 s,透射电镜拍照并保存图像[16]。
1.5.3. MALVERN ZEN3690测定E.coli-OMVs粒径范围
吸取2 mL E.coli-OMVs悬液于测量管中,放入MALVERN ZEN3690测量槽,选定参数:每个样本测量10次取其平均值,每次测量时间5 s。
1.6. MTT检测细胞增殖活力
将SK-N-SH细胞接种于96孔板(5000个细胞/孔),待细胞达到70%~80%融合后,加入E.coli-OMVs继续培养24、48、72 h,吸出培养基并加入80 μL DMEM/F12无血清培养基及20 μL MTT工作液,放入孵箱中继续孵育4 h。吸出工作液,每孔加入DMSO 150 μL,待充分溶解后酶标仪(492 nm波长)测定吸光度。
1.7. TUNEL
细胞接种于24孔板中(200 000细胞/孔),待70%~ 80%融合时给与E.coli-OMVs干预。24 h后用4%多聚甲醛固定15 min,PBS清洗3遍,5% BSA封闭37 ℃ 45 min,TUNEL试剂1:试剂2按1:9的比例配制染液,37 ℃避光染色1 h,PBS清洗3次,Hoechst(1:200)避光染色1 h,PBS清洗3次后封片保存。荧光显微镜下拍摄图像。
1.8. Western blot
RIPA裂解细胞,12 000 g离心10 min,取上清。BCA法测定蛋白浓度。灌制SDS-PAGE凝胶,吸取10 μg总蛋白进行电泳。半干电转将蛋白从SDS-PAGE凝胶转至PVDF膜,5%的脱脂奶粉封闭。一抗小鼠caspase 3抗体(1:1000)、兔GAPDH抗体(1:1000)4 ℃孵育过夜。1:5000稀释二抗,室温孵育1 h,显影后保存图像。Image-J软件分析条带灰度值。
1.9. Transwell
取对数生长期SK-N-SH细胞,0.25%胰酶消化后制成无血清细胞悬液,调整细胞数为1×105/mL。取600 μL不同浓度E.coli-OMVs至24孔板中(下室),置入Transwell小室(8 μm孔径),在上室中加入200 μL SK-NSH细胞悬液,24 h后移除小室,显微镜下(200倍)计数下室5个视野细胞数,取其平均值。迁移抑制率= (1-实验组平均迁移细胞数/对照组平均迁移细胞数) ×100%。
1.10. 统计学分析
采用SPSS 17.0进行统计分析,结果用均数±标准差表示,多组间均数比较采用单因素方差分析,两组间均数比较采用独立样本t检验。P<0.05代表差异有统计学意义。
2. 结果
2.1. E.coli-OMVs形态及粒径分析
透射电镜下可见双层膜、中空、散在分布的圆形或椭圆形膜性囊泡,其内含低密度或高密度物质,具有明显的异质性,结合粒径分析,结果显示其直径介于30~ 450 nm,主要集中在100~300 nm直径范围内,与胞外囊泡定义范围相符[17](图 1)。
1.
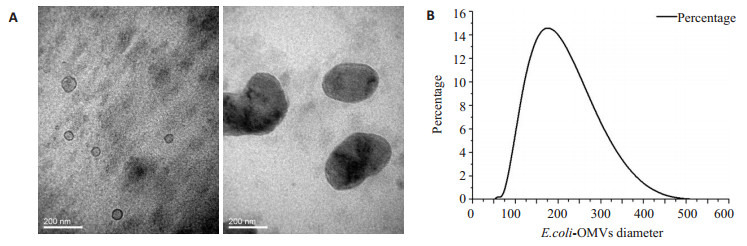
E.coli-OMVs提取及鉴定
Extraction and identification of outer membrane vesicles (OMVs) from wild-type E.coli. A: Observation of the isolated OMVs under transmission electron microscope (Original magnification: × 200 000). The OMVs showed a hollo, circular or elliptical morphology with a double-layer membrane; B: Particle size analysis of the OMVs showed that the diameter of the OMVs ranged from 30 nm to 450 nm.
2.2. E.coli-OMVs抑制SK-N-SH细胞的增殖活性
E.coli-OMVs干预SK-N-SH细胞24、48、72 h后观察各组细胞增殖活力,MTT结果显示,经E.coli-OMVs干预后各组细胞增殖活力均低于对照组,并呈剂量依赖关系(图 2)。
2.
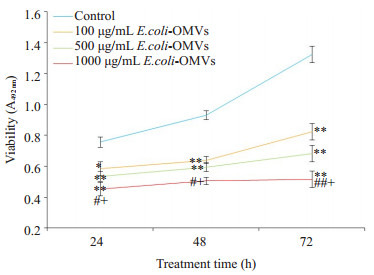
不同浓度E.coli-OMVs对SK-N-SH细胞增殖活力的影响
Effect of E.coli-OMVs on proliferation of human SKN-SH cells. *P < 0.05, **P < 0.01 vs control, #P < 0.05, ##P < 0.01 vs 100 μg/mL E.coli-OMVs, + P < 0.05 vs 500 μg/mL E.coli-OMVs.
2.3. E.coli-OMVs诱导SK-N-SH细胞的凋亡
TUNEL检测各组细胞的凋亡情况,Western blot检测各组细胞中caspase3蛋白表达水平。TUNEL染色结果显示500 μg/mL、1000 μg/mL E.coli-OMVs干预后,SK-N-SH细胞凋亡数量增加,且1000 μg/mL E.coli-OMVs干预后细胞凋亡更明显,视野中可见散在的凋亡小体(图 3),并且与对照组相比,干预后各组细胞中caspase3表达水平增加(图 4)。
3.
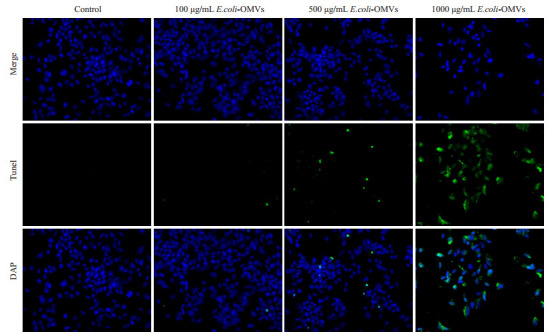
不同浓度E.coli-OMVs干预后Tunel凋亡检测
TUNEL fluorescence for detecting cell apoptosis after treatment with E.coli-OMVs (×200).
4.
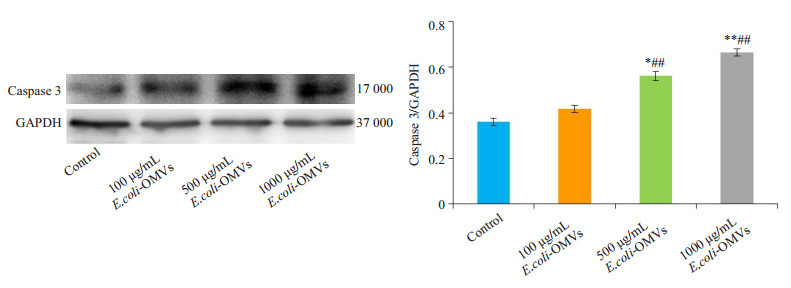
不同浓度E.coli-OMVs干预后caspase3蛋白表达
Relative expression of capsase-3 in different groups. *P < 0.05, **P < 0.01 vs control, ##P < 0.01 vs 100 μg/mL E.coli-OMVs.
2.4. E.coli-OMVs导致SK-N-SH细胞迁移能力降低
与对照组相比,E.coli-OMVs处理后,细胞迁移数量明显降低,100 μg/mL、500 μg/mL和1000 μg/mL E.coli-OMVs组的迁移抑制率分别为63.8%、75.8%和89.8%,呈现剂量依赖关系(P<0.05,图 5)。
5.
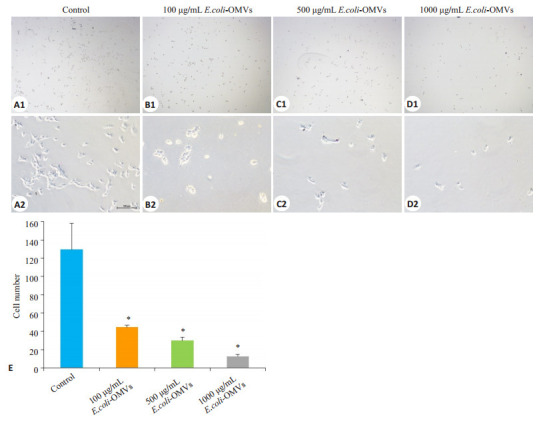
不同浓度E.coli-OMVs对SK-N-SH细胞迁移能力的影响
Effect of E.coli-OMVs on migration capacity of human SK-N-SH cells. A1, B1, C1, D1: × 40; A2, B2, C2, D2: × 200; E: Cell counts in different groups. *P < 0.05 vs control.
3. 讨论
De在19世纪60年代首次在霍乱弧菌中发现具有免疫原性的囊泡性物质,随后Knox首次在电镜下观察到大肠杆菌分泌具有双层膜性结构,直径约20~500 nm的囊泡状物质,将其定义为胞外囊泡(OMVs) [17]。本实验选取野生大肠杆菌进行培养并提取菌液中的OMVs,与真核细胞外泌体不同的是,OMVs来源于细菌,无类似真核细胞外泌体的标志物蛋白,如TSG101,CD63,Alix等[18-20]。故本实验用透射电镜观察E.coli-OMVs的形态,结合粒径分析测量囊泡直径对其定性[14],研究结果显示,透射电镜下E.coli-OMVs呈双层膜,中空,圆形或椭圆形结构,囊泡内含低密度或高密度物质,具有明显的异质性,粒径分析其直径分布在30~450 nm,以上特征均符合细菌OMVs的定义。
OMVs是G-菌产生的囊泡性物质,其内含蛋白质、RNA、LPS等小分子物质,与脂质双分子层的膜性结构有天然的亲和力[21-22]。有研究显示,G-菌OMVs可以诱导小肠上皮细胞和人脑血管上皮细胞发生促炎症反应,从而导致细胞凋亡[4, 23]。亦有研究表明,在小鼠结肠癌模型体内,G-菌OMVs可以抑制肿瘤的生长,囊泡内的蛋白质刺激NK细胞和T细胞产生INF-γ,进而发挥抑制肿瘤生长的作用[13]。该研究表明,G-菌OMVs进入小鼠体内后,可能通过体液免疫间接对肿瘤发挥杀伤作用,但G-菌OMVs是否可以直接作用肿瘤细胞进而影响其生物学行为,还未有文献报道。本研究通过E.coli-OMVs干预SK-N-SH细胞后观察其细胞生物学行为的改变,以明确E.coli-OMVs是否对肿瘤细胞具有直接杀伤作用。MTT结果显示,在给与E.coli-OMVs干预后,不同时间点SK-N-SH细胞增殖活力均降低;Transwell结果显示,随着干预剂量的增加,SK-N-SH细胞迁移数量减少,迁移抑制率越高。因此,E.coli-OMVs可以显著抑制SK-N-SH细胞的增殖及迁移能力,并且存在剂量效应关系。同时,TUNEL和Western blot结果显示,给与高剂量E.coli-OMVs干预后,细胞凋亡数量明显增加,且细胞周围可见散在的凋亡小体,细胞凋亡相关蛋白caspase3的表达显著增加。说明E.coli-OMVs可有效促进SK-N-SH细胞的凋亡。故本研究证实,E.coli-OMVs可直接作用于SK-N-SH细胞,抑制其增殖及侵袭的能力,促进细胞凋亡。但E.coli-OMVs通过何种具体机制对SK-N-SH细胞产生影响还需进一步研究,有研究显示G-菌OMVs可以通过内吞作用直接将OMVs中的LPS运输到细胞内,然后激活细胞内caspase11,进而引起细胞凋亡[24],也有报道称大肠杆菌可以通过OMVs分泌有细胞毒性的细胞溶素A [25],从而对细胞产生杀伤作用。
综上所述,本实验运用E.coli-OMVs干预SK-NSH细胞,结果提示,E.coli-OMVs可直接作用于SK-NSH细胞,抑制细胞的增殖、迁移能力,并促进细胞凋亡。暗示E.coli-OMVs可能成为一种新的神经母细胞瘤治疗方式,其高获得率及对靶细胞的高亲和力使其较传统化疗药物更具潜在优势,但E.coli-OMVs中具体作用成分以及如何提高其靶向性仍需进一步研究。
Biography
唐波,硕士研究生,E-mail: ljsmilekiss@126.com
Funding Statement
国家自然科学基金(81370701);重庆市社会事业与民生保障科技创新专项(cstc2017shmsA130103)
Supported by National Natural Science Foundation of China (81370701)
Contributor Information
唐 波 (Bo TANG), Email: ljsmilekiss@126.com.
何 大维 (Dawei HE), Email: dw.he@163.com.
References
- 1.Salazar BM, Balczewski EA, Ung CY, et al. Neuroblastoma, a paradigm for big data science in pediatric oncology. Int J Mol Sci. 2016;18(1):E37. doi: 10.3390/ijms18010037. [Salazar BM, Balczewski EA, Ung CY, et al. Neuroblastoma, a paradigm for big data science in pediatric oncology[J]. Int J Mol Sci, 2016, 18(1): E37.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De SN. Enterotoxicity of bacteria-free culture-filtrate of Vibrio cholerae. Nature. 1959;183(4674):1533–4. doi: 10.1038/1831533a0. [De SN. Enterotoxicity of bacteria-free culture-filtrate of Vibrio cholerae[J]. Nature, 1959, 183(4674): 1533-4.] [DOI] [PubMed] [Google Scholar]
- 3.Bauman SJ, Kuehn MJ. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. http://www.sciencedirect.com/science/article/pii/S1286457906001808. Microbes Infect. 2006;8(9/10):2400–8. doi: 10.1016/j.micinf.2006.05.001. [Bauman SJ, Kuehn MJ. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response[J]. Microbes Infect, 2006, 8(9/10): 2400-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mondal A, Tapader R, Chatterjee NS, et al. Cytotoxic and inflammatory responses induced by outer membrane VesicleAssociated biologically active proteases from vibrio cholerae. Infect Immun. 2016;84(5):1478–90. doi: 10.1128/IAI.01365-15. [Mondal A, Tapader R, Chatterjee NS, et al. Cytotoxic and inflammatory responses induced by outer membrane VesicleAssociated biologically active proteases from vibrio cholerae[J]. Infect Immun, 2016, 84(5): 1478-90.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ojima Y, Yamaguchi K, Taya M. Quantitative evaluation of recombinant protein packaged into outer membrane vesicles of escherichia coli cells. Biotechnol Prog. 2018;34(1):51–7. doi: 10.1002/btpr.v34.1. [Ojima Y, Yamaguchi K, Taya M. Quantitative evaluation of recombinant protein packaged into outer membrane vesicles of escherichia coli cells[J]. Biotechnol Prog, 2018, 34(1): 51-7.] [DOI] [PubMed] [Google Scholar]
- 6.Dreyer F, Baur A. Biogenesis and functions of exosomes and extracellular vesicles. Methods Mol Biol. 2016;1448:201–16. doi: 10.1007/978-1-4939-3753-0. [Dreyer F, Baur A. Biogenesis and functions of exosomes and extracellular vesicles[J]. Methods Mol Biol, 2016, 1448: 201-16.] [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni HM, Swamy ChV, Jagannadham MV. Molecular characterization and functional analysis of outer membrane vesicles from the Antarctic bacterium Pseudomonas syringae suggest a possible response to environmental conditions. J Proteome Res. 2014;13(3):1345–58. doi: 10.1021/pr4009223. [Kulkarni HM, Swamy ChV, Jagannadham MV. Molecular characterization and functional analysis of outer membrane vesicles from the Antarctic bacterium Pseudomonas syringae suggest a possible response to environmental conditions[J]. J Proteome Res, 2014, 13(3): 1345-58.] [DOI] [PubMed] [Google Scholar]
- 8.Dorward DW, Garon CF, Judd RC. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J Bacteriol. 1989;171(5):2499–505. doi: 10.1128/jb.171.5.2499-2505.1989. [Dorward DW, Garon CF, Judd RC. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae[J]. J Bacteriol, 1989, 171(5): 2499-505.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaweeteerawat C, Na Ubol P, Sangmuang S, et al. Mechanisms of antibiotic resistance in bacteria mediated by Silver nanoparticles. http://www.ncbi.nlm.nih.gov/pubmed/29020531. J Toxicol Environ Health A. 2017;80(23/24):1276–89. doi: 10.1080/15287394.2017.1376727. [Kaweeteerawat C, Na Ubol P, Sangmuang S, et al. Mechanisms of antibiotic resistance in bacteria mediated by Silver nanoparticles[J]. J Toxicol Environ Health A, 2017, 80(23/24): 1276-89.] [DOI] [PubMed] [Google Scholar]
- 10.Jäger J, Keese S, Roessle M, et al. Fusion of legionella pneumophila outer membrane vesicles with eukaryotic membrane systems is a mechanism to deliver pathogen factors to host cell membranes. Cell Microbiol. 2015;17(5):607–20. doi: 10.1111/cmi.12392. [Jäger J, Keese S, Roessle M, et al. Fusion of legionella pneumophila outer membrane vesicles with eukaryotic membrane systems is a mechanism to deliver pathogen factors to host cell membranes[J]. Cell Microbiol, 2015, 17(5): 607-20.] [DOI] [PubMed] [Google Scholar]
- 11.Elmi A, Nasher F, Jagatia H, et al. Campylobacter jejuni outer membrane vesicle-associated proteolytic activity promotes bacterial invasion by mediating cleavage of intestinal epithelial cell Ecadherin and occludin. Cell Microbiol. 2016;18(4):561–72. doi: 10.1111/cmi.v18.4. [Elmi A, Nasher F, Jagatia H, et al. Campylobacter jejuni outer membrane vesicle-associated proteolytic activity promotes bacterial invasion by mediating cleavage of intestinal epithelial cell Ecadherin and occludin[J]. Cell Microbiol, 2016, 18(4): 561-72.] [DOI] [PubMed] [Google Scholar]
- 12.Seo N, Shirakura Y, Tahara Y, et al. Activated CD8 + T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat Commun. 2018;9(1):435. doi: 10.1038/s41467-018-02865-1. [Seo N, Shirakura Y, Tahara Y, et al. Activated CD8 + T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells[J]. Nat Commun, 2018, 9(1): 435.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim OY, Park HT, Dinh NTH, et al. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun. 2017;8(1):626. doi: 10.1038/s41467-017-00729-8. [Kim OY, Park HT, Dinh NTH, et al. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response[J]. Nat Commun, 2017, 8(1): 626.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chutkan H, Macdonald I, Manning A, et al. Quantitative and qualitative preparations of bacterial outer membrane vesicles. Methods Mol Biol. 2013;966:259–72. doi: 10.1007/978-1-62703-245-2. [Chutkan H, Macdonald I, Manning A, et al. Quantitative and qualitative preparations of bacterial outer membrane vesicles[J]. Methods Mol Biol, 2013, 966: 259-72.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lane RE, Korbie D, Trau M, et al. Purification protocols for extracellular vesicles. http://www.ncbi.nlm.nih.gov/pubmed/28828652. Methods Mol Biol. 2017;1660:111–30. doi: 10.1007/978-1-4939-7253-1_10. [Lane RE, Korbie D, Trau M, et al. Purification protocols for extracellular vesicles[J]. Methods Mol Biol, 2017, 1660: 111-30.] [DOI] [PubMed] [Google Scholar]
- 16.Vaswani K, Koh YQ, Almughlliq FB, et al. A method for the isolation and enrichment of purified bovine milk exosomes. Reprod Biol. 2017;17(4):341–8. doi: 10.1016/j.repbio.2017.09.007. [Vaswani K, Koh YQ, Almughlliq FB, et al. A method for the isolation and enrichment of purified bovine milk exosomes[J]. Reprod Biol, 2017, 17(4): 341-8.] [DOI] [PubMed] [Google Scholar]
- 17.Watanabe K. Bacterial membrane vesicles (MVs): novel tools as nature-and nano-carriers for immunogenic antigen, enzyme support, and drug delivery. Appl Microbiol Biotechnol. 2016;100(23):9837–43. doi: 10.1007/s00253-016-7916-7. [Watanabe K. Bacterial membrane vesicles (MVs): novel tools as nature-and nano-carriers for immunogenic antigen, enzyme support, and drug delivery[J]. Appl Microbiol Biotechnol, 2016, 100 (23): 9837-43.] [DOI] [PubMed] [Google Scholar]
- 18.Kowal EJK, Ter-Ovanesyan D, Regev A, et al. Extracellular vesicle isolation and analysis by western blotting. Methods Mol Biol. 2017;1660:143–52. doi: 10.1007/978-1-4939-7253-1. [Kowal EJK, Ter-Ovanesyan D, Regev A, et al. Extracellular vesicle isolation and analysis by western blotting[J]. Methods Mol Biol, 2017, 1660: 143-52.] [DOI] [PubMed] [Google Scholar]
- 19.Hu S, Musante L, Tataruch D, et al. Purification and identification of membrane proteins from urinary extracellular vesicles using triton X-114 phase partitioning. J Proteome Res. 2018;17(1):86–96. doi: 10.1021/acs.jproteome.7b00386. [Hu S, Musante L, Tataruch D, et al. Purification and identification of membrane proteins from urinary extracellular vesicles using triton X-114 phase partitioning[J]. J Proteome Res, 2018, 17(1): 86-96.] [DOI] [PubMed] [Google Scholar]
- 20.Huang W, Wang S, Yao Y, et al. Employing escherichia coli-derived outer membrane vesicles as an antigen delivery platform elicits protective immunity against acinetobacter baumannii infection. Sci Rep. 2016;6:37242. doi: 10.1038/srep37242. [Huang W, Wang S, Yao Y, et al. Employing escherichia coli-derived outer membrane vesicles as an antigen delivery platform elicits protective immunity against acinetobacter baumannii infection[J]. Sci Rep, 2016, 6: 37242.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnington KE, Kuehn MJ. Protein selection and export via outer membrane vesicles. Biochim Biophys Acta. 2014;1843(8):1612–9. doi: 10.1016/j.bbamcr.2013.12.011. [Bonnington KE, Kuehn MJ. Protein selection and export via outer membrane vesicles[J]. Biochim Biophys Acta, 2014, 1843(8): 1612-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.安 泰学, 郑 磊. 细胞外囊泡的分析方法及临床应用进展. http://www.j-smu.com/Upload/html/2017111559.html. 南方医科大学学报. 2017;37(11):1559–62. doi: 10.3969/j.issn.1673-4254.2017.11.23. [安泰学, 郑磊.细胞外囊泡的分析方法及临床应用进展[J].南方医科大学学报, 2017, 37(11): 1559-62.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bielaszewska M, Rüter C, Kunsmann L, et al. Enterohemorrhagic escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS Pathog. 2013;9(12):e1003797. doi: 10.1371/journal.ppat.1003797. [Bielaszewska M, Rüter C, Kunsmann L, et al. Enterohemorrhagic escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis [J]. PLoS Pathog, 2013, 9(12): e1003797.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanaja SK, Russo AJ, Behl B, et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell. 2016;165(5):1106–19. doi: 10.1016/j.cell.2016.04.015. [Vanaja SK, Russo AJ, Behl B, et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation[J]. Cell, 2016, 165(5): 1106-19.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon SY, Lee HM, Kong JN, et al. Secretory expression and enzymatic characterization of recombinant Agarivorans albus β-agarase in Escherichia coli. Prep Biochem Biotechnol. 2017;47(10):1037–42. doi: 10.1080/10826068.2017.1373292. [Yoon SY, Lee HM, Kong JN, et al. Secretory expression and enzymatic characterization of recombinant Agarivorans albus β-agarase in Escherichia coli[J]. Prep Biochem Biotechnol, 2017, 47 (10): 1037-42.] [DOI] [PubMed] [Google Scholar]


