Abstract
目的
通过建立大鼠肺缺血再灌注损伤(IRI)模型,进行低强度脉冲超声(LIPUS)预处理治疗,探讨IRI后高迁移率蛋白1(HMGB1)在肺组织的表达和LIPUS预处理的保护作用。
方法
雄性SD大鼠32只,体质量250~300 g,随机分为4组,每组8只。对照组(A组),开胸游离左肺门,未行阻断;缺血再灌注组(B组),阻断左肺门45 min后再灌注180 min;预处理组(C组)低强度超声波治疗仪超声定位辐照30 min,然后同B组处理;预处理加α7-烟碱型胆碱能受体(α7nAChR)拮抗剂组(D组),LIPUS预处理前30 min腹腔注射α7nAChR拮抗剂甲基牛扁亭2 mg/Kg, 然后同预处理组处理。测量肺组织湿干重比值(W/D)和肺通透指数(LPI),大鼠肺组织病理学观察及评分,ELISA法测定肺组织IL1和IL6的浓度,免疫荧光和Western blot检测HMGB1蛋白表达。
结果
与A组比较,其他三组IR后肺组织W/D值(5.75±0.47)和LPI(2.77±0.18)明显升高(P < 0.05),病理学评分(13.31± 2.82)明显升高(P < 0.05),肺组织IL1(69.13±9.11)和IL6(62.77±8.14)水平明显升高(P < 0.05),免疫荧光检测平均IOD值(0.046± 0.019)和Western blot检测显示HMGB1表达明显增加(P < 0.05)。给于LIPUS预处理后,C组IR后肺组织W/D值(5.07±0.28)和LPI(1.85±0.17)比B组明显降低(P < 0.05),病理学评分(8.13±1.76)比B组明显降低(P < 0.05),同时肺组织IL1和IL6水平以及HMGB1表达也明显降低,给于α7nAChR拮抗剂后该作用明显被抑制。
结论
LIPUS预处理能够减轻IRI后肺损伤,其机制可能是通过激活依赖α7nAChR的胆碱能抗炎通路,从而降低肺组织HMGB1的表达。
Keywords: 低强度脉冲超声, 缺血再灌注损伤, 高迁移率蛋白1, α7-烟碱型胆碱能受体
Abstract
Objective
To observe the effects of low-intensity pulsed ultrasound (LIPUS) pretreatment on pulmonary expression of high mobility group box-1 (HMGB1) in a rat model of lung ischemia-reperfusion (IR).
Methods
Thirty-two male SpragueDawley rats weighing 250-300 g were randomly divided (n=8) into sham-operated group, lung IR group, LIPUS pretreatment group and pretreatment with α7-nicotinic cholinergic receptor (α7nAChR) antagonist group. In the sham-operated group, the left pulmonary hilum was dissociated without occlusion; in the other 3 groups, the left pulmonary hilum was occluded for 45 min followed by reperfusion for 180 min; LIPUS pretreatment for 30 min and intraperitoneal injection of methyllycaconitine (2 mg/kg), an α7nAChR antagonist, were administered before the operation. The wet/dry weight ratio (W/D) and pulmonary permeability index (LPI) of the lung tissue were measured, and the lung histopathology was observed and scored. The contents of interleukin-1 (IL-1) and IL-6 in the lung tissues were measured using ELISA, and the pulmonary expression of HMGB1 protein was detected using immunofluorescence assay and Western blotting.
Results
Compared with those in the sham-operated group, the W/D of the lung tissue, LPI, pathological scores, IL-1 and IL-6 contents in the lung tissue, and pulmonary HMGB1 expression all significantly increased in the other 3 groups (P < 0.05). LIPUS preconditioning significantly lowered the W/D values, LPI, pathological score, IL-1 and IL-6 contents and HMGB1 expression in the lung tissues following lung IR, and these effects were significantly inhibited by administration of methyllycaconitine.
Conclusions
LIPUS preconditioning can reduce lung IR injury possibly by activating α7nAChR-dependent cholinergic anti-inflammatory pathway to reduce lung tissue HMGB1 expression.
Keywords: low intensity pulsed-ultrasound, ischemia and reperfusion injury, high mobility group box-1, α-7nAChR
缺血再灌注损伤(IRI)是临床上肺移植、体外循环、肺的袖式切除、创伤、心肺复苏等情况下引起的急性肺损伤的最常见原因之一[1-2],急性肺损伤患者即便有重症支持疗法,死亡率仍在30%~40%以上[3]。到目前为止, 尚无治疗措施被证明可以特异性预防肺IRI,也没有任何单一治疗手段被证明可以治疗肺IRI [4]。最新研究证实,体外超声处理可以通过激活胆碱能抗炎通路抑制IRI引起的炎症反应[5-6],但该研究仅限于小鼠,所使用超声为诊断性超声,并没有临床试验证据,因此,选择能够运用于临床而且操作简单的预防治疗IRI后肺损伤方法,仍然是我们研究的重点。
低强度脉冲超声(LIPUS)是一种无创伤、操作方便、疗效确切的治疗方法。LIPUS治疗是脉冲超声作用于人体病变部位,影响细胞的物质代谢过程,改善细胞缺血、缺氧状态[7-8]。在组织发生损伤或应激时,组织细胞和或炎性细胞可释放热休克蛋白、透明质酸和高迁移率蛋白1(HMGBl),被称之为“内源性危险信号” [9]。本研究通过建立大鼠肺IRI模型,探讨IRI后HMGB1在肺组织的表达和LIPUS预处理的保护作用及可能机制,以期为LIPUS治疗IRI后肺损伤临床应用提供理论依据。
1. 材料和方法
1.1. 实验材料
雄性SD大鼠36只,体质量250~300 g,置于相对清洁环境中,分笼饲养,予标准饲料,自由饮水。
1.2. 动物模型的建立与分组
所有SD大鼠随机分为4组,每组8只。10%水合氯醛5 mL/kg腹腔注射麻醉后,气管插管接动物小动物呼吸机(HX-300成都泰盟科技),呼吸频率70 b/min,吸呼比为1:2,潮气量:10~12 mL/kg。麻醉维持瑞芬太尼以0.1 μg/kg/h的速度静脉泵入,间隔40 min静脉注射顺苯磺酸阿曲库铵维持肌松,术中生理盐水0.5 mL/h静脉输入。参照文献[10]建立大鼠左肺IR实验模型。于左前胸第5肋间开胸,游离左肺门,生理盐水稀释50 U肝素至500 μL经尾静脉注射,10 min后于肺充盈末用无创微血管夹夹闭左肺门,对照组(A组),开胸游离左肺门,未行阻断;缺血再灌注组(B组),阻断左肺门45 min后再灌注180 min;预处理组(C组)低强度超声波治疗仪超声定位辐照,设定治疗头直径为1.5 cm,频率1.02 MHz,其超声功率为0.08 w/cm2,脉冲波,辐照30 min。辐照过程中保持体温在36±0.5 ℃,然后同B组处理;预处理加α7-烟碱型胆碱能受体(α7nAChR)拮抗剂组(D组)LIPUS预处理前30 min腹腔注射α7nAChR拮抗剂甲基牛扁亭2 mg/Kg,然后同预处理组处理。
所有实验组实验结束后动脉放血处死动物,行支气管灌洗,收集支气管肺泡灌洗液(BALF),并留取肺组织标本。
1.3. 标本检测
1.3.1. 肺血管通透性的观察
取约100 mg肺组织用电子天平测量湿重(W)后,放入烘箱60 ℃烘干48 h至恒重,称干重(D),求得湿干重比值(W/D)(W/D=W/D× l00%);采用考马斯亮蓝染色法(Bradford法)测定血清总蛋白含量和BALF蛋白含量,计算肺通透指数(LPI)。LPI(%)=BALF蛋白含量/血清蛋白含量×100%。
1.3.2. 大鼠肺组织病理学观察及评分
肺组织4%多聚甲醛中固定24 h,石蜡包埋、4 μm连续切片,常规HE染色,光学显微镜观察。肺组织病理评分,组织病理学得分主要依据五方面内容:(1)肺泡腔出血情况;(2)透明膜形成情况;(3)间质水肿情况;(4)呼吸道上皮损伤程度;(5)中性粒细胞浸润情况;每个项目按照0~4分来评分,其中0分代表正常,1分代表极少量变化,2分代表轻微变化,3分代表中度变化,4分代表严重变化。以上得分相加用于评价肺组织损伤程度,满分为20分。所有标本评分均由两位有经验的病理医师独立单盲条件下完成,取两者平均分为最终得分。
1.3.3. 肺组织IL1和IL6检测
取出肺组织标本,按照与生理盐水1:5的比例在EP管中加入生理盐水,在碎冰上进行匀浆,充分匀浆后放入离心机中,以3500 r/min的转速离心10 min。仔细收集上清,用移液器小心将上清移至新的EP管中,然后严格按试剂盒(上海恒远生物技术发展有限公司)说明采用双抗体夹心ELISA法测定IL1和IL6的浓度。
1.3.4. 免疫荧光检测HMGB1蛋白
石蜡切片脱蜡与水化3% H2O2室温孵育10 min以消除内源性过氧化物酶的活性,浸泡3次每次5 min。抗原修复:高压锅中加入0.01 mol/L柠檬酸钠缓冲液,放入组织切片,加热保压100 s后冷却至室温,PBS浸泡2次,5 min/次。正常山羊血清封闭,室温孵育30 min倒去血清,滴加一抗工作液,4 ℃过夜。PBS浸泡3次,5 min/次。滴加适量荧光二抗,室温孵育1 h,PBS浸泡3次,5 min/次。滴加适量的DAPI室温孵育10 min,PBS浸泡3次,5 min/次,中性树脂封片(4 ℃避光保存)荧光显微镜观察拍照。
1.3.5. Western blot蛋白印迹法检测HMGB1蛋白的变化
用-80 ℃保存的肺组织提取总蛋白,Bradford法测定蛋白浓度。总蛋白检测HMGB1、β-actin。取50 μg蛋白以10 %十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS. PAGE)电泳分离。电泳后通过电转移蛋白至聚偏氟乙烯膜,5%牛血清白蛋白常温封闭1 h,分别加入一抗(HMGB1、β-actin)于4 ℃孵育过夜;山羊抗兔近红外二抗(1:15 000)常温避光孵育1 h。Odyssey V3.0扫描仪扫描膜。重复3次。凝胶图像分析系统检测蛋白质印迹条带灰度,测定并计算HMGB1与β-actin的比值。
1.4. 统计学方法
采用SPSS 15.0统计软件进行分析。正态分布计量资料以均数±标准差表示,组内比较采用重复测量方差分析,组间比较采用单因素方差分析。P < 0.05为差异有统计学意义。
2. 结果
2.1. 各组W/D值和LPI的改变
与A组(4.45±0.23)比较,B组(5.75±0.47)、C(5.07± 0.28)组和D组(5.55±0.37)W/D值明显升高(P < 0.05),C组W/D值升高程度明显低于B组和D组(P < 0.05)。再灌注180 min LPI值,与A组(0.82 ± 0.14)比较,B组(2.77±0.18)、C组(1.85±0.17)和D组(2.39±0.15)明显增加(P < 0.05),C组LPI升高的程度明显低于B组和D组(P < 0.05),肺组织IL1和IL6水平,与A组比较,B组、C组和D组均明显增加(P < 0.05),C组则明显低于B组和D组(P < 0.05,表 1)。
1.
各组湿干比重和肺通透指数对比
Wet/dry weight ratio (W/D) and lung permeability index in each group (n=8, Mean±SD)
| Items | Group A | Group B | Group C | Group D |
| *P < 0.05 vs group A, #P < 0.05 vs group B, †P < 0.05 vs group D. | ||||
| W/D | 4.45±0.23 | 5.75±0.47* | 5.07±0.28*#† | 5.55±0.37* |
| LPI (%) | 0.82±0.14 | 2.77±0.18* | 1.85±0.17*#† | 2.39±0.15* |
| IL1 (ng.L-1) | 20.63±6.91 | 69.13±9.11* | 38.73±7.99*#† | 61.33±8.78* |
| IL6 (ng.L-1) | 18.13±7.17 | 62.77±8.14* | 33.13±6.48*#† | 60.61±9.17* |
2.2. 大鼠肺组织病理学检测
A组肺泡、肺间质基本上接近于正常肺结构,仅有少量炎症细胞和肺间质轻度水肿;B组组织结构不清,肺泡腔、间质及支气管腔有大量炎症细胞浸润和红细胞漏出,水肿明显,肺泡间隔破坏(图 1);C组肺泡壁增厚和水肿程度较B组减轻,有部分炎性细胞浸润。与A组比较,B组、C组和D组肺泡壁增厚和水肿程度较大,肺组织病理学评分显著增高(P < 0.05)。C组肺组织病理学评分较B组和D组显著降低(P < 0.05),B组和D组之间无显著差异(P > 0.05,表 2)。
1.
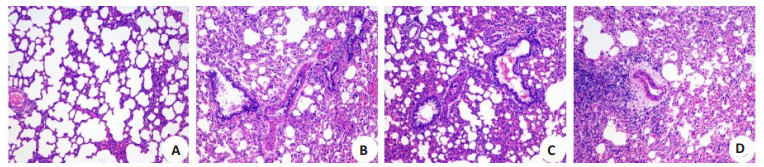
各组大鼠肺组织HE病理切片
HE staining of the lung tissue of the rats in each group (Original magnification: ×100).
2.
各组大鼠肺组织病理学评分
Pathological scores of the lung tissue of the rats in each group (n=8, Mean±SD)
| Items | Group A | Group B | Group C | Group D |
| *P < 0.05 vs group A, #P < 0.05 vs group B, †P < 0.05 vs group D. | ||||
| Scores | 3.21±1.22 | 13.31±2.82* | 8.13±1.76*#† | 12.33±2.07* |
2.3. 免疫荧光检测HMGB1蛋白
荧光显微镜显示HMGBl呈红色发光,细胞核、细胞质及肺间质均有表达,亮蓝色部位为细胞核所在部位。B组HMGBI表达明显增加,肺泡间隔增厚。而C组HMGBl表达较少(图 2)。提示LIPUS预处理可减少缺血再灌注HMGBl的表达。腹腔注射α7nAChR拮抗剂甲基牛扁亭D组HMGBI表达明显增加(表 3)。
2.
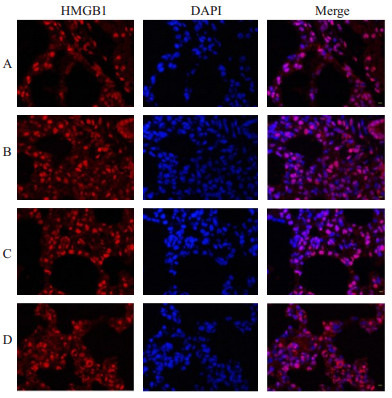
各组大鼠肺组织免疫荧光HMGB1蛋白表达
Immunofluorescence assay of HMGB1 protein expression in the lung tissue of the rats in each group (×400).
3.
各组大鼠肺组织免疫荧光HMGB1蛋白表达平均IOD值
Mean IOD value of immunofluorescence of HMGB1 protein in the lung tissue in each group (n=8, Mean±SD)
| Items | Group A | Group B | Group C | Group D |
| *P < 0.05 vs group A, #P < 0.05 vs group B, †P < 0.05 vs group D. | ||||
| Mean IOD | 0.023±0.011 | 0.046±0.019* | 0.033±0.013*#† | 0.042±0.016* |
2.4. Western blot蛋白印迹法检测HMGB1蛋白表达
与A组(HMGB1/β-actin值为(0.11±0.09)比较,缺血再灌注后B组、C组和D组HMGB1蛋白表达明显增加(HMGB1/β-actin值分别为B组(0.51 ± 0.18)、C组(0.35 ± 0.11)和D组(0.46 ± 0.17)(P < 0.05);但C组HMGB1蛋白表达明显低于B组和D组(P < 0.05);B组和D组间无明显差异(图 3)。
3.
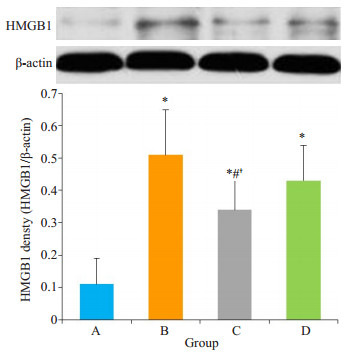
各组大鼠肺组织Western blot检测HMGB1蛋白表达
Detection of HMGB1 protein expression by Western blotting in the lung tissue in each group. Data are presented as Mean±SD. *P < 0.05 vs group A, #P < 0.05 vs group B, †P < 0.05 vs group D.
3. 讨论
本课题研究采用脉冲式LIPUS,其传递给组织的能量小,不会引起组织致热效应,从而避免了以往治疗用超声波致热的不良反应。尽管这种波传递给组织的能量不大,但研究显示,其有利的非致热性细胞生物学效应却不可忽视。体外细胞实验研究发现[11-13],低剂量脉冲式超声波可能具有多种生物学效应,例如能增加蛋白的合成,促使乳突细胞分泌增多,引起成纤维细胞迁移功能的改变,增加第二信使钙离子的摄入以及刺激巨噬细胞产生生长因子等。经过LIPUS预处理,使IRI后肺组织W/D值和LPI均明显下降,同样病理切片也显示其肺组织水肿程度和炎性渗出明显减少,这说明通过LIPUS预处理,能够明显改善IRI后肺组织通透性和炎症渗出,对IRI后肺损伤具有一定的保护作用。
HMGB1是激发和维持IRI后炎性瀑布反应的关键因素[14],越来越多的研究显示,HMGBl作为一种炎性介质,参与了感染性疾病以及多种无菌性炎性疾病(如急性肺损伤、类风湿性关节炎等)的发生和发展过程[15-16],并与疾病的转归和结局有密切联系,因此,HMGBl可能成为一种有效的治疗靶点。在本研究中,IRI后肺组织HMGB1的表达明显增加,通过LIPUS预处理IRI后肺组织HMGB1的表达则明显降低,肺组织通透性和炎症渗出明显减少,这也说明,LIPUS预处理后可以通过抑制IRI后肺组织HMGB1的表达而起到保护作用。
IRI后肺损伤,即使是单侧肺缺血,再灌注后对侧肺也会立即出现同样的生理变化,提示损伤信号可能通过体液分泌[17]。最近的研究已经证明炎症反应同样可以调节先天性免疫反应[18-19],而神经和免疫系统之间的联系目前认为是通过胆碱能抗炎通路来实现的[20-22],其途径为:感染、损伤的组织释放炎症产物发出信号经感觉神经传入孤束核,继而激活迷走传出神经末梢释放乙酰胆碱,作用于炎性细胞表面的α7烟碱型乙酰胆碱受体(α7nAChR),阻碍促炎细胞因子合成和释放,从而抑制局部或全身的炎症反应。而且神经递质乙酰胆碱是目前发现的体内极少数能调控HMGB1释放的生理性物质之一。Wang等[23]研究显示,乙酰胆碱能够作用于α7nAChR,抑制内毒素刺激下巨噬细胞释放的HMGB1。研究已经证实[5-6]以体外超声为基础的治疗方法能防止小鼠肾脏缺血再灌注损伤,这种以治疗方法是通过刺激了脾脏的一种固有的依赖于α7nAChR的胆碱能抗炎通路达到抑制炎症反应,从而减轻组织炎性反应与氧化应激反应,达到保护脏器的目的。肺副交感神经炎症反射弧理论认为迷走神经可以支配到远端气道,甚至肺泡[24-25],支配气道传感器。迷走神经依靠气道传感器或病原体识别受体感知肺内病原体和炎症的变化[26]。感知的信息可以通过迷走神经传入支传递到桥脑的孤束核,整合之后通过肺迷走神经传出支分泌乙酰胆碱,激活肺部炎症细胞α7nAchR,抑制NF-κB活化和下调炎症细胞因子水平,减轻肺部炎症和损伤[27-28],因此切断迷走神经和敲除α7 nAchR可以加重脂多糖或大肠杆菌诱导的急性肺损伤。本研究中,我们观察到,通过LIPUS预处理后IRI肺组织HMGB1的表达则明显降低,IL1和IL6同样明显降低,同时预处理前30 min腹腔注射α7nAChR拮抗剂甲基牛扁亭2 mg/kg后,IRI后肺组织HMGB1的表达明显增加,IL1和IL6也同样明显升高,肺损伤加重。这也同样证实了HMGB1在激发IRI后炎性反应过程中起到关键作用[29],而且LIPUS预处理通过抑制IRI肺组织IL1和IL6的升高以及HMGB1表达依赖于α7nAChR。
因此,本研究证实,LIPUS预处理能够减轻IRI后肺损伤,其机制可能是通过激活依赖α7nAChR的胆碱能抗炎通路,从而降低IRI后肺组织HMGB1的表达。
Biography
曲良超,博士,主治医师,E-mail: 147924554@qq.com
Funding Statement
江西省科技厅重点项目(20171BBG70054);江西省卫计委科技计划(20185111);江西省科技厅自然科学基金(20181BBG78022)
Contributor Information
曲 良超 (Liangchao QU), Email: 147924554@qq.com.
彭 清华 (Qinghua PENG), Email: feeey7@163.com.
References
- 1.Sharma AK, Stone ML, Lau CL, et al. Pulmonary transplantation and ischemia-reperfusion injury. http://d.old.wanfangdata.com.cn/Periodical/zhgdwk201105011. Topics in Thoracic Surgery. 2012;2012:377–401. [Sharma AK, Stone ML, Lau CL, et al. Pulmonary transplantation and ischemia-reperfusion injury[J]. Topics in Thoracic Surgery, 2012, 2012:377-401.] [Google Scholar]
- 2.Zhang J, Wang JS, Zheng ZK, et al. Participation of autophagy in lung ischemia-reperfusion injury in vivo. J Surg Res. 2013;182(2):E79–87. doi: 10.1016/j.jss.2012.11.014. [Zhang J, Wang JS, Zheng ZK, et al. Participation of autophagy in lung ischemia-reperfusion injury in vivo[J]. J Surg Res, 2013, 182(2):E79- 87.] [DOI] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay M. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–49. doi: 10.1056/NEJM200005043421806. [Ware LB, Matthay M. The acute respiratory distress syndrome[J]. N Engl J Med, 2000, 342(18):1334-49.] [DOI] [PubMed] [Google Scholar]
- 4.Bagheri F, Khori V, Alizadeh AM, et al. Reactive oxygen speciesmediated cardiac-reperfusion injury:mechanisms and therapies. Life Sci. 2016;165:43–55. doi: 10.1016/j.lfs.2016.09.013. [Bagheri F, Khori V, Alizadeh AM, et al. Reactive oxygen speciesmediated cardiac-reperfusion injury:mechanisms and therapies[J]. Life Sci, 2016, 165:43-55.] [DOI] [PubMed] [Google Scholar]
- 5.Gigliotti JC, Huang LP, Bajwa A, et al. Ultrasound modulates the splenic neuroimmune axis in attenuating AKI. J Am Soc Nephrol. 2015;26(10):2470–81. doi: 10.1681/ASN.2014080769. [Gigliotti JC, Huang LP, Bajwa A, et al. Ultrasound modulates the splenic neuroimmune axis in attenuating AKI[J]. J Am Soc Nephrol, 2015, 26(10):2470-81.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gigliotti JC, Huang LP, Ye H, et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. JAm Soc Nephrol. 2013;24(9):1451–60. doi: 10.1681/ASN.2013010084. [Gigliotti JC, Huang LP, Ye H, et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway[J]. JAm Soc Nephrol, 2013, 24(9):1451- 60.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schandelmaier S, Kaushal A, Lytvyn L, et al. Low intensity pulsed ultrasound for bone healing:systematic review of randomized controlled trials. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_aa2fc10729ddcb50851b75bdf4cbdc4f. BMJ. 2017;356:j656. doi: 10.1136/bmj.j656. [Schandelmaier S, Kaushal A, Lytvyn L, et al. Low intensity pulsed ultrasound for bone healing:systematic review of randomized controlled trials[J]. BMJ, 2017, 356:j656.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwashina T, Mochida J, Miyazaki T, et al. Low-intensity pulsed ultrasound stimulates cell proliferation and proteoglycan production in rabbit intervertebral disc cells cultured in alginate. Biomaterials. 2006;27(3):354–61. doi: 10.1016/j.biomaterials.2005.06.031. [Iwashina T, Mochida J, Miyazaki T, et al. Low-intensity pulsed ultrasound stimulates cell proliferation and proteoglycan production in rabbit intervertebral disc cells cultured in alginate[J]. Biomaterials, 2006, 27(3):354-61.] [DOI] [PubMed] [Google Scholar]
- 9.Klune JR, Dhupar R, Cardinal J, et al. HMGBI:endogenous danger signaling. http://d.old.wanfangdata.com.cn/Periodical/hnykdx201707005. Mol Med. 2008;14(7-8):476–84. doi: 10.2119/2008-00034.Klune. [Klune JR, Dhupar R, Cardinal J, et al. HMGBI:endogenous danger signaling[J]. Mol Med, 2008, 14(7-8):476-84.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eppinger MJ, Jones ML, Deeb GM, et al. Pattern of injury and the role of neutrophils in reperfusion injury of rat lung. J Surg Res. 1995;58(6):713–8. doi: 10.1006/jsre.1995.1112. [Eppinger MJ, Jones ML, Deeb GM, et al. Pattern of injury and the role of neutrophils in reperfusion injury of rat lung[J]. J Surg Res, 1995, 58(6):713-8.] [DOI] [PubMed] [Google Scholar]
- 11.Korstjens CM, van der Rijt R, Albers G, et al. Low-intensity pulsed ultrasound affects human articular chondrocytes in vitro. Med Biol Eng Comput. 2008;46(12):1263–70. doi: 10.1007/s11517-008-0409-9. [Korstjens CM, van der Rijt R, Albers G, et al. Low-intensity pulsed ultrasound affects human articular chondrocytes in vitro[J]. Med Biol Eng Comput, 2008, 46(12):1263-70.] [DOI] [PubMed] [Google Scholar]
- 12.沈 士浩, 程 凯, 林 强, et al. 低强度脉冲超声波对兔膝骨性关节炎软骨整合素-FAK-MAPKs信号通路蛋白表达的影响. 中国康复医学杂志. 2016;31(2):160–6. doi: 10.3969/j.issn.1001-1242.2016.02.007. [沈士浩, 程凯, 林强, 等.低强度脉冲超声波对兔膝骨性关节炎软骨整合素-FAK-MAPKs信号通路蛋白表达的影响[J].中国康复医学杂志, 2016, 31(2):160-6.] [DOI] [Google Scholar]
- 13.唐 华均, 杨 欢, 范 艺巾, et al. 低强度脉冲超声对环磷酰胺诱导的大鼠卵巢损伤有修复作用. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=2017121632. 南方医科大学学报. 2017;37(12):1632–6. doi: 10.3969/j.issn.1673-4254.2017.12.12. [唐华均, 杨欢, 范艺巾, 等.低强度脉冲超声对环磷酰胺诱导的大鼠卵巢损伤有修复作用[J].南方医科大学学报, 2017, 37(12):1632-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.薛 婷如, 刘 丹彦. 高迁移率族蛋白B1在缺血/再灌注损伤中的研究进展. 医学综述. 2013;19(9):1575–7. doi: 10.3969/j.issn.1006-2084.2013.09.014. [薛婷如, 刘丹彦.高迁移率族蛋白B1在缺血/再灌注损伤中的研究进展[J].医学综述, 2013, 19(9):1575-7.] [DOI] [Google Scholar]
- 15.Yang RK, Zou XP, Tenhunen J, et al. HMGB1 and extracellular histones significantly contribute to systemic inflammation and multiple organ failure in acute liver failure. http://cn.bing.com/academic/profile?id=05946272168cf9903ed6b0ce55508e8b&encoded=0&v=paper_preview&mkt=zh-cn. Mediators Inflamm. 2017;(2017):1–6. doi: 10.1155/2017/5928078. [Yang RK, Zou XP, Tenhunen J, et al. HMGB1 and extracellular histones significantly contribute to systemic inflammation and multiple organ failure in acute liver failure[J]. Mediators Inflamm, 2017(2017):1-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber DJ, Allette YM, Wilkes DS, et al. The HMGB1-RAGE inflammatory pathway:implications for brain injury-induced pulmonary dysfunction. Antioxid Redox Signal. 2015;23(17):1316–28. doi: 10.1089/ars.2015.6299. [Weber DJ, Allette YM, Wilkes DS, et al. The HMGB1-RAGE inflammatory pathway:implications for brain injury-induced pulmonary dysfunction[J]. Antioxid Redox Signal, 2015, 23(17): 1316-28.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palazzo R, Hamvas A, Shuman T, et al. Injury in nonischemic lung after unilateral pulmonary ischemia with reperfusion. J Appl Physiol. 1992;72(2):612–20. doi: 10.1152/jappl.1992.72.2.612. [Palazzo R, Hamvas A, Shuman T, et al. Injury in nonischemic lung after unilateral pulmonary ischemia with reperfusion[J]. J Appl Physiol, 1992, 72(2):612-20.] [DOI] [PubMed] [Google Scholar]
- 18.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209(6):1057–68. doi: 10.1084/jem.20120571. [Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity[J]. J Exp Med, 2012, 209(6):1057-68.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabanyi I, Muller PA, Feighery L, et al. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell. 2016;164(3):378–91. doi: 10.1016/j.cell.2015.12.023. [Gabanyi I, Muller PA, Feighery L, et al. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages[J]. Cell, 2016, 164(3):378-91.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanashiro A, Sonego F, Ferreira RG, et al. Therapeutic potential and limitations of cholinergic anti-inflammatory pathway in sepsis. Pharmacol Res. 2017;117:1–8. doi: 10.1016/j.phrs.2016.12.014. [Kanashiro A, Sonego F, Ferreira RG, et al. Therapeutic potential and limitations of cholinergic anti-inflammatory pathway in sepsis[J]. Pharmacol Res, 2017, 117:1-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millet T, Roger PL Eran N, et al. Role of the α7 nicotinic acetylcholine receptor and RIC-3 in the cholinergic anti-inflammatory pathway. http://cn.bing.com/academic/profile?id=8fd5b8debd210badc5408d0df6b706fe&encoded=0&v=paper_preview&mkt=zh-cn. Cent Nerv SystAgents Med Chem. 2017;17(2):90–9. doi: 10.2174/1871524916666160829114533. [Millet T, Roger PL Eran N, et al. Role of the α7 nicotinic acetylcholine receptor and RIC-3 in the cholinergic anti-inflammatory pathway[J]. Cent Nerv SystAgents Med Chem, 2017, 17(2):90-9.] [DOI] [PubMed] [Google Scholar]
- 22.Reardon C. Neuro-immune interactions in the cholinergic antiinflammatory reflex. Immunol Lett. 2016;178:92–6. doi: 10.1016/j.imlet.2016.08.006. [Reardon C. Neuro-immune interactions in the cholinergic antiinflammatory reflex[J]. Immunol Lett, 2016, 178:92-6.] [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGBl release and improve survival in experimental sepsis. Nat Med. 2004;10(11):1216–21. doi: 10.1038/nm1124. [Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGBl release and improve survival in experimental sepsis[J]. Nat Med, 2004, 10(11):1216-21.] [DOI] [PubMed] [Google Scholar]
- 24.Hertweck MS, Hung KS. Ultrastructural evidence for the innervation of human pulmonary alveoli. Experientia. 1980;36(1):112–3. doi: 10.1007/BF02004006. [Hertweck MS, Hung KS. Ultrastructural evidence for the innervation of human pulmonary alveoli[J]. Experientia, 1980, 36(1):112-3.] [DOI] [PubMed] [Google Scholar]
- 25.曹 腾波. 迷走神经电刺激对肺缺血再灌注损伤的保护作用. http://cdmd.cnki.com.cn/Article/CDMD-10533-2009208162.htm. 上海医学. 2016;(4):229–32. [曹腾波.迷走神经电刺激对肺缺血再灌注损伤的保护作用[J].上海医学, 2016(4):229-32.] [Google Scholar]
- 26.Livermore S, Zhou Y, Pan J, et al. Pulmonary neuroepithelial bodies are polymodal airway sensors:evidence for CO2/H+sensing. Am J Physiol Lung Cell Mol Physiol. 2015;308(8):L807–15. doi: 10.1152/ajplung.00208.2014. [Livermore S, Zhou Y, Pan J, et al. Pulmonary neuroepithelial bodies are polymodal airway sensors:evidence for CO2/H+sensing[J]. Am J Physiol Lung Cell Mol Physiol, 2015, 308(8):L807-15.] [DOI] [PubMed] [Google Scholar]
- 27.Su X, Matthay M, Malik AB. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing gramnegative sepsis-induced acute lung inflammatory injury. J Immunol. 2010;184(1):401–10. doi: 10.4049/jimmunol.0901808. [Su X, Matthay M, Malik AB. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing gramnegative sepsis-induced acute lung inflammatory injury[J]. J Immunol, 2010, 184(1):401-10.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su X, Lee JW, Matthay ZA, et al. Activation of the alpha7nAChR reduces acid-induced acute lung injury in mice and rats. Am J Respir Cell Mol Biol. 2007;37(2):186–92. doi: 10.1165/rcmb.2006-0240OC. [Su X, Lee JW, Matthay ZA, et al. Activation of the alpha7nAChR reduces acid-induced acute lung injury in mice and rats[J]. Am J Respir Cell Mol Biol, 2007, 37(2):186-92.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrassy M, Volz HC, Igwe JC, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117(25):3216–26. doi: 10.1161/CIRCULATIONAHA.108.769331. [Andrassy M, Volz HC, Igwe JC, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart[J]. Circulation, 2008, 117 (25):3216-26.] [DOI] [PubMed] [Google Scholar]


