Abstract
目的
探究尿液细胞外囊泡中足细胞表面标志蛋白podocalyxin(PCX)水平在糖尿病肾病诊断中的应用价值。
方法
收集2017年3月~2017年9月在本院确诊收治的糖尿病患者共57例,其中34例单纯糖尿病患者,23例糖尿病肾病患者,并收集21例其他类型肾病患者,11例健康体检者作为对照。采用透射电镜(TEM)和纳米示踪技术(NTA)验证尿液中细胞外囊泡的分离效果,Western blot法检测分离出的各组细胞外囊泡的分子标记物(TSG101、PCX),ELISA法检测细胞外囊泡中PCX水平。
结果
TEM结果发现尿液中细胞外囊泡含量丰富、形态完整、大小不等,NTA结果发现绝大多数颗粒直径小于300 nm。Western blot法显示4组样本中均可以检测出TSG101表达,且糖尿病肾病患者样本中PCX均呈阳性表达,而在健康体检组、单纯糖尿病患者以及其他肾病患者样本中呈阴性表达。ELISA法显示糖尿病肾病组24 h尿液细胞外囊泡中PCX水平(3.27±2.30 ng/μmol)显著高于健康体检组(1.22±0.36 ng/μmol)、单纯糖尿病组(2.22±1.29 ng/μmol)以及其他肾病组(1.24±0.45 ng/μmol),有统计学意义(P < 0.05)。
结论
糖尿病肾病患者尿液细胞外囊泡中PCX水平明显升高,可以作为临床检测糖尿病肾病的潜在标志物之一,具有重要的临床意义。
Keywords: 尿液细胞外囊泡, 外泌体, podocalyxin, 糖尿病肾病
Abstract
Objective
To explore the value of detecting podocalyxin (PCX) level in urinary extracellular vesicles for the diagnosis of diabetic nephropathy.
Methods
This study was conducted among 57 diabetic patients admitted during the period from March to September, 2017, including 34 with uncomplicated diabetics and 23 with diabetic nephropathy; 21 patients with other types of nephropathy and 11 healthy individuals were also included to serve as the controls. Transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA) were used to verify the separation of urinary extracellular vesicles. The molecular markers of extracellular vesicles (TSG101 and podocalyxin [PCX]) were detected using Western blotting. PCX levels in extracellular vesicles were also detected using ELISA.
Results
TEM reveal the presence of numerous extracellular vesicles in the urine with intact morphology and different sizes, and most of them were below 300 nm in diameter as shown by NTA. TSG101 expression was detected in the samples from all the 4 groups. Positive expression of PCX was detected in the samples from patients with diabetic nephropathy but not in the other groups. In patients with diabetic nephropathy, the mean PCX levels (3.27±2.30 ng/μmol)was significantly higher than those in the healthy control group (1.22±0.36 ng/μmol), uncomplicated diabetes group (2.22±1.29 ng/μmol) and nephropathy group (1.24±0.45 ng/μmol).
Conclusion
PCX level in urinary extracellular vesicles is significantly increased in patients with diabetic nephropathy, suggesting the value of PCX as a potential marker for clinical diagnosis of diabetic nephropathy.
Keywords: urinary extracellular vesicles, exosomes, podocalyxin, diabetic nephropathy
糖尿病现已成为全球最大的健康问题之一,其主要并发症为糖尿病肾病(DN),DN是终末期肾衰竭的主因[1-2]。目前临床上常使用有创伤性的肾活检进行DN的诊断,患者还得经受一定程度的创伤性检测,因此如何有效的减少有创性诊断,开发出无创性且具有临床诊断意义的方法就显得尤为重要。细胞外囊泡是一种纳米级别的微颗粒,在机体内主要是由细胞分泌并带有相应来源细胞生物遗传信息[3],而尿液细胞外囊泡则主要来源于泌尿系统上皮细胞(肾小管细胞、肾小球足细胞等)分泌[4]。目前有研究发现尿液细胞外囊泡可以在病理及生理状态下反应来源细胞的功能情况,并参与了泌尿细胞上皮细胞间的信号传导[5]。已经证实在糖尿病肾病的发生发展过程中足细胞的结构和功能出现了一定程度的损伤,且足细胞的数量和密度均明显降低[6],而podocalyxin(PCX)作为肾小球足细胞的标志蛋白,在足细胞发生病理性损伤时则会出现在尿液中[7]。尿液可重复收集且具有无创性,因此尿液中的足细胞标志物PCX可否作为DN的诊断标志物具有重要的临床诊断意义。本研究采用检测糖尿病肾病患者尿液细胞外囊泡podocalyxin的水平,分析尿液细胞外囊泡podocalyxin在糖尿病肾病临床诊断中的应用价值,现报道如下:
1. 资料和方法
1.1. 资料
选取2017年3月~2017年9月期间在南方医科大学第三附属医院住院的2型糖尿病患者57例(男27例、女30例),年龄(42.53±17.96)岁。按照2014年中华医学会糖尿病学分会微血管并发症学组发布的糖尿病肾病防治专家共识中的诊断标准[8]:出现大量白蛋白尿;糖尿病视网膜病变伴任何一期慢性肾脏病;在10年以上糖尿病病程的糖尿病中出现微量白蛋白尿,将其分为单纯糖尿病组(34例,UAER < 30 mg/24 h)和糖尿病肾病组(23例,UAER≥30 mg/24 h)。另选取已肾穿明确的21例其他肾小球疾病患者,其中5例膜性肾病、2例微小病变性肾病、7例IgA肾病和2例局灶节段性肾小球硬化症,以11例健康体检者作为对照组。所有参与者及家属均签署知情同意书。
1.2. 方法
1.2.1. 透射电镜(TEM)和纳米示踪技术(NTA)
使用液压透析滤过法对收集到的89份24 h尿标本的细胞外囊泡进行分离、富集,将提取的细胞外囊泡进行考马斯亮蓝蛋白定量后备用。在TEM下观察细胞外囊泡的外在形态特征。
根据考马斯蛋白定量结果取10 μg细胞外囊泡,按照1:1000比例用PBS进行稀释,通过NTA法进行细胞外囊泡的粒径和浓度的统计。
1.2.2. Western blot法
在富集的细胞外囊泡样品中加入5×loading buffer,沸水浴5 min,每个样品槽上样量为4 μg蛋白,补齐到40 μL。10% SDS-PAGE凝胶电泳(100V 90 min)分离蛋白。用Bio-Rad转移槽将凝胶中的蛋白条带转移至PVDF膜上,用5%牛奶封闭液封闭3 h。甩去封闭液,TBST洗膜3次,5 min/次;把膜浸入抗体缓冲液中(稀释一抗,1:1000)4 ℃孵育过夜(一抗为家兔抗人Tumor usceptibility Gene101,TSG101和家兔抗人podocalyxin, PCX)。甩去一抗,TBST洗膜3次,10 min/次,后加入1:5000比例稀释的二抗,室温孵育2 h(二抗为山羊抗家兔二抗)。甩去二抗,TBST洗膜4次,15 min/次。加入曝光液A、B(按1:1混合)各1 m L;将膜置于平皿中,取出在滤纸上轻轻滤干,进行定影,并扫描。
1.2.3. ELISA法
取出所需的板条,加入100 μL不同浓度的标准品和待测样品于相应孔中。37 ℃孵育60 min。甩尽孔内液体,每孔加入洗涤液350 μL,静止1~2 min后,甩尽液体,洗5次,每孔加入孵育液A100 μL,37 ℃孵育60 min,甩尽液体,每孔加入洗涤液350 μL,静止1~2 min后,甩尽液体,洗3次。每孔加入孵育液B100 μL,37 ℃孵育30 min,甩尽液体,用上述方法洗5次。每孔加入显色液90 μL,37 ℃孵育15 min,每孔加入终止液50 μL,即刻在酶标仪上450 nm处读数。绘制标准曲线,根据标本A值读出浓度。
1.2.4. 统计分析
本研究采用SPSS 24.0软件进行数据分析,计量资料采用均数±标准差表示,计数资料采用χ2检验,多组间比较采用方差分析,两两比较采用t检验,以α=0.05为检验水准。
2. 结果
2.1. 临床一般资料
对所有纳入研究的对象的一般临床指标进行统计分析,在性别、尿酸和尿量上对比差异没有统计学意义(P > 0.05),而在糖尿病病程、年龄、高血压病例、血清肌酐、尿素氮以及尿蛋白上对比差异有统计学意义(P < 0.05,表 1)。
1.
研究对象一般临床指标
General clinical data of the study subjects (Mean±SD)
| Indicators | Healthy control | Diabetes mellitus | Diabetic nephropathy | Other nephropathy | P |
| -: No data; aP < 0.05 vs healthy control; bP < 0.05 vs diabetes mellitus, compared with diabetic nephropathy; P < 0.05 vs diabetic nephropathy, . | |||||
| Gender (M/F) | 11 (8/3) | 34 (17/17) | 23 (10/13) | 21 (12/9) | 0.322 |
| Duration of diabetes (year) | - | 6.3±1.2 | 8.6±1.4b | 0.5±1.8c | 0.041 |
| Age (year) | 43.91±13.70 | 48.38±13.32 | 55.43±7.28ab | 42.57±13.86 | 0.005 |
| Hypertension cases | 0 | 6 | 14 | 1 | 0.000 |
| Serum creatinine (μmol/L) | 62.55±17.61 | 69.77±15.09 | 131.27±119.38b | 129.19±153.54b | 0.045 |
| Urea nitrogen (mmol/L) | 6.23±0.86 | 5.12±1.33 | 8.06±4.53b | 6.63±3.57 | 0.014 |
| Uric acid (μmol/L) | 397.09±107.5 | 378.11±121.49 | 421.4±96.59 | 441.62±194.04 | 0.434 |
| Urine protein (mg/24 h) | 78.46±16.95 | 85.75±30.4 | 2000.48±2512.98ab | 1996.59±2234.2ab | 0.000 |
| Urine volume (mL) | 1527.5±751.58 | 1873.24±577.99 | 2174.09±881.6 | 2037.14±1005.77 | 0.218 |
2.2. 透射电子显微镜
利用透射电镜观察分离富集到的尿液细胞外囊泡分布情况。可以看到视野内囊泡含量丰富、形态完整、大小不等(图 1)。囊泡分布范围在20~1000 nm,内为均一的低电子密度物质,外周深染的膜结构,包膜完整,一些为具有典型杯状结果的外泌体,也可以看到其他形态各异的囊泡。
1.
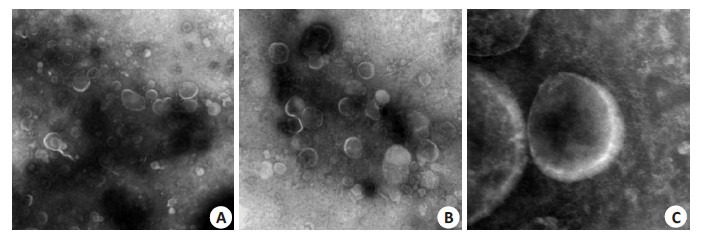
透射电镜放大后尿液细胞外囊泡形态
Morphology of urinary extracellular vesicles under transmission electron microscope (A: Original magnification: ×5000; B: ×10 000; C: ×40 000).
2.3. 纳米颗粒跟踪分析
利用纳米颗粒跟踪分析仪分析纳米颗粒粒径,最大峰值为174.6±10.3 nm,大多数颗粒的直径小于300 nm,符合细胞外囊泡的粒径范围,与透射电镜的结果也吻合(图 2)。
2.
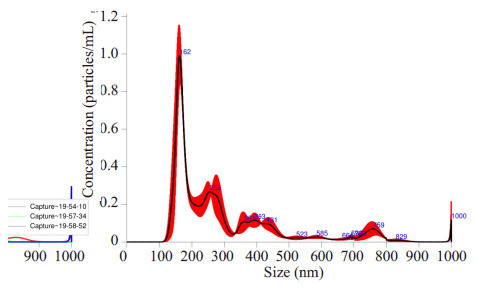
纳米颗粒跟踪分析仪分析
NTAanalysis results Particle Size 174.6±10.3 nm.
2.4. Western blotting
采用Western blotting检测尿液细胞外囊泡的标志蛋白TSG101和足细胞标志蛋白PCX。结果显示糖尿病肾病患者(DN)、糖尿病患者(DM)以及健康体检者(NC)的样本均检出细胞外囊泡的标志蛋白TSG101,而糖尿病肾病患者样本中PCX均阳性表达,健康体检者以及糖尿病患者样本中PCX呈阴性表达(图 3)。
3.
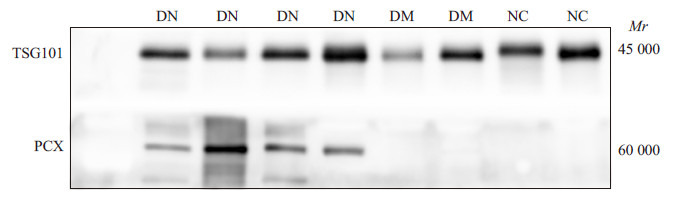
免疫印迹法检测糖尿病肾病、糖尿病以及健康体检者细胞外囊泡TSG101和PCX的表达
Expression of TSG101 and PCX in extracellular vesicular vesicles in DN, DM and NC groups byWestern blotting.
Western blotting结果显示糖尿病肾病(DN)、膜性肾病(MN)、IgA肾病(IgA)以及局灶节段性肾小球硬化症(FSGS)患者的样本中均可以检测出细胞外囊泡的标志蛋白TSG101,而糖尿病肾病患者样本中PCX均阳性表达,其他肾病患者的样本中PCX呈阴性表达(图 4)。
4.

免疫印迹法检测糖尿病肾病和其他肾病患者细胞外囊泡TSG101和PCX的表达
Expression of TSG101 and PCX in extracellular vesicular vesicles in patients with DNand other types of nephropathy detected byWestern blotting.
2.5. ELISA
ELISA实验结果显示糖尿病肾病组24 h尿液细胞外囊泡中PCX水平(3.27±2.30)ng/μmol显著高于健康体检组(1.22±0.36)ng/μmol、单纯糖尿病组(2.22± 1.29)ng/μmol以及其他肾病组(1.24±0.45)ng/μmol,差异有统计学意义(P < 0.05),且单纯糖尿病组的PCX水平也显著高于健康体检组(P < 0.05)。
3. 讨论
糖尿病肾病的早期阶段会发生肾小球基底膜增厚以及系膜基质的扩张,当肾小球的通透性发生改变时会引起足细胞的结构和功能出现障碍,进而出现蛋白尿,这是临床上肾小球滤过屏障出现损伤以及糖尿病肾病的特征性标志之一[9]。PCX是足细胞的特异性标志糖蛋白,带有较高的负电荷。当肾小球发生功能性障碍时会伴有足细胞脱落,并随尿液排出。因此在尿液中对足细胞的碎片分泌物进行检测成为可能。目前已经发现激活或者损伤的细胞能够释放带有生物活性的细胞外囊泡,在囊泡内常包含有来源的特异性生物信息,如蛋白质、mRNA和miRNAs[10]。透射电镜下观察到大鼠肾组织中多种上皮细胞均含有细胞外囊泡[11],细胞外囊泡携带能够反映其分化状态、解剖位置和功能的生物标志物,无论是来源于多囊泡复合体还是直接从细胞膜以出芽方式形成的尿液细胞外囊泡均被认为是反应来源细胞病理生理活动的快照[12]。
本研究通过提取24 h尿液中细胞外囊泡,在透射电镜下观察发现囊泡含量非常丰富,形态完整且大小不等,这与以往报道中细胞外囊泡的形态相吻合[13]。通过纳米示踪技术发现细胞外囊泡粒径,发现绝大多数颗粒的直径小于300 nm,符合细胞外囊泡的粒径范围,这与之前的报道相一致[14]。Aatonen等[15]分析血小板分泌的囊泡,超过90%的囊泡直径低于500 nm,而且在100~ 300 nm范围内的囊泡比例最高。说明本研究中提取富集的尿液细胞外囊泡与绝大多数已经报道的囊泡一致,确保了后续检测的正确性。
PCX正常表达于肾脏足细胞、血管内皮、造血祖细胞及神经元亚群,PCX可以与多种蛋白一起共同形成共免疫复合物,维持着上皮细胞结构的稳定[16]。同时PCX作为足细胞分化成熟的标志蛋白,固定于肾小球足细胞顶膜,参与维持肾小球的正常生理功能[17]。正常肾小球通过电荷屏障和分子屏障阻止血浆白蛋白分子的滤过,而足细胞PCX的表达减少即影响足细胞裂隙隔膜正常结构的维持,也将直接导致肾小球电荷屏障的阴离子分布减少,导致白蛋白尿的产生[18]。研究发现当围生期小鼠在肾脏发育过程出现PCX表达缺失时,会导致小鼠死亡[19]。有学者通过对糖尿病、糖尿病肾病、高血压、肾病综合征等患者和正常人的尿液中的PCX的水平进行分析,发现PCX是诊断肾小球早期损害的一项较为理想的指标[20]。亦有研究发现尿液中的PCX可以提示糖尿病肾病的进展[21]。目前发现,肾小球基底膜出现损伤时尿液中可以检测到PCX水平升高,同时足细胞顶膜区的PCX表达水平也相应升高[22]。研究发现在糖尿病肾病发生的早期阶段,肾小管就已经发生了损伤[23-24]。本研究通过Western blot法发现4组样本中均可以检测出细胞外囊泡标志蛋白-TSG101的表达,且糖尿病肾病患者样本中PCX均呈阳性表达,而在健康体检组、单纯糖尿病患者以及其他肾病患者样本中呈阴性表达。提示在本研究中PCX表达可能是糖尿病肾病患者的临床诊断指标之一。有报道称PCX可以用于微小病变性肾病[25]、局灶节段性肾小球硬化[25]、膜性肾病[26]、IgA肾病[27]、紫癜性肾病[28]、狼疮性肾炎[29]等疾病中足细胞病理改变的检测,这与本研究中发现的在其他肾病患者样本中阴性表达相悖,我们推测是由于尿液中的PCX可来源于脱落的细胞或足细胞碎片或正常凋亡足细胞,故仅仅检测尿液中的PCX水平不能准确体现各种肾病的足细胞损伤情况,而我们检测的是尿液细胞外囊泡中的PCX,会比全尿分析更能反映真实的蛋白变化情况。故本研究中以上疾病尿液细胞外囊泡中PCX的表达有可能确实为阴性,也有可能还未达到可以检测的水平,后期我们将进一步扩大研究样本群,在不同群体中验证该结论。ELISA实验结果发现糖尿病肾病组24 h尿液细胞外囊泡中PCX水平显著高于健康体检组、单纯糖尿病组以及其他肾病组,但在Western blot中的条带中并未能检测到PCX的表达,我们推测可能是由于本研究纳入的研究对象较少,个体间存在一定的差异性引起的,有待我们后期设计实验进一步验证。
综上所述,糖尿病肾病患者尿液细胞外囊泡中PCX水平明显升高,可以作为临床检测糖尿病肾病的潜在标志物之一,具有重要的临床意义。
Biography
吴凡,本科,主治医师,E-mail: 402052712@qq.com
Funding Statement
广东省自然科学基金(2016A030313559);广东省科技厅科技计划项目(2014A020212196);南方医科大学科研启动计划项目(PY2014N094);中国南方智谷引进创新团队和项目(CXTD-04);广州市健康医疗协同创新重大专项(201604020015);欧盟第七框架计划资助项目(UroSense-286386)
Contributor Information
吴 凡 (Fan WU), Email: 402052712@qq.com.
邹 和群 (Hequn ZOU), Email: hequnzou@hotmail.com.
References
- 1.Maruthur NM. The growing prevalence of type 2 diabetes: increased incidence or improved survival? Curr Diab Rep. 2013;13(6):786–94. doi: 10.1007/s11892-013-0426-4. [Maruthur NM. The growing prevalence of type 2 diabetes: increased incidence or improved survival[J]? Curr Diab Rep, 2013, 13(6): 786- 94.] [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Foley RN, Chavers B, et al. US Renal Data System 2013 Annual Data Report. AmJ Kidney Dis. 2014;63(1 Suppl):A7. doi: 10.1053/j.ajkd.2013.11.001. [Collins AJ, Foley RN, Chavers B, et al. US Renal Data System 2013 Annual Data Report[J].AmJ Kidney Dis, 2014, 63(1 Suppl):A7.] [DOI] [PubMed] [Google Scholar]
- 3.Perez A, Loizaga A, Arceo R, et al. A pilot study on the potential of RNA-associated to urinary vesicles as a suitable non-invasive source for diagnostic purposes in bladder cancer. Cancers (Basel) 2014;6(1):179–92. doi: 10.3390/cancers6010179. [Perez A, Loizaga A, Arceo R, et al. A pilot study on the potential of RNA-associated to urinary vesicles as a suitable non-invasive source for diagnostic purposes in bladder cancer[J]. Cancers (Basel), 2014, 6(1): 179-92.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi DS. Urinary extracellular vesicles for biomarker source to monitor polycystic kidney disease. http://www.ncbi.nlm.nih.gov/pubmed/25960355. Proteomics Clin Appl. 2015;9(5/6):447–8. doi: 10.1002/prca.201500053. [Choi DS. Urinary extracellular vesicles for biomarker source to monitor polycystic kidney disease[J]. Proteomics Clin Appl, 2015, 9(5/6): 447-8.] [DOI] [PubMed] [Google Scholar]
- 5.Morrison EE, Bailey MA, Dear JW. Renal extracellular vesicles: from physiology to clinical application. J Physiol. 2016;594(20):5735–48. doi: 10.1113/JP272182. [Morrison EE, Bailey MA, Dear JW. Renal extracellular vesicles: from physiology to clinical application[J]. J Physiol, 2016, 594(20): 5735-48.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hara M, Yamagata K, Tomino Y, et al. Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia. 2012;55(11):2913–9. doi: 10.1007/s00125-012-2661-7. [Hara M, Yamagata K, TominoY, et al. Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: establishment of a highly sensitive ELISA to detect urinary podocalyxin[J]. Diabetologia, 2012, 55(11): 2913-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hara M, Yanagihara T, Kihara I, et al. Apical cell membranes are shed into urine from injured podocytes: a novel phenomenon of podocyte injury. http://d.old.wanfangdata.com.cn/NSTLQK/10.1681-ASN.2004070564/ JAmSoc Nephrol. 2005;16(2):408–16. doi: 10.1681/ASN.2004070564. [Hara M, Yanagihara T, Kihara I, et al. Apical cell membranes are shed into urine from injured podocytes: a novel phenomenon of podocyte injury[J]. JAmSoc Nephrol, 2005, 16(2): 408-16.] [DOI] [PubMed] [Google Scholar]
- 8.胡 仁明. 糖尿病肾病的诊断和防治-中国糖尿病肾病诊断和防治的专家共识解读. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=TNBL201509005&dbname=CJFD&dbcode=CJFQ. 糖尿病天地:临床. 2015;9(9):447–53. [胡仁明.糖尿病肾病的诊断和防治-中国糖尿病肾病诊断和防治的专家共识解读[J].糖尿病天地:临床, 2015, 9(9): 447-53.] [Google Scholar]
- 9.Burger D, Thibodeau JF, Holterman CE, et al. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. http://www.ncbi.nlm.nih.gov/pubmed/24676640. JAmSoc Nephrol. 2014;25(7):1401–7. doi: 10.1681/ASN.2013070763. [Burger D, Thibodeau JF, Holterman CE, et al. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury[J]. JAmSoc Nephrol, 2014, 25(7): 1401-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhayana S, Song F, Jacob J, et al. Urinary miRNAs as biomarkers for noninvasive evaluation of radiation- induced renal tubular injury. http://www.ncbi.nlm.nih.gov/pubmed/28977780. Radiat Res. 2017;188(6):626–35. doi: 10.1667/RR14828.1. [Bhayana S, Song F, Jacob J, et al. Urinary miRNAs as biomarkers for noninvasive evaluation of radiation- induced renal tubular injury[J]. Radiat Res, 2017, 188(6): 626-35.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miranda AM, Lasiecka ZM, Xu Y, et al. Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat Commun. 2018;9(1):291. doi: 10.1038/s41467-017-02533-w. [Miranda AM, Lasiecka ZM, Xu Y, et al. Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures[J]. Nat Commun, 2018, 9(1): 291.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eirin A, Zhu XY, Puranik AS, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92(1):114–24. doi: 10.1016/j.kint.2016.12.023. [Eirin A, Zhu XY, Puranik AS, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation[J]. Kidney Int, 2017, 92(1): 114-24.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woei-A-Jin FJ, Van Der Starre WE, Tesselaar ME, et al. Procoagulant tissue factor activity on microparticles is associated with disease severity and bacteremia in febrile urinary tract infections. Thromb Res. 2014;133(5):799–803. doi: 10.1016/j.thromres.2014.03.007. [Woei-A-Jin FJ, Van Der Starre WE, Tesselaar ME, et al. Procoagulant tissue factor activity on microparticles is associated with disease severity and bacteremia in febrile urinary tract infections[J]. Thromb Res, 2014, 133(5): 799-803.] [DOI] [PubMed] [Google Scholar]
- 14.林 韩翡, 刘 新宇, 徐 小蒙, et al. 健康成人24 h尿液细胞外囊泡定量及大小分布. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=2015111530. 南方医科大学学报. 2015;35(11):1530–4. [林韩翡, 刘新宇, 徐小蒙, 等.健康成人24 h尿液细胞外囊泡定量及大小分布[J].南方医科大学学报, 2015, 35(11): 1530-4+1545.] [PubMed] [Google Scholar]
- 15.Aatonen MT, Ohman T, Nyman TA, et al. Isolation and characterization of platelet-derived extracellular vesicles. http://www.ncbi.nlm.nih.gov/pubmed/25147646. J Extracell Vesicles. 2014;3:10. doi: 10.3402/jev.v3.24692. [Aatonen MT, Ohman T, Nyman TA, et al. Isolation and characterization of platelet-derived extracellular vesicles[J]. J Extracell Vesicles, 2014, 3: 10.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staubach S, Pekmez M, Hanisch FG. Differential proteomics of urinary exovesicles from classical galactosemic patients reveals subclinical kidney insufficiency. J Proteome Res. 2016;15(6):1754–61. doi: 10.1021/acs.jproteome.5b00902. [Staubach S, Pekmez M, Hanisch FG. Differential proteomics of urinary exovesicles from classical galactosemic patients reveals subclinical kidney insufficiency[J]. J Proteome Res, 2016, 15(6): 1754-61.] [DOI] [PubMed] [Google Scholar]
- 17.Wang A, Sankaranarayanan NV, Yanagishita M, et al. Heparin interaction with a receptor on hyperglycemic dividing cells prevents intracellular hyaluronan synthesis and autophagy responses in models of type 1 diabetes. Matrix Biol. 2015;48:36–41. doi: 10.1016/j.matbio.2015.04.003. [Wang A, Sankaranarayanan NV, Yanagishita M, et al. Heparin interaction with a receptor on hyperglycemic dividing cells prevents intracellular hyaluronan synthesis and autophagy responses in models of type 1 diabetes[J]. Matrix Biol, 2015, 48: 36-41.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Echevarria J, Royo F, Pazos R, et al. Microarray-based identification of lectins for the purification of human urinary extracellular vesicles directly from urine samples. Chembiochem. 2014;15(11):1621–6. doi: 10.1002/cbic.201402058. [Echevarria J, Royo F, Pazos R, et al. Microarray-based identification of lectins for the purification of human urinary extracellular vesicles directly from urine samples[J]. Chembiochem, 2014, 15(11): 1621-6.] [DOI] [PubMed] [Google Scholar]
- 19.Gilani SI, Anderson UD, Jayachandran M, et al. Urinary extracellular vesicles of podocyte origin and renal injury in preeclampsia. JAm Soc Nephrol. 2017;28(11):3363–72. doi: 10.1681/ASN.2016111202. [Gilani SI, Anderson UD, Jayachandran M, et al. Urinary extracellular vesicles of podocyte origin and renal injury in preeclampsia[J]. JAm Soc Nephrol, 2017, 28(11): 3363-72.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.司徒 瑞儒, 何 卓雄. 尿液足细胞标志蛋白Podocalyxin在诊断肾脏疾病早期损害中的价值. 中国医药指南. 2011;9(20):92–4. doi: 10.3969/j.issn.1671-8194.2011.20.064. [司徒瑞儒, 何卓雄.尿液足细胞标志蛋白Podocalyxin在诊断肾脏疾病早期损害中的价值[J].中国医药指南, 2011, 9(20): 92-4.] [DOI] [Google Scholar]
- 21.程 亮, 余 伟男, 洪 兵, et al. 尿液足细胞标志蛋白PCX检测在糖尿病肾脏损害程度评估中的意义. http://www.cnki.com.cn/Article/CJFDTOTAL-QKYX201221025.htm. 中国全科医学. 2012;15(7c):2441–3. [程亮, 余伟男, 洪兵, 等.尿液足细胞标志蛋白PCX检测在糖尿病肾脏损害程度评估中的意义[J].中国全科医学, 2012, 15(7c): 2441-3.] [Google Scholar]
- 22.Komers R, Xu B, Schneider J, et al. Effects of xanthine oxidase inhibition with febuxostat on the development of nephropathy in experimental type 2 diabetes. Br J Pharmacol. 2016;173(17):2573–88. doi: 10.1111/bph.v173.17. [Komers R, Xu B, Schneider J, et al. Effects of xanthine oxidase inhibition with febuxostat on the development of nephropathy in experimental type 2 diabetes[J]. Br J Pharmacol, 2016, 173(17): 2573-88.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto Y, Yamagishi S, Mizukami H, et al. Polyol pathway and diabetic nephropathy revisited: Early tubular cell changes and glomerulopathy in diabetic mice overexpressing human aldose reductase. J Diabetes Investig. 2011;2(2):111–22. doi: 10.1111/j.2040-1124.2010.00071.x. [Hashimoto Y, Yamagishi S, Mizukami H, et al. Polyol pathway and diabetic nephropathy revisited: Early tubular cell changes and glomerulopathy in diabetic mice overexpressing human aldose reductase[J]. J Diabetes Investig, 2011, 2(2): 111-22.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen SE, Sugaya T, Tarnow L, et al. Tubular and glomerular injury in diabetes and the impact ofACE inhibition. Diabetes Care. 2009;32(9):1684–8. doi: 10.2337/dc09-0429. [Nielsen SE, Sugaya T, Tarnow L, et al. Tubular and glomerular injury in diabetes and the impact ofACE inhibition[J]. Diabetes Care, 2009, 32(9): 1684-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu B, Li XF, Zhu XL, et al. ELISA analysis of urinary nephrin and podocalyxin standardized by aquaporin-2 in adult patients with nephrotic syndrome. J Nephrol. 2014;27(4):411–7. doi: 10.1007/s40620-014-0066-z. [Zhu B, Li XF, Zhu XL, et al. ELISA analysis of urinary nephrin and podocalyxin standardized by aquaporin-2 in adult patients with nephrotic syndrome[J]. J Nephrol, 2014, 27(4): 411-7.] [DOI] [PubMed] [Google Scholar]
- 26.李 大勇, 曹 海霞, 范 亚平, et al. 膜性肾病肾活检组织中TRPC6和podocalyxin表达改变及其意义. 临床与实验病理学杂志. 2012;28(5):486–9. doi: 10.3969/j.issn.1001-7399.2012.05.003. [李大勇, 曹海霞, 范亚平, 等.膜性肾病肾活检组织中TRPC6和podocalyxin表达改变及其意义[J].临床与实验病理学杂志, 2012, 28(5): 486-9.] [DOI] [Google Scholar]
- 27.Shen P, Shen J, Li W, et al. Urinary podocyte can be an indicator for the pathogenetic condition of patients with IgA nephropathy. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ0233697158. Clin Lab. 2014;60(10):1709–15. doi: 10.7754/clin.lab.2014.131225. [Shen P, Shen J, Li W, et al. Urinary podocyte can be an indicator for the pathogenetic condition of patients with IgA nephropathy[J]. Clin Lab, 2014, 60(10): 1709-15.] [DOI] [PubMed] [Google Scholar]
- 28.Huang D, Wu X, Zheng W, et al. Podocalyxin expression in renal tissues and correlation with the number of urinary podocytes in children with henoch-schonlein purpura nephritis. http://www.ncbi.nlm.nih.gov/pubmed/22561433. Zhong Nan Da Xue Xue BaoYi Xue Ban. 2012;37(2):161–7. doi: 10.3969/j.issn.1672-7347.2012.02.009. [Huang D, Wu X, Zheng W, et al. Podocalyxin expression in renal tissues and correlation with the number of urinary podocytes in children with henoch-schonlein purpura nephritis[J]. Zhong Nan Da Xue Xue BaoYi Xue Ban, 2012, 37(2): 161-7.] [DOI] [PubMed] [Google Scholar]
- 29.Ikuma D, Hiromura K, Kajiyama H, et al. The correlation of urinary podocytes and podocalyxin with histological features of lupus nephritis. Lupus. 2018;27(3):484–93. doi: 10.1177/0961203317734918. [Ikuma D, Hiromura K, Kajiyama H, et al. The correlation of urinary podocytes and podocalyxin with histological features of lupus nephritis[J]. Lupus, 2018, 27(3): 484-93.] [DOI] [PubMed] [Google Scholar]


