Abstract
目的
探讨芦荟苷对人胃癌MKN-28和HGC-27细胞凋亡的诱导作用及可能的分子机制。
方法
胃癌MKN-28和HGC-27细胞用含10%胎牛血清和NEAA(HGC-27细胞)的1640完全培养基常规培养,不同浓度的芦荟苷处理胃癌MKN-28和HGC-27细胞特定的时间,CCK-8实验检测芦荟苷对MKN-28和HGC-27细胞活力的影响;DAPI染色观察凋亡细胞核的形态改变;AnnexinV-FITC/PI双染法检测细胞凋亡率;Western blotting检测凋亡相关蛋白PARP和procaspase-3的表达及MAPKs信号通路p38,ERK及JNK的磷酸化水平;p38,ERK及JNK的特异性抑制剂处理细胞,WB检测抑制剂的对p38,ERK及JNK激活的抑制效果,DAPI染色观察抑制剂对胃癌细胞凋亡的影响。
结果
芦荟苷浓度依赖性地抑制胃癌MKN-28和HGC-27细胞的活力,诱导胃癌细胞凋亡;芦荟苷处理胃癌细胞后,胞内JNK和p38的磷酸化水平明显增加,而ERK的磷酸化水平下降。特异性抑制剂阻断ERK活化,能够增强芦荟苷诱导的细胞凋亡,阻断p38和JNK的激活,能够部分逆转芦荟苷诱发的胃癌细胞凋亡。
结论
芦荟苷通过激活JNK和p38信号途径,抑制ERK信号途径诱导胃癌细胞凋亡。
Keywords: 芦荟苷, 细胞凋亡, HGC-27细胞, MKN-28细胞, MAPKs信号通路
Abstract
Objective
To investigate the effect of aloin on apoptosis of human gastric cancer cells and explore the molecular mechanism.
Methods
Gastric cancer MKN-28 and HGC-27 cells were cultured routinely in 1640 medium supplemented with 10% fetal bovine serum and 10% non-essential amino acids (for HGC-27 cells) and treated with different concentrations of aloin for different durations. The cell viability, cell nuclear morphology, and apoptotic rate of the cells were detected using CCK-8 assay, DAPI staining and AnnexinV-FITC/PI, respectively; Western blotting was used to detect the expression levels of PARP, procaspase 3 and the phosphorylation of p38, ERK and JNK. The cells were treated with specific inhibitors of p38, ERK and JNK, and the inhibitory effects on these pathways were detected with Western blotting; DAPI staining was used to detect the effects of inhibitors on apoptosis of gastric cancer cells.
Results
Aloin dose-dependently inhibited the viability and induced apoptosis of HGC-27 and MKN-28 cells. Alion treatment obvious enhanced the phosphorylation of p38 and JNK but decreased ERK phosphorylation in the cells. Blocking ERK activation with the ERK inhibitor obviously enhanced aloin-induced cell apoptosis, where inhibiting p38 and JNK activation partly reversed alion-induced apoptosis in the cells.
Conclusion
Aloin induces apoptosis of human gastric cancer cells in vitro by activating p38 and JNK signaling pathways and inhibiting ERK signaling pathway.
Keywords: aloin, apoptosis, HGC-27 cells, MKN-28 cells, MAPKs signaling pathway
胃癌的发生率位居世界常见肿瘤的第4位,其死亡率位居全球癌症死亡率的第2位[1-3]。尽管目前临床上用于胃癌治疗的手段较多,包括手术,放疗和化疗等,但是,胃癌的治疗效果仍然不尽如人意[4]。因此,寻找低毒有效的抗肿瘤药物将为胃癌的治疗带来曙光。天然活性产物因其来源广泛,毒性较低的特点引起了科研工作者的关注。目前用于临床癌症治疗的天然活性提取物主要有长春新碱,紫杉醇和喜树碱等[5-6]。此外,国内外亦有很多天然活性产物治疗肿瘤的文献报道,如红景天苷通过抑制PI3K/Akt/mTOR信号途径诱导人克隆癌细胞的凋亡,通过抑制JAK2-STAT3信号途径抑制肾癌细胞的增殖[7-8],二氢杨梅素通过调控p38和ERK信号诱导肝癌细胞凋亡[9]。
芦荟苷是从植物芦荟中提取的天然蒽醌类化合物[10],具有抗炎[11]、解热保肝、抗氧化[12]等功效,临床上主要用于消炎和抗氧化等方面的治疗。近年来,很多研究证明,芦荟苷亦具有抗癌潜力,对肺癌[13]、结肠直肠癌[14]、和乳腺癌[15]的研究发现,芦荟苷能够抑制癌细胞的增殖并诱导其凋亡。然而,芦荟苷在胃癌细胞增殖和凋亡中的作用及内在的分子机制尚不完全明确。
细胞外信号调节蛋白激酶(ERK)、c-Jun氨基末端激酶(JNK)和p38 MAPK,作为丝裂原活化蛋白激酶家族(MAPKs)的3个成员,被证明参与调节细胞的多种生命活动如凋亡、增殖和迁移等[16-17]。已有的研究表明,MAPKs通路的异常激活与多种肿瘤细胞的增殖、凋亡密切相关[18]。因此MAPKs通路可能作为肿瘤治疗的潜在靶点[19]。同样值得注意的是,MAPKs通路的异常激活也与胃癌细胞的异常增殖有关[19],因此,在本研究中,我们的主要目的旨在探究芦荟苷能否通过调控MAPKs信号途径,发挥诱导胃癌细胞凋亡的作用。
本研究以分化程度不同的人胃癌细胞HGC-27(未分化)和MKN-28(中度分化)为研究对象,证明了芦荟苷对胃癌细胞凋亡的诱导作用,并且发现芦荟苷能够通过调控MAPKs信号通路诱导胃癌细胞的凋亡。该研究为胃癌的临床治疗提供了新思路和理论依据,亦为芦荟苷的临床抗肿瘤应用提供了实验支撑。
1. 材料和方法
1.1. 材料
HGC-27和MNK-28细胞(赛库生物);芦荟苷(纯度≥97 %,阿拉丁生化科技股份有限公司);SP600125,SB203580和U0126(selleck);胎牛血清和1640培养基(Gibco);兔单克隆抗体:PARP,pro-caspase 3,p-p38 MAPKs,p38 MAPKs,p-ERK,ERK,p-JNK,JNK,β- actin(CST);DAPI(Sigma);AnnexinⅤ-FITC/PI双染试剂盒及CCK-8试剂盒(凯基);细胞裂解液(碧云天)。流式细胞仪(BD)。
1.2. 细胞培养
MKN-28细胞用含10%胎牛血清,100 U/mL青霉素,100 μg/mL链霉素的1640培养基,HGC-27细胞用含10%胎牛血清,10%NEAA,100 U/mL青霉素,100 μg/mL链霉素的1640培养基,置于37 ℃,5% CO2的细胞培养箱中培养。实验用细胞均为对数生长期。
1.3. CCK-8实验检测细胞活力
将MKN-28和HGC-27细胞提前接种于96孔细胞培养板,2×104/孔,细胞贴壁后,去除培养液,分别设置空白组,对照组和芦荟苷浓度梯度处理组(100, 200, 400 μg/mL),每组3个复孔。培养24 h后弃掉培液,每孔加入含CCK-8试剂10 μL的1640完全培养基100 μL。在二氧化碳培养箱中继续培养4 h,用全波段酶标仪(Thermo)在450 nm波长处检测各组细胞的吸光度(A),数据以均数±标准差表示。细胞存活率=(A实验组-A调零孔)(/ A空白对照组-A调零孔)×100%。
1.4. DAPI染色检测细胞核形态的改变
胃癌细胞HGC-27和MKN-28接种于12孔细胞培养板,次日用不同浓度的芦荟苷(100, 200, 400 μg/mL)分别刺激细胞24 h,同时设置空白对照组。弃去细胞培养基,PBS洗涤细胞1次,4%多聚甲醛室温固定细胞20 min,PBS洗涤细胞后,加入DAPI室温避光染色3 min,PBS洗涤后于荧光倒置显微镜下观察细胞核形态并拍照(Olympus)。
1.5. 流式细胞仪检测细胞凋亡率
将MKN-28和HGC-27细胞提前接种于12孔细胞培养板,密度为2×105/孔。细胞处理同1.4,处理后的胃癌细胞用PBS洗涤1次,然后胰酶消化。离心收集细胞后,PBS洗涤2次,每组细胞加入500 μL bind buffer重悬细胞,制备成单细胞悬液,然后分别加入AnnexinⅤ和PI各5μL,避光染色,最后流式细胞仪检测各组细胞凋亡率。
1.6. Western blotting检测蛋白表达
胃癌细胞处理同1.4,各组细胞处理后,弃去细胞培养基,预冷的PBS洗涤1次,加入含蛋白酶抑制剂的细胞裂解液冰上裂解细胞30 min,期间不断摇晃细胞培养板,使其充分裂解。收集细胞裂解液,12 500 r/min,4 ℃离心10 min,收集上清,加入2×loading buffer混匀,煮沸10 min。蛋白定量后,取等量蛋白样品上样进行SDS-PAGE(12%)。电泳后的蛋白样品转移至硝酸纤维素膜上(PALL,美国),5%脱脂奶粉室温封闭1 h,TBST洗涤3次后,加入相应一抗4 ℃摇床孵育过夜。TBST洗膜3次,加入荧光二抗室温避光孵育2 h,最后用LI-COR双色红外激光成像系统扫膜,并用image J分析实验结果。
1.7. 统计分析实验结果
实验数据均以均数±标准差表示,Graph Pad Prism6软件分析实验结果,统计分析采用单因素方差分析,P < 0.05表示差异具有统计学意义。
2. 结果
2.1. 芦荟苷抑制胃癌细胞的存活
芦荟苷浓度在100~400 μg/mL范围内,能够显著抑制细胞的存活,其抑制效应表现出浓度依赖性(图 1)。根据该实验结果,选取100、200、400 μg/mL的芦荟苷用于后续实验研究。
1.
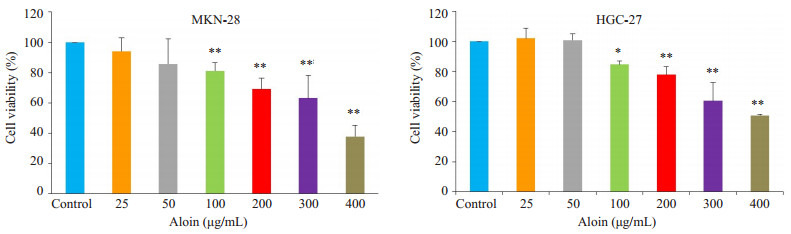
芦荟苷对MKN-28和HGC-27细胞存活率的影响
Effects of aloin on viability of MKN-28 and HGC-27 cells (Mean±SD, n=3), *P < 0.05, **P < 0.01 vs control.
2.2. 芦荟苷诱导胃癌细胞凋亡
空白对照组细胞核形态正常(椭圆形,大小均一,染色均匀蓝染);不同浓度的芦荟苷处理24 h后,细胞核均出现了不同程度的凋亡表现,如细胞核大小不一,核浓缩,核碎裂,染色增强,表现为高亮蓝色,且凋亡程度呈现出明显的浓度依赖性(图 2)。流式细胞仪检测细胞凋亡率发现,对照组MKN-28和HGC-27细胞的凋亡率相对较低,分别为11.92%和2.86%。然而不同浓度芦荟苷处理的MKN-28和HGC-27细胞,其凋亡率明显升高,MKN-28细胞的凋亡率分别为16.42%,24.41%和31.06%。HGC-27细胞的凋亡率为5.37%,7.58%和30.59%,凋亡程度呈现出一定的浓度依赖性(图 3)。WB检测凋亡相关蛋白同样显示,芦荟苷作用于两种胃癌细胞24 h后,PARP切割显著增多,pro-caspase3的表达明显降低(图 4)。
2.
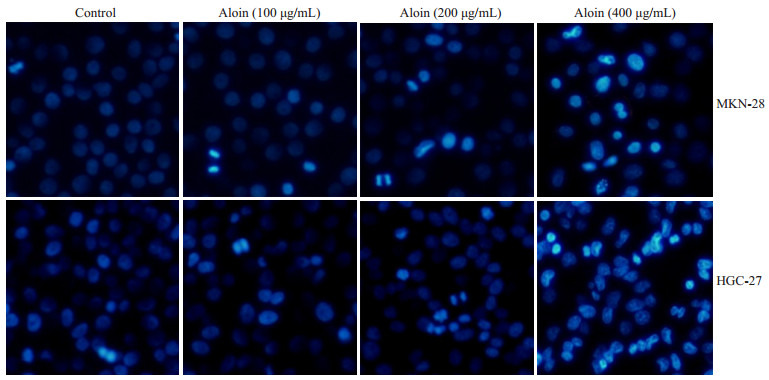
芦荟苷对MKN-28和HGC-27细胞核形态的影响
Effects of aloin on nuclear morphology of MKN-28 and HGC-27 cells (Original magnification: ×100).
3.
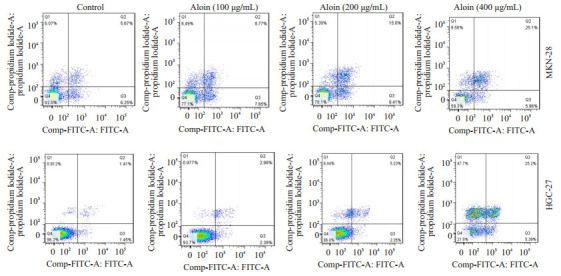
芦荟苷对MKN-28和HGC-27细胞凋亡率的影响
Effects of aloin on apoptotic rate of MKN-28 and HGC-27 cells.
4.
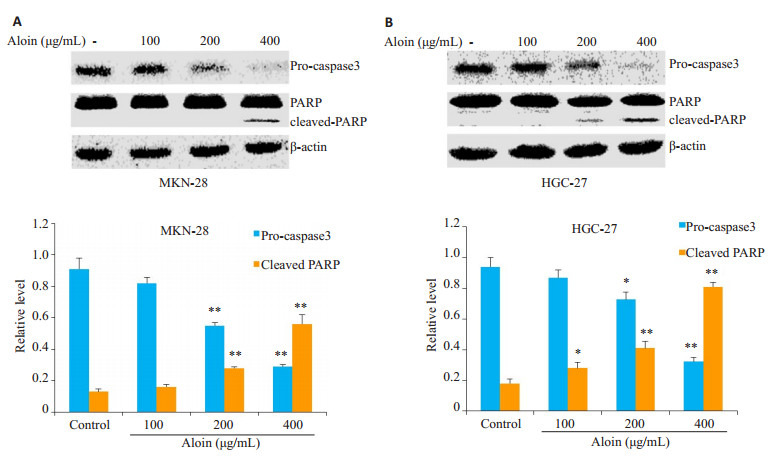
芦荟苷对MKN-28和HGC-27细胞凋亡相关蛋白的影响
Effects of aloin on expression of apoptosis-related proteins in MKN-28 cells (A) and HGC-27 cells (B). *P < 0.05, **P < 0.01 vs control.
2.3. 芦荟苷对MAPKs信号通路的影响
芦荟苷处理组与对照组相比,两种细胞内p-ERK的相对表达水平明显下降,然而p-p38和p-JNK的相对表达水平却显著升高,变化程度亦呈现出浓度依赖性(图 5)。
5.
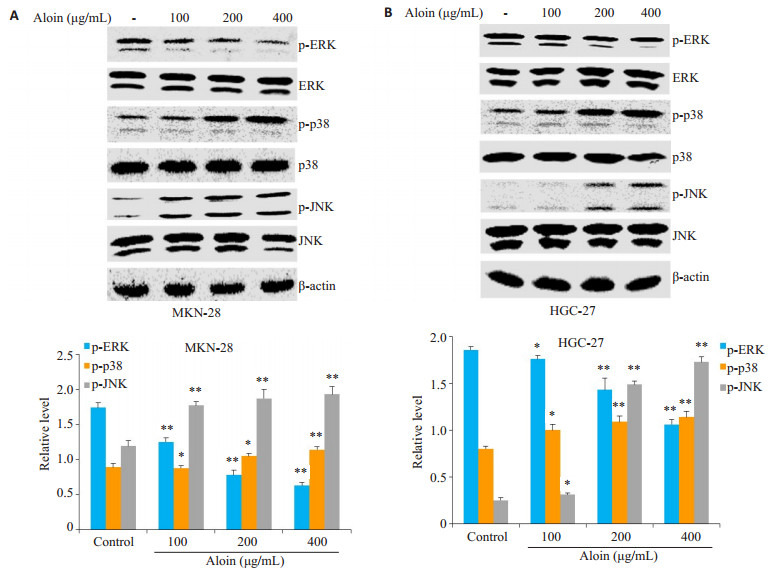
芦荟苷对MKN-28和HGC-27细胞中MAPKs信号通路的影响
Effects of aloin on activation of MAPKs signaling pathway in MKN-28 cells (A) and HGC-27 cells (B).*P < 0.05, **P < 0.01 vs control.
2.4. 抑制MAPKs信号途径对芦荟苷诱导胃癌细胞凋亡的影响
胃癌细胞分别用p38, JNK和ERK的特异性抑制剂SB203580,SP600125和U0126单独处理细胞1 h或抑制剂预处理细胞1 h,然后用芦荟苷刺激24 h。结果表明,SB203580和SP600125能够减弱芦荟苷对p38,JNK磷酸化的激活,U0126则在一定程度上增强了芦荟苷对ERK磷酸化的抑制作用(图 6)。抑制ERK信号的激活,能够一定程度上增强芦荟苷诱导的胃癌细胞凋亡;而p38和JNK的信号途径的抑制,部分逆转了芦荟苷引发的细胞凋亡(图 7)。
6.
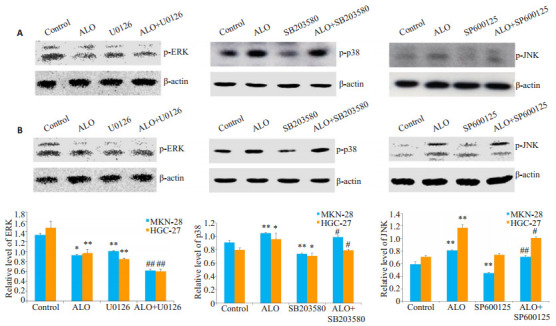
特异性抑制剂对p38, JNK和ERK信号途径活化的影响
Effects of specific inhibitors on the activation of MAPKs signaling pathway in MKN-28 cells (A) and HGC-27 cells (B). *P < 0.05, **P < 0.01 vs control; #P < 0.05, ##P < 0.01 vs ALO. ALO: Aloin.
7.
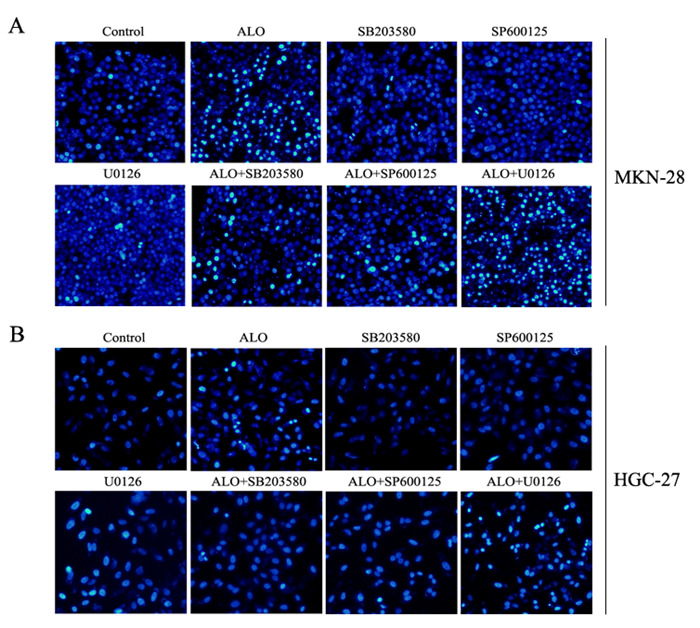
MAPKs信号途径抑制剂对芦荟苷诱导胃癌细胞凋亡的影响
Effects of specific inhibitor of MAPKs signaling pathway on aloin-induced apoptosis in gastric cancer cells.
3. 讨论
芦荟苷在临床主要用于抗炎和抗氧化等方面的治疗,近年来亦有芦荟苷抗肿瘤作用的报道[20-21]。已有的研究发现,不同浓度的芦荟苷处理胃癌细胞后,DAPI染色结果显示,在两种分化程度不同的胃癌细胞中,芦荟苷均能不同程度的诱导细胞核呈现出典型的凋亡形态改变,如核大小不一,核浓缩及核碎裂现象。流式细胞仪分析胃癌细胞的凋亡率发现,芦荟苷作用HGC-27和MKN-28细胞后,胃癌细胞的凋亡率均有不同程度的增加。PARP和caspase3被认为是与细胞凋亡密切相关的蛋白质,凋亡细胞中,PARP的切割明显增强,而pro-caspase3的表达亦因切割而下降[9, 22]。我们的WB结果也证明,芦荟苷能够明显诱导PARP切割,减低pro-caspase3的表达,尤其以400 μg/mL芦荟苷处理组变化最为显著。研究表明,JNK和p38的激活主要发挥凋亡诱导作用[23]。相反,ERK的激活在细胞增殖和分化中扮演着重要角色,被认为是细胞存活调节子[24]。而且,亦有天然活性产物通过调控MAPKs信号通路发挥抗肿瘤作用的文献报道[25-26]。使用特异性抑制剂U0126,SP600125和SB203580分别抑制ERK,JNK和P38的活化,也证明MAPKs信号通路参与了肿瘤细胞凋亡的调控过程[27]。在本研究中,我们也发现,芦荟苷处理后,MKN-28和HGC-27细胞中ERK的磷酸化水平被明显抑制,而p38和JNK的磷酸化水平则显著增强,且三者的磷酸化水平改变均与芦荟苷的用药浓度正相关。为了证明MAPKs信号途径确实参与了芦荟苷对胃癌细胞凋亡的影响,我们用p38,JNK和ERK的抑制剂阻断MAPKs信号途径的活化,DAPI染色实验检测胃癌细胞凋亡的变化。结果表明,阻断p38和JNK的磷酸化,能够部分逆转芦荟苷诱导的胃癌细胞凋亡;ERK信号途径阻断,对芦荟苷诱导的胃癌细胞凋亡呈现出一定的增强作用。总之,本研究显示,芦荟苷通过抑制ERK,诱导p38和JNK信号途径的激活,促进胃癌细胞的凋亡。
肿瘤微环境中,反应活性氧(ROS)和炎症因子的积累,能够诱导癌细胞的增殖和转移等恶性表型,说明氧化应激和炎症因子在肿瘤的发展中发挥着极其重要的作用[28-29]。我们已有的实验结果也证明,在RAW264.7细胞中,芦荟苷能够抑制LPS诱导的ROS产生,进而调控其介导的JAK-STATs信号途径,抑制LPS诱导的炎症因子释放(文章已接收)。在胃癌细胞中,芦荟苷能否影响胞内ROS的含量,通过调控ROS介导的MAPKs信号分子发挥诱导胃癌细胞凋亡的作用呢?芦荟苷能否通过影响MAPKs信号通路或其它信号途径,调控胃癌细胞中炎症因子的释放,进而影响胃癌细胞的增殖和转移呢?以上问题将是我们下一步工作的重心。
综上所述,我们的研究表明,芦荟苷可以通过调控MAPKs信号途径诱导MKN-28和HGC-27胃癌细胞的凋亡。该研究将为芦荟苷在胃癌临床治疗中的应用提供实验依据。然而,芦荟苷是否可以作为一种有效的辅助手段用于胃癌患者的治疗还有待更加深入的研究。
Biography
王子谦,本科,E-mail: 798664036@qq.com
Funding Statement
国家自然科学基金(81601380,31301171);安徽高校自然科学研究项目重大项目(KJ2016SD59);安徽省优秀青年人才支持计划重点项目(gxyqZD2016173);皖南医学院校重点科研项目培育基金(WK2015Z01);皖南医学院博士启动基金(WK2014RC05);2017年大学生创新创业训练计划项目(201710368002,201710368166);2016年皖南医学院大学生科研资助金项目(WK2016S24)
Supported by National Natural Science Foundation of China (81601380, 31301171)
Contributor Information
王 子谦 (Ziqian WANG), Email: 798664036@qq.com.
栗 家平 (Jiaping LI), Email: lijiaping1979@sina.com.
戚 之琳 (Zhilin QI), Email: 422627721@qq.com.
References
- 1.Zhang L, Xu Z, Xu X, et al. SALL4, a novel marker for human gastric carcinogenesis and metastasis. Oncogene. 2014;33(48):5491–500. doi: 10.1038/onc.2013.495. [Zhang L, Xu Z, Xu X, et al. SALL4, a novel marker for human gastric carcinogenesis and metastasis[J]. Oncogene, 2014, 33(48):5491-500.] [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Chen X, Du Z, Li G, Chen M, Liang G, Chen T. Curcumin suppresses gastric tumor cell growth via ROS-mediated DNA polymerase gamma depletion disrupting cellular bioenergetics. J Exp Clin Cancer Res. 2017;36(1):47. doi: 10.1186/s13046-017-0513-5. [Wang L, Chen X, Du Z, Li G, Chen M, Liang G, Chen T. Curcumin suppresses gastric tumor cell growth via ROS-mediated DNA polymerase gamma depletion disrupting cellular bioenergetics[J]. J Exp Clin Cancer Res, 2017, 36(1):47.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–77. doi: 10.1007/978-1-60327-492-0. [Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer[J]. Methods Mol Biol, 2009, 472:467-77.] [DOI] [PubMed] [Google Scholar]
- 4.Van Hagen P, Hulshof M, Van Lanschot J, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84. doi: 10.1056/NEJMoa1112088. [Van Hagen P, Hulshof M, Van Lanschot J, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer[J]. N Engl J Med, 2012, 366(22):2074-84.] [DOI] [PubMed] [Google Scholar]
- 5.Sun KX, Xia HW, Xia RL. Anticancer effect of salidroside on colon cancer through inhibiting JAK2/STAT3 signaling pathway. http://cn.bing.com/academic/profile?id=5c9d4a4c2e24b83ff0716e2d64facf23&encoded=0&v=paper_preview&mkt=zh-cn. Int J Clin Exp Pathol. 2015;8(1):615–21. [Sun KX, Xia HW, Xia RL. Anticancer effect of salidroside on colon cancer through inhibiting JAK2/STAT3 signaling pathway[J]. Int J Clin Exp Pathol, 2015, 8(1):615-21.] [PMC free article] [PubMed] [Google Scholar]
- 6.Paterson I, Anderson EA. The Renaissance of natural products as drug candidates. Science. 2005;310(5747):451–3. doi: 10.1126/science.1116364. [Paterson I, Anderson EA. The Renaissance of natural products as drug candidates[J]. Science, 2005, 310(5747):451-3.] [DOI] [PubMed] [Google Scholar]
- 7.Fan XJ, Wang Y, Wang L, et al. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol Rep. 2016;36(6):3559–67. doi: 10.3892/or.2016.5138. [Fan XJ, Wang Y, Wang L, et al. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway[J]. Oncol Rep, 2016, 36(6):3559-67.] [DOI] [PubMed] [Google Scholar]
- 8.Lv C, Huang Y, Liu ZX, et al. Salidroside reduces renal cell carcinoma proliferation by inhibiting JAK2/STAT3 signaling. Cancer Biomark. 2016;17(1):41–7. doi: 10.3233/CBM-160615. [Lv C, Huang Y, Liu ZX, et al. Salidroside reduces renal cell carcinoma proliferation by inhibiting JAK2/STAT3 signaling[J]. Cancer Biomark, 2016, 17(1):41-7.] [DOI] [PubMed] [Google Scholar]
- 9.Qi S, Kou X, Lv J, et al. Yan L. Ampelopsin induces apoptosis in HepG2 human hepatoma cell line through extrinsic and intrinsic pathways:Involvement of P38 and ERK. http://cn.bing.com/academic/profile?id=9cae3776f1e59960b145cd163e7d8412&encoded=0&v=paper_preview&mkt=zh-cn. Environ Toxicol Pharmacol. 2015;40(3):874–54. doi: 10.1016/j.etap.2015.09.015. [Qi S, Kou X, Lv J, et al. Yan L. Ampelopsin induces apoptosis in HepG2 human hepatoma cell line through extrinsic and intrinsic pathways:Involvement of P38 and ERK[J]. Environ Toxicol Pharmacol, 2015, 40(3):847-54.] [DOI] [PubMed] [Google Scholar]
- 10.Harlev E, Nevo E, Lansky EP, et al. Anticancer potential of aloes: antioxidant, antiproliferative, and immunostimulatory attributes. Planta Med. 2012;78(9):843–52. doi: 10.1055/s-0031-1298453. [Harlev E, Nevo E, Lansky EP, et al. Anticancer potential of aloes: antioxidant, antiproliferative, and immunostimulatory attributes[J]. Planta Med, 2012, 78(9):843-52.] [DOI] [PubMed] [Google Scholar]
- 11.Park MY, Kwon HJ, Aloin SD, et al. Or aloe-gel exerts antiinflammatory activity in a rat colitis model. Life Sci. 2011;88(11/ 12):486–92. doi: 10.1016/j.lfs.2011.01.010. [Park MY, Kwon HJ, Aloin SD, et al. Or aloe-gel exerts antiinflammatory activity in a rat colitis model[J]. Life Sci, 2011, 88(11/ 12):486-92.] [DOI] [PubMed] [Google Scholar]
- 12.Cui Y, Ye Q, Wang H, et al. Aloin protects against chronic alcoholic liver injury via attenuating lipid accumulation, oxidative stress and inflammation in mice. Arch Pharm Res. 2014;37(12):1624–33. doi: 10.1007/s12272-014-0370-0. [Cui Y, Ye Q, Wang H, et al. Aloin protects against chronic alcoholic liver injury via attenuating lipid accumulation, oxidative stress and inflammation in mice[J]. Arch Pharm Res, 2014, 37(12):1624-33.] [DOI] [PubMed] [Google Scholar]
- 13.Wan L, Zhang L, Fan K, et al. Aloin promotes A549 cell apoptosis via the reactive Oxygen species-mitogen activated protein kinase signaling pathway and p53 phosphorylation. Mol Med Rep. 2017;16(5):5759–68. doi: 10.3892/mmr.2017.7379. [Wan L, Zhang L, Fan K, et al. Aloin promotes A549 cell apoptosis via the reactive Oxygen species-mitogen activated protein kinase signaling pathway and p53 phosphorylation[J]. Mol Med Rep, 2017, 16(5):5759-68.] [DOI] [PubMed] [Google Scholar]
- 14.Pan Q, Pan H, Lou H, et al. Inhibition of the angiogenesis and growth of Aloin in human colorectal cancer in vitro and in vivo. Cancer Cell Int. 2013;13(1):69. doi: 10.1186/1475-2867-13-69. [Pan Q, Pan H, Lou H, et al. Inhibition of the angiogenesis and growth of Aloin in human colorectal cancer in vitro and in vivo[J]. Cancer Cell Int, 2013, 13(1):69.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esmat AY, Tomasetto C, Rio MC. Cytotoxicity of a natural anthraquinone (Aloin) against human breast cancer cell lines with and without ErbB-2:topoisomerase Ⅱalpha coamplification. https://www.researchgate.net/publication/7411811_Cytotoxicity_of_a_natural_anthraquinone_Aloin_against_human_breast_cancer_cell_lines_with_and_without_ErbB-2-topoisomerase_IIa_coamplification. Cancer Biol Ther. 2006;5(1):97–103. doi: 10.4161/cbt.5.1.2347. [Esmat AY, Tomasetto C, Rio MC. Cytotoxicity of a natural anthraquinone (Aloin) against human breast cancer cell lines with and without ErbB-2:topoisomerase Ⅱalpha coamplification[J]. Cancer Biol Ther, 2006, 5(1):97-103.] [DOI] [PubMed] [Google Scholar]
- 16.Tao L, Zhang CY, Guo L, et al. MicroRNA-497 accelerates apoptosis while inhibiting proliferation, migration, and invasion through negative regulation of the MAPK/ERK signaling pathway via RAF-1. J Cell Physiol. 2018;233(10):6578–88. doi: 10.1002/jcp.v233.10. [Tao L, Zhang CY, Guo L, et al. MicroRNA-497 accelerates apoptosis while inhibiting proliferation, migration, and invasion through negative regulation of the MAPK/ERK signaling pathway via RAF-1 [J]. J Cell Physiol, 2018, 233(10):6578-88.] [DOI] [PubMed] [Google Scholar]
- 17.Baek SH, Kim C, Lee JH, et al. Cinobufagin exerts anti-proliferative and pro-apoptotic effects through the modulation ROS-mediated MAPKs signaling pathway. Immunopharmacol Immunotoxicol. 2015;37(3):265–73. doi: 10.3109/08923973.2015.1027916. [Baek SH, Kim C, Lee JH, et al. Cinobufagin exerts anti-proliferative and pro-apoptotic effects through the modulation ROS-mediated MAPKs signaling pathway[J]. Immunopharmacol Immunotoxicol, 2015, 37(3):265-73.] [DOI] [PubMed] [Google Scholar]
- 18.Downward J. Targeting ras signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22. doi: 10.1038/nrc969. [Downward J. Targeting ras signalling pathways in cancer therapy[J]. Nat Rev Cancer, 2003, 3(1):11-22.] [DOI] [PubMed] [Google Scholar]
- 19.Sebolt-Leopold JS. Advances in the development of cancer therapeutics directed against the RAS-mitogen-activated protein kinase pathway. Clin Cancer Res. 2008;14(12):3651–6. doi: 10.1158/1078-0432.CCR-08-0333. [Sebolt-Leopold JS. Advances in the development of cancer therapeutics directed against the RAS-mitogen-activated protein kinase pathway[J]. Clin Cancer Res, 2008, 14(12):3651-6.] [DOI] [PubMed] [Google Scholar]
- 20.Esmat AY, El-Gerzawy SM, Rafaat A. DNA ploidy and S phase fraction of breast and ovarian tumor cells treated with a natural anthracycline analog (aloin) http://cn.bing.com/academic/profile?id=3535dd5402350b004a28c6eaa048e062&encoded=0&v=paper_preview&mkt=zh-cn. Cancer Biol Ther. 2005;4(1):108–12. doi: 10.4161/cbt.4.1.1445. [Esmat AY, El-Gerzawy SM, Rafaat A. DNA ploidy and S phase fraction of breast and ovarian tumor cells treated with a natural anthracycline analog (aloin)[J]. Cancer Biol Ther, 2005, 4(1):108- 12.] [DOI] [PubMed] [Google Scholar]
- 21.Nićiforović A, Adzić M, Spasić SD, et al. Antitumor effects of a natural anthracycline analog (Aloin) involve altered activity of antioxidant enzymes in HeLaS3 cellsb. https://www.researchgate.net/publication/6114707_Antitumor_effects_of_a_natural_anthracycline_analog_Aloin_involve_altered_activity_of_antioxidant_enzymes_in_HeLaS3_cells. Cancer Biol Ther. 2007;6(8):1200–5. doi: 10.4161/cbt.6.8.4383. [Nićiforović A, Adzić M, Spasić SD, et al. Antitumor effects of a natural anthracycline analog (Aloin) involve altered activity of antioxidant enzymes in HeLaS3 cellsb[J]. Cancer Biol Ther, 2007, 6 (8):1200-5.] [DOI] [PubMed] [Google Scholar]
- 22.戚 之琳, 刘 银华, 齐 世美, et al. 红景天苷通过抑制NOX2-ROS-MAPKs信号途径保护H2O2诱导的PC12细胞凋亡. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=201702178. 南方医科大学学报. 2017;37(2):178–83. doi: 10.3969/j.issn.1673-4254.2017.02.06. [戚之琳, 刘银华, 齐世美, 等.红景天苷通过抑制NOX2-ROS-MAPKs信号途径保护H2O2诱导的PC12细胞凋亡[J].南方医科大学学报, 2017, 37(2):178-83.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhillon AS, Hagan S, Rath O, et al. MAP kinase signalingpathways in cancer. Oncogene. 2007;26(22):3279. doi: 10.1038/sj.onc.1210421. [Dhillon AS, Hagan S, Rath O, et al. MAP kinase signalingpathways in cancer[J]. Oncogene, 2007, 26(22):3279.] [DOI] [PubMed] [Google Scholar]
- 24.Zhuang SG, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase. J Pharmacol Exp Ther. 2006;319(3):991–7. doi: 10.1124/jpet.106.107367. [Zhuang SG, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase[J]. J Pharmacol Exp Ther, 2006, 319 (3):991-7.] [DOI] [PubMed] [Google Scholar]
- 25.Cao J, Tong C, Liu Y, et al. Ginkgetin inhibits growth of breast carcinoma via regulating MAPKs pathway. Biomed Pharmacother. 2017;96:450–8. doi: 10.1016/j.biopha.2017.09.077. [Cao J, Tong C, Liu Y, et al. Ginkgetin inhibits growth of breast carcinoma via regulating MAPKs pathway[J]. Biomed Pharmacother, 2017, 96:450-8.] [DOI] [PubMed] [Google Scholar]
- 26.Cui Y, Lu P, Song G, et al. Involvement of PI3K/Akt, ERK and p38 signaling pathways in emodin-mediated extrinsic and intrinsic human hepatoblastoma cell apoptosis. Food Chem Toxicol. 2016;92:26–37. doi: 10.1016/j.fct.2016.03.013. [Cui Y, Lu P, Song G, et al. Involvement of PI3K/Akt, ERK and p38 signaling pathways in emodin-mediated extrinsic and intrinsic human hepatoblastoma cell apoptosis[J]. Food Chem Toxicol, 2016, 92:26-37.] [DOI] [PubMed] [Google Scholar]
- 27.Ahmad Hidayat AF, Chan CK, Mohamad J, Abdul Kadir H. Dioscoreabulbifera induced apoptosis through inhibition of ERK 1/2 and activation of JNK signaling pathways in HCT116 human colorectal carcinoma cells. Biomed Pharmacother. 2018;104:806–16. doi: 10.1016/j.biopha.2018.05.073. [Ahmad Hidayat AF, Chan CK, Mohamad J, Abdul Kadir H. Dioscoreabulbifera induced apoptosis through inhibition of ERK 1/2 and activation of JNK signaling pathways in HCT116 human colorectal carcinoma cells[J]. Biomed Pharmacother, 2018, 104:806-16.] [DOI] [PubMed] [Google Scholar]
- 28.Abdul Rahim SN, Ho GY, Coward JI. The role of interleukin-6 in malignant mesothelioma. http://cn.bing.com/academic/profile?id=7930164c872e6bf598d11695f127bec4&encoded=0&v=paper_preview&mkt=zh-cn. Transll Lung Cancer Res. 2015;4(1):55–66. doi: 10.3978/j.issn.2218-6751.2014.07.01. [Abdul Rahim SN, Ho GY, Coward JI. The role of interleukin-6 in malignant mesothelioma[J]. Transll Lung Cancer Res, 2015, 4(1):55- 66.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benedetti S, Nuvoli B, Catalani S, et al. Reactive oxygen species a double-edged sword for mesothelioma. http://cn.bing.com/academic/profile?id=3bdaaa517fd6e5e3a63c5b47749500c4&encoded=0&v=paper_preview&mkt=zh-cn. Oncotarget. 2015;6(19):16848–65. doi: 10.18632/oncotarget.4253. [Benedetti S, Nuvoli B, Catalani S, et al. Reactive oxygen species a double-edged sword for mesothelioma[J]. Oncotarget, 2015, 6(19): 16848-65.] [DOI] [PMC free article] [PubMed] [Google Scholar]


