Abstract
目的
调查南方医院就诊的慢性阻塞性肺疾病(慢阻肺)患者从出现症状至确诊的时间(错失早期诊断时间),探讨其与疾病严重程度的关系。
方法
筛选我院2015年5月~2018年2月临床首次确诊慢阻肺患者,慢阻肺和哮喘慢阻肺重叠(ACO)诊断基于GOLD指南和欧洲标准,按照GLOD指南进行肺功能分级。
结果
入组慢阻肺患者803例,平均年龄61.8±9.9岁,男性726例,女性77例,入组对象平均错失早期诊断时间为3(0.5,8)年;根据GOLD标准,诊断为慢阻肺中度及以上占比85.2%,重度及以上占比为48.3%,其中47.0%为支气管舒张试验阳性,进一步诊断ACO为295例,占36.7%。ACO错失早期诊断时间为3(1,9)年,相比慢阻肺3(0.5,8)年,两者无显著差异(P>0.05);所有入组对象错失早期诊断时间与肺功能显著相关(P<0.05);多元线性回归分析显示:年龄、错失早期诊断时间与疾病严重程度相关。
结论
首诊慢阻肺错失早期诊断时间与疾病严重程度密切相关,临床需加强早期识别。
Keywords: 慢阻肺, 错失早期诊断时间, 严重程度, 慢阻肺和哮喘慢阻肺重叠
Abstract
Objective
To investigate the association of the time of initial diagnosis with the severity of chronic obstructive pulmonary disease (COPD).
Methods
A total of 803 patients who were diagnosed to have COPD for the first time in our hospital between May 2015 to February 2018 were enrolled in this study.The diagnoses of COPD and asthma COPD overlap (ACO) were made according GOLD guidelines and european consensus definition.Lung function of the patients was graded according to the GOLD guidelines.
Results
The patients with COPD had a mean age of 61.8±9.9 years,including 726 male and 77 female patients.The course of the patients (defined as the time from symptom onset to the establishment of a diagnosis) was 3(0.5,8) years.Among these patients,85.2% had a moderate disease severity (FEV1%<80%),and 48.3% had severe or very severe conditions (FEV1%<50%);47.0% of them were positive for bronchial dilation test.In the overall patients,295(36.7%) were also diagnosed to have ACO,and the mean disease course of ACO[3(1,9) years]was similar to that of COPD[3(0.5,8) years](P>0.05).A significant correlation was found between the disease course and the lung function of the patients.Multiple linear regression analysis showed that an older age and a longer disease course were associated with poorer lung functions and a greater disease severity.
Conclusion
The delay of the initial diagnosis is significantly related to the severity of COPD.
Keywords: chronic obstructive pulmonary disease, disease course, severity, asthma chronic obstructive pulmonary disease overlap
慢性阻塞性肺疾病(慢阻肺,COPD)严重危害公众健康。《柳叶刀》杂志发布的2015年慢阻肺全球疾病负担数据显示:1.745亿人罹患慢阻肺,320万人死于慢阻肺,占全球疾病负担的2.6%[1]。而近期我国学者完成的大规模人群研究显示,我国20岁及以上成人的慢阻肺患病率为8.6%,40岁以上则达13.7%,60岁以上人群患病率已超过27%,全国总患病人数约9990万,慢阻肺在中国成年人中非常普遍,已构成我国重大疾病负担[2]。2006年以来全球慢阻肺防治创议(GOLD)指南均强调慢阻肺可防可治,应早诊断早治疗,但慢阻肺早期诊断在临床上的实施非常困难。
临床慢阻肺诊断常依赖于患者慢性咳嗽、咳痰,胸闷及呼吸困难等症状,进一步采用肺功能检查确诊。2014年一项来自英国初级医疗中心回顾性研究共纳入38 859例患者数据,结果发现85%的慢阻肺患者在慢阻肺确诊前5年错失了早期诊断的机会,提示慢阻肺早期诊断是临床面临的重要挑战[3]。2007年我国7个省市随机入选20 245例40岁以上居民的一项研究表明,在所有被确诊慢阻肺的患者中,仅有35.1%以往曾被确诊为肺气肿、哮喘、支气管炎、慢阻肺,表明我国慢阻肺漏诊情况非常普遍[4]。慢阻肺早期诊断仍是临床的热点问题。经纤支镜行光学相干成像技术发现,肺功能正常的重度吸烟患者已经存在显著的小气道重塑[5];一方面,轻度慢阻肺患者第1秒用力呼气容积(FEV1)的下降率明显高于高危人群[6]。另一方面,早期慢阻肺患者FEV1下降较中晚期更为迅猛,在慢阻肺早期进行干预可能会获益更大[7-8]。早期慢阻肺症状隐匿,漏诊率高,同时肺功能下降较其他阶段更快速,需要引起临床重视。
目前我国尚无慢阻肺首次确诊前错失早期诊断时间的研究报道。因此本研究重点验证慢阻肺确诊前错失早期诊断时间是否可以反映慢阻肺的严重程度,探讨与疾病严重程度相关的临床危险因素,从而进一步探索建立有利于慢阻肺早期确诊的方法。
1. 对象和方法
1.1. 研究对象
南方医院2015年5月~2018年2月符合以下入选/排除标准的临床首次确诊慢阻肺患者。
1.2. 疾病诊断及严重程度标准
慢阻肺诊断标准:(1)有或无呼吸困难、慢性咳嗽、咳痰症状;(2)有吸烟、生物燃料、粉尘或其它风险因素暴露史;(3)肺功能提示存在明显的气流受限(舒张后FEV1/FVC<0.7)。
哮喘-慢阻肺重叠(ACO)诊断基于欧洲标准[9]:主要标准:(1)年龄>40岁,持续的气流受限;(2)10年以上的吸烟史或同等的空气污染暴露;(3)患者40岁之前曾患哮喘或吸入支气管舒张剂后第1秒用力呼气容积(FEV1)增加量>400 mL。次要标准:(1)过敏史或过敏性鼻炎病史;(2)随访过程中肺功能2次及以上FEV1可较基线增加≥200 mL,同时改善率≥12%;(3)外周血嗜酸粒细胞≥0.3×109/L。满足3条主要标准和至少1条次要标准即可诊断。疾病严重程度按GOLD指南分为:轻度、中度、重度、极重度。
病例排除标准:既往已确诊的慢性呼吸道疾病,如肺癌、肺转移瘤、间质性肺纤维化,支气管扩张症及重大心血管疾患,近1月明显的呼吸道感染。
1.3. 研究设计及错失早期诊断时间定义
本研究为南方医科大学南方医院单中心、回顾性研究。收集满足入选标准的患者的人口学特征;记录错失早期诊断时间,即从出现症状至确诊的时间;进行肺功能检查,收集FEV1、FEV1占预计值的百分比(FEV1%)、用力肺活量(FVC)等数据;记录呼出气一氧化氮(FENO)、胸片检查等结果。
1.4. 统计学方法
应用SPSS 19.0统计软件进行数据分析。计量资料以均数±标准差描述,独立样本比较采用t检验。不服从正态分布的计量资料以中位数(四分位法),即中位数(25%,75%)描述,采用非参数检验(Mann-Whitney U检验、Kruskal-Wallis检验),多个样本比较采用方差分析;疾病严重程度影响因素采用多元线性回归分析,P<0.05表示差异有统计学意义。
2. 结果
2.1. 患者一般资料
入选慢阻肺患者803例,男女分别为726例和77例,平均年龄61.77±9.86岁。根据GOLD标准,诊断慢阻肺中度及以上占比85.2%,重度以上占比为48.3%,其中47.0%为支气管舒张试验阳性。进一步诊断ACO为295例,占36.7%(表1)。
1.
单纯慢阻肺与ACO患者的流行病学资料
Epidemiological data of patients with COPD and ACO
| Index | COPD | ACO | Total |
| Number | 508 | 295 | 803 |
| Male(%) | 484(95.2%) | 242(82.0%) | 726(90.4%) |
| Age(Mean±SD, year) | 62.76±9.56 | 60.07±10.15 | 61.77±9.86 |
| Smoking history(pack-years) | 30(20, 40) | 30(10, 38) | 30(20, 40) |
| FENO(ppb) | 16(11, 22) | 32(19, 51) | 20(13, 32) |
| FEV1(L) | 1.42±0.69 | 1.40±0.63 | 1.41±0.67 |
| FVC(L) | 2.81±0.84 | 2.81±0.88 | 2.81±0.85 |
| FEV1/FVC(%) | 49.85±14.43 | 50.22±13.63 | 49.99±14.13 |
| FEV1%(%) | 53.76±22.05 | 53.14±19.94 | 53.53±21.29 |
| Mild | 78 | 41 | 119 |
| Moderate | 182 | 114 | 296 |
| Severe | 159 | 111 | 270 |
| Very severe | 89 | 29 | 118 |
| The disease course of initially diagnosed | 3(0.5, 8) | 3(1, 9) | 3(0.5, 8) |
2.2. 单纯慢阻肺与慢阻肺来源ACO与疾病严重程度关系
本研究纳入单纯慢阻肺患者508例,慢阻肺来源ACO 295例,其中ACO错失早期诊断时间为3(1,9)年,相比单纯慢阻肺3(0.5,8)年,两者无明显差异(Z=-1.86,P=0.063)。单纯慢阻肺肺功能FEV1%为53.8%± 22.1%,ACO为53.1%±19.9%,两组无统计学差异(t=0.376,P=0.707)。
2.3. 性别与疾病严重程度关系
所有纳入对象男女性别比例为726:77,女性错失早期诊断时间为3(1.5,10)年,男性为3(0.5,8)年,两组无统计学差异(Z=-1.13,P=0.258)。其中女性的肺功能FEV1%为51.0%±17.7%,男性为53.8%±21.7%,组间没有统计学差异(t=1.21,P=0.231)。
2.4. 年龄与疾病严重程度关系
所有纳入对象平均年龄为(61.6±10.0)年。按年龄分组,分为<50岁、50~60岁、60~70岁、≥70岁,其肺功能指标FEV1%为54.6%±22.7%、57.3%±22.7%、51.8%± 21.5%、51.7%±17.4%,组间有统计学差异(F=3.04,P=0.028)。
2.5. 错失早期诊断时间与疾病严重程度关系
所有纳入对象错失早期诊断时间为3(0.5,8)年。 按错失早期诊断时间分组,分为<3月、3月~1年、1~5年、5~10年、≥10年,其肺功能指标FEV1%分别为64.8%± 18.1%、63.8%±21.6%、53.5%±20.5%、49.3%±22.6%、46.7%± 20.6%,组间具有统计学差异(F=15.36,P<0.001,图1)。
1.
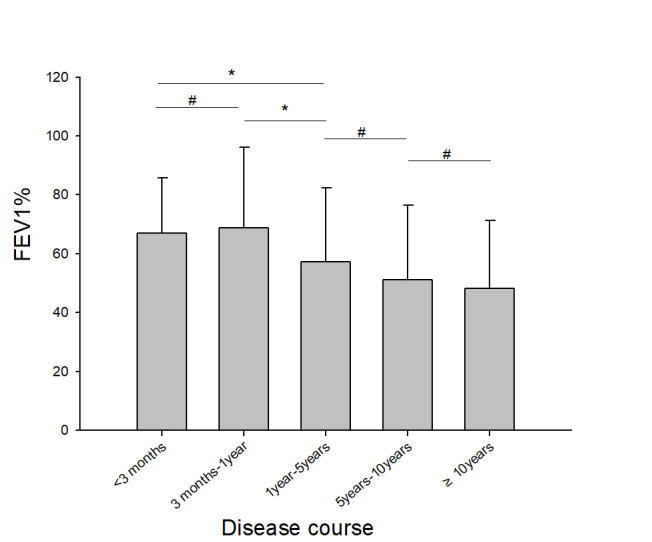
错失早期诊断时间与疾病严重程度关系
Relationship between the disease course and the disease severity *P<0.05, #P>0.05.
2.6. 疾病严重程度的多因素分析
以FEV1%为因变量,把年龄、性别、吸烟指数、是否合并哮喘、错失早期诊断时间作为自变量纳入多元线性回归分析,结果显示,性别、吸烟指数及是否合并哮喘对FEV1%无显著影响(P>0.05),而年龄、错失早期诊断时间对FEV1%有显著影响(P<0.05,表2)。
2.
疾病严重程度FEV1%的影响因素
Factors affecting FEV1% of the patients
| Parameter | Regression coefficient | SE | t | P |
| Constant | 87.166 | 11.114 | 7.843 | < 0.001 |
| Gender | 0.614 | 5.614 | 0.109 | 0.913 |
| Age | -0.286 | 0.133 | -2.161 | 0.031 |
| Smoking history | -0.007 | 0.084 | -0.077 | 0.938 |
| COPD orACO | -1.521 | 2.736 | -0.556 | 0.579 |
| Disease course of initially diagnosed | -4.540 | 0.876 | -5.181 | < 0.001 |
3. 讨论
本研究显示我院慢阻肺首次确诊重度及以上比例约为50%,慢阻肺平均错失早期诊断时间中位数为3年。慢阻肺患者年龄、错失早期诊断时间与疾病严重程度相关,年龄越大,错失早期诊断时间越长,疾病越严重。
慢阻肺早期诊断是近年大家关注的热点问题,在轻度气流受限的慢阻肺患者中,其运动耐量、扩散能力和气体交换可能已经受到损害[10-13],研究表明,通过戒烟或药物等方式早期干预慢阻肺甚至可逆转肺功能下降[14-15],然而临床发现,很多慢阻肺患者诊断时已为重度肺功能障碍[4]。我院慢阻肺首次确诊重度及以上占比约50%,提示慢阻肺早期诊断仍然是其管理的难点。
本研究主要根据病人症状出现至确诊时间(也可理解为病程)来定义错失早期诊断时间,强调的是病人的症状可能对患者就医和医生诊断产生影响,症状反复出现增加就医的意愿和医生进一步肺功能检查的可能性,进而影响慢阻肺的诊断。这与文献报道[4]错失早期诊断概念并不完全一致,后者强调慢阻肺病人在确诊前至少经过一次医疗机构评估,更突出基层医疗机构对慢阻肺筛选的作用,但因为诊疗条件限制,漏诊率相对较高。相对而言,本研究更突出病人的早期诊治意识,也可以反映三甲医院慢阻肺早期诊断的现状。本研究提示病人越早地重视自身疾病并到正规医院就诊,更能早期诊断慢阻肺,这也支持慢阻肺早期诊断与病人症状感知密切相关[16-18]。
本研究显示,单纯慢阻肺与ACO错失早期诊断时间都是3年,两者无明显差异。既往研究显示,ACO患者较单纯慢阻肺患者症状多且重,肺功能更差,急性加重及住院次数增加[19-22]。本研究提示,ACO病人与单纯慢阻肺相比,其错失早期诊断时间并未减少,这似与ACO病人具有更多的症状相矛盾。因为症状多就医机会增加,根据病史,医生判断慢阻肺可能性增加。实际上ACO病人可能因为症状反复,更易耐受,更不易引起病人足够重视,误认为是机体衰老的表现,其确切原因尚不清楚。
本研究显示,在我院首次确诊慢阻肺的患者中女性约占10%,男性FEV1%为53.8%,女性更低为51.0%,虽然两者错失早期诊断时间无明显差异,但女性慢阻肺仍然需引起重视。既往研究显示,基于肺功能判断的相同严重度的慢阻肺患者,女性患者吸烟指数低于男性,咳嗽程度与男性基本相同,较少有咳痰,但更易表现出呼吸困难[4];此外,女性还表现更为严重的精神抑郁[23]。尽管如此,女性患者更易错过早期诊断机会,一个解释是医生可能在女性上更少怀疑慢阻肺[3]。既往认为慢阻肺在男性更为常见,当一个女性患者因咳嗽或呼吸困难前来就诊时,医生往往更多会考虑哮喘或其它疾病。我国近期流行病学调查显示[2],40岁以上女性患病率高达7.5%,而室内被动吸烟及室内生物燃料的使用是女性慢阻肺患者的重要危险因素,女性慢阻肺的早期诊断同样值得重视。
GOLD指南推荐对于有呼吸道症状和危险因素接触史的人群进行肺功能检查确诊慢阻肺,但不推荐对无危险因素接触史和无症状的人群进行肺功能筛查,主要是基于在症状出现前诊断慢阻肺并没有影响疾病病程和患者预后[24]。然而,这一推荐可能存在争议。随访发现,对于未确诊的慢阻肺患者,不管有无呼吸道症状,其急性加重和罹患肺炎的风险都增加[25]。而我国研究显示慢阻肺知晓率及肺功能检查普及率极低,特别需要引起关注的是,60%的慢阻肺患者没有明显的咳嗽、咳痰、喘息等症状[2],对无症状或症状不明显的慢阻肺难以实现早期诊断,说明普及肺功能检查对实现慢阻肺早诊早治的重要性。
来自国外基层医疗机构研究发现,采用性别、年龄、吸烟包年、呼吸困难、咳痰、咳嗽等预测因素构建的简单评分或加权评分量表,有助于发现慢阻肺高危人群,进一步行肺功能检查有助于早期慢阻肺的诊断[26-31]。而我国研究显示,采用由年龄、吸烟包年、体重指数、咳嗽、呼吸困难、呼吸系统疾病家族史、烹调生物燃料烟雾等7个项目组成的COPD筛查问卷(COPD-SQ),对COPD Ⅱ期患者进行筛查,当截断值为16分时,COPD诊断的特异性为85.2%,但敏感性不高(60.6%)[32]。因此,对于长期接触烟雾、生物燃料或其他有害气体、粉尘者;幼年反复呼吸道感染者;出生时低体重或营养不良者;有COPD家族史者,建议常规进行肺功能筛选。
总之,本研究提示慢阻肺错失早期诊断仍然相当严重,慢阻肺错失早期诊断时间与疾病严重度相关,但是否会对慢阻肺长期预后产生影响尚不清楚。加强慢阻肺早期识别,特别是对无症状患者及高危人群进行慢阻肺筛查显得非常重要和必要。
Biographies
彭显如, 在读硕士研究生, E-mail:872651713@qq.com
黄敏於, 在读硕士研究生, E-mail:568368425@qq.com
Funding Statement
国家自然科学基金(81770033, 81470228, 81670026, 81700034);广东省自然科学基金(2015A030313236);广东省科技计划项目(2016A020215117, 2017B020226006);广州市科技计划项目(201804010069);国家重点研发计划(2016YFC0905800)
Supported by National Natural Science Foundation of China (81770033, 81470228, 81670026, 81700034)
Contributor Information
彭 显如 (Xianru PENG), Email: 872651713@qq.com.
黄 敏於 (Minyu HUANG), Email: 568368425@qq.com.
赵 海金 (Haijin ZHAO), Email: haijin99@sina.com.
References
- 1.Theo Vos, Christine A, et al. Prevalence, disability-adjusted Life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015:a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1545–602. doi: 10.1016/S0140-6736(16)31678-6. [Theo Vos, Christine A, et al.Prevalence, disability-adjusted Life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015:a systematic analysis for the global burden of disease study 2015[J].Lancet, 2016, 388(10053):1545-602.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health[CPH]study):a National cross-sectional study. http://www.sciencedirect.com/science/article/pii/S0140673618308419. Lancet. 2018;391(1131):1706–17. doi: 10.1016/S0140-6736(18)30841-9. [Wang C, Xu J, Yang L, et al.Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health[CPH]study):a National cross-sectional study[J].Lancet, 2018, 391(1131):1706-17.] [DOI] [PubMed] [Google Scholar]
- 3.Jones RC, Price D, Ryan D, et al. Opportunities to diagnose chronic obstructive pulmonary disease in routine care in the UK:a retrospective study of a clinical cohort. Lancet Respir Med. 2014;2(4):267–76. doi: 10.1016/S2213-2600(14)70008-6. [Jones RC, Price D, Ryan D, et al.Opportunities to diagnose chronic obstructive pulmonary disease in routine care in the UK:a retrospective study of a clinical cohort[J].Lancet Respir Med, 2014, 2(4):267-76.] [DOI] [PubMed] [Google Scholar]
- 4.Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China:a large, population-based survey. Am J Respir Crit Care Med. 2007;176(8):753–60. doi: 10.1164/rccm.200612-1749OC. [Zhong N, Wang C, Yao W, et al.Prevalence of chronic obstructive pulmonary disease in China:a large, population-based survey[J].Am J Respir Crit Care Med, 2007, 176(8):753-60.] [DOI] [PubMed] [Google Scholar]
- 5.Ding M, Chen Y, Guan WJ, et al. Measuring airway remodeling in patients with different COPD staging using endobronchial optical coherence tomography. Chest. 2016;150(6):1281–90. doi: 10.1016/j.chest.2016.07.033. [Ding M, Chen Y, Guan WJ, et al.Measuring airway remodeling in patients with different COPD staging using endobronchial optical coherence tomography[J].Chest, 2016, 150(6):1281-90.] [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Wang C, Li B, et al. Risk factors for FEV1 decline in mild COPD and high-risk populations. Int J Chron Obstruct Pulmon Dis. 2017;12:435–42. doi: 10.2147/COPD. [Chen S, Wang C, Li B, et al.Risk factors for FEV1 decline in mild COPD and high-risk populations[J].Int J Chron Obstruct Pulmon Dis, 2017, 12:435-42.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decramer M, Celli B, Kesten S, et al. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT):a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374(9696):1171–8. doi: 10.1016/S0140-6736(09)61298-8. [Decramer M, Celli B, Kesten S, et al.Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT):a prespecified subgroup analysis of a randomised controlled trial[J].Lancet, 2009, 374(9696):1171-8.] [DOI] [PubMed] [Google Scholar]
- 8.Jenkins CR, Jones PW, Calverley PM, et al. Efficacy of salmeterol/fluticasone propionate by Gold stage of chronic obstructive pulmonary disease:analysis from the randomised, placebocontrolled TORCH study. Respir Res. 2009;10:59. doi: 10.1186/1465-9921-10-59. [Jenkins CR, Jones PW, Calverley PM, et al.Efficacy of salmeterol/fluticasone propionate by Gold stage of chronic obstructive pulmonary disease:analysis from the randomised, placebocontrolled TORCH study[J].Respir Res, 2009, 10:59.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome?Towards a consensus definition from a round table discussion. Eur Respir. 2016;48:664–73. doi: 10.1183/13993003.00436-2016. [Sin DD, Miravitlles M, Mannino DM, et al.What is asthma-COPD overlap syndrome?Towards a consensus definition from a round table discussion[J].Eur Respir, 2016, 48:664-73.] [DOI] [PubMed] [Google Scholar]
- 10.Rossi A, Butorac-Petanjek B, Chilosi M, et al. Chronic obstructive pulmonary disease with mild airflow limitation:current knowledge and proposal for future research-a consensus document from six scientific societies. Int J Chron Obstruct Pulmon Dis. 2017;12:2593–610. doi: 10.2147/COPD. [Rossi A, Butorac-Petanjek B, Chilosi M, et al.Chronic obstructive pulmonary disease with mild airflow limitation:current knowledge and proposal for future research-a consensus document from six scientific societies[J].Int J Chron Obstruct Pulmon Dis, 2017, 12:2593-610.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'donnell DE, Laveneziana P, Webb K, et al. Chronic obstructive pulmonary disease:clinical integrative physiology. Clin Chest Med. 2014;35(1):51–69. doi: 10.1016/j.ccm.2013.09.008. [O'donnell DE, Laveneziana P, Webb K, et al.Chronic obstructive pulmonary disease:clinical integrative physiology[J].Clin Chest Med, 2014, 35(1):51-69.] [DOI] [PubMed] [Google Scholar]
- 12.Elbehairy AF, Guenette JA, Faisal A, et al. Mechanisms of exertional dyspnoea in symptomatic smokers without COPD. Eur Respir J. 2016;48(3):694–705. doi: 10.1183/13993003.00077-2016. [Elbehairy AF, Guenette JA, Faisal A, et al.Mechanisms of exertional dyspnoea in symptomatic smokers without COPD[J].Eur Respir J, 2016, 48(3):694-705.] [DOI] [PubMed] [Google Scholar]
- 13.Watz H, Pitta F, Rochester CL, et al. An official european respiratory society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–37. doi: 10.1183/09031936.00046814. [Watz H, Pitta F, Rochester CL, et al.An official european respiratory society statement on physical activity in COPD[J].Eur Respir J, 2014, 44(6):1521-37.] [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Zhong N, Li XC, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377(10):923–35. doi: 10.1056/NEJMoa1700228. [Zhou Y, Zhong N, Li XC, et al.Tiotropium in early-stage chronic obstructive pulmonary disease[J].N Engl J Med, 2017, 377(10):923-35.] [DOI] [PubMed] [Google Scholar]
- 15.Pezzuto A, Stellato M, Catania G, et al. Short-term benefit of smoking cessation along with glycopirronium on lung function and respiratory symptoms in mild COPD patients:a retrospective study. J Breath Res. 2018;12(4):046007. doi: 10.1088/1752-7163/aad0a8. [Pezzuto A, Stellato M, Catania G, et al.Short-term benefit of smoking cessation along with glycopirronium on lung function and respiratory symptoms in mild COPD patients:a retrospective study[J].J Breath Res, 2018, 12(4):046007.] [DOI] [PubMed] [Google Scholar]
- 16.Weel C. Underdiagnosis of asthma and COPD:is the general practitioner to blame? http://europepmc.org/abstract/MED/12174705. Monaldi Arch Chest Dis. 2002;57(1):65–8. [Weel C.Underdiagnosis of asthma and COPD:is the general practitioner to blame?[J]Monaldi Arch Chest Dis, 2002, 57(1):65-8.] [PubMed] [Google Scholar]
- 17.Arne M, Emtner M, Janson S, et al. COPD patients perspectives at the time of diagnosis:a qualitative study. Prim Care Respir J. 2007;16(4):215–21. doi: 10.3132/pcrj.2007.00033. [Arne M, Emtner M, Janson S, et al.COPD patients perspectives at the time of diagnosis:a qualitative study[J].Prim Care Respir J, 2007, 16(4):215-21.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enocson A, Jolly K, Jordan RE, et al. Case-finding for COPD in primary care:a qualitative study of patients'perspectives. Int J Chron Obstruct Pulmon Dis. 2018;13:1623–32. doi: 10.2147/COPD. [Enocson A, Jolly K, Jordan RE, et al.Case-finding for COPD in primary care:a qualitative study of patients'perspectives[J].Int J Chron Obstruct Pulmon Dis, 2018, 13:1623-32.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menezes AM, Montes de Oca M, Pérez-Padilla R, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype:COPD-asthma. Chest. 2014;145(2):297–304. doi: 10.1378/chest.13-0622. [Menezes AM, Montes de Oca M, Pérez-Padilla R, et al.Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype:COPD-asthma[J].Chest, 2014, 145(2):297-304.] [DOI] [PubMed] [Google Scholar]
- 20.Nielsen M, Barnes CB, Ulrik C. clinical characteristics of the asthma-COPD overlap syndrome--a systematic review. Int J Chron Obstruct Pulmon Dis. 2015:1443–54. doi: 10.2147/COPD. [Nielsen M, Barnes CB, Ulrik C.clinical characteristics of the asthma-COPD overlap syndrome--a systematic review[J].Int J Chron Obstruct Pulmon Dis, 2015:1443-54.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai JW, Mao B, Yang WL, et al. Asthma-COPD overlap syndrome showed more exacerbations however lower mortality than COPD. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8d05f82a72da0a7db0df659950bef144. QJM. 2017;110(7):431–6. doi: 10.1093/qjmed/hcx005. [Bai JW, Mao B, Yang WL, et al.Asthma-COPD overlap syndrome showed more exacerbations however lower mortality than COPD[J].QJM, 2017, 110(7):431-6.] [DOI] [PubMed] [Google Scholar]
- 22.Mindus S, Malinovschi A, Ekerljung L, et al. Asthma and COPD overlap (ACO) is related to a high burden of sleep disturbance and respiratory symptoms:Results from the Rhine and Swedish GA2LEN surveys. PLoS One. 2018;13(4):e0195055. doi: 10.1371/journal.pone.0195055. [Mindus S, Malinovschi A, Ekerljung L, et al.Asthma and COPD overlap (ACO) is related to a high burden of sleep disturbance and respiratory symptoms:Results from the Rhine and Swedish GA2LEN surveys[J].PLoS One, 2018, 13(4):e0195055.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson L, Vestbo J, Postma DS, et al. Gender differences in the management and experience of chronic obstructive pulmonary disease. Respir Med. 2004;98(12):1207–13. doi: 10.1016/j.rmed.2004.05.004. [Watson L, Vestbo J, Postma DS, et al.Gender differences in the management and experience of chronic obstructive pulmonary disease[J].Respir Med, 2004, 98(12):1207-13.] [DOI] [PubMed] [Google Scholar]
- 24.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report.Gold executive summary. http://onlinelibrary.wiley.com/doi/10.1111/resp.13012/pdf. Eur Respir J. 2017;195(5):557–82. doi: 10.1164/rccm.201701-0218PP. [Vogelmeier CF, Criner GJ, Martinez FJ, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report.Gold executive summary[J].Eur Respir J, 2017, 195(5):557-82.] [DOI] [PubMed] [Google Scholar]
- 25.Çolak Y, Afzal S, Nordestgaard BG, et al. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark:a prospective cohort study. Lancet Respir Med. 2017;5(5):426–34. doi: 10.1016/S2213-2600(17)30119-4. [Çolak Y, Afzal S, Nordestgaard BG, et al.Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark:a prospective cohort study[J].Lancet Respir Med, 2017, 5(5):426-34.] [DOI] [PubMed] [Google Scholar]
- 26.López Varela MV, Montes de Oca M, Rey A, et al. Development of a simple screening tool for opportunistic COPD case finding in primary care in Latin America:The PUMA study. Respirology. 2016;21(7):1227–34. doi: 10.1111/resp.2016.21.issue-7. [López Varela MV, Montes de Oca M, Rey A, et al.Development of a simple screening tool for opportunistic COPD case finding in primary care in Latin America:The PUMA study[J].Respirology, 2016, 21(7):1227-34.] [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi SH, Yanai M, Masaru Y, et al. Early detection of chronic obstructive pulmonary disease in primary care. Intern Med. 2017;56(23):3153–8. doi: 10.2169/internalmedicine.8717-16. [Kobayashi SH, Yanai M, Masaru Y, et al.Early detection of chronic obstructive pulmonary disease in primary care[J].Intern Med, 2017, 56(23):3153-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zubair T, Abbassi A, Khan OA. Early detection of chronic obstructive pulmonary disease in apparently healthy attendants of tertiary care hospital and assessment of its severity. http://europepmc.org/abstract/MED/28599692. J Coll Physicians Surg Pak. 2017;27(5):296–300. [Zubair T, Abbassi A, Khan OA.Early detection of chronic obstructive pulmonary disease in apparently healthy attendants of tertiary care hospital and assessment of its severity[J].J Coll Physicians Surg Pak, 2017, 27(5):296-300.] [PubMed] [Google Scholar]
- 29.Hemmingsen U B, Stycke M, Dollerup J, et al. Guideline-Based early detection of chronic obstructive pulmonary disease in eight Danish municipalities:the TOP-KOM study. http://europepmc.org/articles/PMC5339421/ Pulm Med. 2017:7620397. doi: 10.1155/2017/7620397. [Hemmingsen U B, Stycke M, Dollerup J, et al.Guideline-Based early detection of chronic obstructive pulmonary disease in eight Danish municipalities:the TOP-KOM study[J].Pulm Med, 2017:7620397.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vrbica Ž, Labor M, Gudelj I, et al. Early detection of COPD patients in Gold 0 population:an observational non-interventional cohort study-MARKO study. BMC Pulm Med. 2017;17(1):36. doi: 10.1186/s12890-017-0378-6. [VrbicaŽ, Labor M, Gudelj I, et al.Early detection of COPD patients in Gold 0 population:an observational non-interventional cohort study-MARKO study[J].BMC Pulm Med, 2017, 17(1):36.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu SN, Yu WC, Wong CK, et al. Prevalence of undiagnosed airflow obstruction among People with a history of smoking in a primary care setting. Int J Chron Obstruct Pulmon Dis. 2016;11:2391–9. doi: 10.2147/COPD. [Fu SN, Yu WC, Wong CK, et al.Prevalence of undiagnosed airflow obstruction among People with a history of smoking in a primary care setting[J].Int J Chron Obstruct Pulmon Dis, 2016, 11:2391-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou YM, Chen SY, Tian J, et al. Development and validation of a chronic obstructive pulmonary disease screening questionnaire in China. Int J Tuberc Lung Dis. 2013;17(12):1645–51. doi: 10.5588/ijtld.12.0995. [Zhou YM, Chen SY, Tian J, et al.Development and validation of a chronic obstructive pulmonary disease screening questionnaire in China[J].Int J Tuberc Lung Dis, 2013, 17(12):1645-51] [DOI] [PubMed] [Google Scholar]


