A vaccine against hepatitis C virus (HCV) is crucial for global control of this important pathogen, which induces fatal human liver diseases. Vaccine development has been hampered by the lack of immunocompetent animal models. Discovery of rodent hepacivirus (RHV) enabled establishment of novel surrogate animal models. These allow robust infection and reverse genetic and immunization studies of laboratory animals, which develop HCV-like chronicity. Currently, there are no RHV in vitro systems available to study tropism and molecular virology. Here, we established the first culture systems for RHV, recapitulating the intracellular phase of the virus life cycle in vitro. These replicon systems enabled identification of replication-enhancing mutations and selection of cells highly permissive to RHV replication, which allow study of virus-host interactions. HCV antivirals targeting NS5A, NS5B, and microRNA-122 efficiently inhibited RHV replication. Hence, several important aspects of HCV replication are shared by the rodent virus system, reinforcing its utility as an HCV model.
KEYWORDS: animal models, antiviral agents, hepacivirus, hepatitis C virus, micro-RNA, replication, replicon
ABSTRACT
Animal hepaciviruses represent promising surrogate models for hepatitis C virus (HCV), for which there are no efficient immunocompetent animal models. Experimental infection of laboratory rats with rodent hepacivirus isolated from feral Rattus norvegicus (RHV-rn1) mirrors key aspects of HCV infection in humans, including chronicity, hepatitis, and steatosis. Moreover, RHV has been adapted to infect immunocompetent laboratory mice. RHV in vitro systems have not been developed but would enable detailed studies of the virus life cycle crucial for designing animal experiments to model HCV infection. Here, we established efficient RHV-rn1 selectable subgenomic replicons with and without reporter genes. Rat and mouse liver-derived cells did not readily support the complete RHV life cycle, but replicon-containing cell clones could be selected with and without acquired mutations. Replication was significantly enhanced by mutations in NS4B and NS5A and in cell clones cured of replicon RNA. These mutations increased RHV replication of both mono- and bicistronic constructs, and CpG/UpA-dinucleotide optimization of reporter genes allowed replication. Using the replicon system, we show that the RHV-rn1 NS3-4A protease cleaves a human mitochondrial antiviral signaling protein reporter, providing a sensitive readout for virus replication. RHV-rn1 replication was inhibited by the HCV polymerase inhibitor sofosbuvir and high concentrations of HCV NS5A antivirals but not by NS3 protease inhibitors. The microRNA-122 antagonist miravirsen inhibited RHV-rn1 replication, demonstrating the importance of this HCV host factor for RHV. These novel RHV in vitro systems will be useful for studies of tropism, molecular virology, and characterization of virus-host interactions, thereby providing important complements to in vivo systems.
IMPORTANCE A vaccine against hepatitis C virus (HCV) is crucial for global control of this important pathogen, which induces fatal human liver diseases. Vaccine development has been hampered by the lack of immunocompetent animal models. Discovery of rodent hepacivirus (RHV) enabled establishment of novel surrogate animal models. These allow robust infection and reverse genetic and immunization studies of laboratory animals, which develop HCV-like chronicity. Currently, there are no RHV in vitro systems available to study tropism and molecular virology. Here, we established the first culture systems for RHV, recapitulating the intracellular phase of the virus life cycle in vitro. These replicon systems enabled identification of replication-enhancing mutations and selection of cells highly permissive to RHV replication, which allow study of virus-host interactions. HCV antivirals targeting NS5A, NS5B, and microRNA-122 efficiently inhibited RHV replication. Hence, several important aspects of HCV replication are shared by the rodent virus system, reinforcing its utility as an HCV model.
INTRODUCTION
Hepatitis C virus (HCV) chronically infects ∼70 million people worldwide and leads to hepatic fibrosis, liver cirrhosis, and hepatocellular carcinoma (HCC) (1, 2). The introduction of direct-acting antivirals (DAA) to treat HCV infection has revolutionized therapy, increasing sustained virologic response rates to beyond 90% (3, 4). However, only marginal impact on total HCV cases has been achieved, and chronic infection annually causes at least 400,000 deaths (5). DAA treatment is expensive and remains largely inaccessible in the developing world, where HCV prevalence is highest and most infected individuals remain undiagnosed. Further obstacles include the emergence of resistance and the inability to successfully treat certain patient groups due to, e.g., late-stage liver disease and HCC. Therefore, a vaccine is of paramount importance for global HCV control and eradication (5). HCV vaccine development, however, has been stymied in large part due to the lack of immunocompetent animal models. Natural HCV infection is restricted to humans, and the only permissive experimental animal model, the chimpanzee, is no longer available for research. Mouse models with humanized liver or genetically engineered mice provided important insights into HCV infection in vivo but suffer from blunted immune responses or low robustness, limiting their relevance for analysis of HCV-associated immunity (6).
Following the discovery of HCV, the only other hepacivirus identified was GBV-B, a virus of unknown origin (7), which mostly establishes acute self-resolving infection and can be studied only in primates (8). The discovery of novel HCV-related hepaciviruses has increased since 2011, with identification of the equine nonprimate hepacivirus (NPHV/EqHV) (9), which was found to be endemic in horses (10, 11). EqHV infection is of great interest due to its close relatedness to HCV, and studies of EqHV might shed light on pathogen association with infectious liver disease in horses (12, 13). Additional hepaciviruses were discovered in rodents (14, 15), bats, cows, and monkeys (16, 17). Since diversity among rodents is large and the first discovered rodent hepacivirus (RHV) strains did not infect laboratory mice or rats, the subsequent identification of RHV in Norway rats (Rattus norvegicus) was of particular interest (18). Rat hepacivirus was first discovered in New York City and is distantly related to HCV, with a divergence (70% at the amino acid level) similar to that between HCV and GBV-B but substantially greater than that between HCV and EqHV (14).
In laboratory rats, RHV causes chronic high-titer infection and the propensity to develop life-long chronic infection and liver disease (19). Negative-strand RNA, a hallmark of hepacivirus replication, was exclusively detected in the liver of infected rats, confirming its strict hepatotropism (18). We and collaborators further adapted RHV to infect immunocompetent laboratory mice in order to exploit a broader set of genetic variants and research tools (20). The RHV Rattus norvegicus isolate 1 (RHV-rn1) or Norway rat hepacivirus (NrHV), RHV hereafter, has a positive-sense viral RNA genome of 9,656 nucleotides encoding a single polyprotein consisting of 2,991 amino acids. The polyprotein is predicted to be co- and posttranslationally processed by cellular and viral proteases to produce three structural (core, E1, and E2) and seven nonstructural (p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B) proteins. The 5′ untranslated region (UTR) of 485 nucleotides is predicted to form a type IV internal ribosomal entry site (IRES), similar to that of HCV (19). Binding of microRNA-122 (miR-122) to the HCV 5′ UTR is pivotal for HCV replication (21), as it stimulates IRES-mediated translation and protects the 5′ end from degradation (22–25). In addition, the liver-specific miR-122 contributes to the hepatotropism of HCV. The 5′ UTR of RHV similarly contains two miR-122 seed sites, and propagation also depends on miR-122, as no infection is seen in miR-122 knockout (KO) mice (20) and mutagenesis of seed site 1 abolished RHV infection in rats (19).
There are currently no in vitro culture systems available for RHV. Such model systems are important to study tropism, virus-host interactions, and neutralizing antibodies. Moreover, they could simplify reverse genetic studies and offer a platform for large-scale virus production, which could be crucial for proof-of-concept vaccine development studies using RHV as a surrogate model. In the absence of robust HCV infection in culture, the development of autonomously replicating subgenomic replicons was a major breakthrough (26). HCV replicons have been instrumental in selecting highly permissive cells and replication-enhancing mutations, understanding viral and host factors mediating HCV replication, studying processing of the viral polyprotein, as well as screening for and evaluation of HCV antivirals (3, 26–29).
In the current study, we adapted this strategy and established RHV selectable subgenomic mono- and bicistronic replicons with and without firefly luciferase (FLuc) or enhanced green fluorescent protein (eGFP) reporter genes for direct quantitative analysis of viral replication. These facilitated the identification of replication-enhancing mutations and selection of rodent hepatocyte cell clones or subpopulations with increased permissiveness to RHV replication. Finally, the FLuc reporter replicon was used for testing antiviral compounds relevant for treatment of HCV. These reporter replicons will be useful as molecular tools for RHV studies.
RESULTS
Rodent liver-derived cell lines do not readily support productive RHV infection.
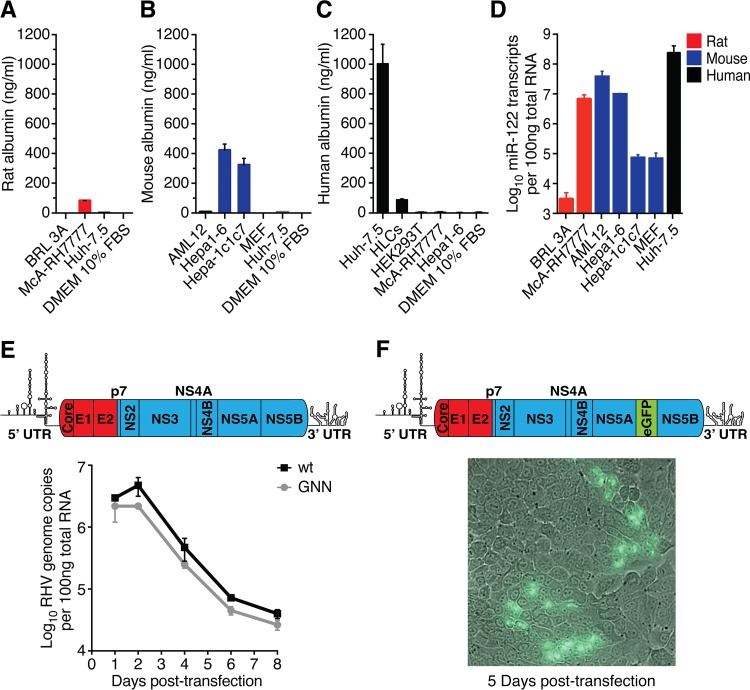
To identify rodent cell lines permissive to RHV infection, we initially screened rat and mouse liver-derived cells for albumin secretion and for miR-122 expression, both important hepatocyte markers (30). Species-specific albumin expression was detected for the McA-RH7777 rat hepatoma cells (Fig. 1A), as well as for Hepa1-6 and Hepa1c1c7 mouse hepatoma cells (Fig. 1B). The albumin levels were 2.5-fold (Hepa1-6 and Hepa1c1c7) to 12-fold (McA-RH7777) lower than those in human Huh-7.5 hepatoma cells but up to 5-fold higher than those in human hepatocyte-like cells (HLCs) following hepatic differentiation of induced pluripotent stem cells (Fig. 1A to C). High levels of mature miR-122 transcripts were observed for the mouse Hepa1-6 and AML12 cells and for the rat McA-RH7777 cells (Fig. 1D). McA-RH7777 and Hepa1-6 cells were prioritized for further experiments due to high expression of both liver markers.
FIG 1.
Rodent liver-derived cell lines do not readily support productive RHV infection. (A to D) Quantification of liver-specific markers in hepatic cell lines. Secreted rat (A), mouse (B), and human (C) albumin was measured in cell culture supernatant. (D) Quantification of miR-122 in relevant cells. HLCs, hepatocyte-like cells derived from human induced pluripotent stem cells; MEF, mouse embryonic fibroblasts (negative control). Data points represent means ± standard deviations (SD) from duplicates. (E) Intracellular viral RNA after transfection of McA-RH7777 cells with full-length genomic RHV-rn1 RNA or the replication-deficient GNN control. Data points represent means ± SD from duplicates. Schematic of the full-length RHV genome depicting predicted protein coding regions and UTR structures is shown above (ORF drawn to scale; genes encoding structural [red] and nonstructural [blue] proteins are indicated). (F) Fluorescence microscopy after transfection of McA-RH7777 cells with full-length genomic RHV-rn1-eGFP RNA. A nonrepresentative picture taken 5 days posttransfection at ×40 magnification shows expression of eGFP in a few clusters of cells. Viral spread could not be detected. A genome schematic of the RHV-rn1-eGFP reporter virus is shown above.
To first probe RHV permissiveness in cultured Norway rat hepatocytes, reflecting its natural host cells, we transfected McA-RH7777 cells with full-length in vitro-transcribed (IVT) RHV wild-type (wt) (19) or replication-deficient NS5B GNN mutant genomic RNA (Fig. 1E). Within 1 week, intracellular RNA levels of both constructs declined below 1% of input, indicating little if any RHV replication (Fig. 1E). Similar results were obtained for an RHV full-length construct with an eGFP reporter inserted between duplicated NS5A/B cleavage sites, an expression strategy used successfully for HCV (31). Clusters of eGFP-expressing cells were observed up to 5 days posttransfection in only one of eight replicate experiments (Fig. 1F), and further evidence of viral (eGFP-positive) spread was not detected.
Selection for RHV replication in rodent hepatocytes.
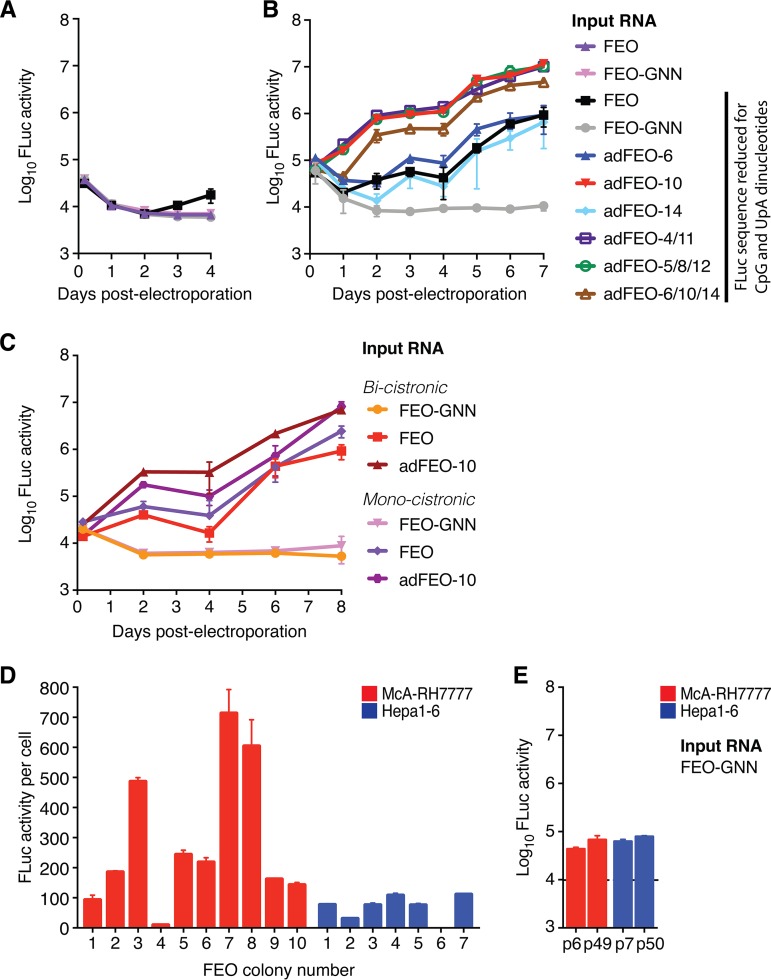
Observations using full-length RNA indicated that a small minority of McA-RH7777 rat liver cells have increased permissiveness to RHV. To select for such cells, we next constructed a selectable subgenomic replicon (RHV-SGR). Sequences encoding the RHV structural proteins, p7, and NS2 were replaced by the neomycin phosphotransferase (NPTII) selection cassette and the encephalomyocarditis virus (EMCV) IRES (Fig. 2A, I). A GNN replication-defective NS5B mutant (RHV-SGR-GNN) served as a negative control. Following electroporation and G418 selection, 150 McA-RH7777, 14 Hepa1-6, and 8 AML12 G418-resistant colonies were obtained with RHV-SGR but not RHV-SGR-GNN RNA (Fig. 2B and C). RHV-SGR did not replicate to levels conferring resistance to G418 in Huh-7.5 cells. RHV led to higher colony formation in rat than mouse hepatoma cell lines, reflecting the origin of the virus. As previously reported, an adapted HCV Con1 replicon was highly efficient in Huh-7.5 cells (28). Replication of this HCV isolate was not observed in rodent cells except for three AML12 colonies, suggesting only limited permissiveness to HCV replication.
FIG 2.
Selection for RHV replication in rodent hepatocytes. (A) Schematic representation of the bicistronic selectable subgenomic RHV replicon construct without (I) or with FLuc (FEO) (II) or eGFP (III) fused to the N terminus of the NPTII selection cassette. The first 12 amino acids of the core region (red) were fused in-frame with NPTII or reporters to ensure optimal translation of the first cistron (26). (IV) Monocistronic FEO replicon having both the reporter/selection cassette and the RHV replicase genes under the control of the RHV 5′ UTR. A short sequence encoding the ribosome-skipping 2A peptide from porcine teschovirus-1 ensures separation of NPTII and NS3. (B) Cell colonies stained using crystal violet following electroporation with RHV-SGR or RHV-SGR-GNN RNA. Representative images of G418-resistant McA-RH7777 rat hepatoma colonies after 2 weeks of selection are shown. (C) Number of selected colonies after electroporation of the RHV-SGR replicon into rat McA-RH7777 or mouse Hepa1-6 or AML12 cells. The number of rat and mouse cell colonies harboring RHV-SGR substitutions identified by sequence analysis is shown. (D) Validation of RHV NS3-4A protease activity in selected replicon-harboring Hepa1-6 cells using the fluorescent cell-based reporter system, leading to nuclear translocation of RFP upon NS3-4A cleavage of a MAVS target sequence (32). Diagram of native MAVS and the reporter is shown above with amino acid numbering. TMD, transmembrane domain; NLS, nuclear localization signal. (E) Comparison of the number of colonies after selection between the original replicon (wt) and those with single and combined mutations. Data from McA-RH7777 and Hepa1-6 cells are shown as fold change compared to levels for the wt in the respective cell line. Data points represent means ± SD from duplicates.
Functionality of the RHV replicon was further validated using a fluorescent cell-based reporter system developed for HCV infection (32). Mitochondrial antiviral signaling protein (MAVS), a cellular innate immune mediator, is cleaved and disabled by hepaciviral NS3-4A proteases (33). In the TagRFP-NLS-MAVS reporter system, red fluorescent protein (RFP) localizes to the outer mitochondrial membrane through coupling to the MAVS cleavage and transmembrane domains, yielding diffuse cytoplasmic staining. Hepacivirus-mediated cleavage allows nuclear translocation of RFP via the nuclear localization signal. Following transient transfection with the reporter-encoding construct, Hepa1-6 cells containing RHV-SGR, but not naive cells, displayed pronounced nuclear translocation (Fig. 2D). This confirmed expression of functional RHV proteins and also suggests that rat hepacivirus uses MAVS cleavage to evade innate immunity. Moreover, this reporter system provides an efficient assay to monitor RHV replication in live cells, thereby constituting a sensitive means of identifying rare infected cells.
Mutations in the nonstructural proteins enhance fitness of the RHV replicon.
Sequencing of the nearly complete replicon genome derived from total RNA extracts of ten McA-RH7777, nine Hepa1-6, and four AML12 colonies yielded information about putative replication-enhancing mutations. Interestingly, only 2/10 rat cell clones but almost all mouse cell clones (8/9 Hepa1-6 and 4/4 AML12) had nonsynonymous mutations, indicating that the RHV replicon underwent higher pressure either for fitness improvement or species adaptation in mouse than in rat cells (Fig. 2C). Similar to HCV replicons (34, 35), mutations primarily localized to the NS3 helicase, NS5A, and NS5B (Table 1). We further amplified the complete RHV genomic ends from three McA-RH7777 rat cell clones, including the two clones with nonsynonymous mutations, and one Hepa1-6 mouse cell clone and observed no further changes. This method identified the RNA termini of both positive- and negative-strand RNA, confirming the presence of replicating RHV RNA. Furthermore, lack of amplification during reverse transcription-PCR (RT-PCR) after omitting reverse transcriptase ruled out the presence of residual DNA template.
TABLE 1.
Nucleotide substitutions identified in RHV repliconsa
| Mutation no. | Nucleotide substitution |
Amino acid substitutionb | Clone | Cell line | Region | |
|---|---|---|---|---|---|---|
| Full-length position (RHV-rn1, GenBank accession no. KX905133) | SGR position | |||||
| 1 | A3822C | A2449C | I171L | 2D4 | McA-RH7777 | NS3c |
| T4147C | T2774C | L279P | 1E4 | Hepa1-6 | ||
| 2 | A4378G | A3005G | N356S | 5A2 | Hepa1-6 | |
| 3 | A4558T | A3185T | Y416F | 1B4 | McA-RH7777 | |
| G5020A | G3647A | G570D | 1E4 | Hepa1-6 | ||
| A5052G | A3679G | R581G | 1E4 | Hepa1-6 | ||
| 4 | T5754G | T4381G | S137A | 5A4 | Hepa1-6 | NS4B |
| 5 | A6873C | A5500C | K261Q | 1E5 | Hepa1-6 | NS5A |
| A6937C | A5564C | D282A | 1E4 | Hepa1-6 | ||
| 6 | A7008G | A5635G | T306A | 4A5 | AML12 | |
| 7 | A7059G | A5686G | T323A | 4A1 | AML12 | |
| T7134C | T5761C | F348L | 1E4 | Hepa1-6 | ||
| 8 | G7152A | G5779A | E354K | 1E5 | Hepa1-6 | |
| 9 | G7512T | G6139T | A474S | 4A1 | AML12 | |
| C7513T | C6140T | A474V | 4A2 | AML12 | ||
| 10 | T7599C | T6226C | W503R | 1E1, 5C4 | Hepa1-6 | |
| 4A5 | AML12 | |||||
| G7600C | G6227C | W503S | 4A4 | AML12 | ||
| 11 | A7602G | A6229G | T504A | 1E6 | Hepa1-6 | |
| 5A4 | ||||||
| G7605A | G6232A | D505N | 4A4 | AML12 | ||
| 12 | A7606G | A6233G | D505G | 1E5 | Hepa1-6 | |
| 1E6 | ||||||
| 4A1 | AML12 | |||||
| T7678C | T6305C | I23T | 1E4 | Hepa1-6 | NS5B | |
| A7714G | A6341G | N35S | 4A4 | AML12 | ||
| C7951A | C6578A | T114N | ||||
| 13 | C7966T | C6593T | T119I | 5A1 | Hepa1-6 | |
| 14 | T8164C | T6791C | V185A | 4A5 | AML12 | |
| A8592G | A7219G | T328A | 1E4 | Hepa1-6 | ||
Boldface indicates mutations that were experimentally tested.
Amino acid position according to the start of the individual viral protein (cleavage sites predicted in reference 19).
NS3 numbering excludes the methionine (Met) residue that has been introduced into RHV-SGR for translation initiation of the RHV replicase (2nd cistron).
A total of 14 putative replication-enhancing mutations (Table 1) and 7 combinations thereof (in which mutations 4/11, 5/8/12, 6/10/14, and 7/9/12 were naturally linked) were engineered into the original RHV replicon. We prioritized coding mutations occurring in several cell clones of different cell lines and considered linkage of mutations, since synergistic, cooperative, and compensatory effects were described for HCV (34, 36). The constructs were initially tested in McA-RH7777 hepatoma cells due to their higher permissiveness. Following electroporation, mutations 4 and 10 to 13 enhanced colony formation by 5 to 15 times relative to that of the original replicon. In particular, two mutations at the C terminus of NS5A (10 and 12) considerably improved replicon fitness (Fig. 2E). Adding naturally linked or unlinked mutations to mutations 10 or 12 did not improve replication further, and neither did combination of other replication-enhancing mutations yield additive effects, except for the combination of 4 and 11, which brought the replication level to that of 10 and 12. We next tested selected mutants in Hepa1-6 mouse hepatoma cells. Mutations 8, 10, and 12 and three of the naturally linked combinations significantly enhanced RHV replication by 3 to 11 times. Nonetheless, whether mutations were introduced or not, colony formation in McA-RH7777 cells remained superior to that in Hepa1-6 cells, in accordance with previous observations. Thus, mutations, although primarily selected in mouse cells, increased colony formation in rat hepatoma cells to a greater extent than that in mouse cells. This suggests that these substitutions are generally replication enhancing rather than specifically mouse adaptive. In conclusion, McA-RH7777 cells showed the greatest permissiveness for replication of both parental and adapted RHV replicons.
A luciferase reporter replicon system enables direct quantification of RHV replication.
To directly quantify RHV replication and to enable selection of cell clones with the highest permissiveness, we modified the replicon to express a chimeric fusion protein of FLuc and NPTII (Fig. 2A, II; FEO), as previously reported for HCV (37). The FEO construct, harboring the FLuc gene sequence N-terminally linked to the NPTII gene via a flexible (Gly)4-Ser linker region, did not lead to RHV replication (Fig. 3A). We therefore altered the FLuc sequence to reduce CpG and UpA dinucleotide frequency (69), which enhanced HCV replication (38), and used an alternative Arg-Arg-Ala linker (39). After dinucleotide optimization, RHV replication was observed and could be directly quantified (Fig. 3A).
FIG 3.
Luciferase reporter replicon systems enable direct quantification of RHV replication. (A) Absolute FLuc activity per well after transfection of parental McA-RH7777 cells with bicistronic FEO replicons with and without dinucleotide optimization. (B) FLuc activity after transfection of McA-RH7777 cells as described for panel A but with comparison of single and combined substitutions. (C) Comparison of FLuc activity per well after transfection of McA-RH7777 cells with mono- or bicistronic versions of the FEO replicon. (D) Replication efficiency of expanded rat McA-RH7777 (red) and mouse Hepa1-6 (blue) FEO cell clones. FLuc activity per cell was measured 4 weeks after electroporation with RHV-adFEO-10 RNA and subsequent G418 selection. (E) Absolute FLuc activity per well in parental rat McA-RH7777 (red) and mouse Hepa1-6 (blue) cells, measured 4 h after electroporation with a replication-deficient FEO-GNN replicon. Different passage numbers are shown, and the background luminescence level is indicated by the dashed line. All data points in panels A to E represent means ± SD from triplicates.
To increase the efficacy of the FEO replicon system further, selected replication-enhancing substitutions were inserted. The best replication-enhancing mutations, 10, 11, and 12, were engineered in combination with the mutations with which they were naturally linked (Table 1) to produce the adapted FEO (adFEO) constructs adFEO-4/11, adFEO-5/8/12, and adFEO-6/10/14. Additionally, FEO constructs with the individual mutations 6, 10, and 14 were engineered for comparison. A 10- to 20-fold increase in RHV replication was observed for all adapted FEO constructs with combinations of mutations, as well as for the single mutation 10 (Fig. 3B). Mutations 6 and 14 did not enhance replication. These data were in line with previous observations, indicating that mutations 5, 6, 8, and 14 most likely were coselected with 10 and 12, whereas 4 and 11 both contributed to increased fitness (compare Fig. 2E and 3B).
We next replaced the EMCV IRES with a short sequence encoding the ribosome-skipping 2A peptide from porcine teschovirus-1 (PTV-1) (40) to create a monocistronic replicon and reduce the amount of foreign RNA (Fig. 2A, IV). This could benefit functional studies of the RHV IRES, since viral translation would be directly controlled by the RHV 5′ UTR. Compared to the bicistronic construct, the monocistronic replicon had similar replication efficacy and kinetics, and mutation 10 also significantly enhanced replication (Fig. 3C).
Thus, irrespective of the replicon architecture, adFEO-10 was among the best-adapted replicons and also incurred the fewest changes to the wt RHV sequence. After G418 selection of adFEO-10 electroporated cells, we found that rat McA-RH7777 cell clones on average were more permissive to RHV replication than Hepa1-6 cell clones (Fig. 3D). This difference was not caused by differences in transfection efficiency, as similar luciferase levels were observed 4 h after electroporation with the replication-deficient FEO-GNN construct (Fig. 3E).
Selection of rat hepatoma cells highly permissive to RHV replication.
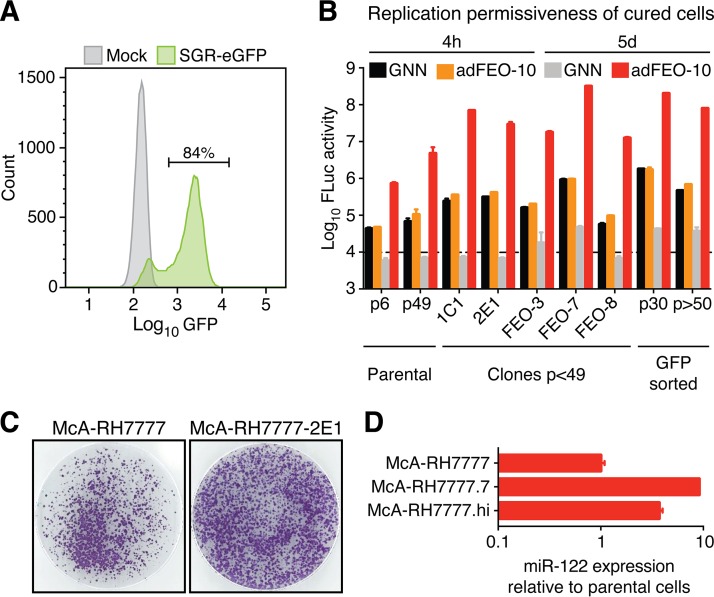
Since most sequenced rat cell clones from the original selection experiment were found not to harbor substitutions (Fig. 2C), we speculated that these originated from a subpopulation of cells with intrinsically higher permissiveness. We therefore used the highly potent pangenotypic HCV DAA sofosbuvir, targeting the viral NS5B RNA-dependent RNA polymerase (RdRp) (3), to cure two McA-RH7777 clones (1C1 and 2E1). Complete cell death after application of G418 confirmed clearance of the replicon. Whereas these clones were selected arbitrarily, quantitative comparison of RHV replication after G418 selection using the FEO replicon might identify even more permissive cell clones (Fig. 3D). As observed for most of the original McA-RH7777 replicon clones (Fig. 2C), the most efficient adFEO-10 replicon clones (FEO-3, -7, and -8) did not select for additional coding mutations. We therefore also cured these clones of replicon RNA using sofosbuvir.
We next modified the replicon system to express a CpG/UpA-depleted eGFP reporter instead of FLuc (Fig. 2A, III). This enabled the use of fluorescent-activated cell sorting (FACS) to select cells with increased RHV permissiveness from a genetically heterogeneous subpopulation (Fig. 4A). McA-RH7777 cells were electroporated with the nonadapted eGFP selectable reporter replicon and subjected to G418 selection for 2 weeks. Subsequently, ∼55,000 cells among the 1.5% with highest eGFP signal were sorted and replated. These sorted high-eGFP RHV-SGR-eGFP cells were also cured of replicon RNA using sofosbuvir.
FIG 4.
Selection of rat McA-RH7777 hepatoma cells with increased permissiveness to RHV replication. (A) Cells containing the eGFP replicon were expanded in selective media for 2 weeks and analyzed by flow cytometry. Mock, parental McA-RH7777 hepatoma cells. (B) Replication permissiveness of cured and parental McA-RH7777 cells as measured by FLuc activity per well 4 h and 5 days after electroporation with adFEO-10 or GNN as a control. Data points represent means ± SD from triplicates. For the parental and GFP-sorted cell populations, different passage numbers are shown. The background luminescence level is indicated by the dashed line. (C) Cell colonies stained using crystal violet following electroporation with RHV-SGR. Representative images of G418-resistant parental or clone 2E1 McA-RH7777 rat hepatoma colonies after 2 weeks of selection in gelatin-coated dishes are shown. (D) Quantification of miR-122 in highly permissive rat cells relative to the parental McA-RH7777 cell line. Quantification of the small noncoding RNA RNU6B was used as an endogenous reference for normalization. Data points represent means ± SD from duplicates.
All cured McA-RH7777 cell clones and the GFP-sorted subpopulation were checked for permissiveness to RHV replication by transfection with RHV-SGR-adFEO-10 RNA. Generally, the replicon-selected cell clones and cured eGFP-high subpopulation had increased permissiveness for RHV replication compared to that of the parental McA-RH7777 cell line (Fig. 4B). Most of the selected and cured cells had increased FLuc activity as early as 4 h after electroporation, suggesting enhanced early translation and/or more efficient RNA delivery. Among the replicon-selected cells, FEO-7- and FACS-sorted RHV-SGR-eGFP cells had the highest permissiveness. FLuc levels of the latter only declined 2.5-fold after >20 passages, indicating that these cells maintained increased permissiveness to RHV replication despite their genetic heterogeneity. Peak levels of FLuc activity exceeded that of the parental cell population up to 50-fold. In summary, the most efficient replicon systems for RHV combined the highly adapted FEO replicon RHV-SGR-adFEO-10 with the McA-RH7777-derived FEO-7 clone cells, McA-RH7777.7, or the FACS-sorted McA-RH7777-eGFP-high subpopulation, McA-RH7777.hi.
Replicon-selected rat hepatoma cells are more permissive to wild-type RHV and express increased miR-122 levels.
Development of infectious culture systems for HCV was made possible by an isolate, JFH-1, replicating efficiently without adaptive mutations (52). We therefore also analyzed replication of the wild-type RHV-SGR in replicon-selected cells and found enhanced colony formation in McA-RH7777-2E1 compared to that of parental cells (Fig. 4C). For HCV, replicon-selected cell clones with elevated replication permissiveness, such as Huh-7.5 and Huh7-Lunet, have been reported to express higher levels of miR-122 than parental Huh-7 cells (41). Therefore, to begin to understand the functional foundation of increased RHV permissiveness, we compared miR-122 expression between the highly permissive McA-RH7777.7 and McA-RH7777.hi cells and the parental rat hepatoma cell line and found 4- to 10-fold increases in miR-122 levels (Fig. 4D). miR-122 abundance therefore may at least partially explain the higher permissiveness of these cells.
A subset of HCV antivirals and miR-122 antagonism efficiently inhibit RHV replication.
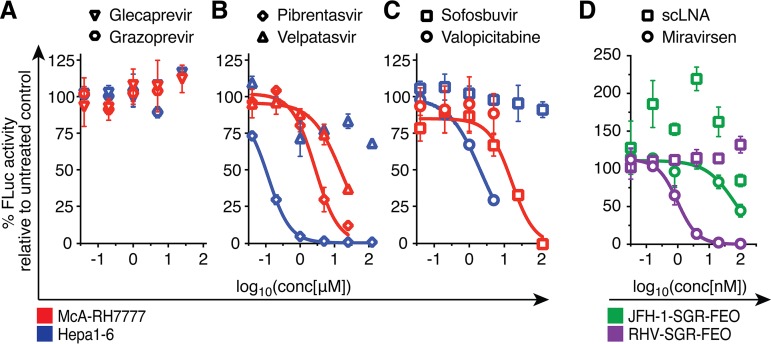
In functional proof-of-principle studies using the RHV replicon, we set out to determine whether leading HCV antiviral compounds are active against RHV. Such small inhibitory compounds would constitute valuable chemical tools for the study of RHV replication and its life cycle. Here, we used selected adFEO-10 rat McA-RH7777 (McA-RH7777.7) and mouse Hepa1-6 (Hepa1-6.7) cell clones with highest FLuc expression as stable reservoirs of RHV replicating cells (Fig. 3D). Approved HCV protease inhibitors glecaprevir and grazoprevir had no measurable activity against RHV (Fig. 5A). The potent HCV NS5A pangenotype inhibitor pibrentasvir (42) was active against RHV in both mouse Hepa1-6.7 (50% effective concentration [EC50], ∼0.12 μM) and rat McA-RH7777.7 (EC50, ∼2.7 μM) hepatoma cells (Fig. 5B). Activity of velpatasvir, another pangenotype NS5A inhibitor (42), against RHV was only observed in rat McA-RH7777.7 cells with an EC50 of ∼17 μM but not in Hepa1-6.7 cells (Fig. 5B). Generally, the EC50 values of the NS5A inhibitors were 104 to 105 times higher than that of HCV, which is comparable to some of the most resistant HCV variants (42).
FIG 5.
HCV antivirals inhibit RHV replication. Treatment of RHV replicon-containing McA-RH7777 (red) or Hepa1-6 (blue) cells with escalating doses of HCV antivirals targeting the NS3-4A protease (A), NS5A (B), or the NS5B RdRp (C). Replication levels after treatment are shown as percentages of FLuc activity compared to levels for the untreated controls. Data points derived from drug treatment above cytotoxic concentrations were excluded, and the highest nontoxic concentration was tested for all drugs. (D) Escalating concentrations of the miR-122 antagonist miravirsen or a scrambled LNA (scLNA) were transfected into RHV or HCV replicon-containing McA-RH7777 and Huh-7.5 cells, respectively. All data are from representative experiments, and data points represent means ± SD from triplicates.
Both the nucleoside analog valopicitabine and the nucleotide prodrug sofosbuvir are efficient inhibitors of the HCV NS5B RdRp (3), the most conserved antiviral target across hepaciviruses (14). Interestingly, their efficacy against RHV replication drastically differed between the tested rodent cell lines. In rat McA-RH7777.7 cells, sofosbuvir was potent against RHV replication, with an EC50 of 16 μM, only ∼25-fold higher than that for HCV and ∼2-fold higher than that for resistant HCV variants (43). In contrast, no inhibition was observed in mouse Hepa1-6.7 hepatoma cells. The opposite was the case for valopicitabine, which efficiently inhibited RHV replication in mouse hepatoma cells only (EC50 of ∼2.1 μM, ∼2-fold higher than for HCV [71]) but was ineffective when applied to rat hepatoma cells (Fig. 5C). Since these antivirals are synthesized as prodrugs and require hydrolysis for conversion into their pharmacologically active state, pharmacokinetics might differ among the tested cell lines, possibly explaining the observed differences. Whereas the McA-RH7777.7 replicon adapted no further than the introduced mutation 10 (NS5A W503R), the Hepa1-6.7 replicon additionally acquired NS5A I77S and NS5B I454F. Although we cannot exclude a contribution of these mutations to antiviral resistance, they do not map to known sites of resistance for HCV. In summary, HCV antivirals, in particular nucleo(s/t)ide analogs, appear to retain activity against highly divergent hepaciviruses.
Due to the critical dependence of HCV on miR-122, locked nucleic acids (LNAs) antagonizing miR-122 have been developed as host-targeting agents (HTAs) against HCV infection (44). The efficacy of the miR-122 antagonist miravirsen has been demonstrated in clinical trials (45) and in cell culture-based assays, where miravirsen inhibited HCV replication with an EC50 of 0.67 μM (46). Propagation of RHV also depends on miR-122, as no infection is seen in miR-122 KO mice (20). To test miR-122 requirements for replication in vitro, we tested the inhibitory potential of miravirsen against RHV in McA-RH7777.7 cells and found it to be highly effective (EC50 of ∼1 nM). Huh-7.5 cells containing the HCV JFH-1-SGR-FEO replicon (39) were included for comparison, since the method of LNA administration significantly influences efficacy. Although our experimental setup identified a lower EC50 (∼65 nM) than that of previous reports, HCV remained less sensitive to miR-122 antagonism than RHV (Fig. 5D). Despite expression of similar miR-122 levels in McA-RH7777.7 and Huh-7.5 cells (compare Fig. 1D and Fig. 4D), a direct comparison is difficult due to the inability of RHV and HCV to replicate in the same cell type. In conclusion, RHV, similarly to HCV, requires miR-122 for productive replication.
DISCUSSION
Establishing infectious, complete-life-cycle cell culture systems for HCV (HCVcc) was a slow process, given that patient isolates did not readily grow in cell culture. In the absence of infectious culture systems, the development of an efficient HCV replicon system (26) paved the way for important in vitro studies. This outstanding achievement finally allowed studies of HCV replication mechanisms using an elegant cell culture-based in vitro system.
Replicon-based screening assays by gene knockdown or genetic complementation using short interfering RNA (siRNA) or cDNA libraries, respectively, permitted the identification of host-mediated innate defense mechanisms against HCV and host factors required for HCV infection (21, 29, 47–49). Further, the replicon system enabled high-throughput drug screening assays which led to the discovery of potent inhibitors of HCV replication (reviewed in references 3 and 4). Ultimately, the HCV replicon system facilitated selection of human hepatoma cells highly permissive to infection with cell culture-adapted full-length virus, including Huh-7.5 (27) and Huh7-Lunet cells (50). Importantly, this remarkable progress contributed to a crucial improvement in treatment options for chronically infected HCV patients (3). Nonetheless, without a vaccine, global prevention of HCV infection currently is not possible. RHV infection in rodents holds great promise as a vaccine model due to striking similarities to HCV infection in tissue tropism, course of infection, host-mediated immunity, and pathogenesis (19, 20, 51). Thus, the most promising vaccine platforms can be validated using the RHV model before moving into testing corresponding HCV vaccine candidates in humans, where accessible high-incidence populations are scarce. Currently, in vitro systems for RHV are lacking but will be of great importance for functional studies of the virus and its host interactions, large-scale virus production, and characterization of the antibody-dependent adaptive immune responses.
Here, we report the first in vitro systems for rat-derived RHV, currently the most promising model for HCV. The development of selectable mono- and bicistronic reporter replicons allowed the selection of cells highly permissive for RHV replication and the identification of replication-enhancing mutations. The resulting systems allowed highly efficient RHV replication in vitro and testing of antiviral compounds used for HCV treatment. These included the HTA miR-122 antagonist miravirsen and the three DAA classes targeting the nonstructural proteins, the NS3-4A protease, NS5A, or the NS5B RdRp. Efficient inhibition of RHV replication, in particular by miravirsen and NS5B inhibitors, proved the functionality of the replicon and emphasizes the utility of HTAs and polymerase inhibitors as broad-spectrum antivirals.
Initial screens of immortalized rodent cell lines for important liver markers identified both rat McA-RH7777 and mouse Hepa1-6 hepatoma cells expressing albumin and miR-122 levels comparable to those seen for HLCs or Huh-7.5 cells. Nonetheless, only marginal RHV replication and no evidence for viral spread was detected upon transfection of McA-RH7777 cells with a full-length RHV clone proven to be infectious in rats (19). Construction of a subgenomic replicon, however, allowed selection of McA-RH7777 cells, as well as Hepa1-6 and AML12 cells, highly permissive for RHV replication. Replicon sequencing revealed that a majority of McA-RH7777 cell clones supported RHV replication without the necessity for adaptive mutations. The development of efficient HCVcc systems ultimately required the identification of the HCV genotype 2a strain JFH-1, which, in contrast to most other isolates, did not depend on mutations for efficient in vitro replication (52). Since replication-enhancing mutations can be detrimental for virus production (53), this isolate was pursued for infectious systems and led to the establishment of the JFH-1 full-length system (54). Although the colony-forming capacity of the unadapted RHV replicon is nowhere near that of JFH-1, these findings suggest that further work also will yield productive infectious culture systems for RHV. The detection of eGFP-expressing cell clusters at 5 days after transfection with a full-length RHV reporter virus further encourages such efforts.
Remarkably, all replicon-selected Hepa1-6 (except for one) and all AML12 mouse cell clones harbored mutations (Fig. 2C and Table 1), suggesting a requirement for cross-species adaptation. However, although replication-enhancing mutations were exclusively selected in mouse cell clones, these substitutions significantly enhanced replication in both mouse Hepa1-6 and rat McA-RH7777 hepatoma cells, indicating that they generally enhance RHV replication rather than solely conferring species-specific adaptation (Fig. 2E). As previously observed for HCV, replication-enhancing mutations primarily localized to the N terminus of the NS3 helicase, NS5A, and the N terminus of the NS5B RdRp (28, 34, 36, 55–57). Interestingly, 3 out of 13 mutations within NS5A led to the removal of threonine residues and another 2 to the addition of serine residues. These may influence the phosphorylation status of the NS5A phosphoprotein. Hypophosphorylation of NS5A is associated with higher RNA replication efficiency in the HCV replicon system (72) and possibly impacts RHV replication as well.
Surprisingly, we observed a cluster of mutations at the very C-terminal end of NS5A, close to the predicted NS5A/NS5B cleavage site at residue 506 (Table 1, mutations 10 to 12). All sequenced AML12 and half of the Hepa1-6 clones were found to harbor mutations at positions 503 to 505 of NS5A. The amino acid residues 503 (W503R/W503S) and 505 (D505N/D505G) were even substituted for different amino acids among various cell clones, suggesting a high adaptive pressure on this region (Table 1). This finding was unexpected, as previous studies characterizing HCV replicons did not report mutation hotspots at the C terminus of NS5A.
In order to directly quantify RHV replication for use in genetic screens or functional studies, we successfully incorporated FLuc and eGFP reporter genes into the RHV replicon system. In the wt FLuc sequence, high CpG and UpA dinucleotide frequencies (38) were shown to substantially restrict replication of an echovirus 7 reporter virus (58). Replacement of the wt FLuc gene with a CpG/UpA-low variant in HCV replicons resulted in a 10- to 100-fold increase in replication (38). Here, we found that diminishing reporter gene CpG and UpA dinucleotide frequency was a necessary prerequisite for efficient RHV replicon replication. The resulting FLuc and eGFP RHV reporter replicons enabled targeted selection of cell clones (Fig. 3D) and subpopulations (Fig. 4A) with increased permissiveness. Following cure of replicon RNA using the RdRp inhibitor sofosbuvir, McA-RH7777.7 and McA-RH7777.hi cells had considerably improved RHV replication efficiency compared to that of the parental McA-RH7777 cell line (Fig. 4B). The eGFP-sorted subpopulation has the potential advantage of allowing studies of RHV replication in a more diverse genetic background compared to that of expanded McA-RH7777 cell subclones. We found that McA-RH7777.7 and McA-RH7777.hi cells express enhanced miR-122 levels, possibly at least partially explaining their higher permissiveness. Future comparative studies of gene expression signatures may further reveal innate immune deficiencies or other host cell differences leading to improved RHV replication. Given the availability of the reporter replicons, important RHV host factors could be identified using siRNA screens or CRISPR-cas9-mediated KO screens.
The reporter replicons further allowed the evaluation of potent HCV antivirals for inhibition of RHV replication. Approved DAA compounds of the three major classes targeting the NS3-4A protease, NS5A, or the NS5B RdRp were examined. The HCV protease inhibitors glecaprevir and grazoprevir were inactive (Fig. 5A). Since HCV NS3-4A protease inhibitors generally are less potent than NS5A inhibitors and can be rendered noneffective at noncytotoxic concentrations by single-amino-acid changes (59), reduced activity against RHV was expected. The approved NS5A DAAs pibrentasvir and velpatasvir were both active against RHV replication in rat McA-RH7777.7 cells. In Hepa1-6.7 mouse hepatoma cells, only pibrentasvir efficiently inhibited RHV replication. The EC50 values of the NS5A inhibitors were 4 to 5 logs higher for RHV than for HCV but were still within noncytotoxic concentrations and in a range similar to those for the most resistant HCV escape mutants (42). Sequence alignment between RHV and HCV revealed that RHV-rn1 already harbors several resistance-associated NS5A substitutions previously described for HCV, which might explain the high EC50 values. Amino acid positions 28, 30, 31, and 93 generally are associated with resistance to NS5A inhibitors. 30K has reduced susceptibility to pibrentasvir (42), 31I in combination with 28S confers resistance to pibrentasvir (42), and 93S reduces susceptibility to velpatasvir (60). The NS5B inhibitors sofosbuvir and valopicitabine were highly efficient against RHV replication in McA-RH7777.7 and Hepa1-6.7 cells, respectively. Due to high conservation of RdRps across viruses, antiviral activity of NS5B inhibitors was expected. Sofosbuvir, the most potent NS5B inhibitor, is broadly effective, exhibiting activity against the flaviviruses Zika (ZIKV), dengue (DENV), and yellow fever (YFV) viruses (61–63) and hepatitis E virus (HEV) (64). Similar to the cases for HCV (EC50 of ∼0.4 to 0.9 μM) (43, 65), HEV (EC50 of ∼1.2 μM), and flaviviruses (EC50 of ∼1.4 to 32 μM) (61), sofosbuvir inhibited RHV replication at noncytotoxic concentrations (EC50 of ∼16 μM) in rat hepatocytes. Surprisingly, sofosbuvir was not active in mouse hepatocytes, while valopicitabine was inactive in rat hepatocytes. This may be related to different metabolism of these prodrugs.
For HCV, interaction with miR-122 is critically required for stabilization of the viral genome and for stimulation of both translation and replication (22–24). RHV also critically depends on miR-122 in vivo, as no infection is seen in miR-122 KO mice (20). Here, we confirmed that miravirsen, an HTA antagonizing miR-122, had high potency against RHV replication in vitro (Fig. 5D) and that the RNA replication phase of the RHV life cycle therefore is highly dependent on miR-122. We found that RHV in McA-RH7777.7 cells is even more sensitive to miR-122 antagonism than HCV in Huh-7.5 cells. Despite similar levels of miR-122 expression in these two cell types (compare Fig. 1D and Fig. 4D), transfection efficiencies could differ, and the strict tropism of hepaciviruses therefore prevents direct comparison of their miR-122 dependency. Further studies will be needed to delineate the relative importance of the RHV miR-122 binding sites in vivo and in vitro. This finding emphasizes that HTAs might have an advantage over DAAs due to broader-spectrum antiviral activity and limited risk of viral escape mediated by the acquisition of resistance-associated substitutions.
In conclusion, the RHV replicons described in this study recapitulate RHV replication in cell culture. Generally, many important aspects of HCV replication are reflected in the rodent system, substantiating its utility as a model for HCV. These RHV replicons allow detailed molecular studies of the mechanism of RHV replication and virus-host interactions. Further efforts in characterizing rodent hepatoma cells with increased permissiveness to RHV replication may reveal novel host factors required for RHV replication that possibly are also relevant for HCV. Moreover, the rodent model enables probing hepacivirus replication and biology in vitro and in vivo such that in vitro studies can be followed up in vivo, which now is rarely the case for HCV. The selection of highly permissive hepatoma cells and the identification of replication-enhancing mutations may pave the way for the development of infectious full-length RHV culture systems. Such systems will be pivotal for complementing ongoing vaccine and pathogenesis studies in rodents, for example, by neutralization assays aimed at defining humoral immune responses that can confer protection against hepacivirus infection.
MATERIALS AND METHODS
Antivirals.
The DAAs glecaprevir, grazoprevir, pibrentasvir, sofosbuvir, valopicitabine, and velpatasvir were synthesized by Acme Bioscience. Miravirsen was from Exiqon (Qiagen).
Cell culture.
Mouse embryonic fibroblasts (MEF) and Hepa1-6, Huh-7.5, and McA-RH7777 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin (P/S; Sigma). AML12 cells were cultured in DMEM-Ham’s F12 medium (1:1) supplemented with 10% FBS, P/S, 10 μg/ml insulin, 5.5 μg/ml transferrin, 5 ng/ml selenium, and 40 ng/ml dexamethasone. BRL 3A cells were maintained in Eagle’s minimum essential medium (EMEM) supplemented with 10% FBS and P/S. Hepa-1c1c7 cells were cultured in alpha minimum essential medium (AMEM) without nucleosides, supplemented with 10% FBS. Human NCRM-5 induced pluripotent stem cells (66) were maintained in mTeSR1 medium (Stemcell Technologies). The stem cells were grown as monolayers in 6-well plates precoated with 2.5% Matrigel hESC-qualified matrix (Corning). For passage, the stem cells were dissociated with Accutase (Life Technologies) and then washed with DMEM-F12 medium and replated in mTeSR1 medium, both containing 10 μM Y-27632 ROCK inhibitor (Calbiochem). Hepatic differentiation was performed as described previously (67). All cell lines were maintained at 37°C and 5% CO2. Cells harboring selectable subgenomic replicons were grown in medium containing 700 μg/ml G418 (Thermo Fisher).
Quantification of albumin.
Secreted albumin was quantified using a human, mouse, and rat albumin enzyme-linked immunosorbent assay detection system (Bethyl Laboratories), using FBS as a control for the species specificity of the assay.
Plasmids and construction of reporter virus and replicons.
To generate a full-length RHV reporter virus, the primer pair TS-O-00305 and TS-O-00306 (Table 2 lists primer sequences) was used for amplification of eGFP from the HCV reporter virus construct pJ6-JFHd40-eGFP (68). TS-O-00306 enabled duplication of the NS5A/B cleavage site with alternative codon usage to prevent recombination. The resulting megaprimer was inserted into the infectious clone of RHV-rn1 (19) by following the two-step QuikChange mutagenesis strategy adapted for large insertions (Agilent).
TABLE 2.
Primer sequences
| No. | ID | Sequence |
|---|---|---|
| 1 | AUAP | GGCCACGCGTCGACTAGTAC |
| 2 | RHV-9020-F | AGCATACACGCCCAGGGAAA |
| 3 | TS-O-00178 | GGCCACGCGTCGACTAGTACTTTTTTTTTTTTTTTTTTTTVN |
| 4 | TS-O-00226 | TGGAGTGTGACAATGGTGTTT |
| 5 | TS-O-00239 | CGCGTCACCCAGCTGGTTGATTTGTGGAAACAATACGGTGTGCATATTTG |
| 6 | TS-O-00240 | CAAATATGCACACCGTATTGTTTCCACAAATCAACCAGCTGGGTGACGCG |
| 7 | TS-O-00259 | GATGGTTTACAGCGGAAACG |
| 8 | TS-O-00305 | TCTGGATCATGGACCGACTGTTCTTGGATGGTGAGCAAGGGCGAGGAGCTG |
| 9 | TS-O-00306 | ACCAATTGAGGAACCGACCATGAGTACGACCAGCTGCAATCCGTCCAGCTGCCGCTCTTGTACAGCTCGTCCATGCCGAG |
| 10 | TS-O-00310 | GGCCACGCGTCGACTAGTACAAAAAAAAAAAAAAAAAAAABN |
| 11 | TS-O-00318 | CCAAGCCCCAATGCCGTCCGGCACCGCTGCCCTTTTCGG |
| 12 | TS-O-00319 | GCTTCCTGGAGCGGGCTAGATACTG |
| 13 | TS-O-00329 | CTCTTTTTCAACTTCCCTTCCACAATGATTGAACAAGATGGATTGC |
| 14 | TS-O-00330 | GTTCTTAAGTTGAAGGGGGCCATGGTATTATCGTGTTTTTCAAAGGAAAACC |
| 15 | TS-O-00335 | ATGCAGGCCTCTGCATC |
| 16 | TS-O-00336 | CATTGTGGAAGGGAAGTTG |
| 17 | TS-O-00337 | CCATGGCCCCCTTCAACT |
| 18 | TS-O-00338 | GTGGTAACTTGCCCAGAT |
| 19 | TS-O-00361 | AGGTGAAGGGGGCATCGATG |
| 20 | TS-O-00383 | CAAATTCGTGAAGCGTTCCAT |
| 21 | TS-O-00561 | TACATGGCTAAGCAATACGG |
| 22 | TS-O-00562 | AAGCGCAGCACCAATTCC |
| 23 | TS-O-00563 | [6-FAM]CTCACGTACATGACGTACGGCATG[BHQ1a–6-FAM] |
| 24 | TS-O-00613 | GCAATCTCTTTTTCAACTTCCCTTCCACAATGGAAGACGCCAAAAACATAAAGAAAGGC |
| 25 | TS-O-00614 | CTGCGTGCAATCCATCTTGTTCAATCATCGAACCTCCTCCACCCAATTTGGACTTTCCGCCCT |
| 26 | TS-O-00978 | GGAAGCGGAGCTACTAACTTCAGCCTGCTGAAGCAGGCTGGAGACGTGGAGGAGAACCCTGGACCTGCCCCCTTCAACTTAAGAACAGTCC |
| 27 | TS-O-00979 | GAAGAACTCGTCAAGAAGGCGATAG |
HCVrep1bBartMan/AvaII is a highly efficient, bicistronic HCV SGR derived from the HCV genotype 1b Con1 sequence (28). The RHV infectious clone pRHV-rn1 (19) served as the starting material to construct a bicistronic RHV-SGR. BglII and SacII were used to digest pRHV-rn1, and the larger fragment containing the plasmid backbone was gel purified. A fragment containing the RHV 5′ UTR and the sequence encoding the first 12 amino acids of RHV core was amplified using TS-O-00335 and TS-O-00336. The NPTII gene followed by EMCV IRES was amplified from pNZP1-SGR (13) using the primer set TS-O-00329 and TS-O-00330. A third PCR fragment was generated using TS-O-00337 and TS-O-00338, spanning the RHV genomic region encoding NS3 to the BglII restriction site. TS-O-00337 was used to introduce a start codon (Met) in order to initiate translation of the RHV replicase genes. The three PCR products and the plasmid backbone were fused by Gibson assembly cloning (NEB). Replication-defective mutants carrying a GNN substitution at the GDD catalytic triad at positions 314 to 316 of the NS5B RdRp were cloned for full-length RHV and the replicon constructs and used as negative controls. The substitutions were introduced by QuikChange mutagenesis (Agilent) using the primer pair TS-O-00239 and TS-O-00240.
In order to clone RHV-SGR-FEO, we amplified the firefly luciferase (FLuc) gene (13) using the primer pair TS-O-00613 and TS-O-00614. The primer TS-O-00614 was used to introduce a flexible (Gly)4-Ser linker between FLuc and NPTII. The amplified PCR product was introduced into RHV-SGR in order to generate RHV-SGR-FEO by following the modified QuikChange (Agilent) protocol for large insertions. In a second approach, we used a synthetic FLuc gene provided by Integrated DNA Technologies (IDT) with optimized sequence depleted for CpG and UpA dinucleotides as previously described (38). In this construct, the flexible (Gly)4-Ser linker sequence was replaced by the Arg-Arg-Ala linker sequence, which has previously been used in the HCV S52/SG-Feo replicon (39). The IDT building block was fused to the 5′ end of NPTII by In-Fusion HD cloning (TaKaRa). Similarly, an RHV-SGR-eGFP replicon was constructed using a synthetic eGFP gene (IDT) with optimized sequence, which was specified using the program Sequence Mutate in the SSE package (69).
In order to generate a monocistronic RHV replicon, the EMCV IRES of the bicistronic RHV replicon was replaced with a short sequence encoding the 2A peptide from PTV-1 using the primers TS-O-00978 and TS-O-00979. The resulting linear PCR fragment was subsequently subjected to the KLD enzyme reaction (NEB). Replication-adaptive point mutations were introduced by standard PCR-based or QuikChange mutagenesis (Agilent). The replicon part of all plasmids was sequence verified (Macrogen).
The protocol for the large-quantity plasmid DNA preparation was modified, as One Shot TOP10 chemically competent bacteria (Thermo Fisher) transformed with pRHV-rn1 or RHV replicon plasmids did not stably grow at 37°C. Following transformation, bacterial colonies were grown overnight at 37°C on LB agar plates supplemented with 100 μg/ml ampicillin. Subsequently, cultures of 150 ml selective LB medium were directly inoculated using a bacteria colony without preceding preparation of an overnight starter culture. The 150-ml cultures were grown at 30°C for 3 days prior to GenElute HP plasmid midiprep preparation (Sigma). To ensure efficient cell lysis, volumes of buffers required for resuspension, cell lysis, neutralization, and binding were increased 1.5 times.
The TRIP-RFP-NLS-IPS plasmid (32) and the JFH-1-SGR-FEO replicon (39) were previously described.
In vitro RNA transcription and electroporation.
RNA was produced from an in vivo-validated clone containing a 5′-flanking T7 promoter and an MluI site at the 3′ end of the RHV genome mediating authentic 5′ and 3′ ends (19). RNA was transcribed from 2.5 μg MluI-linearized plasmid template DNA using RiboMAX T7 RNA polymerase (Promega). Particular care was taken to remove template DNA following IVT to prevent unspecific NPTII integration into transfected cells potentially conferring RHV replication-independent G418 resistance. Therefore, template DNA was degraded for 30 min at 4°C with RQ1 RNase-free DNase. Subsequently, the RNA was purified on RNeasy Mini columns (Qiagen), including an additional on-column DNase I digestion step (Qiagen). Integrity of the RNA was assessed by agarose gel electrophoresis in 3.7% formaldehyde.
For electroporation, cells were washed twice with ice-cold phosphate-buffered saline (PBS) and resuspended at a density of 1.5 × 107 cells/ml. Subsequently, 400 μl of the cell suspension was mixed with 5 μg RNA and transferred to a 2-mm electroporation cuvette (Bio-Rad). The following parameters were used for square wave electroporation: 900 V, 100 μs, and 2 pulses within 5 s (Gene Pulser Xcell electroporation system; Bio-Rad). Following electroporation, the cells were recovered at room temperature for 10 min prior to resuspension in prewarmed medium and transfer to the appropriate plate or dish format. G418 selection (700 μg/ml) was initiated 1 day postelectroporation, and cells were supplied with fresh medium supplemented with G418 every third day.
Detection, quantification, and sequencing of viral RNA and miRNA.
Total RNA was extracted from replicon-containing cells using TRIzol (Thermo Fisher). For reverse transcription, the samples were preincubated with 0.1 μM TS-O-00319 and RNase inhibitors (Promega) in the absence of the RT enzyme at 65°C for 5 min. Following 1 min on ice, the RT reaction was performed using Maxima H Minus reverse transcriptase (Thermo Fisher) in a gradient from 50°C to 55°C for 120 min, followed by an inactivation step at 85°C for 5 min. Subsequently, input RNA was degraded with RNase H and T1 (Thermo Fisher) for 20 min at 37°C. The complete open reading frame (ORF) was amplified as outlined elsewhere (20). Prior to sequencing (Macrogen), amplified DNA was gel purified using a Zymoclean large-fragment DNA recovery kit (Zymo Research).
To determine the sequence of both ends of the RHV replicon, the 3′ ends were tailed with homopolymers of ATP or UTP using yeast poly(A) polymerase (Thermo Fisher). Subsequently, cDNA was synthesized using SuperScript III reverse transcriptase (Thermo Fisher) and the primer TS-O-00178 or TS-O-00310 for RNA tailed with ATP or UTP, respectively. The RNA was preincubated in the presence of primer and deoxynucleoside triphosphates at 65°C for 2 min prior to ramping down the temperature to 48°C; thereafter the RT enzyme was added for reverse transcription in a 48 to 55°C temperature gradient for 1 h. Both UTRs were amplified with Q5 hot start high-fidelity 2× master mix (NEB), using cDNA as a template. The cycling parameters were 98°C for 1 min, followed by 40 cycles of 98°C for 30 s, 52°C for 40 s, and 72°C for 1 min, including a final extension step at 72°C for 5 min. The AUAP primer was combined with TS-O-00259 or RHV-9020-F for amplification of the 5′ and 3′ UTR, respectively. Sequencing analysis revealed the existence of quasispecies at only a few positions, and no dominant substitutions were observed.
Sequencing reactions were performed at Macrogen using standard Sanger sequencing technology. Mutations accounting for >90% of total fluorescence signal were classified as fixed mutations.
Intracellular RHV RNA was detected and quantified from total cellular RNA extracts by one-step RT-quantitative PCR (qPCR) with TaqMan fast virus 1-step master mix (Applied Biosystems), using a LightCycler 96 instrument (Roche) with the following protocol: 50°C for 30 min, 95°C for 5 min, and then 40 cycles of 95°C for 15 s, 56°C for 30 s, and 60°C for 45 s. The RHV NS3-specific primers used for this protocol were TS-O-00561 (sense), TS-O-00562 (antisense), and TS-O-00563 (probe). An RHV standard curve was generated from IVT RHV RNA using a template plasmid which contains partial sequence encoding the RHV nonstructural proteins. After IVT and rigorous DNase treatment, the RNA was quantified and diluted to concentrations ranging from 108 to 101 genome equivalents/μl.
Mature miR-122 and RNU6B were quantified from total cellular RNA extracts using the miScript II RT kit (Qiagen) and qPCR using the TaKaRa one-step SYBR PrimeScript RT-PCR kit (TaKaRa) with forward primers TS-O-00226 and TS-O-00383, respectively. The qPCR reaction was performed on a LightCycler 96 (Roche) at 95°C for 5 min, followed by 40 cycles of 95°C for 15 s, 55°C for 30 s, and 60°C for 30 s. Standard curves were generated by diluting miR-122 mimic (Thermo Fisher) to the range of 108 to 101 copies/μl.
Crystal violet staining and colony counting.
Replicon-containing cells were expanded for 2 weeks in selective culture medium supplemented with 700 μg/ml G418 prior to fixation with 3.7% formaldehyde for 1 h. Subsequently, the formaldehyde solution was replaced with 0.1% crystal violet (Sigma) in water and incubated for 30 min. Following staining, the plates were rinsed with water, and subsequently the dried dishes were scanned. Colonies were automatically counted using the “analyze particles” function of the ImageJ software (70).
Analysis of MAVS cleavage.
To confirm RHV-mediated cleavage of MAVS, 1.5 × 105 naive and replicon-containing Hepa1-6 cells were seeded in 6-well dishes. The next day, 3 μg of the TRIP-RFP-NLS-IPS plasmid (32) was transfected using Lipofectamine 2000 (Thermo Fisher). Living cells were stained with 1 μg/ml Hoechst (Thermo Fisher) 2 days posttransfection and imaged using an AXIO Observer Z1 microscope equipped with a flexible LED Colibri illumination system (Zeiss).
Drug treatment.
Replicon-containing mouse Hepa1-6.7 or rat McA-RH7777.7 hepatoma cells were seeded at a density of 5,000 cells per well of a 96-well plate precoated with 0.1% porcine gelatin (Sigma) dissolved in cell culture-grade endotoxin-free water. The following day, HCV antivirals were added at the indicated concentrations. Medium supplemented with antivirals was replaced every second day. The firefly luciferase assay (Promega) was performed at day seven. EC50 values were calculated from sigmoidal concentration-response curves fitted in GraphPad Prism 7 (GraphPad, La Jolla, CA) using the equation Y = top/(1 + 10[log10EC50 − X] × Hill slope).
LNA transfection.
Reverse transfection was performed for 6 h concomitantly with seeding of 5,000 McA-RH7777.7 or G418-selected JFH-1-SGR-FEO replicon-containing Huh-7.5 cells. The indicated concentrations of miravirsen (Exiqon/Qiagen) or a scrambled LNA (scLNA) were used in combination with Lipofectamine RNAiMAX (Invitrogen). Subsequently, a forward transfection was carried out for 6 h at day four using equimolar concentrations of miravirsen or scLNA in the presence of Lipofectamine RNAiMAX. The FLuc assay was performed on day six.
Firefly luciferase assay.
To measure FLuc activity, replicon cells were washed once with PBS, lysed in passive lysis buffer, and subjected to one freeze-thaw cycle. Luciferase activity was measured with the luciferase assay system (Promega) using a FLUOstar Optima luminometer (BMG Labtech). Such assays were performed in 96-well and 24-well format for drug dose-response and RHV replication kinetics experiments, respectively. In order to ensure optimal adherence of replicon-containing McA-RH7777 or Hepa1-6 hepatoma cells, the plates were precoated with 0.1% gelatin.
Cytotoxicity assay.
To assess cytotoxicity of the used drugs, 5,000 rat or mouse hepatoma cells per well were seeded in 96-well plate format and incubated overnight. The next day, the drugs (reconstituted in DMSO) were diluted in cell culture medium and added to the cells at concentrations identical to those that were used in the experiments. Cells were incubated in the presence of the drug for 3 days. Cell viability was determined using the CellTiter 96 AQueous one-solution cell proliferation assay (MTS) kit (Promega). Absorbance at 490 nm was measured using a FLUOstar Optima luminometer (BMG Labtech).
Flow cytometry and FACS.
For flow cytometry, McA-RH7777 cells were trypsinized and fixed in PBS supplemented with 1% FBS and 0.5% paraformaldehyde. Subsequently, the cells were washed twice with PBS containing 1% FBS. Flow cytometry analysis was performed using a BD LSR Fortessa instrument (BD Bioscience). For cell sorting, McA-RH7777 cells were passed through a cell strainer to remove clumps prior to resuspension in FluoroBrite DMEM (Thermo Fisher) supplemented with 1% FBS. FACS was performed on a BD FACSAria II instrument (BD Bioscience) at the Flow Cytometry Core Facility at the University of Copenhagen.
ACKNOWLEDGMENTS
We thank Jonathan Filskov and Andrea Galli for assisting with fluorescent activated cell sorting (FACS) and flow cytometry analysis and members of the Copenhagen Hepatitis C Program for discussions. Critical support for iPS cell culture was kindly provided by Pamela Gehron Robey, Skeletal Biology Section of the NIDCR and the NIH Stem Cell Unit.
This study was supported by Heads of Department Bjarne Ørskov Lindhardt (Hvidovre Hospital) and Carsten Geisler (University of Copenhagen) and by funding from the European Research Council (ERC Starting Grant 802899), the Independent Research Fund Denmark (4004-00598, 6110-00595, and 6111-00314), the Danish Cancer Society (R204-A12639), the Novo Nordisk Foundation (NNF14OC0012533, NNF15OC0017404, and NNF17OC0029372), Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis' Legat, the Lundbeck Foundation (R192-2015-1154), and the U.S Public Health Service (R01 AI131688, R01 AI116943, and R01 AI137567). R.W. was supported by Swiss National Science Foundation Early Postdoc.Mobility (P2BEP3_178527) and Postdoc.Mobility (P400PB_183952) fellowships.
REFERENCES
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. 2018. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Bisceglie AM. 1997. Hepatitis C and hepatocellular carcinoma. Hepatology 26:34S–38S. doi: 10.1002/hep.510260706. [DOI] [PubMed] [Google Scholar]
- 3.Scheel TK, Rice CM. 2013. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med 19:837–849. doi: 10.1038/nm.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawlotsky JM. 2016. Hepatitis C virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology 151:70–86. doi: 10.1053/j.gastro.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Bartenschlager R, Baumert TF, Bukh J, Houghton M, Lemon SM, Lindenbach BD, Lohmann V, Moradpour D, Pietschmann T, Rice CM, Thimme R, Wakita T. 2018. Critical challenges and emerging opportunities in hepatitis C virus research in an era of potent antiviral therapy: considerations for scientists and funding agencies. Virus Res 248:53–62. doi: 10.1016/j.virusres.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Bukh J. 2012. Animal models for the study of hepatitis C virus infection and related liver disease. Gastroenterology 142:1279–1287. doi: 10.1053/j.gastro.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Simons JN, Pilot-Matias TJ, Leary TP, Dawson GJ, Desai SM, Schlauder GG, Muerhoff AS, Erker JC, Buijk SL, Chalmers ML. 1995. Identification of two flavivirus-like genomes in the GB hepatitis agent. Proc Natl Acad Sci U S A 92:3401–3405. doi: 10.1073/pnas.92.8.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukh J, Apgar CL, Yanagi M. 1999. Toward a surrogate model for hepatitis C virus: an infectious molecular clone of the GB virus-B hepatitis agent. Virology 262:470–478. doi: 10.1006/viro.1999.9941. [DOI] [PubMed] [Google Scholar]
- 9.Kapoor A, Simmonds P, Gerold G, Qaisar N, Jain K, Henriquez JA, Firth C, Hirschberg DL, Rice CM, Shields S, Lipkin WI. 2011. Characterization of a canine homolog of hepatitis C virus. Proc Natl Acad Sci U S A 108:11608–11613. doi: 10.1073/pnas.1101794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burbelo PD, Dubovi EJ, Simmonds P, Medina JL, Henriquez JA, Mishra N, Wagner J, Tokarz R, Cullen JM, Iadarola MJ, Rice CM, Lipkin WI, Kapoor A. 2012. Serology-enabled discovery of genetically diverse hepaciviruses in a new host. J Virol 86:6171–6178. doi: 10.1128/JVI.00250-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaender S, Cavalleri JM, Walter S, Doerrbecker J, Campana B, Brown RJ, Burbelo PD, Postel A, Hahn K, Anggakusuma Riebesehl N, Baumgartner W, Becher P, Heim MH, Pietschmann T, Feige K, Steinmann E. 2015. Clinical course of infection and viral tissue tropism of hepatitis C virus-like nonprimate hepaciviruses in horses. Hepatology 61:447–459. doi: 10.1002/hep.27440. [DOI] [PubMed] [Google Scholar]
- 12.Ramsay JD. 2017. Science-in-brief: equine viral hepatitis. Equine Vet J 49:138–140. doi: 10.1111/evj.12652. [DOI] [PubMed] [Google Scholar]
- 13.Scheel TK, Kapoor A, Nishiuchi E, Brock KV, Yu Y, Andrus L, Gu M, Renshaw RW, Dubovi EJ, McDonough SP, Van de Walle GR, Lipkin WI, Divers TJ, Tennant BC, Rice CM. 2015. Characterization of nonprimate hepacivirus and construction of a functional molecular clone. Proc Natl Acad Sci U S A 112:2192–2197. doi: 10.1073/pnas.1500265112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapoor A, Simmonds P, Scheel TK, Hjelle B, Cullen JM, Burbelo PD, Chauhan LV, Duraisamy R, Sanchez Leon M, Jain K, Vandegrift KJ, Calisher CH, Rice CM, Lipkin WI. 2013. Identification of rodent homologs of hepatitis C virus and pegiviruses. mBio 4:e00216. doi: 10.1128/mBio.00216-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drexler JF, Corman VM, Muller MA, Lukashev AN, Gmyl A, Coutard B, Adam A, Ritz D, Leijten LM, van Riel D, Kallies R, Klose SM, Gloza-Rausch F, Binger T, Annan A, Adu-Sarkodie Y, Oppong S, Bourgarel M, Rupp D, Hoffmann B, Schlegel M, Kummerer BM, Kruger DH, Schmidt-Chanasit J, Setien AA, Cottontail VM, Hemachudha T, Wacharapluesadee S, Osterrieder K, Bartenschlager R, Matthee S, Beer M, Kuiken T, Reusken C, Leroy EM, Ulrich RG, Drosten C. 2013. Evidence for novel hepaciviruses in rodents. PLoS Pathog 9:e1003438. doi: 10.1371/journal.ppat.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheel TK, Simmonds P, Kapoor A. 2015. Surveying the global virome: identification and characterization of HCV-related animal hepaciviruses. Antiviral Res 115:83–93. doi: 10.1016/j.antiviral.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartlage AS, Cullen JM, Kapoor A. 2016. The strange, expanding world of animal hepaciviruses. Annu Rev Virol 3:53–75. doi: 10.1146/annurev-virology-100114-055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Firth C, Bhat M, Firth MA, Williams SH, Frye MJ, Simmonds P, Conte JM, Ng J, Garcia J, Bhuva NP, Lee B, Che X, Quan PL, Lipkin WI. 2014. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. mBio 5:e01933. doi: 10.1128/mBio.01933-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trivedi S, Murthy S, Sharma H, Hartlage AS, Kumar A, Gadi SV, Simmonds P, Chauhan LV, Scheel TKH, Billerbeck E, Burbelo PD, Rice CM, Lipkin WI, Vandegrift K, Cullen JM, Kapoor A. 2018. Viral persistence, liver disease, and host response in a hepatitis C-like virus rat model. Hepatology 68:435–448. doi: 10.1002/hep.29494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billerbeck E, Wolfisberg R, Fahnoe U, Xiao JW, Quirk C, Luna JM, Cullen JM, Hartlage AS, Chiriboga L, Ghoshal K, Lipkin WI, Bukh J, Scheel TKH, Kapoor A, Rice CM. 2017. Mouse models of acute and chronic hepacivirus infection. Science 357:204–208. doi: 10.1126/science.aal1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. 2005. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 22.Sedano CD, Sarnow P. 2014. Hepatitis C virus subverts liver-specific miR-122 to protect the viral genome from exoribonuclease Xrn2. Cell Host Microbe 16:257–264. doi: 10.1016/j.chom.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Masaki T, Yamane D, McGivern DR, Lemon SM. 2013. Competing and noncompeting activities of miR-122 and the 5' exonuclease Xrn1 in regulation of hepatitis C virus replication. Proc Natl Acad Sci U S A 110:1881–1886. doi: 10.1073/pnas.1213515110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimakami T, Yamane D, Jangra RK, Kempf BJ, Spaniel C, Barton DJ, Lemon SM. 2012. Stabilization of hepatitis C virus RNA by an Ago2-miR-122 complex. Proc Natl Acad Sci U S A 109:941–946. doi: 10.1073/pnas.1112263109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henke JI, Goergen D, Zheng J, Song Y, Schuttler CG, Fehr C, Junemann C, Niepmann M. 2008. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J 27:3300–3310. doi: 10.1038/emboj.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 27.Blight KJ, McKeating JA, Rice CM. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol 76:13001–13014. doi: 10.1128/jvi.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blight KJ, Kolykhalov AA, Rice CM. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972–1974. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- 29.Saeed M, Andreo U, Chung HY, Espiritu C, Branch AD, Silva JM, Rice CM. 2015. SEC14L2 enables pan-genotype HCV replication in cell culture. Nature 524:471–475. doi: 10.1038/nature14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ploss A, Dubuisson J. 2012. New advances in the molecular biology of hepatitis C virus infection: towards the identification of new treatment targets. Gut 61(Suppl 1):i25–i35. doi: 10.1136/gutjnl-2012-302048. [DOI] [PubMed] [Google Scholar]
- 31.Horwitz JA, Dorner M, Friling T, Donovan BM, Vogt A, Loureiro J, Oh T, Rice CM, Ploss A. 2013. Expression of heterologous proteins flanked by NS3-4A cleavage sites within the hepatitis C virus polyprotein. Virology 439:23–33. doi: 10.1016/j.virol.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones CT, Catanese MT, Law LM, Khetani SR, Syder AJ, Ploss A, Oh TS, Schoggins JW, MacDonald MR, Bhatia SN, Rice CM. 2010. Real-time imaging of hepatitis C virus infection using a fluorescent cell-based reporter system. Nat Biotechnol 28:167–171. doi: 10.1038/nbt.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anggakusuma Brown RJ, Banda DH, Todt D, Vieyres G, Steinmann E, Pietschmann T. 2016. Hepacivirus NS3/4A proteases interfere with MAVS signaling in both their cognate animal hosts and humans: implications for zoonotic transmission. J Virol 90:10670–10681. doi: 10.1128/JVI.01634-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohmann V, Hoffmann S, Herian U, Penin F, Bartenschlager R. 2003. Viral and cellular determinants of hepatitis C virus RNA replication in cell culture. J Virol 77:3007–3019. doi: 10.1128/jvi.77.5.3007-3019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartenschlager R, Frese M, Pietschmann T. 2004. Novel insights into hepatitis C virus replication and persistence. Adv Virus Res 63:71–180. doi: 10.1016/S0065-3527(04)63002-8. [DOI] [PubMed] [Google Scholar]
- 36.Krieger N, Lohmann V, Bartenschlager R. 2001. Enhancement of hepatitis C virus RNA replication by cell culture-adaptive mutations. J Virol 75:4614–4624. doi: 10.1128/JVI.75.10.4614-4624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokota T, Sakamoto N, Enomoto N, Tanabe Y, Miyagishi M, Maekawa S, Yi L, Kurosaki M, Taira K, Watanabe M, Mizusawa H. 2003. Inhibition of intracellular hepatitis C virus replication by synthetic and vector-derived small interfering RNAs. EMBO Rep 4:602–608. doi: 10.1038/sj.embor.embor840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witteveldt J, Martin-Gans M, Simmonds P. 2016. Enhancement of the replication of hepatitis C virus replicons of genotypes 1 to 4 by manipulation of CpG and UpA dinucleotide frequencies and use of cell lines expressing SECL14L2 for antiviral resistance testing. Antimicrob Agents Chemother 60:2981–2992. doi: 10.1128/AAC.02932-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saeed M, Scheel TK, Gottwein JM, Marukian S, Dustin LB, Bukh J, Rice CM. 2012. Efficient replication of genotype 3a and 4a hepatitis C virus replicons in human hepatoma cells. Antimicrob Agents Chemother 56:5365–5373. doi: 10.1128/AAC.01256-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JH, Lee SR, Li LH, Park HJ, Park JH, Lee KY, Kim MK, Shin BA, Choi SY. 2011. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One 6:e18556. doi: 10.1371/journal.pone.0018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehrhardt M, Leidinger P, Keller A, Baumert T, Diez J, Meese E, Meyerhans A. 2011. Profound differences of microRNA expression patterns in hepatocytes and hepatoma cell lines commonly used in hepatitis C virus studies. Hepatology 54:1112–1113. doi: 10.1002/hep.24366. [DOI] [PubMed] [Google Scholar]
- 42.Gottwein JM, Pham LV, Mikkelsen LS, Ghanem L, Ramirez S, Scheel TKH, Carlsen THR, Bukh J. 2018. Efficacy of NS5A inhibitors against hepatitis C virus genotypes 1-7 and escape variants. Gastroenterology 154:1435–1448. doi: 10.1053/j.gastro.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Ramirez S, Mikkelsen LS, Gottwein JM, Bukh J. 2016. Robust HCV genotype 3a infectious cell culture system permits identification of escape variants with resistance to sofosbuvir. Gastroenterology 151:973–985. doi: 10.1053/j.gastro.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H. 2010. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. 2013. Treatment of HCV infection by targeting microRNA. N Engl J Med 368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 46.Ottosen S, Parsley TB, Yang L, Zeh K, van Doorn LJ, van der Veer E, Raney AK, Hodges MR, Patick AK. 2015. In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrob Agents Chemother 59:599–608. doi: 10.1128/AAC.04220-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sumpter R Jr, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, Gale M Jr. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol 79:2689–2699. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng TI, Mo H, Pilot-Matias T, He Y, Koev G, Krishnan P, Mondal R, Pithawalla R, He W, Dekhtyar T, Packer J, Schurdak M, Molla A. 2007. Identification of host genes involved in hepatitis C virus replication by small interfering RNA technology. Hepatology 45:1413–1421. doi: 10.1002/hep.21608. [DOI] [PubMed] [Google Scholar]
- 49.Tai AW, Benita Y, Peng LF, Kim SS, Sakamoto N, Xavier RJ, Chung RT. 2009. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe 5:298–307. doi: 10.1016/j.chom.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friebe P, Boudet J, Simorre JP, Bartenschlager R. 2005. Kissing-loop interaction in the 3' end of the hepatitis C virus genome essential for RNA replication. J Virol 79:380–392. doi: 10.1128/JVI.79.1.380-392.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartlage AS, Murthy S, Kumar A, Trivedi S, Dravid P, Sharma H, Walker CM, Kapoor A. 2019. Vaccination to prevent T cell subversion can protect against persistent hepacivirus infection. Nat Commun 10:1113. doi: 10.1038/s41467-019-09105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kato T, Date T, Miyamoto M, Furusaka A, Tokushige K, Mizokami M, Wakita T. 2003. Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology 125:1808–1817. doi: 10.1053/j.gastro.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 53.Bukh J, Pietschmann T, Lohmann V, Krieger N, Faulk K, Engle RE, Govindarajan S, Shapiro M, St Claire M, Bartenschlager R. 2002. Mutations that permit efficient replication of hepatitis C virus RNA in Huh-7 cells prevent productive replication in chimpanzees. Proc Natl Acad Sci U S A 99:14416–14421. doi: 10.1073/pnas.212532699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, Bartenschlager R, Liang TJ. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lohmann V, Korner F, Dobierzewska A, Bartenschlager R. 2001. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J Virol 75:1437–1449. doi: 10.1128/JVI.75.3.1437-1449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo JT, Bichko VV, Seeger C. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J Virol 75:8516–8523. doi: 10.1128/jvi.75.18.8516-8523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanford RE, Guerra B, Lee H, Averett DR, Pfeiffer B, Chavez D, Notvall L, Bigger C. 2003. Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, poly(i)-poly(c), tumor necrosis factor alpha, and ribavirin in hepatitis C virus subgenomic replicons. J Virol 77:1092–1104. doi: 10.1128/jvi.77.2.1092-1104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atkinson NJ, Witteveldt J, Evans DJ, Simmonds P. 2014. The influence of CpG and UpA dinucleotide frequencies on RNA virus replication and characterization of the innate cellular pathways underlying virus attenuation and enhanced replication. Nucleic Acids Res 42:4527–4545. doi: 10.1093/nar/gku075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pham LV, Jensen SB, Fahnoe U, Pedersen MS, Tang Q, Ghanem L, Ramirez S, Humes D, Serre SBN, Schonning K, Bukh J, Gottwein JM. 2019. HCV genotype 1-6 NS3 residue 80 substitutions impact protease inhibitor activity and promote viral escape. J Hepatol 70:388–397. doi: 10.1016/j.jhep.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 60.Lawitz EJ, Dvory-Sobol H, Doehle BP, Worth AS, McNally J, Brainard DM, Link JO, Miller MD, Mo H. 2016. Clinical resistance to velpatasvir (GS-5816), a novel pan-genotypic inhibitor of the hepatitis C virus NS5A protein. Antimicrob Agents Chemother 60:5368–5378. doi: 10.1128/AAC.00763-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bullard-Feibelman KM, Govero J, Zhu Z, Salazar V, Veselinovic M, Diamond MS, Geiss BJ. 2017. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antiviral Res 137:134–140. doi: 10.1016/j.antiviral.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu HT, Colby-Germinario SP, Hassounah SA, Fogarty C, Osman N, Palanisamy N, Han Y, Oliveira M, Quan Y, Wainberg MA. 2017. Evaluation of sofosbuvir (beta-D-2'-deoxy-2'-alpha-fluoro-2'-beta-C-methyluridine) as an inhibitor of Dengue virus replication. Sci Rep 7:6345. doi: 10.1038/s41598-017-06612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Freitas CS, Higa LM, Sacramento CQ, Ferreira AC, Reis PA, Delvecchio R, Monteiro FL, Barbosa-Lima G, James Westgarth H, Vieira YR, Mattos M, Rocha N, Hoelz LVB, Leme RPP, Bastos MM, Rodrigues GOL, Lopes CEM, Queiroz-Junior CM, Lima CX, Costa VV, Teixeira MM, Bozza FA, Bozza PT, Boechat N, Tanuri A, Souza T. 2019. Yellow fever virus is susceptible to sofosbuvir both in vitro and in vivo. PLoS Negl Trop Dis 13:e0007072. doi: 10.1371/journal.pntd.0007072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dao Thi VL, Debing Y, Wu X, Rice CM, Neyts J, Moradpour D, Gouttenoire J. 2016. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology 150:82–85. doi: 10.1053/j.gastro.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 65.Ramirez S, Li YP, Jensen SB, Pedersen J, Gottwein JM, Bukh J. 2014. Highly efficient infectious cell culture of three hepatitis C virus genotype 2b strains and sensitivity to lead protease, nonstructural protein 5A, and polymerase inhibitors. Hepatology 59:395–407. doi: 10.1002/hep.26660. [DOI] [PubMed] [Google Scholar]
- 66.Cerbini T, Funahashi R, Luo Y, Liu C, Park K, Rao M, Malik N, Zou J. 2015. Transcription activator-like effector nuclease (TALEN)-mediated CLYBL targeting enables enhanced transgene expression and one-step generation of dual reporter human induced pluripotent stem cell (iPSC) and neural stem cell (NSC) lines. PLoS One 10:e0116032. doi: 10.1371/journal.pone.0116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carpentier A, Nimgaonkar I, Chu V, Xia Y, Hu Z, Liang TJ. 2016. Hepatic differentiation of human pluripotent stem cells in miniaturized format suitable for high-throughput screen. Stem Cell Res 16:640–650. doi: 10.1016/j.scr.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gottwein JM, Jensen TB, Mathiesen CK, Meuleman P, Serre SB, Lademann JB, Ghanem L, Scheel TK, Leroux-Roels G, Bukh J. 2011. Development and application of hepatitis C reporter viruses with genotype 1 to 7 core-nonstructural protein 2 (NS2) expressing fluorescent proteins or luciferase in modified JFH1 NS5A. J Virol 85:8913–8928. doi: 10.1128/JVI.00049-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simmonds P. 2012. SSE: a nucleotide and amino acid sequence analysis platform. BMC Res Notes 5:50. doi: 10.1186/1756-0500-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Einav S, Sobol HD, Gehrig E, Glenn JS. 2010. The hepatitis C virus (HCV) NS4B RNA binding inhibitor clemizole is highly synergistic with HCV protease inhibitors. J Infect Dis 202:65–74. doi: 10.1086/653080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang Y, Staschke K, De Francesco R, Tan SL. 2007. Phosphorylation of hepatitis C virus NS5A nonstructural protein: a new paradigm for phosphorylation-dependent viral RNA replication? Virology 364:1–9. doi: 10.1016/j.virol.2007.01.042. [DOI] [PubMed] [Google Scholar]