Supplemental Digital Content is Available in the Text.
Keywords: blood flow, brain, exercise, stroke, vascular
Background and Purpose:
Previous work demonstrates that older adults have a lower response in the middle cerebral artery velocity (MCAv) to an acute bout of moderate-intensity exercise when compared with young adults. However, no information exists regarding MCAv response to exercise after stroke. We tested whether MCAv response to an acute bout of moderate-intensity exercise differed between participants 3 months after stroke and an age- and sex-matched control group of older adults (CON). A secondary objective was to compare MCAv response between the stroke- and non-stroke-affected MCAv.
Methods:
Using transcranial Doppler ultrasound, we recorded MCAv during a 90-second baseline (BL) followed by a 6-minute moderate-intensity exercise bout using a recumbent stepper. Heart rate (HR), end-tidal CO2 (PETCO2), and beat-to-beat mean arterial blood pressure (MAP) were additional variables of interest. The MCAv response measures included BL, peak response amplitude (Amp), time delay (TD), and time constant (τ).
Results:
The Amp was significantly lower in the stroke-affected MCAv compared with CON (P < 0.01) and in the nonaffected MCAv compared with CON (P = 0.03). No between-group differences were found between TD and τ. No significant differences were found during exercise for PETCO2 and MAP while HR was lower in participants with stroke (P < 0.01). Within the group of participants with stroke, no differences were found between the stroke-affected and non-stroke-affected sides for any measures.
Discussion and Conclusions:
Resolution of the dynamic response profile has the potential to increase our understanding of the cerebrovascular control mechanisms and test cerebrovascular response to physical therapy–driven interventions such as exercise.
Video Abstract available for more insights from the authors (see the Video, Supplemental Digital Content 1, available at: http://links.lww.com/JNPT/A284).
INTRODUCTION
Current exercise recommendations for people after stroke emphasize the need to regularly engage in aerobic exercise to benefit walking endurance, aerobic fitness, and overall cardiovascular health for secondary stroke prevention.1 Exercise has been shown to improve walking performance,2–4 aerobic fitness,2,4–7 and peripheral vascular health.2 However, few studies have examined how aerobic exercise may influence cerebrovascular health after stroke. A study by Ivey et al8 published in 2011 enrolled individuals with chronic stroke (>6 months) to a 6-month aerobic exercise training or a control group. To assess cerebrovascular health, transcranial Doppler (TCD) ultrasound measured middle cerebral artery (MCA) velocity (MCAv) at rest under normocapnia (normal CO2) and in response to hypercapnia (6% inhalation of carbon dioxide, CO2). The response to hypercapnia has been used to assess cerebrovascular reserve, as MCAv typically increases with exposure to CO2. At the end of the study, those individuals in aerobic exercise group demonstrated a higher MCAv response to hypercapnia than the control group, but no differences were reported during the normocapnic condition. These data suggest that aerobic exercise may improve cerebrovascular reserve and could be important for brain health after stroke.
Ivey et al8 were the first to identify cerebral blood flow changes following an aerobic exercise intervention in chronic stroke. Currently, there is no information characterizing MCAv response during an acute bout of exercise in people after stroke. Understanding the MCAv response from rest to a single bout of exercise may uncover important information and underlying control mechanisms that may be unique to people after stroke. Examining the MCAv response to acute exercise has the potential to identify responders or nonresponders to an exercise bout and whether the MCAv response may change following an exercise intervention. Therefore, our initial work focused on the development of a novel method to assess MCAv response to a change in demand from rest to exercise in healthy adults.9 The data revealed the MCAv dynamic response from rest to moderate-intensity exercise could be characterized and modeled. These data were highly reproducible and similar between the left and right MCAs. Therefore, we concluded in healthy adults that either the right or left MCA could be used to assess the dynamic response to exercise, which is important when studying laterality differences in people after stroke. Additional work from our laboratory reported healthy older adults (n = 15) demonstrated a lower baseline (BL) and dynamic MCAv response during moderate-intensity exercise when compared with young adults (n = 15).10 However, evidence is lacking as to how stroke may affect MCAv at rest and during exercise and how this response may differ in people with stroke when compared with older adults.
The primary objective of this study was to examine whether the MCAv dynamic response differed between individuals at 3 months after stroke and a control group of sedentary, age- and sex-matched adults (control, CON). We hypothesized that individuals after stroke would have a lower (1) BL MCAv and (2) dynamic response when compared with the CON participants. A secondary objective was to determine whether differences exist between the stroke-affected and nonaffected MCAv. We hypothesized that the stroke-affected MCA would have a lower dynamic response than the nonaffected MCA in people after stroke.
METHODS
Participants After Stroke
We recruited individuals during their acute or inpatient rehabilitation stay at the University of Kansas Health System. We then followed up with interested participants via either e-mail or phone call. If interested, we scheduled participants to visit the laboratory at 3 months after stroke. Inclusion criteria were (1) unilateral ischemic MCA stroke, (2) stenosis less than 70% of the carotid artery including the internal carotid artery, confirmed in the medical record, (3) 35 to 95 years of age, (4) physician approval to perform an acute bout of moderate-intensity exercise on a recumbent stepper, (5) able to walk more than 10 m with an assistive device but without physical assistance, and (6) ability to travel to the University of Kansas Medical Center for testing. Exclusion criteria were (1) inability to give consent due to aphasia, (2) inability to perform the alternating leg movements on the seated recumbent stepper (T5XR NuStep, Inc, Ann Arbor, Michigan), (3) diagnosis of Parkinson disease, mild cognitive impairment, Alzheimer disease, or multiple sclerosis, (4) pulmonary disease or dependency on supplemental oxygen, or (5) physician determined the individual to have uncontrolled blood pressure or unsafe to exercise. Stenosis greater than 70% was excluded due to the higher velocities produced by the stenosis.8,11 A radiologist (author L.L.) provided stenosis interpretation of either ultrasound imaging or angiogram in the medical record.
Adult Participants (CON)
The CON participants had inclusion/exclusion criteria that were similar to the participants with stroke, except for stroke-specific criteria. For those adults 60 years and older, we recruited the University of Kansas Alzheimer's Disease Center's (KU ADC) ongoing cohort interested in Alzheimer disease research and prevention. These individuals were classified by the KU ADC as not meeting current physical activity guidelines and considered sedentary per the American College of Sports Medicine12 or underactive.13 These individuals were cognitively normal/nondemented based on neuropsychological testing defined as no scores greater than 1.5 SD below the mean on 2 or more tests in the National Alzheimer's Coordinating Center Uniform Data Set and a Clinical Dementia Rating = 0. The older individuals were determined to have no evidence of neurological disease/stroke using magnetic resonance imaging. This information is already collected as part the KU ADC registry (P30AG035982)
Experimental Procedure
Participants were asked to abstain from caffeine for a minimum of 6 hours, a meal for 2 hours, and vigorous exercise at least 12 hours prior to the study visit.9,10 The University of Kansas Medical Center Human Subjects Committee approved all experimental procedures. Institutionally approved written informed consent was obtained from each individual prior to study participation. After informed consent was completed and participants had been sitting for 20 minutes, we acquired resting heart rate (HR) and blood pressure (ProBP 3400, Welch Allyn, Inc, Skaneateles Falls, New York).
Familiarization
The laboratory room in which the experimental protocol took place was dimly lit, quiet, and maintained a constant temperature between 22° and 24°C.9,10 All external stimuli were kept to a minimum. Participants completed the familiarization session on the same day as the study procedure. Each participant was first familiarized with the equipment. Participants practiced using the recumbent stepper and keeping the prescribed step rate of 90 steps/min while the target work rate was determined. The target work rate was identified by setting the resistance to 15 W and then adjusted at a rate of 10 W every 30 seconds until their target HR for the exercise intensity was achieved (See Protocol Set Up for determination of target HR). Once the target HR was reached, participants continued the recumbent stepper exercise for an additional 2 minutes to ensure steady-state HR could be maintained. Participants were familiarized with the nasal cannula and instructed to breathe only through their nose during the protocol. The nasal cannula was placed in the nares and, if needed, we adjusted the nasal cannula position to ensure optimal end-tidal carbon dioxide (PETCO2 in mm Hg) reading. As in our prior work, we monitored PETCO2 to ensure participants did not hyperventilate, since the result would be a lower PETCO2 and induce cerebral artery vasoconstriction.9
After the familiarization session, we obtained height and weight. In a seated position, participants filled out questionnaires regarding their demographics, medical history, physical activity participation, cardiac risk stratification,14 and nonexercise estimated maximal oxygen uptake ( o2max).15 This lasted approximately 30 minutes. After the questionnaires, the setup for the experimental procedure commenced and participants sat quietly on the recumbent stepper for 20 minutes.
o2max).15 This lasted approximately 30 minutes. After the questionnaires, the setup for the experimental procedure commenced and participants sat quietly on the recumbent stepper for 20 minutes.
Protocol Setup
With respect to the exercise response, our previous work demonstrated that there was no significant difference in the right versus left MCAv response to exercise in healthy adults.9 Herein, the left MCA was the primary vessel of interest for the older adults. For the participants with stroke, both MCA signals were acquired in 1 visit. In the seated position on the stepper, individuals were set up with the following equipment: (1) Transcranial Doppler ultrasound (TCD) (Multigon Industries Inc, Yonkers, New York). An adjustable headband and 2-MHz TCD probes with ultrasonic gel were placed on the temporal window (temple region of the head) for acquisition of MCA blood flow velocity cm.s−1.16 The MCA was accurately identified using practice standards for probe positioning and orientation, MCA depth selection, and velocity flow direction.16 The TCD sonographer, who was blinded to the side of stroke, entered the room only after the participant was set up; (2) A 5-lead electrocardiogram (ECG; Cardiocard, Nasiff Associates, Central Square, New York) recorded HR. (3) A nasal cannula and capnography (BCI Capnocheck Sleep 9004 Smiths Medical, Dublin, Ohio) were used to assess end-tidal carbon dioxide (PETCO2). (4) We recorded beat-to-beat mean arterial pressure (MAP; Finometer, Finapres Medical Systems, Amsterdam, the Netherlands) from the left middle finger. Data acquisition of raw data occurred through an analog-to-digital unit (NI-USB-6212, National Instruments) and custom-written software operating in MATLAB (v2014a, The Mathworks Inc, Natick, Massachusetts). (See the Video, Supplemental Digital Content 1, which demonstrates the experimental procedure setup, available at: http://links.lww.com/JNPT/A284.)
Moderate-intensity exercise was defined as 45% to 55% of the participant's HR reserve. We determined the HR range either by using (1) age-predicted (220 − age) HR maximum (HRmax) or (2) for participants using β-blocker medication, we used 164 − 0.72 × age17 to calculate HRmax. We determined the HR range for the moderate-intensity bout using the Karvonen formula,9,10 HR range = [% exercise intensity (age-predicted HRmax − resting HR)] + resting HR.12 After the setup, the BL recording lasted 90 seconds followed by 6 minutes of moderate-intensity exercise at the targeted HR range. The participants were instructed to maintain a step rate of approximately 90 steps per minute throughout the entire exercise bout and resistance was adjusted to obtain the targeted workloads and HR range.9 Work rate increased during the first 30 seconds of exercise until the target work rate was reached. At the end of exercise, recording stopped and the participant engaged in an active cool-down for 2 minutes and then rested until HR returned to BL. Participants then repeated the 90-second BL assessment and 6 minutes of moderate-intensity exercise. Our previous work revealed improved signal-to-noise ratio when the data acquired from multiple bouts of exercise are averaged.9
Variables were sampled at 500 Hz and then interpolated to 2.0 Hz. Three-second averages were calculated and then smoothed using a 9-second sliding window average.10 We used commercial statistical software (R version 3.2.4, R Core team, Vienna, Austria,18 with the “nls” function package) to model the response. Data with ECG R to R intervals greater than 5 Hz or changes in peak blood flow velocity greater than 10 cm/s in a single cardiac cycle were considered artifact and censored. Acquisitions with more than 15% of data points censored were discarded.
MCAv and Kinetics Response
Kinetics were modeled on the MCAv over the entire exercise bout with a monoexponential model:
where MCAV(t) is the cerebral artery velocity at any point in time, BL is the 90-second BL before the onset of exercise, Amp is the peak amplitude of the response above resting BL, TD is the time delay proceeding the increase in MCAV, and τ is the time constant or time to 63% of the steady-state response. Cerebrovascular response (CVR) is the difference between the BL and mean MCAv sampled between minutes 3 to 4.5 during steady-state exercise.
Statistical Analysis
Data are presented as mean ± SD. Participant characteristics were compared using 1-way analysis of variance (ANOVA) and Fisher exact tests for categorical variables. To assess between-group differences, we performed 1-way ANOVA and Mann-Whitney U tests as appropriate following visual inspection of probability plots and Shapiro-Wilk tests. We analyzed the stroke-affected and non-affected MCA (stroke group) with paired t tests. Differences were considered significant when P < 0.05.
RESULTS
Participant Characteristics
We consented 18 participants with MCA stroke into this study. Four individuals were excluded from analysis due to a valid MCAv signal not acquired (n = 2), poor MCAv signal acquisition during exercise (n = 1), and atrial fibrillation resulting in more than 15% of data points censored (n = 1). Therefore, data presented include 14 participants with stroke unless otherwise indicated. All 18 CON participants who provided consent were included in this data analysis. Participant demographics are presented in Table 1. There were no between-group differences for age, sex, or estimated 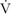 o2max. However, body mass index was significantly higher in the stroke group. No study-related adverse events were reported at any time during the study.
o2max. However, body mass index was significantly higher in the stroke group. No study-related adverse events were reported at any time during the study.
Table 1. Participant Demographicsa.
| CON (n = 18) | CON 95% CI | Stroke (n = 14) | Stroke 95% CI | F Test | P Value | |
|---|---|---|---|---|---|---|
| Age, y | 61.3 ± 12.0 | 55.3-67.2 | 63.6 ± 14.7 | 55.1-72.2 | 0.3 | 0.62 |
| Sex (female), % | 33.0 | 28.6 | 0.54 | 0.54 | ||
| Time after stroke, d | 91.4 ± 7.8 | |||||
| Race/ethnicityb, n | ||||||
| White | 15 | 9 | ||||
| Black | 1 | 5 | ||||
| Native American | 0 | 1 | ||||
| Hispanic/Latino | 1 | 0 | ||||
| Asian | 1 | 0 | ||||
Estimated 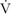 o2max, mL.kg−1.min−1 o2max, mL.kg−1.min−1
|
32.3 ± 9.2 | 27.7-36.9 | 26.0 ± 10.3 | 20.0-31.9 | 3.4 | 0.07 |
| BMI, kg.m−2 | 26.0 ± 3.6 | 24.3-27.8 | 31.1 ± 6.6 | 27.3-34.8 | 7.7 | 0.009 |
| Hypertension, % | 27.8 | 85.7 | 0.001 | |||
| β-Blocker, % | 0 | 50.0 | 0.001 |
Abbreviations: BMI, body mass index; CI, confidence interval; CON, control participants; 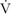 o2max, maximal oxygen uptake.
o2max, maximal oxygen uptake.
aData are presented as mean ± SD unless stated otherwise.
bOne individual selected multiple race categories.
MCAv Kinetics and Moderate-Intensity Exercise
No group differences in BL MCAv in the stroke affected MCAv for participants with stroke (n = 14) and the CON participants (Table 2) were identified. For 2 individuals after stroke, we were unable to clearly obtain a signal for the nonaffected MCA. BL MCAv was not different between the nonaffected BL MCAv in participants with stroke (n = 12) and the CON participants (Table 3).
Table 2. MCAv Kinetic Response Between CON and Stroke-Affected MCAa.
| CON (n = 18) | CON 95% CI | Stroke MCA (n = 14) | Stroke MCA 95% CI | F Test | P Value | |
|---|---|---|---|---|---|---|
| BL, cm.s-1 | 50.4 ± 11.3 | 44.8-56.0 | 49.3 ± 17.5 | 44.8-56.0 | 0.04 | 0.83 |
| TD, s | 59.4 ± 28.4 | 45.3-73.6 | 65.5 ± 32.6 | 45.8-85.2 | 0.30 | 0.59 |
| Amp, cm.s-1 | 10.9 ± 4.4 | 8.7-13.1 | 5.5 ± 4.9 | 2.6-8.4 | 10.7 | <0.01 |
| τ, s | 40.4 ± 33.5 | 23.7-57.1 | 35.6 ± 45.7 | 8.0-63.2 | 89.0 | 0.28 |
| CVR, cm.s-1 | 11.1 ± 4.4 | 8.9-13.3 | 5.0 ± 4.6 | 2.3-7.7 | 14.4 | <0.01 |
Abbreviations: Amp, amplitude; BL, baseline; CI, confidence interval; CON, control participants; CVR, cerebrovascular response; MCAv, middle cerebral artery velocity; τ, time constant; TD, time delay.
aData are presented as mean ± SD.
Table 3. MCAv Kinetic Response Between CON and Nonaffected MCAa.
| CON (n = 18) | CON 95% CI | Nonstroke MCA (n = 12) | Nonstroke MCA 95% CI | F Test | P Value | |
|---|---|---|---|---|---|---|
| BL, cm.s-1 | 50.4 ± 11.3 | 44.8-56.0 | 56.2 ± 15.8 | 45.6-66.8 | 1.3 | 0.26 |
| TD, s | 59.4 ± 28.4 | 43.7-67.5 | 50.3 ± 41.5 | 18.5-82.2 | 0.5 | 0.51 |
| Amp, cm.s-1 | 10.9 ± 4.4 | 8.7-13.1 | 6.4 ± 6.0 | 2.1-10.6 | 5.1 | 0.03 |
| τ, s | 40.4 ± 33.5 | 23.7-57.1 | 28.4 ± 19.8 | 13.2-43.7 | 62.5 | 0.35 |
| CVR, cm.s-1 | 11.1 ± 4.4 | 8.9-13.3 | 7.4 ± 6.0 | 3.3-11.4 | 3.7 | 0.07 |
Abbreviations: Amp, amplitude; BL, baseline; CI, confidence interval; CON, control participants; CVR, cerebrovascular response; MCAv, middle cerebral artery velocity; τ, time constant; TD, time delay.
aData are presented as mean ± SD.
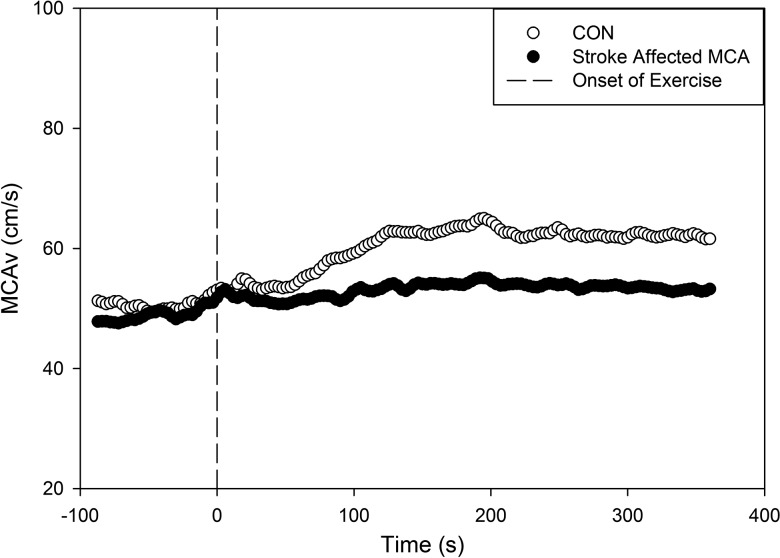
MCAv response increased from rest (BL) to exercise in a close-to-exponential pattern and was fit by the delay + exponential function in all but 2 participants with MCA stroke.9 The Amp was significantly lower in the stroke-affected MCAv compared with the CON participants (P < 0.01) (Table 2 and Figure 1), and was also lower in the nonaffected MCAv and compared with the CON group (P = 0.03). No between-group differences were found for TD and τ. CVR sampled between minutes 3 and 4.5 during steady-state exercise was significantly lower in the stroke-affected MCA (P < 0.01) but not for the nonaffected MCA (P = 0.07) when compared with CON (see Tables 2 and 3).
Figure 1.
MCAv kinetic response in CON (n = 18) and stroke-affected middle cerebral artery (n = 14). CON, control participants; MCAv, middle cerebral artery velocity.
Baseline and Exercise Response for MAP, PETCO2, and HR
The BL and exercise response for MAP, PETCO2, and HR are presented in Table 4. No significant between-groups differences were identified for these values at rest. During the moderate-intensity exercise bout, only HR differed between groups. The moderate-intensity work rate was significantly lower for participants after stroke compared with CON while the rate of perceived exertion (RPE) was not different.
Table 4. Exercise Parameters Comparing CON and Strokea.
| CON (n = 18) | CON 95% CI | Stroke (n = 14) | Stroke 95% CI | F Test | P Value | |
|---|---|---|---|---|---|---|
| BL MAP, mm Hg | 75.6 ± 11.3 | 69.9-81.2 | 77.7 ± 17.6 | 67.1-88.3 | 0.2 | 0.68 |
| SS MAP, mmHg | 100.1 ± 15.5 | 92.4-107.9 | 97.4 ± 20.2 | 85.2-109.6 | 0.2 | 0.68 |
| BL PETCO2, mm Hg | 32.9 ± 4.3 | 30.8-35.1 | 35.1 ± 5.2 | 31.9-38.2 | 1.5 | 0.23 |
| SS PETCO2, mm Hg | 38.0 ± 6.9 | 34.6-41.5 | 38.9 ± 5.1 | 35.8-42.0 | 0.2 | 0.70 |
| BL HR, bpm | 66.3 ± 10.1 | 61.3-71.3 | 65.1 ± 9.7 | 59.2-71.0 | 0.1 | 0.74 |
| SS HR, bpm | 109.0 ± 10.4 | 103.9-114.2 | 93.2 ± 15.1 | 84.0-102.3 | 12.1 | <0.01 |
| Workload, W | 114.1 ± 31.5 | 98.5-129.8 | 52.9 ± 22.2 | 40.0-65.7 | 38.1 | <0.001 |
| RPE | 12.6 ± 1.6 | 11.8-13.3 | 13.0 ± 2.8 | 11.3-14.5 | 0.2 | 0.63 |
Abbreviations: BL, baseline; CI, confidence interval; CON, control participants; HR, heart rate; MAP, mean arterial pressure; PCO2, end-tidal carbon dioxide; RPE, rate of perceived exertion; SS, steady-state exercise.
aData are presented as mean ± SD.
Within-Group Comparison for Participants With Stroke
For the 12 participants with bilateral MCAv data, no significant differences were found between the variables of interest. BL MCAv for the stroke-affected side was 53.3 ± 17.4 cm.s−1 compared with the nonaffected side 56.2 ± 15.8 cm.s−1 (P = 0.27). Following exercise onset, TD (the time delay proceeding the increase in MCAV) for the stroke-affected MCA was 64.7 ± 33.9 seconds compared with the non-stroke-affected side, 50.3 ± 41.5 seconds (P = 0.12). The τ response (time to 63% of the steady-state response) for the stroke-affected side was 39.9 ± 52.4 seconds when compared with non-stroke-affected MCA, 28.4 ± 19.8 seconds (P = 0.56). The large standard deviation of the τ variable for the stroke-affected MCA was primarily driven by one individual who had a τ response of 170 seconds with a low Amp response. The within group comparison showed an increase in Amp above BL MCAv 6.1 ± 5.1 vs 6.4 ± 5.3 cm.s−1 (P = 0.88) for the stroke-affected side versus the nonaffected side, respectively. We report that CVR (steady-state response) was 5.5 ± 4.7 cm.s−1 for the stroke-affected MCAv when compared with the non-stroke-affected MCAv 7.4 ± 6.0 cm.s−1 (P = 0.19).
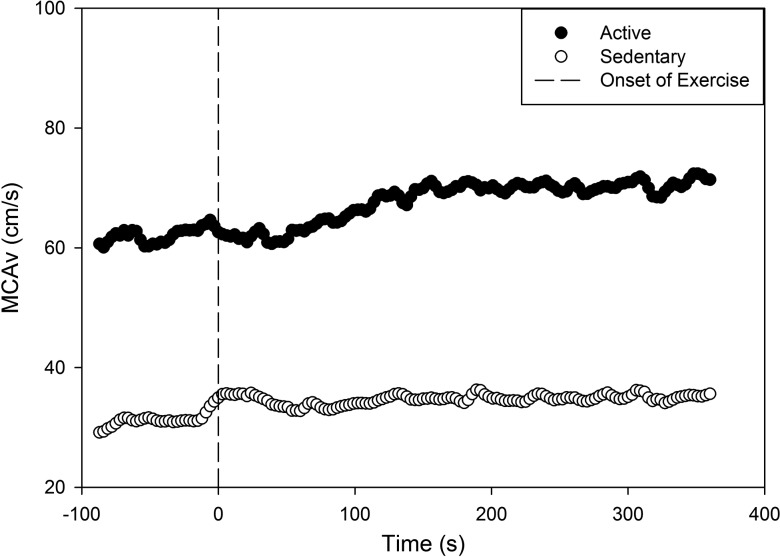
Although no differences were found between the stroke-affected and non-stroke-affected MCAv in the stroke group, in 2 male participants with stroke we observed a noticeable difference in MCAv response despite both participants being of similar age. One was an individual who was physically active and participated in running events prior to stroke; his estimated 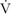 o2max15 was 43.8 mL.kg−1min−1. This individual was starting to return to exercise at the time of the study visit; he had a visibly higher BL and Amp response during moderate-intensity exercise than the other male participant with stroke (estimated
o2max15 was 43.8 mL.kg−1min−1. This individual was starting to return to exercise at the time of the study visit; he had a visibly higher BL and Amp response during moderate-intensity exercise than the other male participant with stroke (estimated 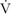 o2max15 = 26.0 mL.kg−1.min−1) whose lifestyle was more sedentary (Figure 2).
o2max15 = 26.0 mL.kg−1.min−1) whose lifestyle was more sedentary (Figure 2).
Figure 2.
MCAv kinetic response in 2 male participants after stroke. The black circle represents a 51-year-old active man prior to stroke. The open circle represents a 53-year-old sedentary man prior to stroke. MCAv, middle cerebral artery velocity.
DISCUSSION
In the current study, we present data using a novel technique for examining the kinetics of cerebrovascular regulation in people with stroke. The present investigation provides novel information regarding the dynamic MCAv response from rest to moderate-intensity exercise in people with stroke. To our knowledge, these data are the first to report the MCAv BL and kinetic parameters (Amp, TD, and τ) in people 3 months after stroke and compare these responses to age- and sex-matched peers. We demonstrate that an increased work rate systematically elevated the MCAv Amp in both groups. However, in those at 3 months after stroke, the Amp was significantly reduced in comparison to healthy controls.
MCAv Kinetics and Moderate-Intensity Exercise
At rest, BL values were not significantly different between the CON group and the stroke-affected and non-stroke-affected MCA. Our data in Figure 1 reveal that Amp was significantly blunted in the bilateral MCA after stroke when compared with CON. While this result may be expected given the event of a stroke, these data provide objective information regarding the dynamic response from rest to moderate-intensity exercise. We observed a slight increase in MCAv just prior to exercise onset in both groups. At 15 seconds prior to exercise onset, we indicate the time remaining before exercise begins. It is possible that there is an anticipatory response to exercise onset with a concomitant increase in brain blood flow. Our future work will explore dynamic responses for HR, MAP, and PETCO2 to determine which physiologic variable influences MCAv response in people with stroke. With the current emphasis on exercise and stroke recovery, this methodology and resultant data could provide evidence regarding cerebrovascular responsiveness during various exercise intensities after stroke.
In addition to typical cardiovascular metrics, (eg, blood pressure, cholesterol, and BMI), the MCAv dynamic response may provide insight regarding which individuals may or may not respond to specific exercise training protocols. Understanding the MCAv dynamic response may help guide exercise prescription for brain health after stroke. As observed in Figure 2, these individuals revealed different BL MCAv and Amp of the response. Future work may be able to determine who benefits from a more tailored exercise intervention versus meeting the physical activity recommendations.12 An important avenue for future research could focus on whether the MCAv response is influenced by an exercise intervention (eg, increases Amp and CVR) and whether this change is related to improved fitness or walking endurance in people after stroke.
As reported in our previous work, TD and τ were highly variable for both the stroke and CON groups.9,10 Mean TD values have been reported at 55.3 seconds for younger adults and 60.7 seconds for the 3 older adults we studied in our previous work.9 In this present investigation, the CON group TD was 59.4 seconds while TD for the stroke-affected and non-stroke-affected MCA was 65.5 and 50.3 seconds, respectively. The slower response in stroke-affected TD could be the direct result of the stroke. Furthermore, the possibility exists that slower kinetic response is a likely combination of reduced partial pressure of oxygen in the microvasculature and impaired cerebral metabolic control.10 Evidence suggests that individuals with acute stroke demonstrate impaired cerebrovascular regulation.19 A recent study examined cerebral autoregulation at rest and during changes in head of bed positioning during the acute hospital stay. The study concluded that impairments in cerebral autoregulation do exist and those with worse autoregulation showed the most impairment in the CVR during head of bed postural changes. These findings and those presented in the current study provide evidence of impaired CVR especially with challenges to the cerebrovascular system such as posture change and exercise. Therefore, there is a clear need to increase our understanding in this area of research and whether activities (ie, early mobilization or exercise) along the continuum of stroke recovery would improve the MCAv dynamic response profile. We believe understanding cerebral blood flow and the kinetic response to exercise after stroke has the potential to provide novel information regarding brain health and, as we stated previously, is an important future direction of scientific inquiry.
MCAv, and Work Rate, MAP, PETCO2, and HR
Across the aging spectrum, it is well established that MAP increases from higher peripheral resistance20 and the potential exists to observe important differences between individuals after stroke and their peers. There is evidence for sex differences in the exercise-induced response for MAP, with older women exhibiting a greater pressure response.21,22 Therefore, a strength of this study was closely matching participants for age and sex. The data in Table 4 demonstrate no significant differences were observed at rest or during exercise for MAP and PETCO2. Resting HR was not significant between the 2 groups while HR during steady-state exercise was significantly different. One potential rationale for the lower HR response in the people after stroke is the use of β-blockers, which are known to blunt the exercise response. Approximately 50% of the participants in the stroke group reported taking β-blockers, while none of the CON participants used these medications. For those taking β-blockers, the equation, HRmax = 164 − 0.72 × age, was used, which may not represent an accurate maximum HR response. Future work should consider using an exercise test to obtain maximal HR for exercise prescription. All of our participants exercised within age-predicted HR range. However, we acknowledge that the significantly lower workload in the participants with stroke may have contributed to the lower HR response since both the stroke-affected and non-stroke-affected MCAv Amp were significantly lower than CON. This is intriguing since MAP was not different, which has a strong influence on MCAv.9 We also report that PETCO2 and RPE were not different between groups during the acute bout of moderate-intensity exercise. These findings offer support for future research examining whether an exercise intervention could improve exercise tolerance, thereby increasing the MCAv dynamic response to exercise in people with stroke.
Within-Group Comparison for Participants With Stroke
Once individuals are discharged from stroke care, there is little information available regarding cerebrovascular regulation and the long-term implications for maintaining optimal brain health. While the brain's metabolic demand increases during exercise and cerebral blood flow increases, evidence suggests differences exist between young and older adults,10,23,24 while our understanding after stroke is still in its infancy. In a first of kind analysis, we present objective information regarding MCAv dynamics comparing both the stroke-affected and nonaffected sides. In this group of people 3 months after MCA stroke, our hypothesis was not supported.
The data showed that the MCAV BL and kinetics analysis was not different between the stroke-affected and nonaffected sides. The possibility exists that the participants had a small lesion size, which may have contributed to our findings. Future work may benefit from collecting magnetic resonance imaging data to obtain stroke lesion size, which likely impacts the MCA hemodynamic response to exercise. We did ensure that the carotid arteries were patent and did not have stenosis greater than 70%, as this has the potential to impact MCA blood flow velocity after stroke.8 We also acknowledge that the data are limited by a small sample size.
We present data on 2 participants with stroke, similar in age and sex, with differing levels of prestroke physical activity. We observed a noticeable difference (Figure 2) in MCAv response in one individual who was physically active and his estimated 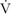 o2max15 was 43.8 mL.kg−1.min−1 while the other individual was sedentary with a lower estimated
o2max15 was 43.8 mL.kg−1.min−1 while the other individual was sedentary with a lower estimated 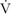 o2max (26.0 mL.kg−1.min−1). We find these results novel and they provide a compelling impetus to further explore exercise and aerobic fitness for brain health after stroke. In humans, evidence exists that individuals across the aging spectrum who are physically active and demonstrate higher
o2max (26.0 mL.kg−1.min−1). We find these results novel and they provide a compelling impetus to further explore exercise and aerobic fitness for brain health after stroke. In humans, evidence exists that individuals across the aging spectrum who are physically active and demonstrate higher 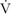 o2max values have better cerebral hemodynamics than sedentary individuals.25,26 Further, physically fit individuals demonstrate better white matter integrity,27 brain volume,28 and cerebral perfusion,29 which further supports the notion that aerobic exercise benefits overall brain health.
o2max values have better cerebral hemodynamics than sedentary individuals.25,26 Further, physically fit individuals demonstrate better white matter integrity,27 brain volume,28 and cerebral perfusion,29 which further supports the notion that aerobic exercise benefits overall brain health.
Perspective for Stroke Recovery
It is well established that moderate-intensity exercise benefits walking distance (6-minute walk test),2–4 aerobic fitness,2,4–7 and peripheral vascular health.2 We now provide a potential method for assessing CVR during exercise and following exercise interventions. Transcranial Doppler ultrasound combined with beat-to-beat blood pressure, as studied herein, allows for the assessment of rapid blood pressure changes with a concomitant MCAv response. Further, the MCAv kinetic response profile has the potential to reveal new insights for cerebrovascular health in response to stroke rehabilitation and exercise interventions. There is a clear need to test innovative methods and outcomes to identify potential cerebrovascular changes that affect optimal brain health. We believe that the physical challenge of exercise during the assessment of CVR may provide unique information regarding cerebrovascular control mechanisms versus resting conditions or steady-state exercise.9 Using a dynamic, physiologic challenge would allow for early identification of cerebrovascular impairment that could have meaningful clinical and global implications for brain health. This information may also be valuable for guiding aerobic exercise prescription for individuals with stroke. Studies by our group and others have shown implementation of aerobic exercise after stroke is lacking. This work highlights the need for further research into use of aerobic exercise post stroke and brain health.30,31
Limitations
Several limitations in the study design should be considered. First, as with all studies using transcranial Doppler ultrasound, the assumption of constant MCA diameter is important in order for the MCAV to be used as a direct proxy for cerebral blood flow. Therefore, as in our previous work9,32 and others,33–35 we assume that MCA diameter remains constant throughout the experimental procedure. Second, maximal exercise testing was not performed to determine maximal HR to more accurately reflect the moderate-intensity exercise session. Rather, we used an age-predicted maximal HR equation instead. Future investigation should consider how the MCAv kinetic response profile is impacted by age, stroke lesion size, and multiple comorbid conditions. Finally, all of our participants were community ambulators and could walk with/without an assistive device, which limits generalizability to all individuals after stroke. We did not study lower-extremity strength or motor function, which may be a factor in our results. However, all participants completed the 6-minute bout of moderate-intensity exercise with no adverse events.
CONCLUSIONS
The present investigation demonstrates that MCAv and its kinetic response profile can be characterized in people after stroke and has the potential to reveal novel information about cerebrovascular health. Bilateral MCAv in the participants with stroke demonstrated lower Amp and CVR when compared with CON, suggesting the presence of altered cerebrovascular control at 3 months after stroke. These results support the idea that characterization of the dynamic cerebral blood flow response to exercise is an important and valuable assessment with the potential to assess improvements in these measures to various therapeutic interventions.
Supplementary Material
Footnotes
Dr Billinger was supported by K01HD067318 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Ms Whitaker and Ms Kempf were supported in part by T32HD057850 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr Billinger received support from the Wohlgemuth Faculty Scholar Award. Dr Perdomo received partial support from the University of Kansas Alzheimer's Disease Center (P30AG035982). REDCap at University of Kansas Medical Center is supported by Clinical and Translational Science Awards (CTSA) Award # ULTR000001 from National Center for Research Resources (NCRR) and National Center for Advancing Translational Sciences (NCATS) awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research. This work was supported by a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (# UL1TR002366). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS. The Georgia Holland Research in Exercise and Cardiovascular Health (REACH) laboratory space was supported by the Georgia Holland Endowment Fund.
A portion of this data set was presented at Combined Sections Meeting, APTA, January 2019.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal's Web site (www.jcrpjournal.com).
The authors declare no conflicts of interest.
REFERENCES
- 1.Billinger SA, Arena R, Bernhardt J, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532–2553. [DOI] [PubMed] [Google Scholar]
- 2.Billinger SA, Mattlage AE, Ashenden AL, Lentz AA, Harter G, Rippee MA. Aerobic exercise in subacute stroke improves cardiovascular health and physical performance. J Neurol Phys Ther. 2012;36(4):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83(12):1703–1707. [DOI] [PubMed] [Google Scholar]
- 4.Tang A, Sibley KM, Thomas SG, et al. Effects of an aerobic exercise program on aerobic capacity, spatiotemporal gait parameters, and functional capacity in subacute stroke. Neurorehabil Neural Repair. 2009;23(4):398–406. [DOI] [PubMed] [Google Scholar]
- 5.Mackay-Lyons M, McDonald A, Matheson J, Eskes G, Klus MA. Dual effects of body-weight supported treadmill training on cardiovascular fitness and walking ability early after stroke: a randomized controlled trial. Neurorehabil Neural Repair. 2013;27(7):644–653. [DOI] [PubMed] [Google Scholar]
- 6.Globas C, Becker C, Cerny J, et al. Chronic stroke survivors benefit from high-intensity aerobic treadmill exercise: a randomized control trial. Neurorehabil Neural Repair. 2012;26(1):85–95. [DOI] [PubMed] [Google Scholar]
- 7.Macko RF, DeSouza CA, Tretter LD, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients. A preliminary report. Stroke. 1997;28(2):326–330. [DOI] [PubMed] [Google Scholar]
- 8.Ivey FM, Ryan AS, Hafer-Macko CE, Macko RF. Improved cerebral vasomotor reactivity after exercise training in hemiparetic stroke survivors. Stroke. 2011;42(7):1994–2000. [DOI] [PubMed] [Google Scholar]
- 9.Billinger SA, Craig JC, Kwapiszeski SJ, et al. Dynamics of middle cerebral artery blood flow velocity during moderate intensity exercise. J Appl Physiol (1985). 2017;122(5):1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward JL, Craig JC, Liu Y, et al. Effect of healthy aging and sex on middle cerebral artery blood velocity dynamics during moderate intensity exercise. Am J Phys Heart Circ Physiol. 2018;315(3):H492–H501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo ZN, Liu J, Xing Y, et al. Dynamic cerebral autoregulation is heterogeneous in different subtypes of acute ischemic stroke. PLoS One. 2014;9(3):e93213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American College of Sports Medince Riebe D, Ehrman JK, Liguori G, Magal M. ACSM's Guidelines for Exercise Testing and Prescription. India-napolis, IN: Wolters Kluwer; 2018. [Google Scholar]
- 13.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4):A118. [PMC free article] [PubMed] [Google Scholar]
- 14.Pescatello LS, American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 9th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. [Google Scholar]
- 15.Jurca R, Jackson AS, LaMonte MJ, et al. Assessing cardiorespiratory fitness without performing exercise testing. Am J Prev Med. 2005;29(3):185–193. [DOI] [PubMed] [Google Scholar]
- 16.Alexandrov AV, Sloan MA, Wong LK, et al. Practice standards for transcranial Doppler ultrasound: part I—test performance. J Neuroimaging. 2007;17(1):11–18. [DOI] [PubMed] [Google Scholar]
- 17.Brawner CA, Ehrman JK, Schairer JR, Cao JJ, Keteyian SJ. Predicting maximum heart rate among patients with coronary heart disease receiving beta-adrenergic blockade therapy. Am Heart J. 2004;148(5):910–914. [DOI] [PubMed] [Google Scholar]
- 18.A Language and Environment for Statistical Computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 19.Reinhard M, Roth M, Guschlbauer B, et al. Dynamic cerebral autoregulation in acute ischemic stroke assessed from spontaneous blood pressure fluctuations. Stroke. 2005;36(8):1684–1689. [DOI] [PubMed] [Google Scholar]
- 20.Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol. 2012;590(9):2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin WH, 3rd, Ogawa T, Kohrt WM, et al. Effects of aging, gender, and physical training on peripheral vascular function. Circulation. 1991;84(2):654–664. [DOI] [PubMed] [Google Scholar]
- 22.Trinity JD, Layec G, Hart CR, Richardson RS. Sex-specific impact of aging on the blood pressure response to exercise. Am J Physiol Heart Circ Physiol. 2018;314(1):H95–H104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsden KR, Haykowsky MJ, Smirl JD, et al. Aging blunts hyperventilation-induced hypocapnia and reduction in cerebral blood flow velocity during maximal exercise. Age (Dordr). 2012;34(3):725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarumi T, Zhang R. Cerebral blood flow in normal aging adults: cardiovascular determinants, clinical implications, and aerobic fitness. J Neurochem. 2018;144(5):595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ainslie PN, Cotter JD, George KP, et al. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586(16):4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey DM, Marley CJ, Brugniaux JV, et al. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke. 2013;44(11):3235–3238. [DOI] [PubMed] [Google Scholar]
- 27.Tseng BY, Gundapuneedi T, Khan MA, et al. White matter integrity in physically fit older adults. Neuroimage. 2013;82:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng BY, Uh J, Rossetti HC, et al. Masters athletes exhibit larger regional brain volume and better cognitive performance than sedentary older adults. J Magn Reson Imaging. 2013;38(5):1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu N, Jacobs DR, Jr, Schreiner PJ, et al. Cardiorespiratory fitness and brain volume and white matter integrity: The CARDIA Study. Neurology. 2015;84(23):2347–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyne P, Billinger S, MacKay-Lyons M, et al. Aerobic exercise prescription in stroke rehabilitation: a web-based survey of US physical therapists. J Neurol Phys Ther. 2017;41(2):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathoo C, Buren S, El-Haddad R, et al. Aerobic training in Canadian stroke rehabilitation programs. J Neurol Phys Ther. 2018;42(4):248–255. [DOI] [PubMed] [Google Scholar]
- 32.Sisante JV, Vidoni ED, Kirkendoll K, et al. Blunted cerebrovascular response is associated with elevated beta-amyloid. J Cereb Blood Flow Metab. 2019;39(1):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher JP, Ogoh S, Young CN, Raven PB, Fadel PJ. Regulation of middle cerebral artery blood velocity during dynamic exercise in humans: influence of aging. J Appl Physiol (1985). 2008;105(1):266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ide K, Horn A, Secher NH. Cerebral metabolic response to submaximal exercise. J Appl Physiol (1985). 1999;87(5):1604–1608. [DOI] [PubMed] [Google Scholar]
- 35.Ide K, Pott F, Van Lieshout JJ, Secher NH. Middle cerebral artery blood velocity depends on cardiac output during exercise with a large muscle mass. Acta Physiol Scand. 1998;162(1):13–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.