Abstract
Introduction:
Multiple studies have reported higher rates of glioma in areas with higher socioeconomic status (SES) but have not stratified by other factors, including race/ethnicity or urban versus rural location.
Methods:
We identified the average annual age-adjusted incidence rates and calculated hazard ratios for death for glioma of various subtypes, stratified by a county-level index for SES, race/ethnicity, US region, and rural/urban status.
Results:
Rates of glioma were highest in counties with higher SES (rate ratio=1.18, 95%CI=1.15-1.22 comparing highest to lowest quintiles, p<0.001). Stratified by race/ethnicity, higher rates in high SES counties persisted for White non-Hispanic individuals. Stratified by rural/urban status, differences in incidence by SES were more pronounced among urban counties. Survival was higher for residents of high SES counties after adjustment for age and extent of resection (HR=0.82, 95%CI=0.76-0.87 comparing highest to lowest quintile of SES, p<0.001). Survival was higher among White Hispanic, Black, and Asian or Pacific Islander individuals compared to White non-Hispanic individuals, after adjustment for age, SES, extent of resection, and when restricted to those with glioblastoma who received radiation and chemotherapy.
Conclusion:
Incidence of glioma was higher in US counties of high compared to low SES. These differences were most pronounced among White non-Hispanic individuals and White Hispanic individuals, in urban areas. We observed better survival in high SES counties, even when adjusting for extent of resection, and when restricting to those who received radiation and chemotherapy for glioblastoma. Differences in incidence and survival were associated with SES and race, rather than rural/urban status.
Keywords: incidence, survival, glioma, glioblastoma, socioeconomic, disparity
Precis:
This study of nearly all tumors diagnosed in the United States over a five year period demonstrated higher incidence of glioma in counties of high SES compared to low SES, although these differences were generally restricted to White non-Hispanic and White Hispanic individuals, in urban rather than rural areas. In a survival analysis including approximately 28% of the cases from the incidence analysis, survival was better in high SES compared to low SES counties, even when adjusting for extent of resection, and when restricting to those who received radiation and chemotherapy for glioblastoma.
Introduction
Socioeconomic status (SES) is associated with risk of multiple cancers, which may be due to its correlation with increased case ascertainment or causal risk factors.1-4 Whereas higher SES is associated with better access to healthcare, cancer screening, and diagnostic tools, lower SES is associated with many factors that affect risk of cancer, such as exposure to environmental and occupational pollutants.5, 6
Glioma incidence is higher in areas of higher SES in the US and Europe.7-11 These disparities have been observed for over 30 years. In 1991, Demers et al. reported a pattern of higher rates of brain tumors, including glioma and astrocytoma, with increasing SES among White men, using data from 1969 to 1978.12 In 2006, Deorah et al. reported disparities in glioma incidence by race/ethnicity, with highest rates among White men living in metropolitan counties.13 More recently, several studies in the US and Europe have reported relative risks of glioma comparing high to low SES areas ranging from 1.1 to 1.2, using a variety of definitions of SES.8, 9 These findings are unlikely to be solely the result of ascertainment bias, given that glioma, and especially its high grade form, glioblastoma (GBM), typically is rapidly progressive and fatal.7, 14 Although some cancer sites are diagnosed at higher rates in areas of high SES simply due to better access to screening programs and healthcare, there are no screening strategies for glioma and the vast majority of cases present suddenly and characteristically, without preceding symptoms. Therefore, while lower SES is expected to correlate with shorter survival due to worse access to comprehensive healthcare, the association between higher SES and higher incidence of glioma is less easily explained. Few risk factors for glioma have currently been identified, and the risk factors that commonly explain variations in incidence by SES for cancers of other sites, such as prevalence of infectious diseases, access to screening and preventive treatments, and specific occupational and environmental exposures (e.g., lead), have not been shown to be associated with glioma incidence.1, 4, 14-16
Because the factors that drive the existing disparities in glioma incidence by SES have not been adequately explained, further exploration of these disparities with stratification by race/ethnicity and urbanicity may aid researchers in identifying underlying causal factors and focusing prevention efforts. With additional information on possible population characteristics associated with glioma incidence, researchers may be better equipped to identify and intervene on causal factors underlying these disparities. Similarly, identifying associations between sociodemographic variables and survival after glioma diagnosis can help target strategies to eliminate previously observed disparities.
To accomplish these aims, in this study, we used data from the Central Brain Tumor Registry of the United States (CBTRUS), the American Community Survey (ACS), and the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) program to examine the association between county-level SES and incidence and survival for glioma. We also stratified by race/ethnicity, geographic region, and rural versus urban status to identify variation in incidence and survival by SES and these factors. Our goal was to summarize the associations between several sociodemographic features, including SES, race/ethnicity, and urbanicity, and glioma incidence and survival.
Methods
Data Sources
This study was approved as an exempt study by the University Hospitals Cleveland Medical Center Institutional Review Board. Data were obtained from the CBTRUS through a data release agreement with the CDC’s National Program of Cancer Registries (NPCR), which includes incidence data from 100% of the US population.17, 18 These data are derived from 51 central cancer registries (50 state registries and Washington D.C.). Incidence data were obtained via CBTRUS, while survival data were obtained only via SEER.
Socioeconomic Metrics and Urban/Rural Status
Data were collected on multiple county-level socioeconomic factors from the 2006-2010 ACS five year estimates, including percentage of residents ≥25 years old with less than high school education, percentage of residents with at least a bachelor’s degree, percentage of families whose incomes are below the poverty level, median household income, percentage of persons aged ≥16 who are unemployed, and percentage of persons who work in management, business, science, and arts occupations.19 A county-level SES metric was constructed by normalizing each of these variables, assigning negative values to percentage of residents with less than high school education, percentage of families below the poverty level, and unemployment rate.3 The variables were then summed, generating a SES index with a mean of 0, which was then dichotomized into high (0 or higher) versus low (below 0), and also categorized by quintiles (Supplementary Figure 1). This procedure was based on a previously published method.3 The United States Department of Agriculture’s 2013 Rural Urban Continuum Codes (RUCCs), which classify counties by population size and proximity to a metropolitan area, were used to classify counties as rural or urban (rural RUCC=4-9; urban RUCC=1-3) (Supplementary Figure 2).20 Counties were categorized into U.S. regions (New England, Middle Atlantic, East North Central, East South Central, West South Central, Mountain, Pacific, West North Central, or South Atlantic) according to the official census divisions of the United States.
Statistical Analysis
Average annual age-adjusted incidence rates (AAAIR) with 95% confidence intervals (95%CIs) were generated using SEER*Stat 8.3.521 from 2011-2015, by SES, histology, urban/rural location of residence, region of the US, and race/Hispanic ethnicity22). To adjust for differences in age distribution between populations, all rates were standardized to the 2000 US population and reported per 100,000. Histologic groups were classified using International Classification of Diseases for Oncology 3rd edition (ICD-O-3) codes, and were defined based on the CBTRUS histologic grouping scheme (Supplementary Table 1).17, 23,24 Incidence rate ratios (IRRs) generated using the stratified age-adjusted incidence rates were used to compare groups, and p values were calculated using the formulas described by Fay et al. to test if IRR were significantly different from 125 IRRs were considered statistically significantly different when the p-value was less than 0.05. Linear regression was used to model the association between county-level incidence rates (including counties with >5 cases during the time period) and SES, urban vs. rural status, and percentage of the county that is White non-Hispanic in both univariable and multivariable analyses adjusted for these factors and state of diagnosis.
Most central cancer registries in the US routinely collect limited data on clinical variables. The SEER program, which represents about 28% of the cases included in the CBTRUS dataset, collects a broader range of information relevant to clinical outcomes.26-28 Adults 18 years or older at diagnosis with histologic confirmation from 2000-2015 were included in survival analyses (followed until December 31, 2015 regardless of year of diagnosis), representing a limited subset of the overall dataset used for incidence analyses. A custom dataset was obtained containing additional treatment data fields, including chemotherapy and radiation.28 While treatment information in SEER has previously been shown to have high positive predictive value (95% for radiation and 90% for chemotherapy),29 sensitivity is only moderate. SEER strongly recommends that ‘no’ values be treated as missing data and that comparisons not be made between individuals identified as having treatment and those not so identified. As a result, treatment groups were defined using ‘yes’ values for beam radiation and/or chemotherapy. Additional survival analyses were performed using Cox proportional hazard models in R version 3.5.0,30 and were adjusted for known prognostic variations including age of diagnosis and extent of resection. These Cox models were further adjusted for SES, urban vs. rural status, and percentage of the county that is White non-Hispanic, in both univariable and multivariable models.
Results
Incidence of Glioma by Socioeconomic Status
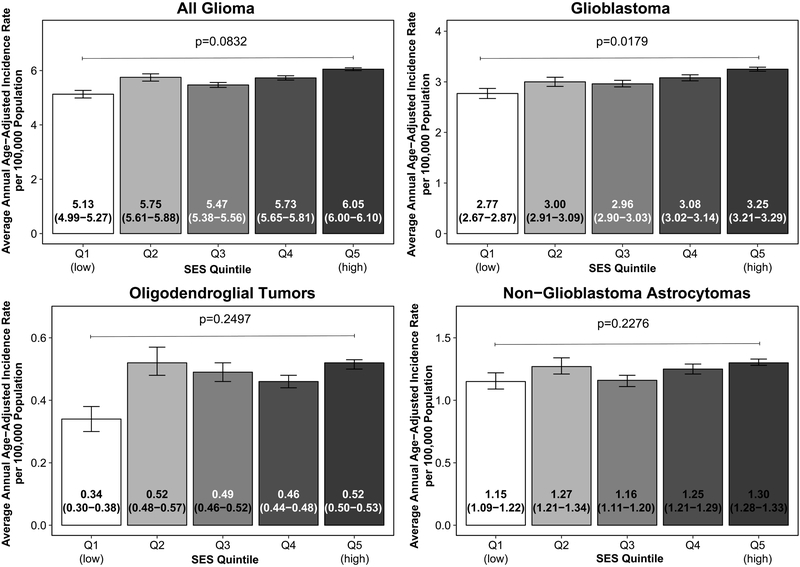
From 2011 to 2015 there were 97,810 cases of glioma in CBTRUS, of which more than 90% were histologically confirmed (Supplementary Table 2). Glioma incidence was 18% higher in the highest quintile of SES (AAAIR=6.05, 95%CI: 6.00-6.10 compared to 5.13, 95%CI: 4.99-5.27 in the lowest quintile, IRR=1.18, 95%CI: 1.15-1.22, p<0.001, Figure 1). Incidence of GBM, other astrocytoma, oligodendroglial tumors, and other gliomas were each significantly higher in the highest quintile of SES compared to the lowest; p-trend across all quintiles was significant only for GBM (p=0.02).
Figure 1.
Incidence of glioma of various subtypes by SES quintile, p-value indicates p-trend, CBTRUS 2011-2015
When stratified by SES and race/ethnicity, the incidence of glioma overall and GBM was higher in counties of high SES compared to low SES among White non-Hispanic individuals (AAAIR=6.69, 95%CI: 6.63-6.74 in high SES compared to 6.44, 95%CI: 6.33-6.56 in low SES counties for glioma overall, IRR=1.04, 95%CI: 1.02-1.06, p=<0.001). However, among Asian or Pacific Islander (API) individuals, rates were significantly lower in counties of high SES compared to low SES (Table 1, Supplementary Figure 3).
Table 1.
Incidence rates of glioma of various subtypes by SES and race/ethnicity, CBTRUS 2011-2015
| Histologic group | Race/ethnicity | High SES | Low SES | Low SES: High SES Incidence Rate Ratio (95%CI) |
P-value | ||
|---|---|---|---|---|---|---|---|
| Count† | AAAIR (95%CI) | Counta | AAAIR (95%CI) | ||||
| All glioma | All races | 80,763 | 5.88 (5.84-5.92) | 18,238 | 5.52 (5.44-5.61) | 0.94 (0.92-0.95) | <0.001 |
| White non-Hispanic | 64,953 | 6.69 (6.63-6.74) | 13,668 | 6.44 (6.33-6.56) | 0.96 (0.94-0.98) | 0.001 | |
| White Hispanic | 6,502 | 4.57 (4.45-4.69) | 2,305 | 4.48 (4.30-4.68) | 0.98 (0.93-1.03) | 0.48 | |
| Black | 5,454 | 3.49 (3.40-3.59) | 1,738 | 3.45 (3.28-3.62) | 0.99 (0.93-1.04) | 0.67 | |
| Asian or Pacific Islander | 2,831 | 3.40 (3.28-3.53) | 285 | 4.27 (3.78-4.81) | 1.26 (1.10-1.42) | <0.001 | |
| American Indian or Alaska Native | 325 | 2.62 (2.31-2.95) | 192 | 2.92 (2.50-3.38) | 1.11 (0.92-1.35) | 0.29 | |
| Glioblastoma | All races | 45,696 | 3.16 (3.13-3.19) | 10,469 | 2.95 (2.89-3.01) | 0.93 (0.91-0.96) | <0.001 |
| White non-Hispanic | 38,462 | 3.52 (3.49-3.56) | 8,294 | 3.38 (3.30-3.45) | 0.96 (0.93-0.98) | <0.001 | |
| White Hispanic | 2,897 | 2.48 (2.38-2.57) | 1,091 | 2.39 (2.24-2.54) | 0.96 (0.90-1.04) | 0.32 | |
| Black | 2,645 | 1.79 (1.72-1.86) | 862 | 1.73 (1.61-1.85) | 0.97 (0.89-1.05) | 0.44 | |
| Asian or Pacific Islander | 1,229 | 1.52 (1.44-1.61) | 113 | 1.83 (1.50-2.21) | 1.20 (0.97-1.47) | 0.09 | |
| American Indian or Alaska Native | 138 | 1.33 (1.10-1.60) | 79 | 1.41 (1.10-1.77) | 1.06 (0.78-1.43) | 0.75 | |
| Non-glioblastoma astrocytomas | All races | 16,294 | 1.26 (1.24-1.28) | 3,703 | 1.22 (1.18-1.26) | 0.96 (0.93-1.00) | 0.03 |
| White non-Hispanic | 12,375 | 1.48 (1.45-1.50) | 2,545 | 1.44 (1.38-1.50) | 0.97 (0.93-1.02) | 0.22 | |
| White Hispanic | 1,667 | 0.95 (0.90-1.00) | 563 | 0.98 (0.90-1.07) | 1.03 (0.93-1.14) | 0.59 | |
| Black | 1,295 | 0.77 (0.73-0.82) | 452 | 0.87 (0.79-0.96) | 1.13 (1.01-1.26) | 0.04 | |
| Asian or Pacific Islander | 709 | 0.85 (0.79-0.91) | 72 | 0.97 (0.76-1.23) | 1.15 (0.88-1.47) | 0.30 | |
| American Indian or Alaska Native | 80 | 0.54 (0.42-0.68) | 60 | 0.82 (0.62-1.07) | 1.52 (1.05-2.18) | 0.03 | |
| Oligodendroglial tumors | All races | 6,521 | 0.50 (0.49-0.52) | 1,316 | 0.45 (0.42-0.47) | 0.88 (0.83-0.94) | <0.001 |
| White non-Hispanic | 5,086 | 0.60 (0.58-0.62) | 1,012 | 0.59 (0.55-0.63) | 0.98 (0.91-1.05) | 0.61 | |
| White Hispanic | 624 | 0.38 (0.35-0.42) | 161 | 0.29 (0.25-0.34) | 0.76 (0.64-0.91) | 0.003 | |
| Black | 381 | 0.24 (0.22-0.27) | 97 | 0.19 (0.16-0.24) | 0.80 (0.63-1.01) | 0.06 | |
| Asian or Pacific Islander | 320 | 0.36 (0.32-0.40) | 24 | 0.34 (0.22-0.52) | 0.96 (0.60-1.47) | 0.95 | |
| American Indian or Alaska Native | 27 | 0.18 (0.12-0.26) | 19 | 0.26 (0.15-0.41) | 1.47 (0.75-2.78) | 0.29 | |
| Ependymoma | All races | 5,406 | 0.42 (0.40-0.43) | 1,223 | 0.41 (0.38-0.43) | 0.98 (0.92-1.04) | 0.46 |
| White non-Hispanic | 4,027 | 0.47 (0.46-0.49) | 828 | 0.47 (0.44-0.51) | 1.00 (0.92-1.08) | 0.96 | |
| White Hispanic | 624 | 0.36 (0.33-0.39) | 221 | 0.37 (0.33-0.43) | 1.04 (0.88-1.22) | 0.70 | |
| Black | 413 | 0.25 (0.23-0.28) | 122 | 0.24 (0.20-0.28) | 0.95 (0.77-1.17) | 0.67 | |
| Asian or Pacific Islander | 255 | 0.29 (0.25-0.33) | 30 | 0.44 (0.29-0.63) | 1.53 (1.00-2.25) | 0.05 | |
| American Indian or Alaska Native | 38 | 0.26 (0.18-0.37) | 18 | 0.24 (0.14-0.39) | 0.91 (0.47-1.69) | 0.90 | |
| Other gliomas | All races | 6,846 | 0.54 (0.53-0.55) | 1,527 | 0.51 (0.48-0.53) | 0.93 (0.88-0.99) | 0.02 |
| White non-Hispanic | 5,003 | 0.62 (0.60-0.63) | 989 | 0.57 (0.54-0.61) | 0.93 (0.86-1.00) | 0.04 | |
| White Hispanic | 690 | 0.40 (0.36-0.43) | 269 | 0.45 (0.40-0.51) | 1.14 (0.98-1.33) | 0.09 | |
| Black | 720 | 0.44 (0.41-0.47) | 205 | 0.42 (0.36-0.48) | 0.95 (0.80-1.11) | 0.53 | |
| Asian or Pacific Islander | 318 | 0.39 (0.34-0.43) | 46 | 0.68 (0.50-0.92) | 1.78 (1.27-2.44) | 0.001 | |
| American Indian or Alaska Native | 42 | 0.30 (0.21-0.42) | 16 | 0.19 (0.11-0.31) | 0.61 (0.32-1.16) | 0.15 | |
Abbreviations: AAAIR=average annual age-adjusted incidence rate, SES=socioeconomic status
Sum of cases among all races may not equal total of cases in individual races combined due to missing information on race/ethnicity
Incidence of glioma overall and GBM was lower in rural counties than urban counties (IRR=0.98, 95%CI 0.97-1.00, p=0.06; IRR=0.97, 95%CI 0.95-0.99, p=0.007 for glioma and GBM, respectively, Supplementary Table 3); the difference for GBM was statistically significant. Generally, these differences were driven by differences in incidence for White non-Hispanic (IRR=0.91, 95%CI 0.90-0.93, p<0.001 for urban versus rural counties for glioma overall) and White Hispanic individuals (IRR=0.79, 95%CI 0.72-0.87, p<0.001 for urban versus rural counties for glioma overall) with no difference for Black individuals and higher incidence in rural areas for API and American Indian or Alaska Native individuals. When stratified by both SES and urban versus rural status, rates of each of these tumor types were generally higher in counties of high SES versus low SES among urban counties (IRR comparing low SES counties to high SES counties for glioma overall=0.92, 95%CI: 0.90-0.94, p<0.001 for urban counties), but there were no significant differences comparing SES among rural counties except for oligodendroglial tumors (Supplementary Figure 4, Supplementary Table 4).
When stratified by SES and region, rates of glioma and glioma subtypes were generally higher in counties of high versus low SES, although these differences were not observed in New England or the East South Central and Pacific regions (Supplementary Table 5). The West North Central region had higher rates of each tumor type among low SES counties than high SES counties.
In order to assess the association between individual county-level characteristics and glioma incidence, univariable and multivariable adjusted models for county-level incidence of glioma are presented in Supplementary Table 6. On univariate analysis, both SES and race/ethnicity (measured as percentage of the county that is White non-Hispanic, a population that is known to be at elevated risk of glioma) were significantly associated with higher county-level incidence of glioma overall (p<0.001), but urban versus rural location was not associated with county-level incidence (p=0.48). On mutual adjustment for SES, urban versus rural status, race, and state of diagnosis, both race (p<0.001) and SES (p<0.001) were predictive of higher county-level incidence, but urban versus rural status was not (p=0.59). Similar results were observed for GBM and for non-GBM astrocytoma, but for oligodendroglial tumors, all three measures were independently predictive of incidence.
Survival after Glioma Diagnosis by Socioeconomic Status
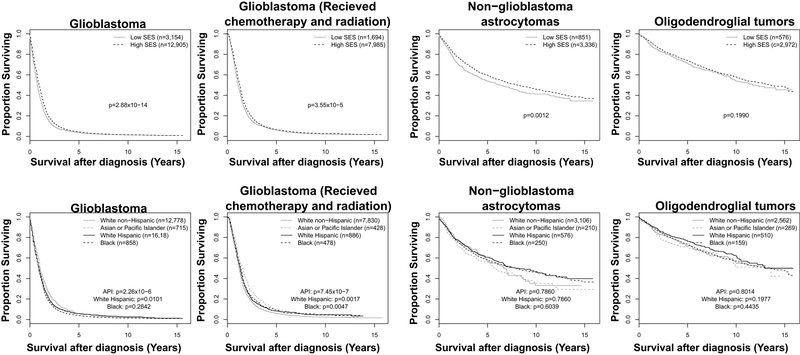
The results of Cox proportional hazards models for death demonstrate that survival after GBM diagnosis was higher among individuals from counties of higher SES quintiles when adjusted for age at diagnosis and extent of resection (EOR; subtotal versus gross total) (Table 2, Figure 2). Compared to the lowest quintile of SES, residence in a county of the highest quintile of SES was associated with a hazard ratio of 0.82 (95%CI: 0.76-0.87, p<0.001). These differences persisted when the population was restricted to those who were diagnosed with GBM and received radiation and chemotherapy (hazard ratio=0.85, 95%CI: 0.78-0.94 comparing highest quintile to the lowest quintile, p<0.001). Similar hazard ratios were observed for non-GBM astrocytoma and oligodendroglial tumors when comparing counties of the highest to lowest quintile.
Table 2.
Median survival, hazard ratios, and 95% confidence intervals for individuals diagnosed with glioma that received resection by SES quintile, adjusted for age at diagnosis and extent of resection (subtotal vs. gross total) (SEER18 2000-2015).
| Group | SES | N | Deaths | Median survival in months (95% CI) |
SES Hazard ratio (95% CI)† |
SES P-value |
|---|---|---|---|---|---|---|
| Glioblastoma | Q1 | 1143 | 986 | 9 (8-10) | Ref. | Ref. |
| Q2 | 1352 | 1136 | 10 (9-11) | 0.90 (0.82-1.00) | 0.01 | |
| Q3 | 2929 | 2491 | 10 (9-10) | 0.93 (0.87-1.00) | 0.06 | |
| Q4 | 1712 | 1482 | 10 (10-11) | 0.90 (0.83-0.98) | 0.01 | |
| Q5 | 8923 | 7490 | 12 (11-12) | 0.82 (0.76-0.87) | <0.001 | |
| Glioblastoma (received radiation and chemotherapy) | Q1 | 495 | 496 | 13 (12-15) | Ref. | Ref. |
| Q2 | 799 | 648 | 14 (12-15) | 0.91 (0.81-1.02) | 0.10 | |
| Q3 | 11461 | 1191 | 15 (14-16) | 0.88 (0.79-0.98) | 0.02 | |
| Q4 | 1014 | 847 | 14 (13-15) | 0.92 (0.82-1.03) | 0.14 | |
| Q5 | 5810 | 4753 | 15 (15-15) | 0.85 (0.78-0.94) | <0.001 | |
| Non-glioblastoma astrocytoma | Q1 | 313 | 171 | 59 (42-86) | Ref. | Ref. |
| Q2 | 343 | 163 | 88 (64-162) | 0.81 (0.65-1.00) | 0.06 | |
| Q3 | 863 | 418 | 72 (55-110) | 0.83 (0.69-1.00) | 0.04 | |
| Q4 | 408 | 198 | 72 (55-110) | 0.82 (0.67-1.10) | 0.06 | |
| Q5 | 2260 | 930 | 113 (97-129) | 0.67 (0.59-0.83) | <0.001 | |
| Oligodendroglial tumors | Q1 | 179 | 115 | 115 (76-**) | Ref. | Ref. |
| Q2 | 267 | 94 | 169 (122-**) | 0.70 (0.52-0.95) | 0.02 | |
| Q3 | 691 | 258 | 129 (119-**) | 0.85 (0.66-1.09) | 0.21 | |
| Q4 | 342 | 134 | 131 (113-**) | 0.77 (0.53-1.03) | 0.07 | |
| Q5 | 2069 | 680 | 181 (163-**) | 0.68 (0.53-0.86) | 0.001 |
not able to be calculated
Adjusted for age at diagnosis and extent of resection (subtotal vs. gross total)
Abbreviations: CI=confidence interval, SES=socioeconomic status
Figure 2.
Survival after diagnosis for glioma of various subtypes, by SES and race/ethnicity, SEER18 2000-2015
Hazard ratios of death by race/ethnicity, adjusted for county-level SES (continuous variable), age, and EOR are presented in Table 3. For GBM, White Hispanic individuals and API individuals, but not Black individuals, had improved survival compared to White non-Hispanic individuals (for White Hispanic individuals, HR=0.92, 95%CI: 0.87-0.98, p=0.01, for API individuals, HR=0.81, 95%CI: 0.75-0.89, p<0.001). When restricted to those diagnosed with GBM who received radiation and chemotherapy, the differences in survival were strengthened. Compared to White non-Hispanics, White Hispanic (HR=0.88, 95%CI: 0.81-0.95, p=0.002), Black (HR=0.86, 95%CI: 0.77-0.95, p=0.005), and API (HR=0.75, 95%CI: 0.67-0.84, p<0.001) individuals each had improved survival. There were no significant differences in survival by race for non-GBM astrocytomas or oligodendroglial tumors.
Table 3.
Median survival, hazard ratios, and 95% confidence intervals for individuals diagnosed with glioma that received resection by race/ethnicity, adjusted for SES (continuous measure), age at diagnosis, and extent of resection (subtotal vs. gross total) (SEER18 2000-2015).
| Group | Race/ethnicity | N | Deaths | Median survival in months (95% CI) |
Race/ethnicity Hazard Ratio† |
Race/ethnicity p value |
|---|---|---|---|---|---|---|
| Glioblastoma | White non-Hispanic | 12778 | 11049 | 11 (11-11) | Ref. | |
| White Hispanic | 1618 | 1232 | 11 (10-12) | 0.92 (0.87-0.98) | 0.01 | |
| Black | 858 | 686 | 11 (10-13) | 0.96 (0.89-1.04) | 0.28 | |
| Asian or Pacific Islander | 715 | 558 | 13 (12-14) | 0.81 (0.75-0.89) | <0.001 | |
| Glioblastoma (received radiation and chemotherapy) | White non-Hispanic | 7830 | 6567 | 14 (14-15) | Ref. | |
| White Hispanic | 886 | 648 | 16 (15-17) | 0.88 (0.81-0.95) | 0.002 | |
| Black | 478 | 364 | 16 (15-18) | 0.86 (0.77-0.95) | 0.005 | |
| Asian or Pacific Islander | 428 | 320 | 18 (16-20) | 0.75 (0.67-0.84) | <0.001 | |
| Non-glioblastoma astrocytoma | White non-Hispanic | 3106 | 1443 | 95 (84-107) | Ref. | |
| White Hispanic | 576 | 224 | 99 (79-132) | 0.96 (0.84-1.11) | 0.60 | |
| Black | 250 | 118 | 68 (52-102) | 1.20 (1.00-1.45) | 0.05 | |
| Asian or Pacific Islander | 210 | 86 | 80 (59-107) | 1.03 (0.83-1.28) | 0.79 | |
| Oligodendroglial tumors | White non-Hispanic | 2562 | 943 | 163 (141-185) | Ref. | |
| White Hispanic | 510 | 144 | ** (134-**) | 0.89 (0.75-1.06) | 0.20 | |
| Black | 159 | 58 | 163 (106-**) | 1.11 (0.85-1.45) | 0.44 | |
| Asian or Pacific Islander | 269 | 84 | 156 (117-**) | 1.03 (0.82-1.29) | 0.81 |
not able to be calculated
Adjusted for SES (continuous), age at diagnosis, and extent of resection (subtotal vs. gross total)
Abbreviations: CI=confidence interval, GTR=gross total resection, SES=socioeconomic status
Hazard ratios of death by urban versus rural status, adjusted for county-level SES (continuous variable), age, and EOR are presented in Supplementary Table 7. For GBM, individuals in urban areas had improved survival compared to those in rural areas, but this finding was attenuated and no longer significant after adjustment for SES (HR=0.90, 95%CI: 0.85-0.95, p<0.001 unadjusted for SES, HR=0.96, 95%CI: 0.91-1.02, p=0.16 adjusted for SES). When restricted to those diagnosed with GBM who received radiation and chemotherapy, the differences in survival persisted both unadjusted and adjusted for SES (HR=0.87, 95%CI: 0.81-0.93, p<0.001 unadjusted for SES, HR=0.91, 95%CI: 0.84-0.98, p=0.01 adjusted for SES). There were no significant differences in survival by urban versus rural status for non-GBM astrocytomas, but improved survival in urban areas persisted for oligodendroglial tumors regardless of SES (HR=0.76, 95%CI: 0.64-0.91, p=0.003).
Mutual adjustment of these models for SES, urban versus rural status, and percentage of the county that is White non-Hispanic is presented in Supplementary Table 8. On univariate analysis, both SES and urban versus rural status were associated with improved survival for glioma overall (p<0.001), but race was not (p=0.52). On mutual adjustment, SES remained a significant predictor (p<0.001), but urban versus rural status was attenuated (p=0.12), and race remained non-significant (p=0.26). For non-GBM astrocytoma and oligodendroglial tumors, findings were similar. For GBM, univariate analysis again showed that SES (p<0.001) and urban versus rural status (p<0.001), but not race (p=0.07) were significantly associated with survival. Mutual adjustment resulted in attenuation of urban versus rural status (p=0.25), while SES (p<0.001) and race (p=0.03) were each significantly predictive of improved survival.
Discussion
This study of nearly all glioma cases diagnosed in the US over a five year period demonstrates higher incidence rates of glioma in counties of high SES when compared to counties of lower SES. The overall findings are consistent with prior reports that have showed higher incidence in areas of high SES in the United States and Europe.7-9 These prior studies have estimated statistically significant relative risks of glioma ranging from 1.1 to 1.2 comparing areas of high SES with areas of low SES,8, 9 with one study estimating a relative risk of glioblastoma of 1.45.7 These studies have used a variety of definitions of SES, however, with some comparing incidence rates by SES proxies, such as educational level or income.7-9 Our aim in the study was to use data from the entire US and a comprehensive, county-level composite metric for SES to assess associations between SES and glioma incidence. We further stratified by possible confounders of the association between SES and glioma, including urbanicity and race/ethnicity, to evaluate if any of these sociodemographic characteristics may be independently associated with glioma incidence, and may have contributed to the observed differences by SES in prior studies on this topic.7-9, 12 This study also extends these analyses to survival after glioma diagnosis, using data from SEER, to assess the extent to which survival after glioma diagnosis varies by these sociodemographic factors.
Survival was significantly higher among counties of high SES compared to counties of low SES, and among White Hispanic and Black individuals compared to White non-Hispanic individuals. These differences in survival persisted when adjusted for EOR and when restricted to GBM patients who received radiation and chemotherapy. When stratified by race/ethnicity and urban versus rural status, higher rates of glioma were most prominent among White non-Hispanic individuals and to a lesser extent, White Hispanic individuals, among urban rather than rural counties. Mutual adjustment for SES, urban versus rural location, and race demonstrated that while SES and race independently predict county-level glioma incidence, differences in survival are mostly attributable to SES, independently of race and urban versus rural location.
Incidence of Glioma by Socioeconomic Status
Currently, there is little evidence for factors that modify the risk for glioma. Exposure to ionizing radiation has been shown to increase risk,31, 32 whereas atopic disease may have protective effects.6 Although both of these may be correlated with SES, this confounding is unlikely to explain the relatively large differences in incidence reported here. For example, in this study, we identified an 18% increased incidence rate of glioma in high SES counties compared to low SES counties. It is unlikely that atopic diseases, which have approximately a 5% prevalence in the United States, or ionizing radiation, which are both generally more common in high SES areas, could explain such a large difference.14, 33, 34
For some cancers of other sites, differential rates of incidence by SES can be attributed to ascertainment bias.5, 35, 36 For glioma, however, ascertainment bias is unlikely to explain differential rates by levels of SES because the disease is nearly universally diagnosed in the US, particularly for GBM, the most aggressive form.7, 14, 37 The preclinical period for these tumors is likely short and they are rapidly progressive, with distinctive symptoms that prompt medical care.38 It is possible that histologic diagnosis may be more accurate in areas of high SES than in areas of low SES because of access to higher quality medical facilities, but this would be more likely to cause erroneous incidence of subtypes of glioma rather than overall rates of glioma. Individuals of low SES may be less likely to undergo surgical resection or biopsy, the data presented here include tumors diagnosed histologically as well as radiographically.
Stratification by race demonstrated that rates of glioma are similar between high SES and low SES counties for API and Black individuals, but differ for White Hispanic and especially White non-Hispanic individuals, with higher rates in high SES counties. This suggests that the overall findings of higher incidence in high SES areas are mainly driven by differential rates among White non-Hispanic individuals and, to a lesser extent, White Hispanic individuals, rather than other racial and ethnic groups.37
A prior study identified increased risk of brain cancer among those living in a metropolitan county.13 In our study, incidence rates of glioma were largely similar in urban counties and rural counties when stratified by SES. This suggests that any increased risk due to farming and pesticide exposure, which have previously been suggested as risk factors, is either small or is matched by risk factors that operate more strongly in urban environments.39 Additionally, incidence rates were more disparate comparing high SES and low SES counties in urban areas than in rural areas, suggesting that overall differences by SES are driven mainly by differential rates in urban environments.
Perhaps most importantly, mutual adjustment for SES, urban versus rural status, and race demonstrated that whereas SES and race were independently predictive of county-level incidence, urban location was not. This indicates that the increased risk of glioma that has previously been reported among urban versus rural environments likely operates through SES, race, or both.
Survival after Diagnosis of Glioma by Socioeconomic Status
Whereas incidence data remain difficult to explain by currently understood risk factors, differential survival after glioma diagnosis by SES can more easily be explained. High SES is associated with better access to healthcare, including access to surgery, radiation, chemotherapy, and other adjunctive treatments, all of which have been shown to improve survival after diagnosis.5, 35, 37 Additionally, living in high SES areas is associated with improved survival from death from any cause, due to a variety of factors including fewer environmental exposures that increase risk of death.5, 15 In the present study, survival after diagnosis of glioma was higher among those from high SES counties compared to low SES counties for all tumor types.
Stratification by race revealed increased survival for Black and White Hispanic individuals compared to White non-Hispanic individuals for most tumor subtypes. Within each race, survival was generally higher in areas of high SES compared to areas of low SES, suggesting that the factors that result in poorer survival in areas of low SES are not isolated to a single racial identity.
In multivariable analyses, SES in the highest quintile was associated with lower risk of death compared to the lowest quintile for all tumor types, even among patients who all received radiation and chemotherapy. Although access to radiation and chemotherapy is only a proxy for overall healthcare quality, this suggests that while access to care may play a role in reduced survival for those of low SES, the factors driving the improved survival among those from high SES locations may not operate solely through access to care.
When adjusted for race, age, EOR, and a continuous measure of county-level SES, survival after diagnosis of GBM remained superior for White Hispanic and API individuals compared to White non-Hispanic individuals. When restricted to GBM patients who received radiation and chemotherapy, these differences were strengthened and Black individuals also had improved survival compared to White non-Hispanic individuals. Because Black individuals have improved survival compared to White non-Hispanic individuals only when restricted to those who received chemotherapy and radiation for GBM, this suggests that access to these treatments may be worse among Black individuals, independently of SES, and that this adversely affects survival after GBM diagnosis.40, 41 Future studies should continue to examine this disparity, as it is suggestive that individuals from racial and ethnic minorities receive worse access to these treatments by the US medical system, independent of SES level.
The fact that adjustment for county-level SES does not fully attenuate the differential survival by race/ethnicity suggests that other factors drive the improved survival among Black, White Hispanic, and API individuals. One possibility is that worse survival in White non-Hispanic individuals may be due to a higher preponderance of wild-type isocitrate dehydrogenase 1 (IDH1/2) in these patients, as mutation in IDH1/2 is associated with improved prognosis, however in this present study, we could not ascertain genetic mutation status.42, 43
Notably, in a multivariable Cox model mutually adjusted for SES, urban versus rural location, and race (measured as percentage of the county that is White non-Hispanic), only SES was independently predictive of survival for glioma overall. For GBM, both SES and race were independently predictive. This suggests that observed differences in survival by urban versus rural location may be confounded by SES or race.
Strengths and Limitations
The strengths of this study include the nearly universal representation of glioma cases from the US population over multiple years. This is the first study of its kind to use the 100% population coverage of CBTRUS to examine rates of glioma by SES, and one of the few to stratify by race/ethnicity and rural versus urban status. Additionally, while other studies have examined incidence by socioeconomic status for certain tumor subtypes, this study analyzes both incidence and survival for glioma overall, and its various subtypes.
Additionally, the data include information on treatment, including radiation, chemotherapy, and EOR, which allows for additional examination of factors that have particular influence over survival after glioma diagnosis. However, we lack data on other factors that may be related to differences in survival, including co-morbidities, tumor volume/location, EOR, Karnofsky performance status, and treatment pattern.44, 45 There is no central pathology review of tumor samples so it is possible that ‘true’ histologic classification could be confounded with SES, urban/rural location of residence, and/or race/ethnicity. As noted, important molecular classifications that predict survival—including IDH1/2, MGMT, ATRX, and 1p/19q—were not incorporated into this analysis, and these may confound the association between the sociodemographic variables and survival. Although these data were not available for this analysis, the hallmark molecular features have started to be collected in the US starting in 2018.46 The most prominent limitation is the crude nature of county-level metrics of SES. Counties are large and can be fairly heterogeneous, incorporating both rural and urban areas and areas of high and low SES. Unfortunately, our data use agreement with the CDC does not permit access to census tract level data for analyses of this type, due to the rarity of glioma and some of its subtypes leading to potential identifiability of cases in sparse strata. Perhaps more accurate identification of individual SES would demonstrate even larger differences.
Conclusion
Incidence of glioma was higher in US counties of high SES compared to counties of low SES. These differences were most pronounced among White non-Hispanic individuals and, to a lesser extent, White Hispanic individuals, in urban rather than rural areas. Survival was worse in low SES counties, even when adjusting for EOR, and when restricting to those who received radiation and chemotherapy for GBM. Differences in incidence and survival were driven by both SES and race, rather than urban versus rural status.
Supplementary Material
Acknowledgments
Funding: National Institutes of Health (NIH) Training Grant T32 CA 009001 (DJC). QTO is supported by a Research Training Grant from the Cancer Prevention and Research Institute of Texas (CPRIT; RP160097T). Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under Contract No. 2016-M-9030, the American Brain Tumor Association, The Sontag Foundation, Novocure, National Brain Tumor Society, the Zelda Dorin Tetenbaum Memorial Fund, AbbVie, the Musella Foundation, as well as private and in kind donations. Contents are solely the responsibility of the authors and do not necessarily reflect the official views of the CDC.
Footnotes
Disclosures: Dr. Stampfer serves as an expert witness for Verizon in litigation regarding cell phones and brain cancer.
References
- 1.Baquet CR, Horm JW, Gibbs T, Greenwald P. Socioeconomic factors and cancer incidence among blacks and whites. J Natl Cancer Inst. 1991;83: 551–557. [DOI] [PubMed] [Google Scholar]
- 2.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20: 417–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Megwalu UC. Impact of County-Level Socioeconomic Status on Oropharyngeal Cancer Survival in the United States. Otolaryngol Head Neck Surg. 2017;156: 665–670. [DOI] [PubMed] [Google Scholar]
- 4.Diez-Roux AV, Kiefe CI, Jacobs DR Jr., et al. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Ann Epidemiol. 2001;11: 395–405. [DOI] [PubMed] [Google Scholar]
- 5.Mackenbach JP, Stirbu I, Roskam AJ, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358: 2468–2481. [DOI] [PubMed] [Google Scholar]
- 6.Uphoff E, Cabieses B, Pinart M, Valdes M, Anto JM, Wright J. A systematic review of socioeconomic position in relation to asthma and allergic diseases. Eur Respir J. 2015;46: 364–374. [DOI] [PubMed] [Google Scholar]
- 7.Porter AB, Lachance DH, Johnson DR. Socioeconomic status and glioblastoma risk: a population-based analysis. Cancer Causes Control. 2015;26: 179–185. [DOI] [PubMed] [Google Scholar]
- 8.Khanolkar AR, Ljung R, Talback M, et al. Socioeconomic position and the risk of brain tumour: a Swedish national population-based cohort study. J Epidemiol Community Health. 2016. [DOI] [PubMed] [Google Scholar]
- 9.Plascak JJ, Fisher JL. Area-based socioeconomic position and adult glioma: a hierarchical analysis of surveillance epidemiology and end results data. PLoS One. 2013;8: e60910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wigertz A, Lonn S, Hall P, Feychting M. Non-participant characteristics and the association between socioeconomic factors and brain tumour risk. J Epidemiol Community Health. 2010;64: 736–743. [DOI] [PubMed] [Google Scholar]
- 11.Deb S, Pendharkar AV, Schoen MK, Altekruse S, Ratliff J, Desai A. The effect of socioeconomic status on gross total resection, radiation therapy and overall survival in patients with gliomas. J Neurooncol. 2017;132: 447–453. [DOI] [PubMed] [Google Scholar]
- 12.Demers PA, Vaughan TL, Schommer RR. Occupation, socioeconomic status, and brain tumor mortality: a death certificate-based case-control study. J Occup Med. 1991;33: 1001–1006. [PubMed] [Google Scholar]
- 13.Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20: E1. [DOI] [PubMed] [Google Scholar]
- 14.Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16: 896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pappas G, Queen S, Hadden W, Fisher G. The increasing disparity in mortality between socioeconomic groups in the United States, 1960 and 1986. N Engl J Med. 1993;329: 103–109. [DOI] [PubMed] [Google Scholar]
- 16.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12: 703–711. [DOI] [PubMed] [Google Scholar]
- 17.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2010-2014. Neuro-oncology. 2017;19: v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Cancer Statistics Working Group. . United States Cancer Statistics: 1999-2015 Incidence and Mortality Web-based Report. Available from URL: http://www.cdc.gov/uscs.
- 19.United States Census Bureau. 2010 – 2015 American Community Survey. Available from URL: http://factfinder2.census.gov [accessed August 13, 2018].
- 20.United States Department of Agriculture. 2013 Rural Urban Continuum Codes. Available from URL: https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/documentation.aspx.
- 21.Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat software version 8.3.2. Available from URL: www.seer.cancer.gov/seerstat.
- 22.NAACCR Race and Ethnicity Work Group. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1]. .
- 23.Fritz APC, Jack A, Shanmugaratnam K, Sobin L, Perkin DM, Whelan S International Classification of Diseases for Oncology, Third edition: World Health Organization, 2000. [Google Scholar]
- 24.Melin BS, Barnholtz-Sloan JS, Wrensch MR, et al. Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat Genet. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fay MP, Tiwari RC, Feuer EJ, Zou Z. Estimating average annual percent change for disease rates without assuming constant change. Biometrics. 2006;62: 847–854. [DOI] [PubMed] [Google Scholar]
- 26.Surveillance Epidemiology and End Results (SEER) Program. Overview of the SEER Program. Available from URL: http://seer.cancer.gov/about/overview.html.
- 27.Surveillance Epidemiology and End Results (SEER) Program. Number of Persons by Race and Hispanic Ethnicity for SEER Participants (2010 Census Data). Available from URL: http://seer.cancer.gov/registries/data.html.
- 28.Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 18 Regs Custom Data (with additional treatment fields), Nov 2016 Sub (2000-2014) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission.2017.
- 29.Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER Treatment Data With Medicare Claims. Med Care. 2016;54: e55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team. R: A language and environment for statistical computing. Available from URL: http://www.R-project.org/.
- 31.Freeman K, Strauchler D, Miller TS. Impact of socioeconomic status on ionizing radiation exposure from medical imaging in children. J Am Coll Radiol. 2012;9: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strauchler D, Freeman K, Miller TS. The impact of socioeconomic status and comorbid medical conditions on ionizing radiation exposure from diagnostic medical imaging in adults. J Am Coll Radiol. 2012;9: 58–63. [DOI] [PubMed] [Google Scholar]
- 33.Ofenloch RF, Schuttelaar MLA, Svensson A, et al. Socioeconomic Status and the Prevalence of Skin and Atopic Diseases in Five European Countries. Acta Derm Venereol. 2018. [DOI] [PubMed] [Google Scholar]
- 34.Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: A Cross-Sectional Study Examining the Prevalence and Disease Burden of Atopic Dermatitis in the US Adult Population. J Invest Dermatol. 2018. [DOI] [PubMed] [Google Scholar]
- 35.Kilpelainen TP, Makinen T, Karhunen PJ, et al. Estimating bias in causes of death ascertainment in the Finnish Randomized Study of Screening for Prostate Cancer. Cancer Epidemiol. 2016;45: 1–5. [DOI] [PubMed] [Google Scholar]
- 36.Rawshani A, Svensson AM, Zethelius B, Eliasson B, Rosengren A, Gudbjornsdottir S. Association Between Socioeconomic Status and Mortality, Cardiovascular Disease, and Cancer in Patients With Type 2 Diabetes. JAMA Intern Med. 2016;176: 1146–1154. [DOI] [PubMed] [Google Scholar]
- 37.Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult Glioma Incidence and Survival by Race or Ethnicity in the United States From 2000 to 2014. JAMA Oncol. 2018: e181789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cote DJ, Downer MK, Smith TR, Smith-Warner SA, Egan KM, Stampfer MJ. Height, waist circumference, body mass index, and body somatotype across the life course and risk of glioma. Cancer Causes Control. 2018;29: 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruder AM, Carreon T, Butler MA, et al. Exposure to farm crops, livestock, and farm tasks and risk of glioma: the Upper Midwest Health Study. Am J Epidemiol. 2009;169: 1479–1491. [DOI] [PubMed] [Google Scholar]
- 40.Curry WT Jr., Carter BS, Barker FG 2nd. Racial, ethnic, and socioeconomic disparities in patient outcomes after craniotomy for tumor in adult patients in the United States, 1988-2004. Neurosurgery. 2010;66: 427–437; discussion 437-428. [DOI] [PubMed] [Google Scholar]
- 41.Curry WT Jr., Barker FG 2nd. Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009;93: 25–39. [DOI] [PubMed] [Google Scholar]
- 42.Myung JK, Cho HJ, Park CK, Kim SK, Phi JH, Park SH. IDH1 mutation of gliomas with long-term survival analysis. Oncol Rep. 2012;28: 1639–1644. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka K, Sasayama T, Mizukawa K, et al. Combined IDH1 mutation and MGMT methylation status on long-term survival of patients with cerebral low-grade glioma. Clin Neurol Neurosurg. 2015;138: 37–44. [DOI] [PubMed] [Google Scholar]
- 44.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine. 2005;352: 987–996. [DOI] [PubMed] [Google Scholar]
- 45.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95: 190–198. [DOI] [PubMed] [Google Scholar]
- 46.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131: 803–820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.