Abstract
Canine glaucoma is a group of disorders that are generally associated with increased intraocular pressure (IOP) resulting in a characteristic optic neuropathy. Glaucoma is a leading cause of irreversible vision loss in dogs and may be either primary or secondary. Despite the growing spectrum of medical and surgical therapies, there is no cure, and many affected dogs go blind. Often eyes are enucleated because of painfully high, uncontrollable IOP. While progressive vision loss due to primary glaucoma is considered preventable in some humans, this is mostly not true for dogs. There is an urgent need for more effective, affordable treatment options. Because newly developed glaucoma medications are emerging at a very slow rate and may not be effective in dogs, work toward improving surgical options may be the most rewarding approach in the near term. This Viewpoint Article summarizes the discussions and recommended research strategies of both a Think Tank and a Consortium focused on the development of more effective therapies for canine glaucoma; both were organized and funded by the American College of Veterinary Ophthalmologists Vision for Animals Foundation (ACVO‐VAF). The recommendations consist of (a) better understanding of disease mechanisms, (b) early glaucoma diagnosis and disease staging, (c) optimization of IOP‐lowering medical treatment, (d) new surgical therapies to control IOP, and (e) novel treatment strategies, such as gene and stem cell therapies, neuroprotection, and neuroregeneration. In order to address these needs, increases in research funding specifically focused on canine glaucoma are necessary.
Keywords: aqueous humor, canine, glaucoma, intraocular pressure, optic nerve, surgery
1. INTRODUCTION
Canine glaucoma is an often painful, complex group of blinding optic neuropathies that have in common elevated intraocular pressure (IOP) leading to loss of retinal ganglion cells (RGCs) and their axons, associated with degeneration of optic nerve head (ONH) and retina. Glaucoma is a leading cause of irreversible vision loss in both humans and dogs.1, 2, 3, 4 Impaired aqueous humor drainage through the physiologic outflow pathways is responsible for increases in IOP. Canine glaucoma is defined as either primary or secondary, the latter being caused by a clinically or histopathologically detectable underlying disease process. Secondary glaucoma is among the most feared complications following canine cataract surgery with an estimated incidence of 5%‐19% over a 2‐year postoperative period; in some breeds, such as Boston Terriers, Shih Tzus, and Labrador Retrievers, this glaucoma incidence can rise to 29%‐38%, suggesting a possible hereditary component.5, 6, 7, 8, 9, 10, 11 Current medical and surgical treatments aim at slowing vision loss by maintaining IOP at a healthy level. The range of such a nondamaging IOP is poorly understood and likely varies between individuals based on factors such as the biomechanical properties of the eye. For many forms of canine glaucoma, there is no cure with vision loss progressing despite intensive and costly medical and surgical treatments. This is in contrast to primary glaucoma in human patients where vision loss is manageable and can be prevented in some with early diagnosis and intervention.12 Glaucoma therapies in dogs frequently fail within months with rebounding IOP elevation and blindness, thus illustrating a need for more effective, affordable treatment options. During a recent survey performed by the American College of Veterinary Ophthalmologists Vision for Animals Foundation (ACVO‐VAF), a majority of responding ACVO Diplomates (board certified veterinary ophthalmologists) consider research toward this goal one of the most pressing needs based on their clinical practice. Because newly developed glaucoma medications are emerging at a very slow rate, are optimized for the human eye, and may not show enhanced efficacy in dogs, the survey revealed that focus on improved surgical therapies may be the most rewarding approach in the near term.
On November 5, 2016, the ACVO‐VAF organized and funded its second Think Tank at the Detroit Metropolitan Airport Westin Hotel in Michigan (USA) to develop recommendations for research and clinical strategies toward improvement of canine glaucoma therapies, with a special focus on surgical treatments. The event was followed by the establishment of a Canine Glaucoma Consortium to (a) review and update nomenclature, (b) create toolkits for data collection, (c) coordinate research efforts, (d) review and compile clinical and research data and samples, (e) establish more accurate glaucoma classifications, and (f) review emerging discoveries. This Viewpoint Article summarizes the discussions and recommended strategies of both Think Tank and Consortium; they are grouped under the following main topics: (a) Better understanding of disease mechanisms, (b) early glaucoma diagnosis and disease staging, (c) optimization of IOP‐lowering medical treatment, (d) new surgical therapies to control IOP, and (e) novel treatment strategies, such as gene and stem cell therapies, neuroprotection, and neuroregeneration. Potential differences in depth of focus between these topics correlate with how they were weighed during our deliberations.
2. BETTER UNDERSTANDING OF DISEASE MECHANISMS
Despite recent experimental advances in the protection and regeneration of RGCs and their axons, lowering IOP to prevent or slow ONH damage will remain the main focus of canine glaucoma therapy in the foreseeable future. In order to develop more effective treatments that target specific disease mechanisms, it is critical that the anatomy and physiology of aqueous humor outflow pathways are evaluated in greater detail in normal and glaucomatous eyes (Figure 1). Frequently, assumptions are made that these pathways in dogs are similar to human eyes; however, this is not true in all aspects. For example, the pectinate ligament, dysplasia of which is considered a risk factor for the development of canine primary angle‐closure glaucoma (PACG), is present in dogs but not humans. Another example is the differences in the post‐trabecular meshwork outflow pathways, including human Schlemm's canal vs canine angular aqueous plexus. While the human Schlemm's canal has been studied in detail, much remains to be learned about the canine angular aqueous plexus and its role in glaucoma pathogenesis. We also need to improve our knowledge of canine glaucoma risk factors and pathogenesis, including differences between the dog and other species and between individual canine breeds, to allow earlier diagnosis and treatment to prevent continued RGC loss and blindness. We believe that early disease recognition will be facilitated by the increased accessibility and affordability of powerful diagnostic technologies, including high‐resolution imaging tools (eg, high‐resolution ultrasonography [HRUS]/ultrasound biomicroscopy [UBM], optical coherence tomography [OCT], and anterior segment angiography) and more frequent IOP measurements by telemetric devices or home monitoring. Furthermore, as shown by recent advances in canine glaucoma genetics, improving molecular laboratory tools, such as next‐generation sequencing and proteomics, facilitate the detailed investigation of genetic risk factors as well as molecular and cellular disease mechanisms. Table 1 lists specific topics related to canine primary and secondary glaucoma that we identified as important. The list represents our discussions in that it focuses on the anterior segment and IOP. We avoided further prioritization because choice of research topic and wording of specific aims will depend on the expertise and resources available to an individual investigator or team. While multiple forms of secondary glaucoma exist, our discussions focused on postphacoemulsification glaucoma.
Figure 1.
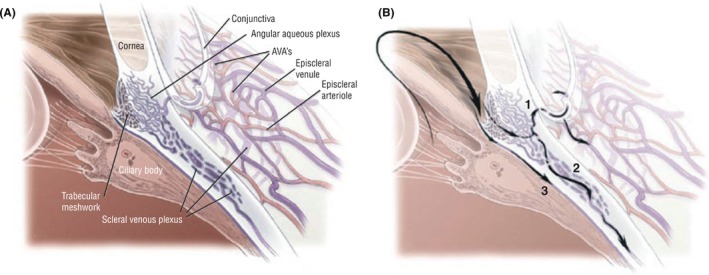
Cross‐sectional anatomy (A) and aqueous humor drainage routes (B) in the canine eye. Once through the trabecular meshwork, the aqueous can pass into the angular aqueous plexus and is directed either anteriorly into the more superficial episcleral venules (1) or posteriorly into the scleral venous plexus and the vortex venous system (2). An alternative aqueous humor drainage pathway (3) is the diffusion through the ciliary muscle interstitium to the suprachoroidal space and through the sclera (ie, uveoscleral flow). Abbreviation: AVAs, arteriovenous anastomosis (from Tsai et al14; with permission)
Table 1.
High‐priority research topics toward the better understanding of canine glaucoma disease mechanisms
|
Canine ocular anatomy and physiology Characterization of:
|
|
Pathogenesis of elevated IOP in canine breed‐specific, primary glaucoma Description of (including potential age‐effect):
Pathogenesis of postphacoemulsification glaucoma Improve our understanding of:
|
3. EARLY GLAUCOMA DIAGNOSIS AND DISEASE STAGING
Closely associated with the incomplete understanding of disease mechanisms is our inability to diagnose preclinical disease stages and to predict disease onset, especially in canine PACG. Addressing this shortcoming is critical to determine when more effective treatment should be initiated to delay or prevent vision loss (Table 2). This challenge may be addressed by novel and/or improving diagnostic technologies that will allow a more detailed structural and functional assessment of the eye and for an evaluation of the effect of various glaucoma drugs on the outflow pathways in glaucoma.13, 14 Many of these technologies are being developed in a laboratory setting and/or for application in human patients, and they need to be validated for dogs. It is beyond the scope of this article to list pros and cons for all of these methods, but continual assessment of their usefulness is needed since they are constantly evolving. Technological advances have been most dramatic in high‐resolution imaging, such as OCT and HRUS/UBM (Figures 2 and 3), and functional testing such as chromatic pupillary light reflex and advanced electroretinography.15, 16, 17, 18, 19, 20 As prices for many of these technologies decrease, and they become more user‐friendly, their application will be more realistic for the veterinary practice. To go even further, improvements of monitoring by dog owners will likely become possible in the not too distant future thanks to smartphone applications, user‐friendly home tonometry, and continuous IOP monitoring with telemetric technologies.21, 22 The development of tools to recognize and identify a healthy IOP range with its individual variability will be important for early diagnosis. Progress in canine glaucoma genetics has been made, especially for primary open‐angle glaucoma (POAG), but more work needs to be done to identify reliable disease markers that help in risk assessment and diagnosis, especially for canine PACG, which is more challenging to investigate because of its complex nature.23, 24 The ACVO‐VAF Canine Glaucoma Consortium is initiating and coordinating a large‐scale, multicenter project to collect DNA and tissue samples, and gonioscopy and UBM iridocorneal angle measurements from glaucoma‐affected and control dogs to develop improved biomarkers that allow reliable identification of early, preclinical glaucoma stages and/or dogs at risk of developing disease. For example, the development of a chip‐based diagnostic DNA test may facilitate the early detection and treatment of hereditary glaucoma based on the presence of specific genetic markers years before the emergence of clinical signs. Early therapeutic intervention could result in significant delay or prevention of advanced disease and vision loss.
Table 2.
Potential strategies toward improved early diagnosis, staging, and response to therapy of canine glaucoma
| Detailed clinical and genetic definition of breed‐specific forms of primary glaucoma with similarities and differences with human forms of glaucoma. |
| Development of more accurate classification of canine glaucoma. |
| Standardization and development of grading scheme for ultrasound biomicroscopy measurements and ciliary cleft width. |
| Facilitation of routine direct and indirect measurement of aqueous humor dynamics, such as episcleral venous pressure, tonography, and fluorophotometry, allowing estimation of conventional and unconventional outflow. |
| Development of continuous tonometry and determination of its value for early diagnosis.* |
| Definition of safe, healthy target IOP, and its potential individual variability. |
| Routine assessment of iridocorneal angle morphology and regional variability, including pectinate ligament dysplasia and width of ciliary cleft. |
| Review and revision of relevant and definable iridocorneal angle classification, including the effect of age and disease. |
| Validation and comparison of high‐resolution imaging technologies, for both anterior and posterior segment.* |
| Development of functional techniques, such as electroretinography and pupillometry, for early detection of retinal and optic nerve damage.* |
| Development of molecular and genetic glaucoma markers for clinical application. |
| Determination of inter‐individual differences in responsiveness to glaucoma drugs and nonresponder rates. |
Many of these technologies already exist but need to be validated for canine glaucoma.
Figure 2.
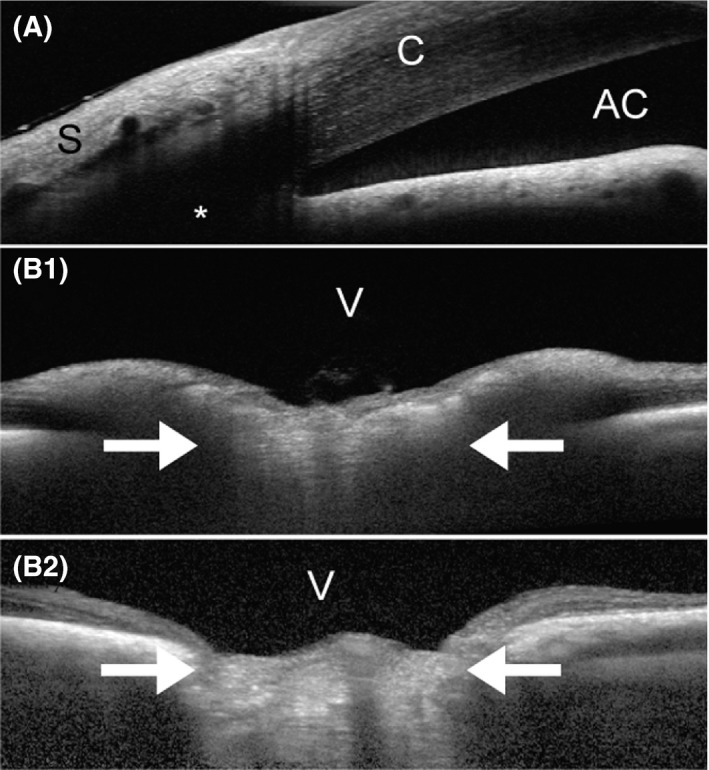
Optical coherence tomography (OCT) images of the canine eye taken with the Spectralis® (Heidelberg Engineering GmbH, Heidelberg, Germany). A, Iridocorneal angle of a 2.5‐year old, female Beagle with POAG (IOP during imaging: 23 mm Hg). OCT often provides higher resolution than routine high‐resolution ultrasonography (Figure 3), but there are still limits when imaging deeper tissues, such as the aqueous humor outflow pathways (*). B, ONH images of a normal (B1; 6.5‐years old female) and POAG‐affected (B2; 9.5‐years old female) Beagle. While the nondegenerated, well‐myelinated normal canine ONH bulges into the vitreous (B1; IOP during imaging: 15 mm Hg), the chronically glaucomatous ONH appears cupped (B2; IOP during imaging: 19 mm Hg). The white arrows indicate the location of the lamina cribrosa. Unless there is extensive degeneration, the canine lamina cribrosa is difficult to visualize, even with the current enhanced depth imaging (EDI) technology, due to the thick, myelinated prelaminar ONH. AC, anterior chamber; C, cornea; S, sclera; V, vitreous
Figure 3.
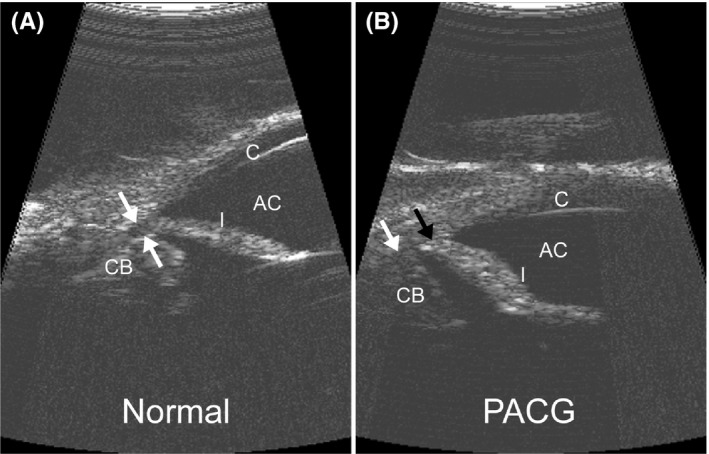
High‐resolution ultrasound (HRUS) images of the canine iridocorneal angle. Compared to a normal eye with physiologic IOP (A) with flat iris and open ciliary cleft (white arrows), the iris has a sigmoidal shape with increased corneal contact (black arrow) and a collapsed ciliary cleft (white arrow) in an eye with acute PACG and IOP of 55 mm Hg (B). AC, anterior chamber; C, cornea; CB, ciliary body; I, iris. (From Miller143; with permission)
4. OPTIMIZATION OF IOP‐LOWERING MEDICAL TREATMENT
Medical therapies continue to play an important role either separately or concurrent with surgical treatments. To date, the only treatable glaucoma risk factor is IOP; additional medical treatment options may be identified in the future. Current drugs are aimed at either decreasing aqueous humor production or improving drainage through conventional and unconventional pathways (Figure 1). Unfortunately, the development of new, IOP‐lowering medications has been slow and most of these medications are optimized to treat human rather than canine glaucoma. Different forms of canine glaucoma may respond differently to specific medications. For example, results of IOP studies performed in Beagle POAG may not translate to other forms of canine glaucoma. Furthermore, we have observed inter‐individual differences in dogs' responsiveness to glaucoma drugs. Recent efforts are geared toward the development of mechanistic‐based therapies with the hope that they would be more effective. Two topical IOP‐lowering medications recently approved by the US Food and Drug Administration (FDA) are latanoprostene bunod (Vyzulta™; Bausch & Lomb Incorporated) and netarsudil (Rhopressa™; Aerie Pharmaceuticals). Latanoprostene bunod is a nitric oxide‐donating prostaglandin F2α agonist with improved IOP‐lowering effect in ADAMTS10‐mutant beagles with POAG compared to latanoprost.25, 26, 27 The enhanced effectiveness of latanoprostene bunod is based on the beneficial effect on both uveoscleral and trabecular aqueous humor outflow.28 Netarsudil is a Rho kinase (ROCK) inhibitor and the first drug specifically designed to target the trabecular meshwork cells. Based on this mechanism of action, the drug is expected to be more effective in POAG than PACG. ROCK inhibition reduces cell contractility and cell stiffness, and it decreases expression of fibrosis‐related proteins, resulting in increased trabecular outflow facility.29, 30, 31 In addition, netarsudil has norepinephrine transporter (NET) inhibitory activity, which may be responsible for the documented reduction of aqueous humor production and decrease in episcleral venous pressure, thereby further contributing to the lowering of IOP.29, 32, 33 Testing of netarsudil 0.02% ophthalmic solution (corresponding to commercial Rhopressa™) resulted in IOP reduction of ~5 mm Hg in normal Dutch Belted rabbits and Formosan Rock monkeys, but there are no published reports on its effectiveness in dogs.29 Canine studies of netarsudil were limited to corneal metabolic assays, and the ROCK inhibitor Y27632 has been shown to stimulate corneal endothelial wound healing in normal dogs following experimental transcorneal freezing.29, 34 Netarsudil has been combined with latanoprost (Roclatan™, Aerie Pharmaceuticals) with an improvement in IOP reduction in human patients with POAG or ocular hypertension.35
Poor adherence to eye drop administration is a major factor contributing to the progression of glaucomatous optic neuropathy in human patients. An estimated 50% of patients do not adhere to their medication over 75% of the time.36 In addition, only 60%‐70% of prescribed doses of eye drops are taken by glaucoma patients.37 Drug administration adherence in canine glaucoma has rarely been studied, but also could be a concern. In one study evaluating the capability of demecarium bromide or betaxolol to prevent/delay the onset of PACG in the normotensive fellow eyes of dogs with unilateral PACG, 78%‐94% of clients self‐reported that they administered the medications at least 90% of the time, and 93%‐97% reported that they administered it at least 50% of the time.38 To address this problem in human patients, several drug companies have developed devices for long‐term, sustained drug release, either onto the corneal surface or into the anterior chamber. Most of these drug implants release prostaglandin analogs; some were moved from preclinical testing into clinical application in human patients. Externally placed devices include the OTX‐TP travoprost punctal plugs (Ocular Therapeutix, Inc) and the Helios™ bimatoprost periocular ring (Allergan plc) for placement into the conjunctival fornix.39, 40 Intracameral implants include Bimatoprost SR (Allergan), ENV515 travoprost (Envisia Therapeutics, Inc), OTX‐TIC travoprost (Ocular Therapeutix, Inc), and iDose travoprost (Glaukos®).39, 41 Intracameral, biodegradable latanoprost‐, bimatoprost‐, and travoprost‐releasing devices have been tested successfully in normal dogs and dogs with POAG, but we are not aware of any plans to move these devices into veterinary clinical application.41, 42, 43, 44 The use of ocular, slow‐releasing drug implants is not new in veterinary ophthalmology since cyclosporine devices are being used in both horses and dogs for recurrent uveitis, immune‐mediated keratitis, and keratoconjunctivitis sicca.45, 46, 47
Our discussions on medical management of canine glaucoma also included the evaluation of compounds to decrease the rate of secondary glaucoma following phacoemulsification surgery. The detailed functional and morphological assessment of the aqueous humor outflow pathways following canine cataract surgery should be continued (Figure 4).48, 49 The ability of cholinergics (eg, carbachol) or prostaglandin analogs (eg, latanoprost) to reverse some of the anatomic alterations in ciliary cleft morphology associated with lens extraction needs to be further evaluated in randomized and adequately powered, prospective clinical trials.50 Additionally, there are clear indications that the formation of pre‐iridal fibrovascular membranes (PIFVMs) impairs aqueous humor drainage and elevates IOP.7 It is suspected that upregulation of vascular endothelial growth factor (VEGF) expression associated with lens‐induced uveitis is one of the factors resulting in PIFVM formation.51 The intravitreal administration of anti‐VEGF compounds, such as ranibizumab (Lucentis®, Genentech, Roche Group) or bevacizumab (Avastin®, Genentech), is performed routinely in human patients with age‐related macular degeneration and diabetic retinopathy and also could be considered for the treatment of dogs following cataract surgery.52, 53 These injections are mostly safe in human patients, but adverse effects, including immune‐mediated uveitis directed at a humanized protein and decrease in aqueous humor outflow facility, have been reported in few human patients and also observed by some of us following injection in canine eyes (unpublished).54, 55, 56 Clearly, other anti‐neovascular strategies, such as gene therapies, are needed to address some of these limitations in the dogs, including the need for repeated intraocular injections.57
Figure 4.
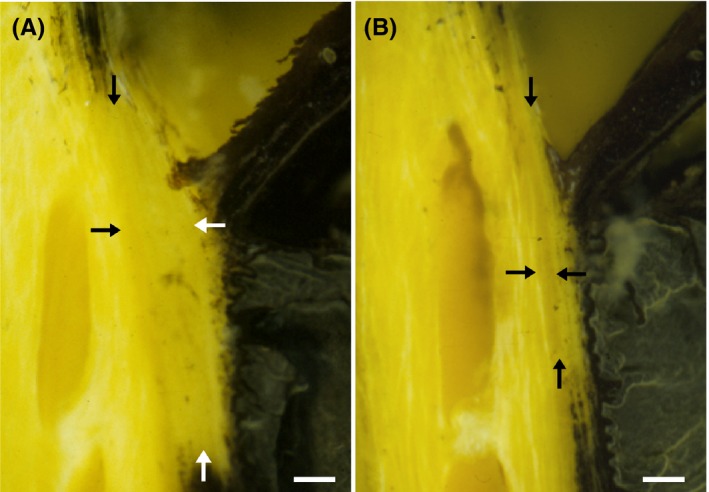
The canine ciliary cleft may collapse following lens removal by phacoemulsification. Tissue cross‐sections of the iridocorneal angle in normal Bouin's fixed globes show that compared to the normal, unoperated eye (A) the ciliary cleft is severely reduced in an eye 24 h after phacoemulsification (B). The IOP in this eye reached 52 mm Hg 3 h after surgery and decreased to 15 mm Hg at 24 h. Despite the normalization of IOP, the ciliary cleft remained reduced. The arrows denote the approximate boundaries of the ciliary cleft. Bars = 0.2 mm. (from Miller et al49; with permission)
Until specific therapeutic targets can be identified in dogs, the use of better anti‐inflammatory drugs may be the most promising option currently available. These drugs need to inhibit lens‐induced uveitis and re‐establish the blood‐aqueous barrier following cataract surgery. Compounds/methods that have been discussed, and are already used by some veterinary ophthalmologists, include the intraocular administration of corticosteroids by injection or implant (eg, Retisert® fluocinolone acetonide intravitreal implant, Bausch & Lomb), and highly potent steroidal and nonsteroidal anti‐inflammatory ophthalmic solutions/suspensions, such as difluprednate (Durezol®, Alcon Laboratories, A Novartis Division) and nepafenac (Nevanac®, Alcon Laboratories), respectively. Prospective randomized clinical trials are required to objectively evaluate the effectiveness of these compounds in dogs.
5. NOVEL SURGICAL THERAPIES TO CONTROL IOP
The development of new glaucoma medications is slow, arduous, costly, and optimized for the human rather than canine eye. Therefore, the improvement of surgical management of canine glaucoma may offer more promise in the foreseeable future for long‐term IOP control and sight preservation.
Currently, the most commonly used surgical techniques for canine glaucoma are placement of drainage implants to shunt aqueous humor to equatorial bleb‐promoting reservoirs (Figure 5), cyclodestructive techniques to reduce aqueous humor production, and combinations of these two methods.58, 59 Even though recently introduced surgical methods appear promising with increased success rates, they are often associated with intensive postoperative care and considerable expense, and a substantial number of affected dogs still go blind. The method of choice is based on clinician preference and cost to owner, and is influenced by factors such as breed, type and stage of glaucoma, and surgeon experience. Based on these factors, long‐term success rates for IOP control and preservation of sight vary considerably, but tend to improve with more advanced technologies and modifications of surgical techniques. The most recently published 1‐year success rates of canine glaucoma surgery for both IOP control and sight preservation are ~90% for Ahmed valved drainage implants,21, 60 65%‐75% for Baerveldt nonvalved glaucoma drainage device,61, 62 41%‐92% for transscleral cyclophotocoagulation (TSCP) alone or in combination with the placement of Ahmed valved drainage implants,62, 63, 64, 65, 66, 67 and 72%‐74% for endolaser cyclophotocoagulation (ECP).58 Even though we have to be careful when comparing human and canine studies because of different study designs, inclusion/exclusion criteria, and specific outcome measures, these canine success rates are comparable to published results in human patients for Ahmed and Baerveldt drainage devices (ClinicalTrials.gov Identifier: NCT00376363).68 Most of the published canine studies are severely limited because of small sample size and short follow‐up period; these are shortcomings that need to be addressed in the future, for example, by taking advantage of multicenter studies. This has been recognized as a high priority during our discussions. The most common complications which can result in vision loss and which should be addressed in future research projects include ocular hypertension, intracameral fibrin formation, ocular hypotony and phthisis bulbi, cataract formation, and corneal ulceration.61, 62 The confounding effect of inflammation in canine eyes with PACG has to be considered in the development and improvement of surgical therapies.69
Figure 5.
Tube positioning of an Ahmed VS‐2 valved drainage implant (New World Medical Inc, Rancho Cucomonga, CA, USA) in the anterior chamber of two dogs (A and B). B, Subconjunctival filtering bleb is shown underneath the upper eyelid
Improvements in implant design, surgical technique, modulation of wound healing, patient selection, and postoperative management, including IOP home monitoring to detect early implant failure, have resulted in improved success rates using the Ahmed valved device as a single surgical treatment option.21, 60 Other drainage implants that are being used in dogs include Molteno and Baerveldt devices, as well as frontal sinus shunts.61, 62, 70 Scar formation over the subconjunctival bleb is one of the main reasons for implant failure in both human and canine patients. For reasons that remain to be determined, bleb fibrosis appears much more exaggerated in dogs than humans. Currently, the main approach to inhibit scar formation continues to be the intraoperative treatment of the bleb site with antimitotic compounds, such as mitomycin‐C (MMC) or 5‐fluorouracil (5‐FU).21, 59, 61, 62 These reagents have to be handled with great care because of their potential toxic effect and possible conjunctival necrosis.59, 60 Whenever capsule fibrosis has developed and IOP starts to increase, repeated bleb revision of the overlying conjunctiva is indicated by removing a portion of the fibrotic capsule over the implant and/or injection of an antimitotic reagent such as 5‐FU.59 The search for new, improved anti‐fibrotic treatment strategies (“scar wars”) has been ongoing for many years and continues to be a high priority.71, 72, 73, 74 The use of new compounds and molecular therapies are being considered for targeting of specific profibrotic molecular pathways.71, 72, 73, 74, 75
In humans, a two‐stage drainage implant technique is oftentimes useful to reduce complications related to hypotony and inflammation and may be applicable for some forms of canine glaucoma. This method was proposed in 1979 by Molteno et al.76 In the first stage, the episcleral plate of the drainage device is positioned without intracameral tube insertion. A capsule is allowed to form around the plate over ~6‐week period. Subsequently, the silicone tube is inserted into the anterior chamber during the second stage. The already formed capsule provides resistance to aqueous outflow, allowing a more controlled IOP decrease.76
Despite the recent emergence of ECP (see below), diode laser TSCP is still widely used by veterinary ophthalmologists, either as a sole surgical tool or in combination with the placement of a drainage implant.21, 60, 63, 64, 65, 66, 67 While TSCP is relatively noninvasive and easy to perform, the long‐term success rate tends to be lower (50%‐92% IOP control and 50%‐53% vision at 1‐year post‐TSCP), unless the laser treatment is combined with drainage implants.58, 59, 63, 64, 65 Vision‐threatening complications associated with conventional TSCP include immediate IOP elevation, corneal ulceration, retinal detachment, hemorrhage, and hypotony with resulting phthisis bulbi. Most of these adverse effects are due to the nonselective destruction of adjacent tissues because of the high temperatures reached in the target tissue.58, 77 A novel technique currently being evaluated for TSCP is the use of micropulse laser (Figure 6; MicroPulse® Cyclo G6; Iridex).78 The proposed main advantage of micropulse TSCP is the short wave of energy followed by an off cycle that allows the adjacent nonpigmented tissue to cool off, thereby minimizing any collateral thermal damage to adjacent tissues.78, 79 The exact mechanism of action of micropulse TSCP still needs to be determined, but may consist not only of ciliary ablation but also improved conventional and uveoscleral aqueous humor outflow.78, 79 Preliminary results about the effectiveness of the micropulse laser in dogs are mixed, with a need to refine protocols and patient selection to improve long‐term IOP‐lowering treatment effect and reduce the rate of complications, such as corneal ulceration.79, 80
Figure 6.
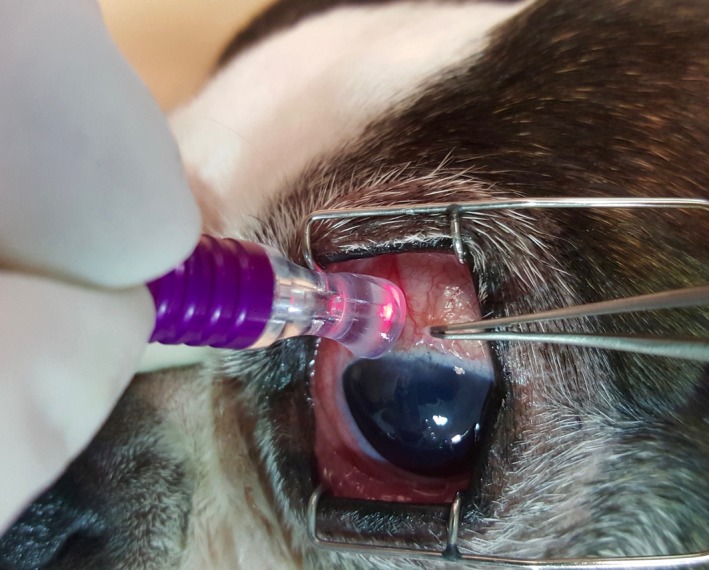
Positioning of the MicroPulse® Cyclo G6 probe (Iridex) 3 mm posterior to the limbus of a dog during transscleral cyclophotocoagulation (TSCP)
Diode ECP is an attractive alternative to TSCP in the treatment of canine glaucoma. It is combined with lens removal by phacoemulsification, or it also can be used for prophylactic treatment combined with cataract removal in dogs at increased risk of glaucoma development.58 The main advantage of ECP over TSCP is that the ciliary processes and the laser treatment effect can be visualized directly through an endoscope, allowing the use of more controlled application of significantly less laser energy (Figure 7). Even though ECP has been used by veterinary ophthalmologists for over 10 years, peer‐reviewed publications of large case series are lacking and considered of high priority by our group. The use of laser cyclophotocoagulation may be potentially less effective in color‐diluted dogs with blue irises and no pigment in the ciliary musculature possibly resulting in less absorption of laser energy for photocoagulation.81
Figure 7.
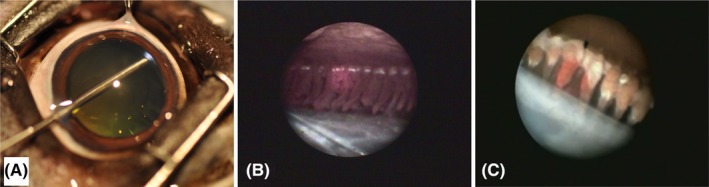
Endolaser cyclophotocoagulation (ECP) in the canine eye. A, The laser endoscope is inserted through a limbal incision and the pupil to access the ciliary processes. The endoscopic view shows the red aiming beam on the ciliary processes before (B) and following laser treatment when they appear white and shrunken (C). The lens capsule is shown on the bottom and the posterior iris surface on the top (B and C)
The introduction of microinvasive or minimally invasive glaucoma surgeries (MIGS) in the surgical management of open‐angle glaucoma in humans represents an innovative development. The impact of the various MIGS remains to be determined with long‐term outcomes of effective IOP reduction and complications, and with appropriate comparative effectiveness trials and meta‐analyses.82 Because of their minimal invasiveness with moderate IOP reductions, these aqueous humor draining techniques are being considered more often as alternatives for the treatment of early glaucoma stages, instead of medical therapies.83 Even though MIGS are being developed specifically for human patients, some could be considered for application in dogs, especially if they are not targeting the Schlemm's canal which does not exist in canines. For example, the EX‐PRESS® Mini Glaucoma Shunt (Alcon, A Novartis Division) allows aqueous humor drainage from the anterior chamber beneath a scleral flap. Some of the authors as well as others have used the EX‐PRESS® Mini Glaucoma Shunt in selected cases in combination with ECP or cataract surgery for temporary IOP relief, with a 1‐year vision survival rate of up to 80% (Saito 2018, personal communication).84 Other approved devices and techniques used in human patients include InnFocus MicroShunt® (InnFocus, Inc), iStent® (Glaukos®), and Gonioscopy‐Assisted Transluminal Trabeculotomy (GATT). The XEN® Gel Stent (Allergan Plc) is a microfistula implant that consists of a glutaraldehyde cross‐linked porcine gelatin tube that is placed ab interno under direct gonioscopic visualization from the anterior chamber through the trabecular meshwork and sclera into the subconjunctival space; its biocompatibility was successfully tested over 1 year in normal Beagles, but we are not aware of any applications in glaucomatous dogs.85 Recently, the nanoengineered SalVO/Brown Glaucoma Implant (MicroOptx) was proposed for use in dogs.86, 87 This MIGS device drains aqueous humor onto the ocular surface, and it was safe and effective to lower IOP in normal Yucatan pigs.87 Safety and efficacy remain to be determined in humans and dogs.
6. NOVEL TREATMENT STRATEGIES FOR THE FUTURE
6.1. Gene and stem cell therapies to control IOP
Major advances have been made over the past 20 years in ocular gene therapy, and a few retinal and optic nerve treatments have been translated into clinical application for human patients.57, 88, 89 We anticipate that gene therapy of both the anterior and posterior segments of the eye will eventually benefit glaucoma patients. The identification of molecular disease pathways and genetic risk factors affecting the aqueous humor outflow pathways will allow us to target and correct the disease pathogenesis very specifically, potentially resulting in long‐term, effective IOP control. Major advances in this direction already have been made by robust and safe targeting of transgene expression to the trabecular meshwork in several animal species, including dogs: Treatment has been done by aqueous paracentesis and intracameral administration of adenovirus, lentivirus, and adeno‐associated virus (AAV) gene therapy vectors.90, 91, 92, 93, 94 The recent development of novel capsid mutated virus particles resulted in an expanded AAV vector toolkit for targeting of the trabecular meshwork and other tissues within the anterior segment of the eye that may contribute to increased aqueous humor outflow resistance in glaucoma.91, 93, 94, 95 The good safety and efficacy record of AAV within the eye renders it a very attractive option for therapy and long‐term IOP control in primary glaucoma.
Recently, trabecular meshwork‐like cells were created from induced pluripotent stem cells (iPSCs) and injected into the anterior chamber of transgenic, myocilin (MYOC)‐mutant mice with POAG.96, 97 Subsequently, the conventional outflow pathway was replenished with new TM cells, resulting in improved outflow facility, IOP control, and halted RGC loss, even in animals with advanced stages of glaucoma.96, 97 These proof‐of‐concept studies indicate that stem cell‐based therapy also may become an option for long‐term IOP control in dogs with primary glaucoma.
6.2. Modification of the eye's biomechanical properties
The biomechanical properties of the eye, most importantly its fibrous layer (cornea, sclera, and lamina cribrosa), determine the susceptibility to various levels of IOP.98 Even physiologic IOP can damage RGC axons as they pass through the lamina cribrosa if the surrounding connective tissue does not provide the necessary protective support. This may contribute to the disease process in nearly half of human patients with open‐angle glaucoma who are normotensive with IOP measurements consistently lower than 21mmHg.99, 100 A 30% IOP reduction in these normotensive glaucoma patients can prevent progression of visual field loss.101 As veterinary ophthalmologists we have a unique opportunity to study how the biomechanical properties of the ocular tissues affect susceptibility to IOP. For example, we observe that ADAMTS10‐mutant beagles102 and ADAMTS17‐mutant Chinese shar‐peis103 with POAG maintain eye sight longer with slower progression of ONH atrophy than many other glaucomatous dog breeds with comparable pressures.16 While proof still needs to be provided that tissue properties are linked to IOP susceptibility in dogs, initial biomechanical studies in ADAMTS10‐mutant beagles showed that their posterior sclera is weaker with reduced fibrous collagen density.104, 105, 106 Similarly, some genetically altered mice are resistant to glaucoma damage, while treatment of the sclera with cross‐linking agents worsens IOP‐related damage to the RGC axons.98 Once we define advantageous biomechanical properties of the fibrous layer of the eye, therapeutic tools can be developed to modify these properties in order to achieve a protective effect.
6.3. Neuroprotection
The final common pathway of all forms of glaucoma is the progressive loss of RGCs and their axons, even when IOP is effectively controlled. Considerable effort has been put into the study of IOP‐independent disease mechanisms responsible for RGC death in animal models and human patients, so that neuroprotective treatments can be developed. Some of these disease pathways, which may or may not be triggered by IOP, include: excitotoxicity caused by excessive excitatory amino acid release, such as glutamate and aspartate107, 108; neurotrophin deprivation from blockage of retrograde axonal transport109, 110, 111, 112; excessive intracellular calcium113; compromised blood flow to the ONH and retina114, 115, 116, 117, 118, 119, 120; oxidative stress121, 122; inflammation and autoimmunity against retinal and optic nerve antigens123, 124, 125; and reactive gliosis.126, 127, 128, 129 Unfortunately, most of these mechanisms have not yet been investigated in dogs, and there may be profound species differences. For example, the unique vascular anatomy in dogs may render their retina and ONH more susceptible to ischemia with IOP variation.130
While many available compounds address some of these previously listed disease pathways resulting in significant RGC protection in experimental animal models of glaucoma, none of them has been moved successfully into clinical application. Two neuroprotective therapies that have undergone clinical trials for glaucoma are memantine, given by oral route, and ciliary neurotrophic factor (CNTF), continuously released into the vitreous (Figure 8). Memantine is an Nmethyl‐Daspartate (NMDA) receptor antagonist that counters the toxic effect of excessive glutamate in the extracellular space; it is used traditionally for the treatment of Alzheimer's disease. Memantine reduces RGC death and functional loss in experimental glaucoma in rats and primates.131, 132, 133 Unfortunately, protection of visual function by memantine could not be demonstrated in human glaucoma patients enrolled in two phase 3 clinical trials.134 The intravitreal administration of CNTF reportedly slows RGC death in rats with experimental glaucoma.135 The continuous release of CNTF by intravitreal encapsulated cell therapy was tested recently in phase 1 clinical trials in patients with POAG (ClinicalTrials.gov NCT01408472) and ischemic optic neuropathy (NCT01411657), but results have not yet been published. Some veterinary ophthalmologists, including some authors of this article, are using the calcium channel blocker amlodipine systemically in selected canine glaucoma patients based on its documented beneficial effects on ocular blood flow in normal dogs and potential neuroprotection.136 Currently, there is no data showing advantages of amlodipine in glaucomatous dogs, and it is possible that the blood pressure lowering effect of the drug may negatively affect ocular perfusion pressure. The development of neuroprotective therapies for glaucoma continues to be a high‐priority goal, which may also take advantage of tools such as gene and stem cell therapies.137, 138
Figure 8.
Sustained intraocular delivery of ciliary neurotrophic factor (CNTF) by encapsulated cell technology (ECT) in a canine eye. The NT‐501 implant containing CNTF‐secreting human cells (Neurotech Pharmaceuticals, Inc) is located within the vitreous and anchored to the pars plana of the ciliary body
6.4. Neuroregeneration
In mammals, RGCs do not regenerate once they are lost; this is contrary to other classes of animals, such as fish and amphibians, where retina and optic nerve can regenerate naturally. A number of animal studies have shown that under the right circumstances, mammalian RGCs are able to regenerate their axons and connect to the proper targets within the brain, resulting in some functional recovery of eye sight.139, 140 The replacement of lost RGCs and the regeneration of their axons are high priorities in glaucoma research, and our dog patients may also benefit from these efforts in the future. The National Eye Institute (NEI) within the National Institutes of Health (NIH) predicts that these goals are achievable within 10‐15 years and has made them high priorities for research funding.141 Furthermore, transplantation of RGCs by intravitreal injection is one method to replace lost RGCs.142 Ultimately, even transplantation of whole eyes may become an option with improvements in optic nerve regeneration.
7. SUMMARY
While much progress has been made in the understanding and treatment of canine glaucoma, there is still no cure and many affected dogs go blind. The improved knowledge of disease mechanisms and the development of reliable biomarkers are critical so that animals at risk or in early stages of disease can be identified more readily. Early diagnosis facilitates effective, mechanism‐based treatment before the occurrence of any clinically appreciable optic nerve damage and vision loss. To achieve these goals, we recommend research priorities for clinicians and basic scientists. One of the main limitations in these efforts is the scarcity of major research funding specifically dedicated to canine disease.
CONFLICTS OF INTEREST
The authors have the following potential conflicts of interest (in alphabetic order): Aerie Pharmaceutical (RLF and SEM: research funding, clinical trial grant), Allergan (SEM: research funding, clinical trial grant), Bausch and Lomb (RLF: research funding), Beaver‐Visitec (RLF: consultant), Cara Life (DWE: previous consultant), Elsevier (PEM: book royalties), Ivantis (CBT: research funding), Nicox (CBT: research funding), OSOD (PEM: consultant), PolyActiva (AMK: research funding), Santen (CBT: research funding), and Wolters Kluwer (SEM: book royalties). The opinions expressed in this article are the authors' own and do not necessarily reflect the view of the United States Food and Drug Administration (FDA) or the United States Government. Special thanks go to Ms. Jen Gazdacko (ACVO‐VAF) for her technical support.
ACKNOWLEDGMENTS
The Canine Glaucoma Think Tank and Consortium were supported by the American College of Veterinary Ophthalmologists Vision for Animals Foundation (ACVO‐VAF). Additional funding of the authors' glaucoma research was provided by the ACVO‐VAF (AMK), Bouvier Health Foundation (PEM), BrightFocus Foundation (AMK: G2017185), Michigan State University College of Veterinary Medicine Endowed Research Funds (AMK), and National Eye Institute/National Institutes of Health (AMK: R01‐EY025752; SEM: R01‐EY022124).
Komáromy AM, Bras D, Esson DW, et al. The future of canine glaucoma therapy. Vet Ophthalmol. 2019;22:726–740. 10.1111/vop.12678
REFERENCES
- 1. Gelatt KN, MacKay EO. Prevalence of the breed‐related glaucomas in pure‐bred dogs in North America. Vet Ophthalmol. 2004;7:97‐111. [DOI] [PubMed] [Google Scholar]
- 2. Gelatt KN, MacKay EO. Secondary glaucomas in the dog in North America. Vet Ophthalmol. 2004;7:245‐259. [DOI] [PubMed] [Google Scholar]
- 3. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta‐analysis. Ophthalmology. 2014;121:2081‐2090. [DOI] [PubMed] [Google Scholar]
- 5. Newbold GM, Kelch WJ, Chen T, et al. Phacoemulsification outcomes in Boston terriers as compared to non‐Boston terriers: a retrospective study (2002–2015). Vet Ophthalmol. 2018;21:353‐361. [DOI] [PubMed] [Google Scholar]
- 6. Foote BC, Pederson SL, Welihozkiy A, et al. Retinal detachment and glaucoma in the Boston Terrier and Shih Tzu following phacoemulsification (135 patients): 2000–2014. Vet Ophthalmol. 2018;21:240‐248. [DOI] [PubMed] [Google Scholar]
- 7. Scott EM, Esson DW, Fritz KJ, et al. Major breed distribution of canine patients enucleated or eviscerated due to glaucoma following routine cataract surgery as well as common histopathologic findings within enucleated globes. Vet Ophthalmol. 2013;16(Suppl 1):64‐72. [DOI] [PubMed] [Google Scholar]
- 8. Moeller E, Blocker T, Esson D, et al. Postoperative glaucoma in the Labrador Retriever: incidence, risk factors, and visual outcome following routine phacoemulsification. Vet Ophthalmol. 2011;14:385‐394. [DOI] [PubMed] [Google Scholar]
- 9. Sigle KJ, Nasisse MP. Long‐term complications after phacoemulsification for cataract removal in dogs: 172 cases (1995–2002). J Am Vet Med Assoc. 2006;228:74‐79. [DOI] [PubMed] [Google Scholar]
- 10. Biros DJ, Gelatt KN, Brooks DE, et al. Development of glaucoma after cataract surgery in dogs: 220 cases (1987–1998). J Am Vet Med Assoc. 2000;216:1780‐1786. [DOI] [PubMed] [Google Scholar]
- 11. Lannek EB, Miller PE. Development of glaucoma after phacoemulsification for removal of cataracts in dogs: 22 cases (1987–1997). J Am Vet Med Assoc. 2001;218:70‐76. [DOI] [PubMed] [Google Scholar]
- 12. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. J Am Med Assoc. 2014;311:1901‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsai S, Almazan A, Lee SS, et al. The effect of topical latanoprost on anterior segment anatomic relationships in normal dogs. Vet Ophthalmol. 2013;16:370‐376. [DOI] [PubMed] [Google Scholar]
- 14. Tsai S, Miller PE, Struble C, et al. Topical application of 0.005% latanoprost increases episcleral venous pressure in normal dogs. Vet Ophthalmol. 2012;15(Suppl 1):71‐78. [DOI] [PubMed] [Google Scholar]
- 15. Dubin AJ, Bentley E, Buhr KA, et al. Evaluation of potential risk factors for development of primary angle‐closure glaucoma in Bouviers des Flandres. J Am Vet Med Assoc. 2017;250:60‐67. [DOI] [PubMed] [Google Scholar]
- 16. Grozdanic SD, Kecova H, Harper MM, et al. Functional and structural changes in a canine model of hereditary primary angle‐closure glaucoma. Invest Ophthalmol Vis Sci. 2010;51:255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hasegawa T, Kawata M, Ota M. Ultrasound biomicroscopic findings of the iridocorneal angle in live healthy and glaucomatous dogs. J Vet Med Sci. 2016;77:1625‐1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kagemann L, Wollstein G, Ishikawa H, et al. Visualization of the conventional outflow pathway in the living human eye. Ophthalmology. 2012;119:1563‐1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kagemann L, Wollstein G, Ishikawa H, et al. 3D visualization of aqueous humor outflow structures in‐situ in humans. Exp Eye Res. 2011;93:308‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Almazan A, Tsai S, Miller PE, et al. Iridocorneal angle measurements in mammalian species: normative data by optical coherence tomography. Vet Ophthalmol. 2013;16:163‐166. [DOI] [PubMed] [Google Scholar]
- 21. Saito A, Kazama Y, Iwashita H, et al. Outcome of anterior chamber shunt procedure in 104 eyes of dogs (abstract). 48th Annual Conference of the American College of Veterinary Ophthalmologists. 2017;41. [Google Scholar]
- 22. Meier‐Gibbons F, Berlin MS, Toteberg‐Harms M. Twenty‐four hour intraocular pressure measurements and home tonometry. Curr Opin Ophthalmol. 2018;29:111‐115. [DOI] [PubMed] [Google Scholar]
- 23. Komaromy AM, Petersen‐Jones SM. Genetics of canine primary glaucomas. Vet Clin North Am Small Anim Pract. 2015;45:1159‐1182. [DOI] [PubMed] [Google Scholar]
- 24. Graham KL, McCowan C, White A. Genetic and biochemical biomarkers in canine glaucoma. Vet Pathol. 2017;54:194‐203. [DOI] [PubMed] [Google Scholar]
- 25. Krauss AH, Impagnatiello F, Toris CB, et al. Ocular hypotensive activity of BOL‐303259‐X, a nitric oxide donating prostaglandin F2alpha agonist, in preclinical models. Exp Eye Res. 2011;93:250‐255. [DOI] [PubMed] [Google Scholar]
- 26. Borghi V, Bastia E, Guzzetta M, et al. A novel nitric oxide releasing prostaglandin analog, NCX 125, reduces intraocular pressure in rabbit, dog, and primate models of glaucoma. J Ocul Pharmacol Ther. 2010;26:125‐132. [DOI] [PubMed] [Google Scholar]
- 27. Impagnatiello F, Borghi V, Gale DC, et al. A dual acting compound with latanoprost amide and nitric oxide releasing properties, shows ocular hypotensive effects in rabbits and dogs. Exp Eye Res. 2011;93:243‐249. [DOI] [PubMed] [Google Scholar]
- 28. Cavet ME, DeCory HH. The role of nitric oxide in the intraocular pressure lowering efficacy of latanoprostene bunod: review of nonclinical studies. J Ocul Pharmacol Ther. 2017;34:52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin CW, Sherman B, Moore LA, et al. Discovery and preclinical development of netarsudil, a novel ocular hypotensive agent for the treatment of glaucoma. J Ocul Pharmacol Ther. 2017;34:40‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rao PV, Pattabiraman PP, Kopczynski C. Role of the Rho GTPase/Rho kinase signaling pathway in pathogenesis and treatment of glaucoma: Bench to bedside research. Exp Eye Res. 2017;158:23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang SK, Chang RT. An emerging treatment option for glaucoma: Rho kinase inhibitors. Clin Ophthalmol. 2014;8:883‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rao PV, Deng PF, Kumar J, et al. Modulation of aqueous humor outflow facility by the Rho kinase‐specific inhibitor Y‐27632. Invest Ophthalmol Vis Sci. 2001;42:1029‐1037. [PubMed] [Google Scholar]
- 33. Kiel JW, Kopczynski CC. Effect of AR‐13324 on episcleral venous pressure in Dutch belted rabbits. J Ocul Pharmacol Ther. 2015;31:146‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miyagi H, Kim S, Li J, et al. Topical Rho‐associated kinase inhibitor, Y27632, accelerates corneal endothelial regeneration in a canine cryoinjury model. Cornea. 2019;38:352‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lewis RA, Levy B, Ramirez N, et al. Fixed‐dose combination of AR‐13324 and latanoprost: a double‐masked, 28‐day, randomised, controlled study in patients with open‐angle glaucoma or ocular hypertension. Br J Ophthalmol. 2016;100:339‐344. [DOI] [PubMed] [Google Scholar]
- 36. Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid study. Ophthalmology. 2009;116:191‐199. [DOI] [PubMed] [Google Scholar]
- 37. Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS). Invest Ophthalmol Vis Sci. 2007;48:5052‐5057. [DOI] [PubMed] [Google Scholar]
- 38. Miller PE, Schmidt GM, Vainisi SJ, et al. The efficacy of topical prophylactic antiglaucoma therapy in primary closed angle glaucoma in dogs: a multicenter clinical trial. J Am Anim Hosp Assoc. 2000;36:431‐438. [DOI] [PubMed] [Google Scholar]
- 39. Aref AA. Sustained drug delivery for glaucoma: current data and future trends. Curr Opin Ophthalmol. 2017;28:169‐174. [DOI] [PubMed] [Google Scholar]
- 40. Brandt JD, DuBiner HB, Benza R, et al. Long‐term safety and efficacy of a sustained‐release bimatoprost ocular ring. Ophthalmology. 2017;124:1565‐1566. [DOI] [PubMed] [Google Scholar]
- 41. Lee SS, Burke J, Shen J, et al. Bimatoprost sustained‐release intracameral implant reduces episcleral venous pressure in dogs. Vet Ophthalmol. 2018;21:376‐381. [DOI] [PubMed] [Google Scholar]
- 42. Komaromy AM, Koehl KL, Harman CD, et al. Long‐term intraocular Pressure (IOP) control by means of a novel biodegradable intracameral (IC) latanoprost free acid (LFA) implant (abstract). Annual Meeting of the Association for Research in Vision and Ophthalmology. 2017;58:4591. [Google Scholar]
- 43. Robeson R, Verhoeven RS, Garcia A, et al. A 12‐month study of the ENV515 (travoprost) intracameral implant on intraocular pressure in beagle dogs (abstract). Annual Meeting of the Association for Research in Vision and Ophthalmology.2017;58:1072. [Google Scholar]
- 44. Seal JR, Robinson MR, Burke J, et al. Intracameral sustained‐release bimatoprost implant delivers bimatoprost to target tissues with reduced drug exposure to off‐target tissues. J Ocul Pharmacol Ther 2018;35:50‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barachetti L, Rampazzo A, Mortellaro CM, et al. Use of episcleral cyclosporine implants in dogs with keratoconjunctivitis sicca: pilot study. Vet Ophthalmol. 2015;18:234‐241. [DOI] [PubMed] [Google Scholar]
- 46. Gilger BC, Wilkie DA, Clode AB, et al. Long‐term outcome after implantation of a suprachoroidal cyclosporine drug delivery device in horses with recurrent uveitis. Vet Ophthalmol. 2010;13:294‐300. [DOI] [PubMed] [Google Scholar]
- 47. Gilger BC, Stoppini R, Wilkie DA, et al. Treatment of immune‐mediated keratitis in horses with episcleral silicone matrix cyclosporine delivery devices. Vet Ophthalmol. 2014;17(Suppl 1):23‐30. [DOI] [PubMed] [Google Scholar]
- 48. Rose MD, Mattoon JS, Gemensky‐Metzler AJ, et al. Ultrasound biomicroscopy of the iridocorneal angle of the eye before and after phacoemulsification and intraocular lens implantation in dogs. Am J Vet Res. 2008;69:279‐288. [DOI] [PubMed] [Google Scholar]
- 49. Miller PE, Stanz KM, Dubielzig RR, et al. Mechanisms of acute intraocular pressure increases after phacoemulsification lens extraction in dogs. Am J Vet Res. 1997;58:1159‐1165. [PubMed] [Google Scholar]
- 50. Stuhr CM, Miller PE, Murphy CJ, et al. Effect of intracameral administration of carbachol on the postoperative increase in intraocular pressure in dogs undergoing cataract extraction. J Am Vet Med Assoc. 1998;212:1885‐1888. [PubMed] [Google Scholar]
- 51. Sandberg CA, Herring IP, Huckle WR, et al. Aqueous humor vascular endothelial growth factor in dogs: association with intraocular disease and the development of pre‐iridal fibrovascular membrane. Vet Ophthalmol. 2012;15(Suppl 1):21‐30. [DOI] [PubMed] [Google Scholar]
- 52. Lim LS, Mitchell P, Seddon JM, et al. Age‐related macular degeneration. Lancet. 2012;379:1728‐1738. [DOI] [PubMed] [Google Scholar]
- 53. Simunovic MP, Maberley DA. Anti‐vascular endothelial growth factor therapy for proliferative diabetic retinopathy: A systematic review and meta‐analysis. Retina. 2015;35:1931‐1942. [DOI] [PubMed] [Google Scholar]
- 54. Wen JC, Reina‐Torres E, Sherwood JM, et al. Intravitreal anti‐VEGF injections reduce aqueous outflow facility in patients with neovascular age‐related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:1893‐1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Biagi C, Conti V, Montanaro N, et al. Comparative safety profiles of intravitreal bevacizumab, ranibizumab and pegaptanib: the analysis of the WHO database of adverse drug reactions. Eur J Clin Pharmacol. 2014;70:1505‐1512. [DOI] [PubMed] [Google Scholar]
- 56. Cunningham MA, Tlucek P, Folk JC, et al. Sequential, acute noninfectious uveitis associated with separate intravitreal injections of bevacizumab and ranibizumab. Retin Cases Brief Rep. 2013;7:355‐358. [DOI] [PubMed] [Google Scholar]
- 57. Campochiaro PA, Lauer AK, Sohn EH, et al. Lentiviral vector gene transfer of endostatin/angiostatin for macular degeneration (GEM) study. Hum Gene Ther. 2017;28:99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bras D, Maggio F. Surgical treatment of canine glaucoma: cyclodestructive techniques. Vet Clin North Am Small Anim Pract. 2015;45:1283‐1305. [DOI] [PubMed] [Google Scholar]
- 59. Maggio F, Bras D. Surgical treatment of canine glaucoma: filtering and end‐stage glaucoma procedures. Vet Clin North Am Small Anim Pract. 2015;45:1261‐1282. [DOI] [PubMed] [Google Scholar]
- 60. Westermeyer HD, Hendrix DV, Ward DA. Long‐term evaluation of the use of Ahmed gonioimplants in dogs with primary glaucoma: nine cases (2000–2008). J Am Vet Med Assoc. 2011;238:610‐617. [DOI] [PubMed] [Google Scholar]
- 61. Graham KL, Donaldson D, Billson FA, et al. Use of a 350‐mm2 Baerveldt glaucoma drainage device to maintain vision and control intraocular pressure in dogs with glaucoma: a retrospective study (2013–2016). Vet Ophthalmol. 2017;20:427‐434. [DOI] [PubMed] [Google Scholar]
- 62. Graham KL, Hall E, Caraguel C, et al. Comparison of diode laser trans‐scleral cyclophotocoagulation versus implantation of a 350‐mm(2) Baerveldt glaucoma drainage device for the treatment of glaucoma in dogs (a retrospective study: 2010–2016). Vet Ophthalmol. 2018;21:487‐497. [DOI] [PubMed] [Google Scholar]
- 63. Cook C, Davidson M, Brinkmann M, et al. Diode laser transscleral cyclophotocoagulation for the treatment of glaucoma in dogs: results of six and twelve month follow‐up. Vet Comp Ophthalmol. 1997;7:148‐154. [Google Scholar]
- 64. Hardman C, Stanley RG. Diode laser transscleral cyclophotocoagulation for the treatment of primary glaucoma in 18 dogs: a retrospective study. Vet Ophthalmol. 2001;4:209‐215. [DOI] [PubMed] [Google Scholar]
- 65. O'Reilly A, Hardman C, Stanley RG. The use of transscleral cyclophotocoagulation with a diode laser for the treatment of glaucoma occurring post intracapsular extraction of displaced lenses: a retrospective study of 15 dogs (1995–2000). Vet Ophthalmol. 2003;6:113‐119. [DOI] [PubMed] [Google Scholar]
- 66. Sapienza JS, van der Woerdt A. Combined transscleral diode laser cyclophotocoagulation and Ahmed gonioimplantation in dogs with primary glaucoma: 51 cases (1996–2004). Vet Ophthalmol. 2005;8:121‐127. [DOI] [PubMed] [Google Scholar]
- 67. Bentley E, Miller PE, Murphy CJ, et al. Combined cycloablation and gonioimplantation for treatment of glaucoma in dogs: 18 cases (1992–1998). J Am Vet Med Assoc. 1999;215:1469‐1472. [PubMed] [Google Scholar]
- 68. Budenz DL, Barton K, Gedde SJ, et al. Five‐year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015;122:308‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reilly CM, Morris R, Dubielzig RR. Canine goniodysgenesis‐related glaucoma: a morphologic review of 100 cases looking at inflammation and pigment dispersion. Vet Ophthalmol. 2005;8:253‐258. [DOI] [PubMed] [Google Scholar]
- 70. Cullen CL, Allen AL, Grahn BH. Anterior chamber to frontal sinus shunt for the diversion of aqueous humor: a pilot study in four normal dogs. Vet Ophthalmol. 1998;1:31‐39. [DOI] [PubMed] [Google Scholar]
- 71. Esson DW, Neelakantan A, Iyer SA, et al. Expression of connective tissue growth factor after glaucoma filtration surgery in a rabbit model. Invest Ophthalmol Vis Sci. 2004;45:485‐491. [DOI] [PubMed] [Google Scholar]
- 72. Esson DW, Popp MP, Liu L, et al. Microarray analysis of the failure of filtering blebs in a rat model of glaucoma filtering surgery. Invest Ophthalmol Vis Sci. 2004;45:4450‐4462. [DOI] [PubMed] [Google Scholar]
- 73. Yu‐Wai‐Man C, Spencer‐Dene B, Lee R, et al. Local delivery of novel MRTF/SRF inhibitors prevents scar tissue formation in a preclinical model of fibrosis. Sci Rep. 2017;7:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Martorana GM, Schaefer JL, Levine MA, et al. Sequential therapy with saratin, bevacizumab and ilomastat to prolong bleb function following glaucoma filtration surgery in a rabbit model. PLoS ONE. 2015;10:e0138054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sriram S, Robinson P, Pi L, et al. Triple combination of siRNAs targeting TGFbeta1, TGFbetaR2, and CTGF enhances reduction of collagen I and smooth muscle actin in corneal fibroblasts. Invest Ophthalmol Vis Sci. 2013;54:8214‐8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Molteno AC, Van Biljon G, Ancker E. Two‐stage insertion of glaucoma drainage implants. Trans Ophthalmol Soc N Z. 1979;31:17‐26. [PubMed] [Google Scholar]
- 77. Nadelstein B, Wilcock B, Cook C, et al. Clinical and histiologic effects of diode transscleral cyclophotocoagulation in the normal canine eye. Vet Comp Ophthalmol. 1997;7:155‐162. [Google Scholar]
- 78. Lee JH, Shi Y, Amoozgar B, et al. Outcome of micropulse laser transscleral cyclophotocoagulation on pediatric versus adult glaucoma patients. J Glaucoma. 2017;26:936‐939. [DOI] [PubMed] [Google Scholar]
- 79. Sapienza JS, Kim K, Rodriguez E, DiGirolamo N. Preliminary findings in 30 dogs treated with micropulse transscleral cyclophotocoagulation for refractory glaucoma. Vet Ophthalmol. 2018; 10.1111/vop.12622. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 80. Sebbag L, Allbaugh RA, Strauss RA, et al. MicroPulse™ transscleral cyclophotocoagulation in the treatment of canine glaucoma: Preliminary results (12 dogs). Vet Ophthalmol 2018. 10.1111/vop.12603 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 81. Newkirk KM, Haines DK, Calvarese ST, et al. Distribution and amount of pigment within the ciliary body and iris of dogs with blue and brown irides. Vet Ophthalmol. 2010;13:76‐80. [DOI] [PubMed] [Google Scholar]
- 82. Lavia C, Dallorto L, Maule M, et al. Minimally‐invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta‐analysis. PLoS ONE. 2017;12:e0183142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fingeret M, Dickerson JE Jr. The role of minimally invasive glaucoma surgery devices in the management of glaucoma. Optom Vis Sci. 2018;95:155‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lutz EA, Sapienza JS. Combined diode endoscopic cyclophotocoagulation and Ex‐Press™ shunt gonioimplantation in four cases canine glaucoma (abstract). 40th Annual Conference of the American College of Veterinary Ophthalmologists. 2009;80.
- 85. Shute TS, Dietrich UM, Baker JF, et al. Biocompatibility of a novel microfistula implant in nonprimate mammals for the surgical treatment of glaucoma. Invest Ophthalmol Vis Sci. 2016;57:3594‐3600. [DOI] [PubMed] [Google Scholar]
- 86. Larocca RD, Martin RC. Early results of the veterinary implant glaucoma registry (VIGOR) a multicenter evaluation of the Brown glaucoma implant in canines (abstract). 49th Annual Conference of the American College of Veterinary Ophthalmologists. 2018;137.
- 87. Martin RC, Baker SR, Render JA, et al. Safety and efficacy evaluation of a nanoengineered, externally comunicating, aqueous humor shunt in Yucatan swine (abstract). 49th Annual Conference of the American College of Veterinary Ophthalmologists. 2018;136.
- 88. Guy J, Feuer WJ, Davis JL, et al. Genet for Leber hereditary optic neuropathy: low‐ and medium‐dose visual results. Ophthalmology. 2017;124:1621‐1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bennett J. Taking stock of retinal gene therapy: looking back and moving forward. Mol Ther. 2017;25:1076‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Buie LK, Rasmussen CA, Porterfield EC, et al. Self‐complementary AAV virus (scAAV) safe and long‐term gene transfer in the trabecular meshwork of living rats and monkeys. Invest Ophthalmol Vis Sci. 2010;51:236‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bogner B, Boye SL, Min SH, et al. Capsid mutated adeno‐associated virus delivered to the anterior chamber results in efficient transduction of trabecular meshwork in mouse and rat. PLoS ONE. 2015;10:e0128759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dang Y, Loewen R, Parikh HA, et al. Gene transfer to the outflow tract. Exp Eye Res. 2017;158:73‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang L, Xiao R, Andres‐Mateos E, et al. Single stranded adeno‐associated virus achieves efficient gene transfer to anterior segment in the mouse eye. PLoS ONE. 2017;12:e0182473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Oh A, Harman CD, Koehl K, et al. Targeting of gene expression to the wildtype and ADAMTS10‐mutant canine trabecular meshwork by non‐self‐complementary AAV2 (abstract). Annual Meeting of the Association for Research in Vision and Ophthalmology. 2014;55:5669. [Google Scholar]
- 95. Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther. 2012;20:699‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhu W, Gramlich OW, Laboissonniere L, et al. Transplantation of iPSC‐derived TM cells rescues glaucoma phenotypes in vivo. Proc Natl Acad Sci USA. 2016;113:E3492‐E3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhu W, Jain A, Gramlich OW, et al. Restoration of aqueous humor outflow following transplantation of iPSC‐derived trabecular meshwork cells in a transgenic mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2017;58:2054‐2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Quigley HA. The contribution of the sclera and lamina cribrosa to the pathogenesis of glaucoma: diagnostic and treatment implications. Prog Brain Res. 2015;220:59‐86. [DOI] [PubMed] [Google Scholar]
- 99. Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090‐1095. [DOI] [PubMed] [Google Scholar]
- 100. Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992;99:1499‐1504. [DOI] [PubMed] [Google Scholar]
- 101. Collaborative Normal‐Tension Glaucoma Study Group . Comparison of glaucomatous progression between untreated patients with normal‐tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487‐497. [DOI] [PubMed] [Google Scholar]
- 102. Kuchtey J, Olson LM, Rinkoski T, et al. Mapping of the disease locus and identification of ADAMTS10 as a candidate gene in a canine model of primary open angle glaucoma. PLoS Genet. 2011;7:e1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Oliver J, Rustidge S, Pettitt L, et al. Evaluation of ADAMTS17 in Chinese Shar‐Pei with primary open‐angle glaucoma, primary lens luxation, or both. Am J Vet Res. 2018;79:98‐106. [DOI] [PubMed] [Google Scholar]
- 104. Boote C, Palko JR, Sorensen T, et al. Changes in posterior scleral collagen microstructure in canine eyes with an ADAMTS10 mutation. Mol Vis. 2016;22:503‐517. [PMC free article] [PubMed] [Google Scholar]
- 105. Palko JR, Iwabe S, Pan X, et al. Biomechanical properties and correlation with collagen solubility profile in the posterior sclera of canine eyes with an ADAMTS10 mutation. Invest Ophthalmol Vis Sci. 2013;54:2685‐2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Palko JR, Morris HJ, Pan X, et al. Influence of age on ocular biomechanical properties in a canine glaucoma model with ADAMTS10 mutation. PLoS ONE. 2016;11:e0156466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Seki M, Lipton SA. Targeting excitotoxic/free radical signaling pathways for therapeutic intervention in glaucoma. Prog Brain Res. 2008;173:495‐510. [DOI] [PubMed] [Google Scholar]
- 108. Brooks DE, Garcia GA, Dreyer EB, et al. Vitreous body glutamate concentration in dogs with glaucoma. Am J Vet Res. 1997;58:864‐867. [PubMed] [Google Scholar]
- 109. Pease ME, McKinnon SJ, Quigley HA, et al. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000;41:764‐774. [PubMed] [Google Scholar]
- 110. Knox DL, Eagle RC Jr, Green WR. Optic nerve hydropic axonal degeneration and blocked retrograde axoplasmic transport: histopathologic features in human high‐pressure secondary glaucoma. Arch Ophthalmol. 2007;125:347‐353. [DOI] [PubMed] [Google Scholar]
- 111. Salinas‐Navarro M, Alarcon‐Martinez L, Valiente‐Soriano FJ, et al. Ocular hypertension impairs optic nerve axonal transport leading to progressive retinal ganglion cell degeneration. Exp Eye Res. 2010;90:168‐183. [DOI] [PubMed] [Google Scholar]
- 112. Fahy ET, Chrysostomou V, Crowston JG. Mini‐review: impaired axonal transport and glaucoma. Curr Eye Res. 2016;41:273‐283. [DOI] [PubMed] [Google Scholar]
- 113. Ward NJ, Ho KW, Lambert WS, et al. Absence of transient receptor potential vanilloid‐1 accelerates stress‐induced axonopathy in the optic projection. J Neurosci. 2014;34:3161‐3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Agarwal R, Gupta SK, Agarwal P, et al. Current concepts in the pathophysiology of glaucoma. Indian J Ophthalmol. 2009;57:257‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Flammer J, Haefliger IO, Orgul S, et al. Vascular dysregulation: a principal risk factor for glaucomatous damage? J Glaucoma. 1999;8:212‐219. [PubMed] [Google Scholar]
- 116. Michelson G, Langhans MJ, Harazny J, et al. Visual field defect and perfusion of the juxtapapillary retina and the neuroretinal rim area in primary open‐angle glaucoma. Graefes Arch Clin Exp. 1998;236:80‐85. [DOI] [PubMed] [Google Scholar]
- 117. Chung HS, Harris A, Kagemann L, et al. Peripapillary retinal blood flow in normal tension glaucoma. Br J Ophthalmol. 1999;83:466‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gelatt KN, Miyabayashi T, Gelatt‐Nicholson KJ, et al. Progressive changes in ophthalmic blood velocities in Beagles with primary open angle glaucoma. Vet Ophthalmol. 2003;6:77‐84. [DOI] [PubMed] [Google Scholar]
- 119. Gelatt‐Nicholson KJ, Gelatt KN, MacKay EO, et al. Comparative Doppler imaging of the ophthalmic vasculature in normal Beagles and Beagles with inherited primary open‐angle glaucoma. Vet Ophthalmol. 1999;2:97‐105. [DOI] [PubMed] [Google Scholar]
- 120. Brooks DE, Samuelson DA, Gelatt KN. Ultrastructural changes in laminar optic nerve capillaries of beagles with primary open‐angle glaucoma. Am J Vet Res. 1989;50:929‐935. [PubMed] [Google Scholar]
- 121. Mozaffarieh M, Grieshaber MC, Orgul S, et al. The potential value of natural antioxidative treatment in glaucoma. Surv Ophthalmol. 2008;53:479‐505. [DOI] [PubMed] [Google Scholar]
- 122. Liu Q, Ju WK, Crowston JG, et al. Oxidative stress is an early event in hydrostatic pressure induced retinal ganglion cell damage. Invest Ophthalmol Vis Sci. 2007;48:4580‐4589. [DOI] [PubMed] [Google Scholar]
- 123. Wax MB, Tezel G. Immunoregulation of retinal ganglion cell fate in glaucoma. Exp Eye Res. 2009;88:825‐830. [DOI] [PubMed] [Google Scholar]
- 124. Bell K, Gramlich OW, Von Thun Und Hohenstein‐Blaul N, et al. Does autoimmunity play a part in the pathogenesis of glaucoma? Prog Retinal Eye Res. 2013;36:199‐216. [DOI] [PubMed] [Google Scholar]
- 125. Pumphrey SA, Pizzirani S, Pirie CG, et al. Western blot patterns of serum autoantibodies against optic nerve antigens in dogs with goniodysgenesis‐related glaucoma. Am J Vet Res. 2013;74:621‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bringmann A, Pannicke T, Grosche J, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397‐424. [DOI] [PubMed] [Google Scholar]
- 127. Son JL, Soto I, Oglesby E, et al. Glaucomatous optic nerve injury involves early astrocyte reactivity and late oligodendrocyte loss. Glia. 2010;58:780‐789. [DOI] [PubMed] [Google Scholar]
- 128. Inman DM, Horner PJ. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia. 2007;55:942‐953. [DOI] [PubMed] [Google Scholar]
- 129. Neufeld AH, Liu B. Glaucomatous optic neuropathy: when glia misbehave. Neuroscientist. 2003;9:485‐495. [DOI] [PubMed] [Google Scholar]
- 130. Fick CM, Dubielzig RR. Short posterior ciliary artery anatomy in normal and acutely glaucomatous dogs. Vet Ophthalmol. 2016;19:43‐49. [DOI] [PubMed] [Google Scholar]
- 131. Hare WA, WoldeMussie E, Lai RK, et al. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, I: Functional measures. Invest Ophthalmol Vis Sci. 2004;45:2625‐2639. [DOI] [PubMed] [Google Scholar]
- 132. Hare WA, WoldeMussie E, Weinreb RN, et al. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, II: Structural measures. Invest Ophthalmol Vis Sci. 2004;45:2640‐2651. [DOI] [PubMed] [Google Scholar]
- 133. WoldeMussie E, Yoles E, Schwartz M, et al. Neuroprotective effect of memantine in different retinal injury models in rats. J Glaucoma. 2002;11:474‐480. [DOI] [PubMed] [Google Scholar]
- 134. Weinreb RN, Liebmann JM, Cioffi GA, et al. Oral memantine for the treatment of glaucoma: design and results of 2 randomized, placebo‐controlled, phase 3 studies. Ophthalmology. 2018;125:1874‐1885. [DOI] [PubMed] [Google Scholar]
- 135. Pease ME, Zack DJ, Berlinicke C, et al. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50:2194‐2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kallberg ME, Brooks DE, Komaromy AM, et al. The effect of an L‐type calcium channel blocker on the hemodynamics of orbital arteries in dogs. Vet Ophthalmol. 2003;6:141‐146. [DOI] [PubMed] [Google Scholar]
- 137. Jutley G, Luk SM, Dehabadi MH, et al. Management of glaucoma as a neurodegenerative disease. Neurodegener Dis Manag. 2017;7:157‐172. [DOI] [PubMed] [Google Scholar]
- 138. Becker S, Eastlake K, Jayaram H, et al. Allogeneic transplantation of Müller‐derived retinal ganglion cells improves retinal function in a feline model of ganglion cell depletion. Stem Cells Transl Med. 2016;5:192‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Benowitz LI, He Z, Goldberg JL. Reaching the brain: advances in optic nerve regeneration. Exp Neurol. 2017;287:365‐373. [DOI] [PubMed] [Google Scholar]
- 140. Laha B, Stafford BK, Huberman AD. Regenerating optic pathways from the eye to the brain. Science. 2017;356:1031‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Goldberg JL, Guido W, AGI Workshop Participants . Report on the National Eye Institute Audacious Goals Initiative: Regenerating the optic nerve. Invest Ophthalmol Vis Sci. 2016;57:1271‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tanaka T, Yokoi T, Tamalu F, et al. Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Sci Rep. 2015;5:8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Miller PE. The glaucomas In: Maggs DJ, Miller PE, Ofri R. eds. Slatter's Fundamentals of Veterinary Ophthalmology. 5th edition St. Louis, MO: Elsevier; 2013; 258. [Google Scholar]


