Summary:
The placement of eyes on insect head is an important evolutionary trait. The stalk-eyed fly, Cyrtodopsis whitei (C. whitei), exhibits a hypercephaly phenotype where compound eyes are located on lateral extension from the head while the antennal segments are placed inwardly on this stalk. This stalk-eyed phenotype is characteristic of the family Diopsidae in the Diptera order and dramatically deviates from other dipterans, such as Drosophila. Like other insects, the adult eye and antenna of stalk-eyed fly develop from a complex eye-antennal imaginal disc. We analyzed the markers involved in proximo-distal (PD) axis of the developing eye imaginal disc of the stalk-eyed flies. We used homothorax (hth) and distalless (dll), two highly conserved genes as the marker for proximal and distal fate, respectively. We found that lateral extensions between eye and antennal field of the stalk-eyed fly’s eye-antennal imaginal disc exhibit robust Hth expression. Hth marks the head specific fate in the eye- and proximal fate in the antenna-disc. Thus, the proximal fate marker Hth expression evolves in the stalk-eyed flies to generate lateral extensions for the placement of the eye on the head. Moreover, during pupal eye metamorphosis, the lateral extension folds back on itself to place the antenna inside and the adult compound eye on the distal tip. Interestingly, the compound eye in other insects does not have a prominent proximo-distal axis as observed in the stalk-eyed fly.
Keywords: Stalk-eyed fly, Drosophila, eye development, eye-antennal imaginal disc, proximo-distal axis, Homothorax, Distalless, Dacshund
Introduction
During evolution, morphological diversity is an outcome of modification of body plans due to changes in the existing developmental programs. Some Diptera have extensive head hypercephaly, forming protrusions called antlers and stalk-eyes (Schutze et al., 2007; Sivinski, 1997; Wilkinson and Dodson, 1997). Evolutionarily, eye development is a relatively new trait. Most flies have characteristic compound eyes and antennae on their head, but the stalk-eyed flies have their eye located at the end of long lateral extension, or stalks. Unlike other stalk-eye flies where only the eye is located on the stalk, the stalk-eyed fly from the Diopsidae family have the antenna, eye and optic lobe located at the end of the stalk (Buschbeck and Hoy, 2005; Buschbeck et al., 2001). The “stalk-eyed” morphology is a dramatic deviation from other dipterans, including Drosophila (Buschbeck and Hoy, 1998). It is known that the length of the stalk or eye span, a sexually dimorphic trait, varies among different Dipteran species (Baker et al., 2001; Buschbeck et al., 2001). Stalk length plays an important role in the selection of a male mating partner, where males with longer stalks have an advantage over other males (Cotton et al., 2014; Wilkinson and Reillo, 1994). The role of these extreme sexual dimorphic structures in sexual selection and meiotic drive have been discussed (Baker et al., 2001; Baker and Wilkinson, 2001; Cotton et al., 2014; Grimaldi and Fenster, 1989; Warren and Smith, 2007; Wilkinson and Dodson, 1997; Wilkinson and Reillo, 1994).
In insects, adult appendages develop from a group of cells that are set aside during embryonic development, which undergo patterning and growth during larval stages, and then metamorphose to adult appendages during pupal development. Drosophila melanogaster, a holometabolous insect, has blue print for its adult organs housed inside the larva as a monolayer epithelium called as imaginal discs (Cohen et al., 1993; Held, 2002). Upon differentiation, the larval eye-antennal imaginal disc gives rise to an adult compound eye, antenna and head capsule (Atkins and Mardon, 2009; Hurley et al., 2002; Peters, 2002; Poulson, 1950; Ready et al., 1976; Singh et al., 2005a; Singh et al., 2012; Tsachaki and Sprecher, 2012). In Drosophila, a core cascade of genes referred to as the retinal determination and differentiation machinery is required to form an eye. The core genetic machinery is comprised of Pax-6 homolog eyeless (ey), eyes absent (eya), dacshund (dac) and sineoculis (so) (Atkins and Mardon, 2009; Kango-Singh et al., 2003; Kumar, 2018; Singh et al., 2012; Tare et al., 2013a). During eye development, both positive and negative regulators are involved in determination and differentiation of the eye field. Among the negative regulators, include homothorax (hth) (Moskow et al., 1995) and wingless (wg), a morphogen, which serves as the ligand for the evolutionarily conserved Wingless/Wnt signaling pathway. Hth is a TALE type homeodomain protein and interacts physically with homeodomain protein Extradenticle (Exd) to promote its nuclear localization (Jaw et al., 2000; Kurant et al., 1998; Pai et al., 1998; Rieckhof et al., 1997). Hth expresses uniformly in the first instar larval eye-antennal disc, but it retracts from the posterior region of the second instar larval eye-antennal imaginal disc. In third instar larval eye-antennal imaginal disc, Hth expression is in the proximal region of antenna and in the anterior region of the eye disc (Bessa et al., 2002; Lebreton et al., 2008; Pai et al., 1998; Singh et al., 2004; Singh et al., 2002; Singh et al., 2011; Wang and Sun, 2012). Apart from these, several other genes are involved in eye development (Jang et al., 2003; Kumar, 2018). These genes also include the ones that regulate axial patterning (Cavodeassi et al., 1999; Chern and Choi, 2002; Cho and Choi, 1998; Dominguez and de Celis, 1998; Maurel-Zaffran and Treisman, 2000; Oros et al., 2010; Papayannopoulos et al., 1998; Sato and Tomlinson, 2007; Tare et al., 2013a).
Axial patterning is a crucial developmental program where the monolayer organ primordium transitions into a three–dimensional organ by delineating the antero-posterior (AP), dorso-ventral (DV) and proximo-distal (PD) axis. Interestingly, in Drosophila dorso-ventral axis is the first axis defined, which is followed by delineation of antero-posterior axis (Oros et al., 2010; Singh et al., 2005a; Singh and Choi, 2003; Singh et al., 2005b; Singh et al., 2012; Tare et al., 2013a). Majority of insects, including Drosophila, the adult eye is present in a socket on the head. Therefore, there is no well-defined PD axis in these insect eyes. However, in the case of the stalk-eyed fly, these lateral extensions from the head may be due to the generation of a novel PD axis. In Drosophila, PD axis in other appendages is defined by function of genes like hth for the proximal fate (Gonzalez-Crespo and Morata, 1996; Wu and Cohen, 1999), dac for the medial (Mardon et al., 1994), and dll for the distal fate (Cohen et al., 1989; Cohen and Jurgens, 1989).
We wanted to study the developmental mechanism guiding the generation of the “stalk-eyed” morphology. To do this, we looked into the imaginal development of the stalk-eyed flies and compared it to that of Drosophila. It has been reported that the stalk-eye like pattern results from the extension of the optic vesicle (Buschbeck and Hoy, 2005; Buschbeck et al., 2001). It is interesting how this optic vesicle extension and lateral extension from the head contribute towards the generation of final stalk eye phenotype. We analyzed eye-antennal imaginal disc development during late larval and pupal stages. We found that organization of the stalk-eyed fly eye disc is similar to the organization of Drosophila eye disc (Buschbeck and Hoy, 1998; Buschbeck et al., 2001). However, there is an exception in terms of a tube-like projection between the eye and the antenna disc. Interestingly, in larval eye disc, the eye region is posterior and antennal region is anterior. Moreover, in the adult eye, the arrangement of the eye with respect to the antenna is reverse, where the eye is at the anterior tip and the antenna is placed posteriorly on the stalk. Since genetic machinery is highly conserved across the species, the antibodies from one species can cross react with others. We used antibodies against Drosophila proteins to study the expression of the patterning genes in stalk-eyed fly eye development. We used markers for axonal targeting from the retina to brain, proneural fate markers to determine the eye fate, and finally the proximal and distal fate markers like Dll and Hth to mark the fate of the developing eye-antennal disc. Here we present that intervening region between the eye and antennal field of larval eye-antennal imaginal disc exhibits robust expression of proximal fate marker Hth. The changes in the final morphology is due to the cell movements during the time window of 24-48 hours of pupal development.
Material and Methods
Fly culture:
We used the stalk-eyed fly Cyrtodopsis whitei in this study. The stalk-eyed fly colony was maintained at 25°C on a 16h: 8h light and dark cycle in population cages. A population cage is a transparent plastic chamber (60cm × 60cm, commonly used for rearing a large Drosophila colony). Each cage has around 200 flies. Stalk-eyed flies were reared on Drosophila food supplemented with ground sweet corn food source, activated yeast, and liberal supply of water. The stalk-eyed flies laid eggs on decayed Araceae leaves that were covered with a nylon mesh in box. Larvae that hatched from the egg moved to the decayed leaves. Larvae were reared in uncrowded conditions (Baker et al., 2001; Baker and Wilkinson, 2001) until third instar larval or early pupal stage. The white pupae were transferred to dry vials to prevent newly emerged flies from sticking to the wet decayed leaves.
We used the wild-type Canton-S stock of D. melanogaster in this study. Fly stocks were maintained at 25oC on a cornmeal, molasses food. The third instar larvae at the wandering stages were collected from the culture bottles for immunohistochemistry.
Immunohistochemistry
Both stalk-eyed fly and Drosophila eye-antennal imaginal discs were dissected out from wandering third instar larvae in 1× Phosphate Buffered Saline (PBS) and fixed in freshly prepared 4% paraformaldehyde (in PBS) for 20 minutes (Singh et al., 2002; Tare et al., 2013b). Eye imaginal discs were washed (thrice) in PBST (1× PBS+2% Triton-X-100) for 10 minute per wash. After washes, eye-imaginal discs were blocked for one hour in 10% normal goat serum (NGS) in PBST. The discs were incubated overnight at 4oC in one or a combination of the following primary antibodies viz., mouse anti-22C10 (1:100) (Developmental Studies Hybridoma Bank, DSHB), mouse anti-Dac (1:100) (DSHB), rat anti-ELAV (1:200) (DSHB), and rabbit anti-Hth (1:200) (Pai et al., 1998). Following primary antibody incubation, the discs were washed three times in PBST for 10 minute each and blocked for 30 minute in 10% goat serum in PBST. The discs were incubated with secondary antibodies for about 2 hour at room temperature and washed three times in PBST for 10 minute. Secondary antibodies (Jackson Laboratories) used were donkey anti-rat IgG conjugated with Cy5 (1:200), donkey anti-rabbit IgG conjugated to Cy3 (1:400) or goat anti-mouse IgG conjugated to FITC or Alexa Fluor 488 (1:200). The stalk-eyed fly eye-antennal imaginal discs were mounted in DABCO (Sigma) mountant in 90% glycerol. While mounting stalk-eyed flies precautions were taken to prevent folding of the lateral extension between the eye and antennal discs. The stalk-eyed imaginal disc were photo-documented on a Zeiss LSM510 confocal microscope. While the Drosophila eye-antennal imaginal discs were mounted in Vectashield (Vector labs), and images were taken on Olympus Fluoview Laser scanning Confocal Microscope (Tare et al., 2016). The final images and figures were prepared using Adobe Photoshop CS6 software.
Scanning Electron Microscopy
The stalk-eyed flies’ heads were excised from their body, and fixed overnight at 4oC in freshly prepared 4% paraformaldehyde in the phosphate buffered saline (PBS). The tissues were then post-fixed in OsO4.dH2O. Following dehydration in an ethanol series, the samples were processed for critical drying in CO2 and coated with gold using a sputter coater. The adult heads were mounted on carbon tape covered aluminum stubs. Mounting of stalk-eyed fly tissue requires special attention, as the stalk-eyed head tend to roll over on the carbon tape. Images were captured using JEOL scanning electron microscope. For Drosophila, the sample was dehydrated through an acetone series, and then treated with 1:1 acteone/HMDS (Hexa Methyl Di Silazane, Electron Microscopy Sciences) solution for 24 hours. After which the samples were treated with 100% HMDS and were set aside to air dry in the hood. Dehydrated flies were mounted on Electron Microscopy Sciences (EMS) stubs and coated with gold using a Denton vacuum sputter coater (Tare and Singh, 2009). Images were captured using a Hitachi S-4800 High Resolution Scanning Electron Microscope (HRSEM). The final images and figures were prepared using Adobe Photoshop CS6 software.
Adult Eye Imaging
Adult flies were prepared for imaging by freezing at −20ºC for approximately 2 hours (Sarkar et al., 2018a; Wittkorn et al., 2015). Images were captured on a stereomicroscope attached to a camera. The final images were prepared using Adobe Photoshop CS6 software.
Results
Spatial organization of eye
The stalk-eyed fly, like Drosophila, are holometabolous insect. The Drosophila, adult eyes are embedded in the adult fly head (Figure 1A, C). The Drosophila eye-antennal imaginal disc consists of eye- and antennal- region with small intervening region between the two domains (Figure 1B). The Drosophila adult eye comprises of nearly 600–800 unit eyes or ommatidia (Kumar, 2018; Ready et al., 1976; Singh et al., 2012; Tare et al., 2013a; Wolff and Ready, 1993). The Drosophila adult eye has small mechanosensory bristles between ommatidia called inter-ommatidial bristles (Figure 1H). In comparison, the stalk-eyed fly exhibits a unique morphological deviation in the placement of the compound eye and antenna on the adult fly head (Figure 1E). Adult stalk-eyed fly’s compound eye (Figure 1G), develops from the larval eye-antennal imaginal disc (Figure 1F). The eye-antennal imaginal disc of stalk-eyed fly consists of three distinct domains. The eye- and antennal-region are connected by a long intervening region in stalk-eyed fly imaginal disc (arrowhead, Figure 1F). The adult eye is present on the distal tip of the lateral extension, which projects from the head, and the antenna is present inside of this lateral extension (Figure 1G). The adult compound eye of stalk-eyed fly comprises of several unit eyes called ommatidia (Figure 1H). Moreover, the stalk-eyed fly adult eye lacks the inter-ommatidial bristles (Figure 1H). Even though the basic pattern is similar between the eyes of Drosophila and stalk-eyed fly, presence of a lateral extension in the imaginal disc, the placement of eye on the adult head, and absence of interommatidial bristle remain the key differences.
Figure 1. (A) Eye development in stalk-eyed fly and Drosophila.
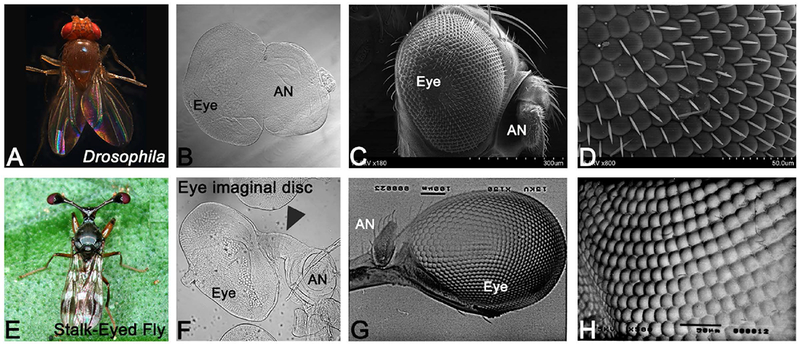
(A-D) The Drosophila melanogaster, a holometabolous insect, (A) adult. The adult eye compound eye of Drosophila develops from (B) larval eye imaginal disc. (C, D) The SEM images of the Drosophila adult compound eye comprising of 600-800 unit eyes. The inter-ommatidial bristles are present in the Drosophila eye. In Drosophila adult fly, eye is present in a socket on the head where eye is posterior and antenna is positioned anteriorly. There is no distinct proximo-distal (PD) axis. (E) Stalk-eyed fly, C. whitei (F) larval eye-antennal imaginal disc, which metamorphose into (G) the adult compound eye. (F) The eye-antennal imaginal disc of stalk-eyed fly has a distinct lateral extension, which connects the eye with the antennal field (arrowhead). The compound eye of stalk-eyed fly is present on a lateral extension from the adult head, which exhibits a prominent proximo-distal (PD) axis. Antenna is located posterior to the eye on the inner side of the stalk. (H) The compound eye comprises of many unit eyes, which lack the inter-ommatidial bristles. (The orientation of all imaginal discs in the figure is posterior to left and dorsal up. [AN: antenna].
Retinal axonal targeting in Drosophila and Stalk-eyed fly
During visual system development, connections are formed between the retinal photoreceptor neurons and the brain. The differentiating neurons send out the axons that innervate different parts of the brain (Newsome et al., 2000). Since the genetic machinery is conserved, some of the antibodies against Drosophila protein also cross-react with other insects. We used an antibody against the Drosophila Futsch protein called 22C10 that marks all axons in the nervous system (Zipursky et al., 1984). The rationale behind this approach was to study the gross organization of photoreceptors clusters in the stalk-eyed fly and Drosophila. In Drosophila, the axonal projections from ommatidia fasciculate and pierces the basement membrane of the eye disc, which then extends through the optic stalk into different layers of brain like lamina and medulla (Figure 2A, B) (Cutler et al., 2015; Moran et al., 2013; Sarkar et al., 2018b; Zipursky et al., 1984). In stalk-eyed fly, 22C10 marks axons from photoreceptor neurons that organize into an axonal bundle. The axonal bundle projections from the eye antennal disc innervate the entire depth of the brain (Figure 2C, D) (Buschbeck and Hoy, 1998). The Futsch (22C10) antibody also marks the Bolwig’s nerve, which extends from the larval photoreceptor organ along the length of eye-antennal disc and then innervates the optic stalk as seen in the eye-antennal imaginal disc of stalk-eyed fly (Figure 2C, D) as well as the Drosophila eye imaginal disc (Figure 2A, B).
Figure 2. Retinal axon targeting in Stalk-eyed flies and Drosophila.
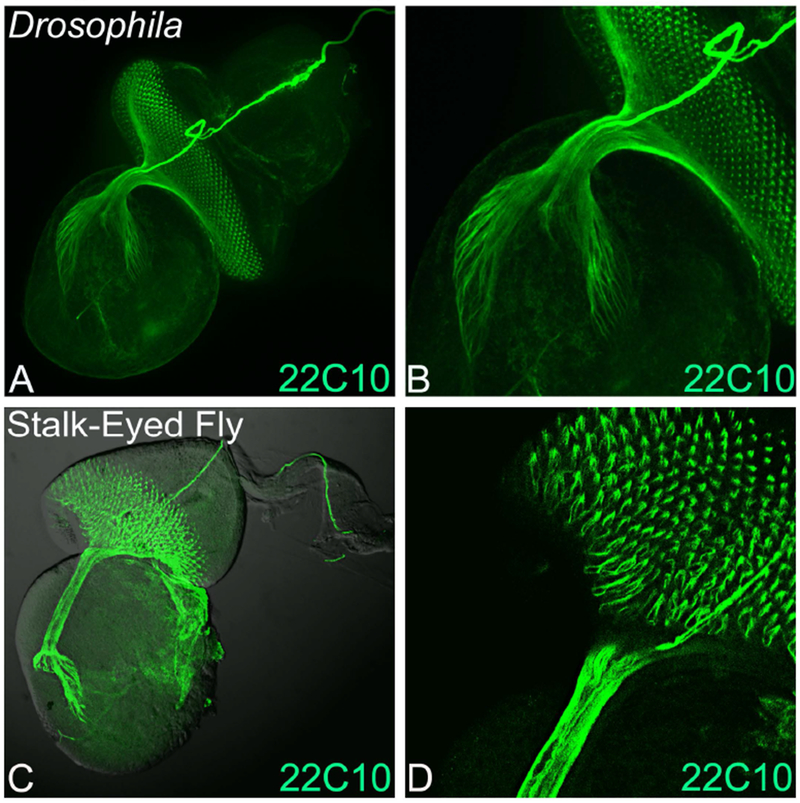
Monoclonal antibody 22C10 marks all axonal sheath of the photoreceptors in the developing eye. Expression of 22C10 antibody in larval eye antennal imaginal disc of (A, B) Drosophila melanogaster, and (C, D) Stalk-eyed fly. (B) and (D) are magnified images (40×) of A and C. The 22C10 staining marks the axons, axonal targets and its innervation in the brain both in the stalk-eyed fly and Drosophila. Note that all the retinal neurons axons fasciculate into an axonal tract, which innervate the brain of stalk-eyed fly and Drosophila. The orientation of all imaginal discs in the figure is posterior to left and dorsal up.
Expression of Retinal Determination gene
The Drosophila antibody against Dac protein, encoded by a retinal determination and differentiation gene, can cross react with stalk-eyed fly proteins. In Drosophila eye-antennal disc, Dac expression is in a crescent moon pattern within the dorso-lateral regions of the third antennal segment (Figure 3A, A’) (Mardon et al., 1994). In the eye disc, Dac expression initiates at the posterior margin prior to initiation of the morphogenetic furrow (MF) (Figure 3A, arrow). Strong Dac expression is observed in the unpatterned epithelium preceding the MF as it moves anteriorly across the eye disc. Posterior to the MF, Dac expression is primarily in photoreceptors R1, R6 and R7 and cone cells (Figure 3A, A’). Dac is expressed only in the part of the eye disc, which is fated to become retina (Mardon et al., 1994), whereas the periphery of the disc that is destined to become head cuticle does not express Dac (Figure 3A, A’). Dac expression in the stalk-eyed fly eye imaginal disc is similar. It is expressed in the unpatterned epithelium preceding the eye field boundary (Figure 3B, C, marked by white arrow). Moreover, like Drosophila eye imaginal disc, Dac expression is also posterior to the MF (Figure. 3F, white arrow). Dac expression is different in antennal disc as it is expressed strongly in medial region but has some weaker expression in the distal region too (Figure 3B, D). During pupal eye development, Dac expression is no longer present in retina, although it is expressed in a band of cells in the optic lobe (Figure 3D). Thus, Dac expression in eye imaginal disc is comparable between Drosophila and stalk-eyed fly.
Figure 3. Retinal determination gene dacshund marks the zone anterior to the eye field in stalk-eyed fly.
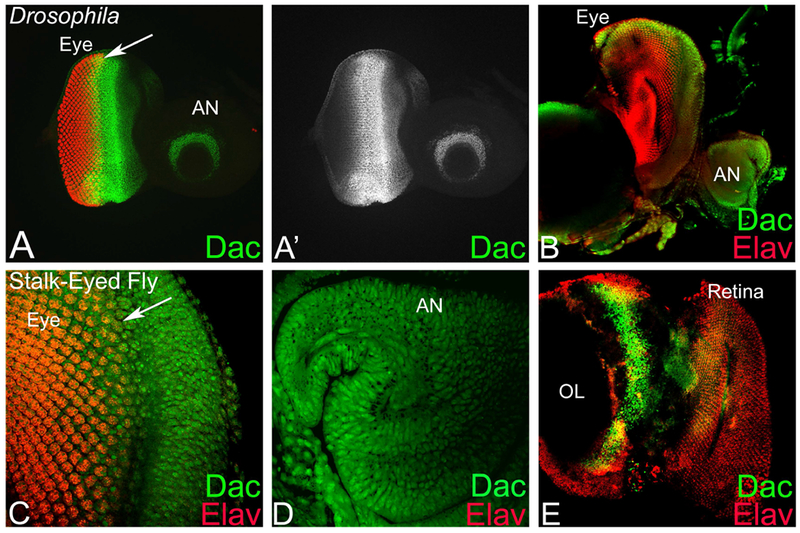
(A, A’) Dac expression in Drosophila third instar larval eye-antennal imaginal disc. (A’) Split channel showing Dac expression alone in the eye imaginal disc. Note that arrow marks the MF in the eye imaginal disc. (B-E) In stalk-eyed fly, Dac expression in (B-D) eye-antennal imaginal disc, (E) pupal retina. (C, D) Magnified view of (C) eye and (D) antennal region. The stalk of the stalk-eyed fly eye imaginal disc is not visible due to folding. (E) In stalk-eyed fly, Dac is not expressed in the pupal retina, its expression is restricted to a band of cells in the optic lobe. Note that eye discs are stained for Dac (Green) and proneural marker Elav (Red). [AN: antenna, OL: optic lobe].
Stalk-eyed fly exhibit distinct proximo-distal axis
During Drosophila larval eye antennal disc development, Hth expression is restricted anterior to the MF in the developing eye, which corresponds to the head fate. In the third instar antennal disc, Hth is expressed Hth expression is in the proximal ring and also served as a proximal fate marker (Pai et al., 1998; Rieckhof et al., 1997; Singh et al., 2011). In the third instar eye-antennal imaginal disc, Hth expression is anterior to the MF, and in the peripodial membrane, which corresponds to the head cuticle of adult fly (Figure 4A, A’). Interestingly, third instar larval eye–antennal disc of stalk-eyed fly has a long extension, which connects the eye and antennal field (Figure 1E, 4B, black arrow). This extension is not present in the Drosophila eye-antennal imaginal disc (Figure 1B). Moreover, the proximal fate marker Hth showed a robust expression in proximal concentric rings in antenna, and head region, which is anterior to the eye field marked by the proneural marker Elav (Figure 4C). Surprisingly, there was robust expression of Hth in the extension that connects the eye field to the antennal field of the stalk-eyed fly (Figure 4D, white arrow). In the developing antennal disc of stalk-eyed fly, Hth was expressed in the outermost antennal ring that corresponds to the proximal fate whereas Dll, a marker for distal fate, was expressed robustly in the inner concentric rings of the antennal disc (Figure 4E). Dll was not expressed in the eye region of stalk-eyed fly eye-antennal disc. During early pupal development, Hth expression marked the antenna and lateral extensions as well as the peripodial membrane of the eye-antennal imaginal disc of stalk-eyed fly (Figure 4 F, arrow). During later stages of pupal development, Hth expression was restricted to the margin of the eye field with optic lobe (Figure 4G). Thus, Hth, a proximal fate marker, exhibits a robust expression in the lateral extension of stalk-eyed eye-antennal imaginal disc. This data suggests that stalk-eyed fly eye antennal disc has a distinct PD, which is absent in Drosophila and other Dipterans.
Figure 4. Homothorax (Hth) expression marks the elongated extension between the antenna and eye imaginal disc in stalk-eyed fly.
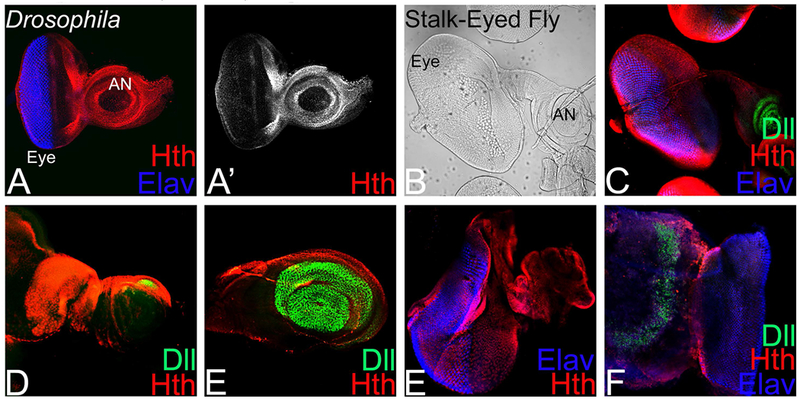
(A. A’) In Drosophila eye-antennal imaginal disc, Hth (Red) and proneural fate marker, Elav (Blue), expression. (A’) Split channel showing Hth expression. Note that Hth expression is restricted to the proximal ring in the antenna and in region anterior to the morphogenetic furrow (MF) in the eye disc. (B) Stalk-eyed fly eye-antennal imaginal disc bright field image showing a lateral extension (marked by black arrow) between the eye and the antennal field. (C) Stalk-eyed fly eye antennal disc with characteristic morphology of intervening stalk region separating the eye and antennal region (marked by white arrow) showing proximal fate marker Hth (Red), distal fate marker Distalless (Dll, Green) and ELAV (Blue) expression. (D) Note that Hth exhibits nuclear localization and marks the extension (marked by white arrow) between the eye and antennal field, whereas (E) Dll marks the distal tip of the antenna in the larval eye-antennal imaginal disc. (F, G) Hth staining in the early pupa and late pupa. [AN: antenna, OL: optic lobe].
Development of stalk-eyed phenotype from eye-antennal imaginal disc
We studied retinal fate using an antibody against Elav protein (Robinow and White, 1991). ELAV, a proneural marker, allows us to determine how the eye (based on retinal neuron population) undergoes metamorphosis from a larval eye imaginal disc to an adult eye in the stalk-eyed fly (Figure 5). Here, the rationale was to gather the bright field image of each developmental stage from the third instar larval eye-antennal disc attached to the optic lobe and compare it with the expression of proneural marker Elav during those stages. The larval eye-antennal imaginal disc of stalk-eyed fly resembles that of any other insect eye-antennal imaginal disc except for the presence of an intermediate stalk, which connects the eye field with the antennal field (Figure 5A, B). The larval eye imaginal disc is connected to an optic lobe through the axonal tract (Figure 2C, 5L). During pre-pupal development in stalk-eyed fly, the developing eye field, marked by proneural marker Elav, begins to envelope the optic lobe (Fig. 5C, D, L). At this stage, the antennal field along with the lateral extension begins to fold back. By 48 hours of pupal development, the optic lobe is encapsulated/covered by the developing eye field marked by Elav (Figure 5F). At this stage, the antennal field is not visible as it is behind the eye field due to folding back of eye-antennal disc (Figure 5E, F, L). At the 72 hour stage, Elav marks both the retinal neurons as well as the neurons in the optic lobe (Figure 5H), while antenna is present behind the optic lobe and eye field (Figure 5G, H, L). The antennal field is difficult to see in the same plane since it is covered by the optic lobe and eye field and the lateral extension or stalk has folded back with the antennal field along with it (Figure 5L). At 96 hour of pupal development, the optic lobe is covered by the retina and the antenna is now folded back along with the lateral stalk, which connects the larval eye field and the antennal field (Figure 5 I, J, L). The adult stalk-eyed fly emerges from the pupa after pupal metamorphosis, and exhibits that characteristic stalk-eyed morphology with hypercephaly phenotype (Figure 5K, L).
Figure 5. Position of adult eye and antenna on stalk-eyed fly head is due to inversion or folding of eye-antennal imaginal disc during development.
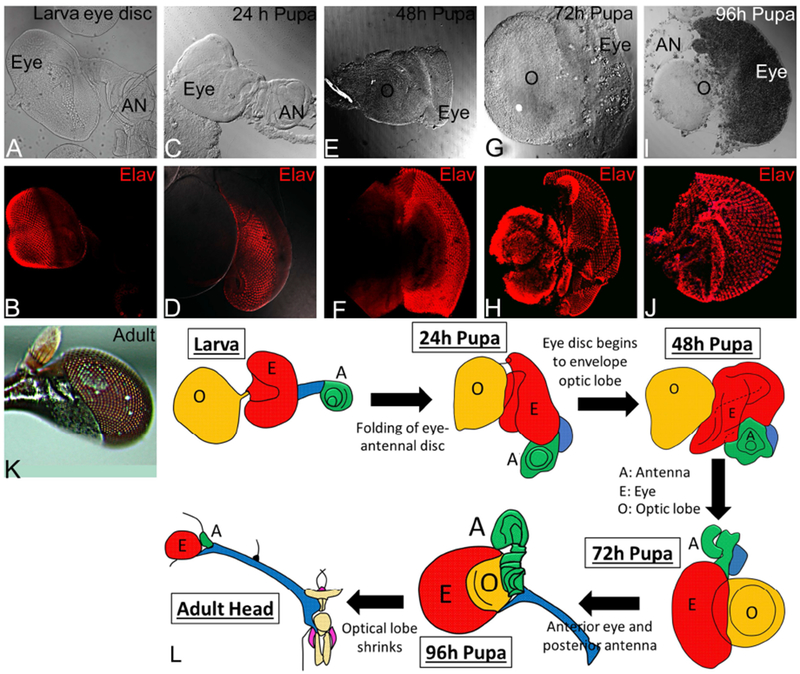
Eye development in stalk-eyed fly during various stages (A, B) Larval eye-antennal imaginal disc (C, D) pupal eye at 24hr, (E, F) pupa eye at 48hr, (G, H) pupal eye at 72 hr, (I, J) pupal eye at 96 hr, and (K) the adult eye. Note that (A, C, E, G, I) are bright field images of the developing eye and (B, D, F, H, J) are same developmental; stages stained with proneural marker ELAV (red). L. Cartoon showing transition of larval eye-antennal imaginal disc to an adult eye. The hypercephalic phenotype of placement of eye on a lateral extension is generated by folding of the eye-antennal imaginal disc, which initiates during transition of larval eye disc to 24 hr of pupal development.
Discussion
Use of comparative developmental biology approaches have facilitated evolutionary studies on patterning and form (Carroll, 2005). Insect vision provides an opportunity to study the evolution of animal form and function. In majority of insects, the compound eye is the most prominent visual organ and presents an evolutionary conservation paradigm due to high level of similarity in its design. The basic Bauplan of the dipteran visual system evolved at least 200 million years ago, prior to the radiation of the cyclorrhapan Diptera (Hennig, 1973). Despite the unusual placement of the eyes on the stalk, the members of Diopsidae have a visual system that is anatomically similar to other flies. One of the dramatic deviation is the presence of hypercephalization or placement of adult eye on a stalk, which is extending laterally from the adult head. In the basal Sphyracephela group, stalk-eyes are sexually monomorphic. The length of these lateral extensions is pronounced in males, which suggests a functional role in sexual selection. However, the stalk-eyed trait is present both in males and females. Thus, it can be better explained as a functional adaptation, which allows these flies to have increased field of view. Here we suggest that this morphology is accompanied with emergence of a new proximo-distal (PD) axis, which is absent in other fly eyes.
During development, the generation of a lineage restriction boundary results in two differently determined cell populations called compartments (Garcia-Bellido et al., 1973; Singh et al., 2005b; Singh et al., 2012). All adult appendages like wing, leg, eye, head and antenna develops from their larval imaginal discs. Interestingly, early organ or appendage primordia are monolayer epithelium where delineation of antero-posterior (AP), Dorso-ventral (DV) and proximo-distal (PD) lineage restriction is crucial for its transition into a three-dimensional organ (Blair, 2001; Cavodeassi et al., 1999; Garcia-Bellido and Merriam, 1969; Garcia-Bellido et al., 1973; Morata and Lawrence, 1975). This process is highly conserved across the multicellular organisms. In Drosophila antenna, wing and leg imaginal disc, the AP boundary is the first lineage restriction. This is followed by the generation of DV boundary, which results in the formation of the dorsal and ventral compartment midway through the growth phase of the disc (Blair, 2001; Milan and Cohen, 2003; Morata and Lawrence, 1975; Singh and Choi, 2003; Tare et al., 2013a). These appendages also develop a distinct PD domain. Therefore, antenna, wing, and leg have distinct AP, DV and PD axes. However, the Drosophila eye disc does not follow this sequence of events. In the developing Drosophila eye, the DV axis is the first lineage restriction event. The formation of antero-posterior (AP) compartments occurs later in late second or early third instar of larval eye development when morphogenetic furrow (MF) is formed at the posterior margin of the larval eye imaginal disc. MF progresses anteriorly as a synchronous wave of differentiation resulting in formation of the anterior and posterior domains (Kumar, 2013; Ready et al., 1976; Wolff and Ready, 1993). Interestingly, there is no distinct PD lineage in the Drosophila eye where the adult eye is present in a socket on the head (Singh and Choi, 2003; Singh et al., 2004; Singh et al., 2012). This suggests that axial patterning in Drosophila and other insect eyes that do not have any lateral extension have lost one of the co-ordinate viz., PD axis formation during development. The presence of a lateral extension in stalk-eyed fly provides raises an interesting possibility that this third PD axis is restored in developmental programming of axial patterning of these flies eye-antennal disc patterning. Alternatively, it could be a newly developed trait in these flies.
Earlier fate map studies on various species of stalk-eyed flies have revealed that expression of four key regulator genes -defective proventriculus (dve), dll , engrailed (en) and wingless (wg) in the eye imaginal disc give rise to the head which is remarkably similar to Drosophila and other Diopsids during larval development (Hurley et al., 2001). Comparison of antenna specific gene Dll, medial cuticle specific gene en and wg expression in Drosophila and stalk-eyed fly species exhibited several similarities. Interestingly, Dll marks the distal fate of the stalk-eyed fly antenna, however, the fate of the intervening region that connects the eye and the antennal region in the eye-antennal imaginal disc of the stalk-eyed fly was not known (Hurley et al., 2001; Hurley et al., 2002).
It was not clear whether this lateral extension adds a PD axis on an otherwise eye primordium, which has distinct DV and AP axis. Since Drosophila and other insects that do not have lateral extensions from the head, the only structure missing in their eye-antennal disc is the intervening region. Earlier studies have shown that the entire eye and dorsal head capsule is derived from the posterior portion of the disc, and that the antenna and palpus are derived from the anterior portion of stalk-eyed eye-antennal imaginal disc. Moreover, it has been shown that the medial and lateral fate markers of the head capsule are expressed in the posterior region (Hurley et al., 2001). However, until recently there was no marker identified that marks the intervening region of the stalk-eyed fly eye-antennal imaginal disc. Our studies demonstrated that Hth, a proximal fate marker is expressed robustly in the intervening stalk region of the stalk-eyed fly.
Previously, it has been shown that nervous system of the stalk-eyed fly is modified where a relatively long optic nerve is formed. The functional adaptation of placement of the adult eye on the lateral extension is due to the enlargement of the optic tract, which connects the retinal neurons to the brain, and the lateral extension of proximal fate, as evident from robust Hth expression, encapsulating this structure. This intervening region, positively marked by Hth, then folds back on the eye, which then encapsulates the optic lobe (Figure 5).
In summary, this study shows that stalk-eyed fly have a distinct proximo-distal axis in the developing eye, which is a dramatic deviation from other dipteran insects. Furthermore, presence of an intervening region of proximal fate in larval eye-antennal imaginal disc provides a basis for this distinct hypercephalic phenotype observed in the adult fly.
Acknowledgment
We thank the Bloomington Stock Center and the Developmental Studies Hybridoma Bank (DSHB) for the antibodies. The authors also thank Dr. Madhuri Kango-Singh, Aditi Singh and the members of Singh lab for critical comments on the manuscript. Confocal microscopy was supported by Biology Department central core facility. NG is supported by Graduate program of Biology. AS is supported by National Institute of General Medical Sciences (NIGMS) - 1 R15 GM124654-01, STEM Catalyst Grant from University of Dayton and start-up support from UD to AS. YHS is supported by the National Science Council of the Republic of China (NSC-88-2312-B-001-016).
Footnotes
Competing financial interests:
The author(s) declare no competing interests.
References Cited
- Atkins M, Mardon G, 2009. Signaling in the third dimension: the peripodial epithelium in eye disc development. Dev Dyn 238, 2139–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RH, Ashwell RIS, Richards TA, Fowler K, Chapman T, Pomiankowski A, 2001. Effects of multiple mating and male eye span on female reproductive output in the stalk-eyed fly, Cyrtodiopsis dalmanni. Behavioral Ecology 12, 732–739. [Google Scholar]
- Baker RH, Wilkinson GS, 2001. Phylogenetic analysis of sexual dimorphism and eye-span allometry in stalk-eyed flies (Diopsidae). Evolution 55, 1373–1385. [DOI] [PubMed] [Google Scholar]
- Bessa J, Gebelein B, Pichaud F, Casares F, Mann RS, 2002. Combinatorial control of Drosophila eye development by eyeless, homothorax, and teashirt. Genes Dev 16, 2415–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SS, 2001. Cell lineage: compartments and Capricious. Curr Biol 11, R1017–1021. [DOI] [PubMed] [Google Scholar]
- Buschbeck EK, Hoy RR, 1998. Visual system of the stalk-eyed fly, Cyrtodiopsis quinqueguttata (Diopsidae, Diptera): an anatomical investigation of unusual eyes. J Neurobiol 37, 449–468. [DOI] [PubMed] [Google Scholar]
- Buschbeck EK, Hoy RR, 2005. The development of a long, coiled, optic nerve in the stalk-eyed fly Cyrtodiopsis whitei. Cell Tissue Res 321, 491–504. [DOI] [PubMed] [Google Scholar]
- Buschbeck EK, Roosevelt JL, Hoy RR, 2001. Eye Stalks or No Eye Stalks: A Structural Comparison of Pupal Development in the Stalk-Eyed Fly Cyrtodiopsis and in Drosophila. J Comp Neurol 433, 486–498. [DOI] [PubMed] [Google Scholar]
- Carroll SB, 2005. Endless forms most beautifuk: The new science of evo devo and the making of animal kingdom. W.W. Norton & Company, NewYork. [Google Scholar]
- Cavodeassi F, Diez Del Corral R, Campuzano S, Dominguez M, 1999. Compartments and organising boundaries in the Drosophila eye: the role of the homeodomain Iroquois proteins. Development 126, 4933–4942. [DOI] [PubMed] [Google Scholar]
- Chern JJ, Choi KW, 2002. Lobe mediates Notch signaling to control domain-specific growth in the Drosophila eye disc. Development 129, 4005–4013. [DOI] [PubMed] [Google Scholar]
- Cho KO, Choi KW, 1998. Fringe is essential for mirror symmetry and morphogenesis in the Drosophila eye. Nature 396, 272–276. [DOI] [PubMed] [Google Scholar]
- Cohen B, Simcox AA, Cohen SM, 1993. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development 117, 597–608. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Bronner G, Kuttner F, Jurgens G, Jackle H, 1989. Distal-less encodes a homoeodomain protein required for limb development in Drosophila. Nature 338, 432–434. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Jurgens G, 1989. Proximal-distal pattern formation in Drosophila: cell autonomous requirement for Distal-less gene activity in limb development. EMBO J 8, 2045–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton AJ, Foldvari M, Cotton S, Pomiankowski A, 2014. Male eyespan size is associated with meiotic drive in wild stalk-eyed flies (Teleopsis dalmanni). Heredity (Edinb) 112, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler T, Sarkar A, Moran M, Steffensmeier A, Puli OR, Mancini G, Tare M, Gogia N, Singh A, 2015. Drosophila Eye Model to Study Neuroprotective Role of CREB Binding Protein (CBP) in Alzheimer’s Disease. PLoS One 10, e0137691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez M, de Celis JF, 1998. A dorsal/ventral boundary established by Notch controls growth and polarity in the Drosophila eye. Nature 396, 276–278. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A, Merriam JR, 1969. Cell lineage of the imaginal discs in Drosophila gynandromorphs. J Exp Zool 170, 61–75. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A, Ripoll P, Morata G, 1973. Developmental compartmentalisation of the wing disk of Drosophila. Nat New Biol 245, 251–253. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Crespo S, Morata G, 1996. Genetic evidence for the subdivision of the arthropod limb into coxopodite and telopodite. Development 122, 3921–3928. [DOI] [PubMed] [Google Scholar]
- Grimaldi D, Fenster G, 1989. Evolution of Extreme Sexual Dimorphisms: Structural and Behavioral Convergence Among Broad-Headed Male Drosophilidae (Diptera). American Museum Novitates, 1–25. [Google Scholar]
- Held LIJ, 2002. The eye disc, in: Held LI (Ed.), Imaginal Disc. Cambridge University Press, pp. 197–236. [Google Scholar]
- Hennig W, 1973. Ordnung Diptera (Zweifluegler) , in: Beier M (Ed.), Kuekenthals’sHandbuch der Zoologie, 2 ed. Walter de Gruyter, Berlin, pp. 1–200. [Google Scholar]
- Hurley I, Fowler K, Pomiankowski A, Smith H, 2001. Conservation of the expression of Dll, en, and wg in the eye-antennal imaginal disc of stalk-eyed flies. Evol Dev 3, 408–414. [DOI] [PubMed] [Google Scholar]
- Hurley I, Pomiankowski A, Fowler K, Smith H, 2002. Fate map of the eye-antennal imaginal disc in the stalk-eyed fly Cyrtodiopsis dalmanni. Dev Genes Evol 212, 38–42. [DOI] [PubMed] [Google Scholar]
- Jang CC, Chao JL, Jones N, Yao LC, Bessarab DA, Kuo YM, Jun S, Desplan C, Beckendorf SK, Sun YH, 2003. Two Pax genes, eye gone and eyeless, act cooperatively in promoting Drosophila eye development. Development 130, 2939–2951. [DOI] [PubMed] [Google Scholar]
- Jaw TJ, You LR, Knoepfler PS, Yao LC, Pai CY, Tang CY, Chang LP, Berthelsen J, Blasi F, Kamps MP, Sun YH, 2000. Direct interaction of two homeoproteins, homothorax and extradenticle, is essential for EXD nuclear localization and function. Mechanisms of development 91, 279–291. [DOI] [PubMed] [Google Scholar]
- Kango-Singh M, Singh A, Sun YH, 2003. Eyeless collaborates with Hedgehog and Decapentaplegic signaling in Drosophila eye induction. Developmental Biology 256, 48–60. [DOI] [PubMed] [Google Scholar]
- Kumar JP, 2013. Catching the next wave: patterning of the Drosophila eye by the morphogenetic furrow., in: Singh A, and, Kango-Singh M (Eds.), In Molecular genetics of axial patterning, growth and disease in the Drosophila eye. Springer, NewYork, pp. 75–97. [Google Scholar]
- Kumar JP, 2018. The fly eye: Through the looking glass. Dev Dyn 247, 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurant E, Pai CY, Sharf R, Halachmi N, Sun YH, Salzberg A, 1998. Dorsotonals/homothorax, the Drosophila homologue of meis1, interacts with extradenticle in patterning of the embryonic PNS. Development 125, 1037–1048. [DOI] [PubMed] [Google Scholar]
- Lebreton G, Faucher C, Cribbs DL, Benassayag C, 2008. Timing of Wingless signalling distinguishes maxillary and antennal identities in Drosophila melanogaster. Development 135, 2301–2309. [DOI] [PubMed] [Google Scholar]
- Mardon G, Solomon NM, Rubin GM, 1994. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120, 3473–3486. [DOI] [PubMed] [Google Scholar]
- Maurel-Zaffran C, Treisman JE, 2000. pannier acts upstream of wingless to direct dorsal eye disc development in Drosophila. Development 127, 1007–1016. [DOI] [PubMed] [Google Scholar]
- Milan M, Cohen SM, 2003. A re-evaluation of the contributions of Apterous and Notch to the dorsoventral lineage restriction boundary in the Drosophila wing. Development 130, 553–562. [DOI] [PubMed] [Google Scholar]
- Moran MT, Tare M, Kango-Singh M, Singh A, 2013. Homeotic Gene teashirt (tsh) has a neuroprotective function in amyloid-beta 42 mediated neurodegeneration. PLoS One 8, e80829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morata G, Lawrence PA, 1975. Control of compartment development by the engrailed gene in Drosophila. Nature 255, 614–617. [DOI] [PubMed] [Google Scholar]
- Moskow JJ, Bullrich F, Huebner K, Daar IO, Buchberg AM, 1995. Meis1, a PBX1-related homeobox gene involved in myeloid leukemia in BXH-2 mice. Mol Cell Biol 15, 5434–5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome TP, Asling B, Dickson BJ, 2000. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127, 851–860. [DOI] [PubMed] [Google Scholar]
- Oros SM, Tare M, Kango-Singh M, Singh A, 2010. Dorsal eye selector pannier (pnr) suppresses the eye fate to define dorsal margin of the Drosophila eye. Dev Biol 346, 258–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai CY, Kuo TS, Jaw TJ, Kurant E, Chen CT, Bessarab DA, Salzberg A, Sun YH, 1998. The Homothorax homeoprotein activates the nuclear localization of another homeoprotein, extradenticle, and suppresses eye development in Drosophila. Genes Dev 12, 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V, Tomlinson A, Panin VM, Rauskolb C, Irvine KD, 1998. Dorsal-ventral signaling in the Drosophila eye. Science 281, 2031–2034. [DOI] [PubMed] [Google Scholar]
- Peters MA, 2002. Patterning the neural retina. Curr Opin Neurobiol 12, 43–48. [DOI] [PubMed] [Google Scholar]
- Poulson D,F , 1950. Histogenesis, oogenesis, and differentiation in the embryo of Drosophila melanogaster meigen, in: Demerec M (Ed.), Biology of Drosophila. Wiley, New York, pp. 168–274. [Google Scholar]
- Ready DF, Hanson TE, Benzer S, 1976. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol 53, 217–240. [DOI] [PubMed] [Google Scholar]
- Rieckhof GE, Casares F, Ryoo HD, Abu-Shaar M, Mann RS, 1997. Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell 91, 171–183. [DOI] [PubMed] [Google Scholar]
- Robinow S, White K, 1991. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J Neurobiol 22, 443–461. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Gogia N, Farley K, Payton L, Singh A, 2018a. Characterization of a morphogenetic furrow specific Gal4 driver in the developing Drosophila eye. PLoS One 13, e0196365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Gogia N, Glenn N, Singh A, Jones G, Powers N, Srivastava A, Kango-Singh M, Singh A, 2018b. A soy protein Lunasin can ameliorate amyloid-beta 42 mediated neurodegeneration in Drosophila eye. Sci Rep 8, 13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Tomlinson A, 2007. Dorsal-ventral midline signaling in the developing Drosophila eye. Development 134, 659–667. [DOI] [PubMed] [Google Scholar]
- Schutze MK, Yeates DK, Graham GC, Dodson GN, 2007. Phylogenetic relationships of antlered flies, Phytalmia Gerstaecker (Diptera:Tephritidae): the evolution of antler shape and mating behaviour. Australian Journal of Entomology 46, 281–293. [Google Scholar]
- Singh A, Chan J, Chern JJ, Choi KW, 2005a. Genetic interaction of Lobe with its modifiers in dorsoventral patterning and growth of the Drosophila eye. Genetics 171, 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Choi KW, 2003. Initial state of the Drosophila eye before dorsoventral specification is equivalent to ventral. Development 130, 6351–6360. [DOI] [PubMed] [Google Scholar]
- Singh A, Kango-Singh M, Choi KW, Sun YH, 2004. Dorso-ventral asymmetric functions of teashirt in Drosophila eye development depend on spatial cues provided by early DV patterning genes. Mechanisms of development 121, 365–370. [DOI] [PubMed] [Google Scholar]
- Singh A, Kango-Singh M, Sun YH, 2002. Eye suppression, a novel function of teashirt, requires Wingless signaling. Development 129, 4271–4280. [DOI] [PubMed] [Google Scholar]
- Singh A, Lim J, Choi K-W, 2005b. Dorso-ventral boundary is required for organizing growth and planar polarity in the Drosophila eye, in: Mlodzik M (Ed.), “Planar Cell Polarization during Development: Advances in Developmental Biology and Biochemistry”. Elsevier Science & Technology Books; , pp. 59–91. [Google Scholar]
- Singh A, Tare M, Kango-Singh M, Son WS, Cho KO, Choi KW, 2011. Opposing interactions between homothorax and Lobe define the ventral eye margin of Drosophila eye. Dev Biol 359, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Tare M, Puli OR, Kango-Singh M, 2012. A glimpse into dorso-ventral patterning of the Drosophila eye. Dev Dyn 241, 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivinski J, 1997. Ornaments in the Diptera. Florida Entomologist 80, 142–164. [Google Scholar]
- Tare M, Puli OR, and, Singh A, 2013a. Molecular Genetic Mechanisms of Axial Patterning: Mechanistic Insights into Generation of Axes in the Developing Eye, in: Singh A, and, Kango-Singh M (Eds.), Molecular Genetics of Axial Patterning, Growth and Disease in the Drosophila Eye. Springer, Springer NewYork Heidelberg Dordrecht London, pp. 37–75. [Google Scholar]
- Tare M, Puli OR, Moran MT, Kango-Singh M, Singh A, 2013b. Domain specific genetic mosaic system in the Drosophila eye. Genesis 51, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tare M, Sarkar A, Bedi S, Kango-Singh M, Singh A, 2016. Cullin-4 regulates Wingless and JNK signaling-mediated cell death in the Drosophila eye. Cell Death Dis 7, e2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tare M, Singh A, 2009. Drosophila adult eye model to teach Scanning Electron Microscopy in an undergraduate cell biology laboratory. Dros. Infor. Serv 91, 174–180. [Google Scholar]
- Tsachaki M, Sprecher SG, 2012. Genetic and developmental mechanisms underlying the formation of the Drosophila compound eye. Dev Dyn 241, 40–56. [DOI] [PubMed] [Google Scholar]
- Wang CW, Sun YH, 2012. Segregation of eye and antenna fates maintained by mutual antagonism in Drosophila. Development 139, 3413–3421. [DOI] [PubMed] [Google Scholar]
- Warren I, Smith H, 2007. Stalk-eyed flies (Diopsidae): modelling the evolution and development of an exaggerated sexual trait. Bioessays 29, 300–307. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Dodson GN, 1997. Function and evolution of antlers and eye stalks in flies, in: Crespi J.C.a.B. (Ed.), The Evolution of Mating Systems in Insects and Arachnids. Cambridge University Press, Cambridge, pp. 310–328. [Google Scholar]
- Wilkinson GS, Reillo PR, 1994. Female choice response to artificial selection on an exaggerated male trait in a stalk-eyed fly. Proceedings of Royal Society of London B 255, 1–6. [Google Scholar]
- Wittkorn E, Sarkar A, Garcia K, Kango-Singh M, Singh A, 2015. The Hippo pathway effector Yki downregulates Wg signaling to promote retinal differentiation in the Drosophila eye. Development 142, 2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T, Ready DF, 1993. Pattern formation in the Drosophila retina., in: Martinez-Arias M.B.a.A. (Ed.), The Development of Drosophila melanogaster. Cold-Spring Harbor: Cold Spring Harbor Laboratory Press, pp. 1277–1325. [Google Scholar]
- Wu J, Cohen SM, 1999. Proximodistal axis formation in the Drosophila leg: subdivision into proximal and distal domains by Homothorax and Distal-less. Development 126, 109–117. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Venkatesh TR, Teplow DB, Benzer S, 1984. Neuronal development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell 36, 15–26. [DOI] [PubMed] [Google Scholar]


