Abstract
Objective
The effects of temporal lobe epilepsy (TLE) on subcortical arousal structures remain incompletely understood. Here, we evaluate thalamic arousal network functional connectivity in TLE and examine changes after epilepsy surgery.
Methods
We examined 26 adult patients with TLE and 26 matched control participants and used resting-state functional MRI (fMRI) to measure functional connectivity between the thalamus (entire thalamus and 19 bilateral thalamic nuclei) and both neocortex and brainstem ascending reticular activating system (ARAS) nuclei. Postoperative imaging was completed for 19 patients >1 year after surgery and compared with preoperative baseline.
Results
Before surgery, patients with TLE demonstrated abnormal thalamo-occipital functional connectivity, losing the normal negative fMRI correlation betweenthe intralaminar central lateral (CL) nucleus and medial occipital lobe seen in controls (p < 0.001, paired t-test). Patients also had abnormal connectivity between ARAS and CL, lower ipsilateral intrathalamic connectivity, and smaller ipsilateral thalamic volume compared with controls (p < 0.05 for each, paired t-tests). Abnormal brainstem–thalamic connectivity was associated with impaired visuospatial attention (ρ = −0.50, p = 0.02, Spearman’s rho) while lower intrathalamic connectivity and volume were related to higher frequency of consciousness-sparing seizures (p < 0.02, Spearman’s rho). After epilepsy surgery, patients with improved seizures showed partial recovery of thalamo-occipital and brainstem–thalamic connectivity, with values more closely resembling controls (p < 0.01 for each, analysis of variance).
Conclusions
Overall, patients with TLE demonstrate impaired connectivity in thalamic arousal networks that may be involved in visuospatial attention, but these disturbances may partially recover after successful epilepsy surgery. Thalamic arousal network dysfunction may contribute to morbidity in TLE.
INTRODUCTION
Temporal lobe epilepsy (TLE) is the most common form of epilepsy, and 40% of patients have debilitating medication-resistant seizures originating in mesial temporal limbic structures.1 In drug-resistant TLE, surgery results in seizure-freedom in 60%−70% of patients.1,2 While TLE is a focal epilepsy, it engenders widespread deleterious functional and structural brain changes that cannot be explained by abnormalities in temporal or limbic structures.3–6 We hypothesise that recurrent focal seizures in TLE may incite aberrant changes in subcortical arousal networks.7 This may lead to perturbed connectivity between arousal structures, such as the thalamus and neocortex, which may contribute to neuropsychological deficits common in TLE.3,5,8 Recently, we have shown using MRI that TLE is associated with connectivity perturbations between brainstem ascending reticular activating system (ARAS) structures and neocortex, which, in turn, may be associated with neurocognitive problems.5,9 We have also shown that patients with TLE who achieve seizure-freedom after epilepsy surgery may demonstrate recovery of connectivity between ARAS and neocortex.10 However, other subcortical arousal centres with ascending excitatory projections, such as the thalamic arousal network, have not yet been well studied in preoperative or postoperative patients with TLE.
Intralaminar thalamic nuclei project broadly to the cortex and are known to be central for maintenance of arousal and neurocognitive functions.11,12 Of particular interest is the central lateral (CL) thalamic nucleus which is the largest of the intralaminar thalamic nuclei and plays an important role in awareness and visuospatial attention.13,14 The CL receives dense anatomic input from two brainstem ARAS structures, median raphe (MR) and parabrachial complex (PBC),15,16 and the CL projects heavily to medial occipital regions, including visual cortex.13,14 Interestingly, thalamo-occipital connectivity is associated with posterior dominant rhythm which is a hallmark of healthy resting-state adult electroencephalography (EEG),17–20 but has been shown to be slowed in patients with epilepsy.21,22 Furthermore, in rodent epilepsy models, aberrant activity in CL is associated with behavioural arrest and abnormal neocortical activity during limbic seizures.23,24 While there are some indications of abnormalities in thalamic arousal system function in epilepsy, long-term effects of seizures on thalamic arousal networks and their potential clinical implications remain poorly understood in TLE. In this study, we will examine thalamic arousal network functional connectivity in patients with TLE using MRI, relate network parameters to important clinical variables such as visuospatial attention, and then evaluate how thalamic arousal network connectivity may change after epilepsy surgery.
MATERIALS AND METHOD
Participants
Participants included 26 adult patients with TLE who received evaluation for epilepsy surgery at Vanderbilt University Medical Center from 2012 to 2016. Details of these patients have been described previously.9 In these patients, diagnosis of mesial TLE was established according to standard clinical care at our institution by a multidisciplinary process including neurologists, neurosurgeons, neuropsychologists, and other practitioners. This process included a detailed patient history, analysis of seizure semiology, anatomical MRI, inpatient video EEG, positron emission tomography, eloquent function localisation by functional MRI (fMRI) or Wada testing, and neuropsychological testing by a licensed neuropsychologist. Based on these tests, the multi-disciplinary epilepsy committee felt confident in recommending proceeding to surgery with a diagnosis of TLE without intracranial EEG monitoring. Then, 25 patients elected to undergo epilepsy surgery, and 19 of these patients underwent a second research MRI 35.7±13.8 (mean±SD) months after surgery. Additionally, 26 healthy controls were recruited and individually matched to patients by age (±3 years), sex, and handedness (table 1). One-to-one matching is performed at a date prior to any analyses, and therefore investigators are blinded to results of any analyses while matching controls. All participants gave written informed consent to participate in this study and all procedures were approved by the Vanderbilt University Institutional Review Board.
Table 1.
Participant demographics and disease factors
| Patients (mean±SD) |
Controls (mean±SD) |
P value | |
|---|---|---|---|
| Age, years | 37.3±12.5 | 38.4±12.6 | 0.75 |
| Gender, female | 13 (50) | 13 (50) | 0.99 |
| Handedness, right | 23 (88.4) | 23 (88.4) | 0.99 |
| Epilepsy duration, years | 20.1±14.5 | ||
| Seizure frequency, monthly | |||
| FACS | 2.8±11.4 | ||
| FICS | 6.7±7.8 | ||
| FBTC | 0.4±1.0 | ||
| History of FBTC, yes | 14 (53.8) | ||
| Epileptogenic side, right | 18 (69.2) | ||
| MTS on MRI, yes | 19 (73.0) | ||
| Postoperative patients: time between surgery and postoperative MRI, months | 35.7±13.8 | ||
| Surgery type | |||
| SAH | 12 | ||
| ATL | 7 |
For continuous variables, data shown are: mean±SD and the statistical test is the paired t-test. For categorical variables, data are N (%) and the statistical test is χ2.
N=26 preoperative patients with TLE and N=26 controls, and N=19 postoperative patients with repeat imaging.
ATL, anterior temporal lobectomy; FACS, focal aware conscious seizures; FBTC, focal to bilateral tonic-clonic (secondarily generalised) seizures; FICS, focal impaired consciousness seizures; MTS, mesial temporal sclerosis; SAH, selective amygdalohippocampectomy.
Imaging
MRI was acquired using Philips Achieva 3T MRI scanner (Philips Healthcare, Best, Netherlands) with 32-channel head coil. As in previous studies,9 imaging performed consisted of (i) three-dimensional T1-weighted whole-brain images for inter-participant normalisation and tissue segmentation (gradient echo, repetition time (TR)=9.10 ms, echo time (TE)=4.60 ms, 192 shots, flip-angle=8.0°, matrix=256×256, 1.0×1.0×1.0 mm3), (ii) two-dimensional, T1-weighted axial images for functional to structural image coregistration (1.0×1.0×4.0 mm3), (iii) two resting-state eyes closed 10-minute T2*-weighted blood oxygenation level dependent (BOLD) fMRI (field of view (FOV)=240.0 mm, TE=35.0 ms, TR=2.0 s, 34 axial slices, slice thickness=3.50 mm/0.50 mm gap, matrix=80×80, 3.0×3.0×4.0 mm3), with 300 volumes acquired during both 10 min acquisitions.5 Identical resting-state conditions for all participants included instructions prior to fMRI acquisition to lay at rest with eyes closed for the entire scan. Physiological signals, respiratory and cardiac rates, were acquired at 500 Hz.
Regions of interest
Regions of interest (ROIs) for connectivity measurements included 105 cortical and subcortical regions from Harvard-Oxford atlas (http://www.fmrib.ox.ac.uk/fsl), which includes a mask of the entire thalamus. This Harvard-Oxford atlas whole thalamus mask was used when calculating connectivity of the entire thalamus. For occipital regions, the medial occipital lobe was defined to include bilateral cuneal cortex, intracalcarine cortex, lingual gyrus, occipital fusiform gyrus, occipital pole, and supracalcarine cortex, whereas the lateral occipital lobe included bilateral inferior lateral occipital cortex and superior lateral occipital cortex. In addition, a hierarchical active shape model25 guided by the Morel stereotactic atlas26 was used to segment 23 bilateral (46 total) intrathalamic nuclei on T1 MRI and create a custom thalamic atlas for each participant.27 Masks for four of these intrathalamic nuclei were smaller than two fMRI voxels (mean across participants), and were therefore excluded, leaving 19 bilateral (38 total) intrathalamic nuclei. Finally, we also examined an ROI incorporating the MR and PBC ARAS nuclei from the Harvard Ascending Arousal Network Atlas (https://www.martinos.org/resources/aan-atlas),15 given known projections between MR/PBC and intralaminar thalamus. Coregistration processes for these regions have been published.9
Functional connectivity analysis
SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and MATLAB 2017a (The MathWorks, Natick, MA, USA) were used to preprocess fMRI data. Preprocessing steps included slice-timing correction, motion correction, correction for respiratory and cardiac noise using RETROspective Image CORrection,28 segmentation into white and grey matters and cerebrospinal fluid, and spatial normalisation to the Montreal Neurological Institute template. SPM was used to normalise and coregister fMRI through T1 MRI to the cortical/subcortical atlas. fMRIs were band-pass filtered between 0.0067 and 0.1 Hz. For each of the two fMRI sessions in each participant, functional connectivity was computed between ROIs outlined above by partial Pearson correlation between each region’s time series, with six motion time series (three measures of translation: x, y, and z and three measures of rotation: roll, pitch, and yaw) and mean white matter BOLD signal serving as confounds. Pearson correlations were transformed using Fisher z-transformation for each participant and were averaged across both fMRI sessions. For visualisation of functional connectivity differences between participant groups, we employed CONN toolbox 17 (https://www.nitrc.org/projects/conn/).29 Patients’ functional images were oriented according to epileptogenic side, and images of matched controls were reoriented accordingly.
Volume calculations
The T1-weighted images were parcellated into regions using a multiatlas approach, for which technical details have previously been described.30 All resulting segmentations were visually inspected for accuracy and no conspicuous flaws were found. From this analysis, volume of the thalamus was calculated.
Neuropsychological performance and epilepsy measures
Participant demographics and patient epilepsy measures— including seizure type and frequency, duration of epilepsy, history of focal to bilateral tonic-clonic (secondarily generalised) seizures, epileptogenic side, antiepileptic medication doses and MRI evidence of mesial temporal sclerosis (MTS)— were determined using treating epileptologist’s clinical assessments (table 1). For patients with postoperative imaging, seizure outcomes were designated at time of postoperative MRI by epileptologist’s clinical assessment using Engel classification.31 Additionally, a licensed neuropsychologist administered a preoperative standardised set of neuropsychological testing to patients. While there was some variability in tests administered to patients (see our previous full description5), the trail-making test part A was administered to all patients, and was of specific interest given the potential role of the thalamic arousal network in visuospatial attention. The neuropsychological report for one patient was not available; therefore, this patient was excluded from neurocognitive analyses.
Statistical analyses
Parametric tests were utilised for normally distributed data, as defined using Anderson-Darling test. Demographics in preoperative patients versus controls were compared with paired t-tests for continuous variables and χ2 for categorical variables. Paired t-tests with post-hoc Bonferroni-Holm correction for multiple comparisons were used to compare functional connectivity in preoperative patients versus controls, to compare connectivity between each patient and their individually matched control. Analysis of variance (ANOVA), with post-hoc Fisher’s least significant difference (LSD) procedure, was used to compare functional connectivity between preoperative patients, postoperative patients, and control participants. Spearman’s rho was used to compare functional connectivity, neuropsychological testing, disease measures, and thalamic volume. Statistical analyses were performed with MATLAB 2017a and SPSS V.23 (Armonk, NY, USA). Statistical significance was prospectively defined as p<0.05 for all tests.
RESULTS
Normal thalamo-occipital functional correlations are lost in patients with TLE
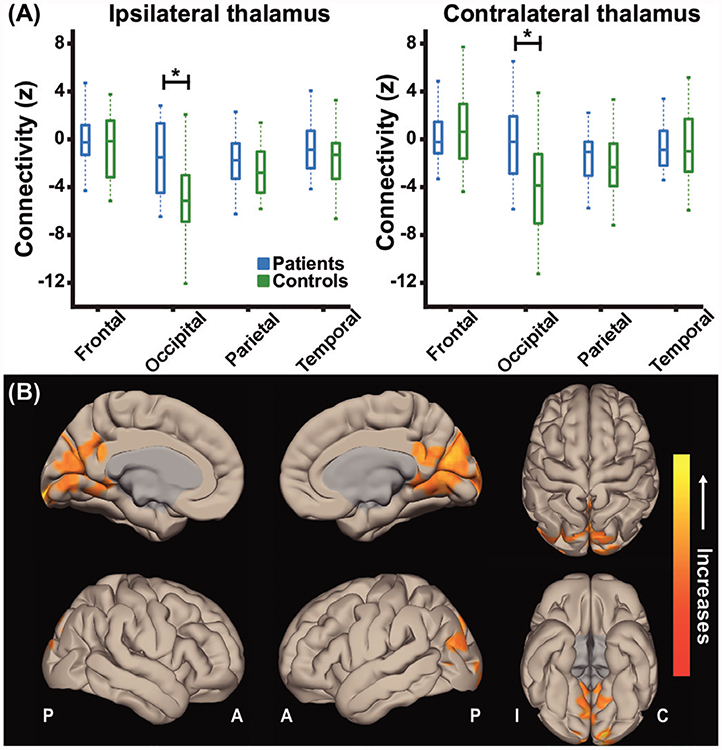
Prior to evaluating thalamic arousal networks, we first examined whether overall functional connectivity between the entire thalamus and frontal, occipital, parietal, or temporal lobes differed between preoperative patients with TLE and healthy control participants. While a negative correlation between thalamic and occipital fMRI signals is typically expected during the resting state,17,19,32,33 and was observed in controls, this negative connectivity was not present in patients either ipsilateral or contralateral to the epileptogenic side (figure 1A; p<0.01 for each side, paired t-tests with Bonferroni-Holm correction). In a voxel-wise comparison of thalamic functional connectivity between patients and controls, it was noted that altered thalamic connectivity in patients was primarily observed in medial occipital lobe bilaterally while no connectivity differences were seen in lateral occipital lobe or other lobes (figure 1B). These findings suggest overall aberrant connectivity between the thalamus and medial occipital cortex in TLE.
Figure 1.
Patients lose negative thalamo-occipital functional connectivity seen in controls. (A) Mean thalamo-occipital functional connectivity ismore positive in patients with TLE compared with control subjects while no difference in thalamo-cortical connectivity is detected for frontal, parietal ,or temporal lobes. (B) Cortical surface views are shown, demonstrating functional connectivity increases in the medial occipital lobe in patients with TLE seeded from bilateral thalami. Data represent seed-to-voxel functional connectivity maps (bivariate correlation) comparing preoperative patients versus matched control subjects fMRI (paired t-test, cluster threshold level p<0.05, FDR correction). Positive contrasts are shown, no connectivity decreases were observed in grey matter on negative contrasts. fMRIs are oriented with respect to epileptogenic side for patients with TLE and matched controls were flipped accordingly. N=26 patients with TLE before surgery and 26 matched control subjects. *p<0.01 paired t-test with Bonferroni-Holm correction. Centre bar shows median value, bottom and top of box designate 25th and 75th percentiles, respectively, and whiskers indicate data extremes. A: anterior; C: contralateral; FDR: false discovery rate; I: ipsilateral; P: posterior; TLE, temporal lobe epilepsy.
Patients with TLE exhibit perturbed connectivity in the thalamic arousal network
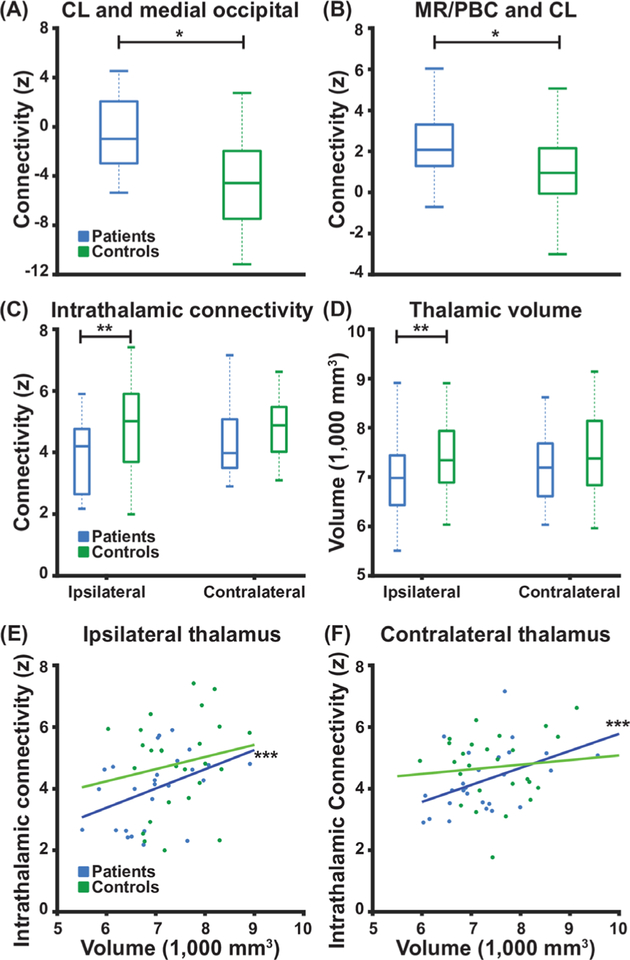
We next investigated connectivity between individual thalamic nuclei and the medial occipital lobe, with a particular focus on the intralaminar thalamic nucleus CL (highlighted in figure 2), given its potential role in visuospatial attention14 and possible involvement in limbic epilepsy.23 For these analyses, we utilised a custom atlas of 19 bilateral (38 total) thalamic nuclei (figure 2). We found that while controls demonstrated a normal negative functional correlation between CL and medial occipital lobe, this was lost in patients with TLE (figure 3A; p<0.001, paired t-test, uncorrected). Overall, of the bilateral thalamic nuclei, CL demonstrated the largest difference in connectivity with medial occipital lobe between patients and controls. Given that the CL is an intralaminar nucleus13 important for maintaining cortical activation and arousal,15 with major afferent projections originating in the MR and PBC brainstem ARAS nuclei,34 we next measured functional connectivity between MR/PBC and CL in all participants. Functional connectivity between MR/PBC and CL was found to be altered in patients with TLE compared with controls, with more positive connectivity observed in patients (figure 3B; p=0.003, paired t-test, uncorrected). Together, these findings suggest abnormal connectivity in thalamic arousal networks in TLE.
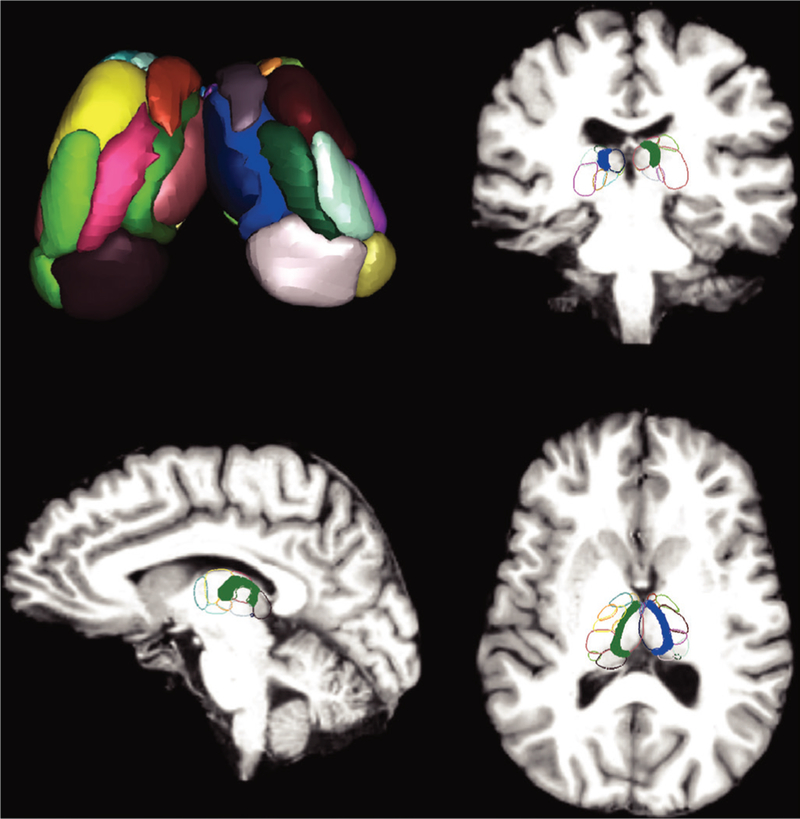
Figure 2.
Thalamic atlas shown here is the active shape modelthalamic atlas with all 23 bilateral intrathalamic nuclei (46 total nuclei) volumetrically rendered in the top left. Four nuclei with masks smaller than two voxels (mean across participants) were excluded, and 19 bilateral (38 total) nuclei were used for analyses. MRI coronal, sagittal, and axial slices show the same atlas overlaid on a standard Montreal Neurological Institute space brain with each nucleus outlined in colour. In the coronal, sagittal, and axial slices, the CL intralaminar thalamic nuclei are highlighted in solid blue and green. For analyses involving the whole thalamus, the Harvard-Oxford atlas entire thalamus was used, whereas for connectivity analyses using CL the highlighted nuclei were used. CL, central lateral.
Figure 3.
Patients with TLE exhibit perturbed thalamic connectivity and decreased ipsilateral thalamic volume. (A) Patients exhibit loss of negative connectivity between CL intralaminar thalamic nucleus and medial occipital lobe when compared with control subjects. (B) Patients exhibit abnormally increased functional connectivity between CL and MR/PBC as compared with control subjects. (C) Compared with control subjects, patients exhibit decreased intrathalamic connectivity and (D) decreased thalamic volume, on the side ipsilateral to the epileptogenic temporal lobe but not the contralateral side. (E, F) For patients, but not control subjects, higher thalamic volume is correlated with higher intrathalamic connectivity. N=26 patients with TLE before surgery and 26 matched control subjects. *p<0.01 paired t-test, **p<0.05 paired t-test with Bonferroni-Holm correction, and ***p<0.05 Spearman’s Rho with Bonferroni-Holm correction. Centre bar shows median, bottom and top of box designate 25th and 75th percentiles, respectively, and whiskers indicate data extremes. CL: central lateral; MR: median raphe; PBC: parabrachial complex; TLE, temporal lobe epilepsy.
Furthermore, to ensure robustness of our findings, we examined correlations of functional connectivity values calculated for the first and second resting-state epochs in preoperative patients and matched controls. We found that all thalamic connectivity measures (whole thalamus and CL) from each resting state were correlated (ρ=0.54−0.78, p<0.01 for all, Spearman’s rho). We also repeated all comparisons of preoperative patients versus matched controls connectivity measures independently for each resting-state epoch and found the same relationships as the averaged comparisons for thalamic (whole thalamus and CL) connectivity (p<0.01 for all, paired t-tests).
Ipsilateral intrathalamic connectivity and thalamic volume are reduced in patients with TLE
We next evaluated intrathalamic connectivity, which we defined as mean functional connectivity between all 19 individual thalamic nuclei in each side of the thalamus (figure 2). Intrathalamic connectivity was reduced in patients with TLE compared with controls on the side ipsilateral to the epileptogenic zone (p=0.04), but there was no discernible difference in intrathalamic connectivity on the contralateral side (figure 3C; p=0.10, paired t-tests with Bonferroni-Holm correction). We also examined volume of the thalamus. We found that thalamic volume on the ipsilateral side was smaller in patients than in controls (p=0.02), but there was no difference detected in contralateral thalamic volume (figure 3D; p=0.26, paired t-tests with Bonferroni-Holm correction). As prior studies have described differences in thalamic atrophy in patients with TLE with MTS versus those without MTS, we compared thalamic volume between these groups.35 We found no differences between ipsilateral thalamic volume in patients with MTS (N=19, 6986±838.7 mm3, mean±SD) versus those without MTS (N=7, 6922.9±596.1 mm3, p=0.85, unpaired t-test). Also, we did not find differences in contralateral thalamic volume between patients with MTS (7310.2±888.0 mm3) versus those without MTS (7098.7±691.7 mm3, p=0.57, unpaired t-test). Interestingly, in both the ipsilateral (figure 3E) and contralateral (figure 3F) thalamus, higher intrathalamic connectivity was correlated with greater thalamic volume in patients (ρ =0.48, p=0.02 ipsilateral; ρ=0.46, p=0.03 contralateral; Spearman’s rho with Bonferroni-Holm correction) but not in controls (ρ =0.13, p=0.52 ipsilateral; ρ =0.04, p=0.81 contralateral; Spearman’s rho with Bonferroni-Holm correction). Neither ipsilateral nor contralateral thalamic volume were correlated with occipital lobe connectivity (ρ =0.13−0.17, p=0.39−0.51, Spearman’s rho). In addition, to ensure intrathalamic connectivity is not simply a reflection of thalamic volume, we examined correlation of intrathalamic connectivity with thalamic volume in an expanded group of 40 controls. In this larger group of healthy participants, left thalamic volume was not correlated with left intrathalamic connectivity (ρ=0.20 p=0.19, Spearman’s rho) nor was right thalamic volume correlated with right intrathalamic connectivity (ρ =0.27, p=0.09, Spearman’s rho). These observations further suggest functional and structural thalamic abnormalities in TLE, particularly ipsilateral to the epileptogenic zone.
Clinical correlates of network alterations
Next, we examined potential associations between thalamic network disturbances in preoperative patients with TLE and clinical variables related to visuospatial attention and epilepsy severity. Greater positive connectivity between MR/PBC and CL (ie, more abnormal connectivity further from healthy control values) was associated with lower percentile score on trail-making part A test of visuospatial attention (ρ=−0.50, p=0.02), although connectivity between CL and medial occipital lobe was not associated with trail-making score (ρ=−0.09, p=0.67, Spearman’s rho, Bonferroni-Holm correction). In examining disease severity, frequency of neither focal aware conscious seizures (FACS) nor focal impaired consciousness seizures (FICS) was related to connectivity between MR/PBC and CL nor between CL and medial occipital lobe (ρ=−0.29−0.08, p=0.28−0.78, Spearman’s rho, Bonferroni-Holm correction). However, a higher frequency of FACS was associated with lower intrathalamic connectivity in the ipsilateral (ρ=−0.55, p<0.01) and contralateral thalamus (ρ=−0.58, p<0.01, Spearman’s rho, Bonferroni-Holm correction), and smaller volume of the ipsilateral (ρ=−0.50, p=0.02) and contralateral thalamus (ρ=−0.50, p=0.01, Spearman’s rho, Bonferroni-Holm correction). No relationships were observed between frequency of FICS or duration of epilepsy and the aforementioned thalamic parameters (p>0.60 for each, Spearman’s rho). Additionally, there were no differences detected in any of the parameters in patients with versus without a history of secondarily generalised (focal to bilateral tonic-clonic) seizures (p>0.05, unpaired t-tests). Overall, these findings demonstrate some negative associations between perturbed thalamic arousal network properties and disease severity.
Thalamic arousal network connectivity perturbations may improve after epilepsy surgery
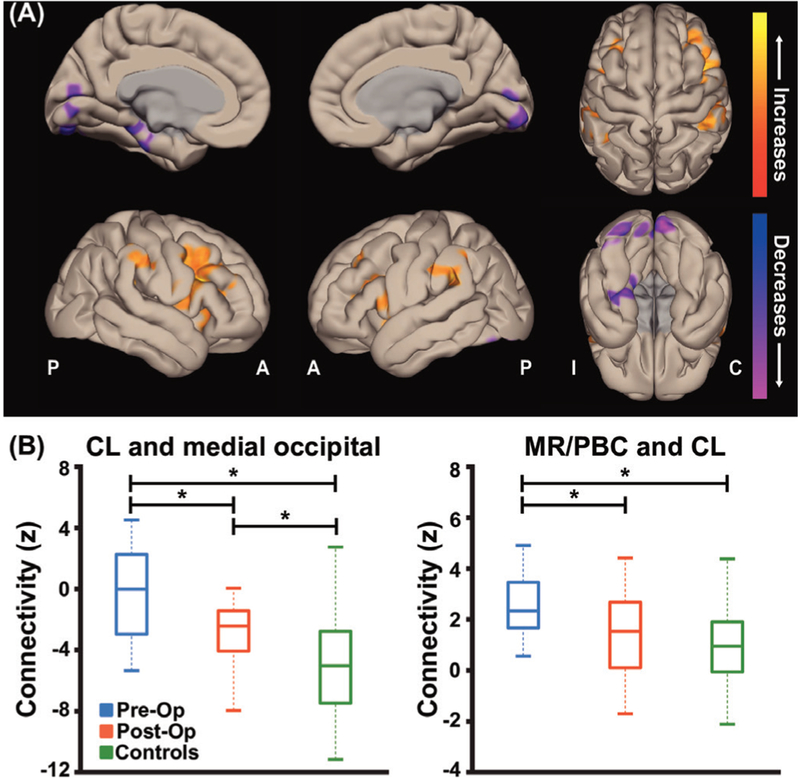
Postoperative fMRI data were available in 19 patients 35.7±13.8 (mean±SD) months after epilepsy surgery. At the time of the postoperative scan, seizure outcome was Engel I-A in seven patients, Engel I-B in two patients, Engel I-D in two patients, Engel II-C in two patients, Engel III-A in four patients, Engel III-B in one patient, and Engel IV-C in one patient. As we are most interested in the potential relationship between seizure improvement and connectivity changes after surgery, we excluded the one patient who did not improve after surgery (Engel IV) and included the remaining 18 patients for postoperative connectivity analyses. We first examined voxel-wise maps of functional connectivity changes, seeded from the entire bilateral thalami, in postoperative patients compared with their own preoperative values. We noted connectivity decreases between the thalamus and medial occipital cortex after surgery (figure 4A), in areas showing connectivity increases in preoperative patients compared with controls (figure 1B). Connectivity increases between the thalamus and fronto-parietal association cortex were also observed (figure 4A). Examining functional connectivity between CL and medial occipital cortex, which was altered in preoperative patients (figure 3A), we saw a decrease in postoperative connectivity towards normal connectivity values in controls (figure 4B left; p<0.001, ANOVA with Fisher’s LSD). Similarly, connectivity between MR/PBC and CL in postoperative patients more closely resembled connectivity in controls than the patients’ preoperative baseline (figure 4B right; p<0.01, ANOVA with Fisher’s LSD). While we saw overall differences between preoperative and postoperative thalamic connectivity in all patients with seizure improvement (Engel I–III), no preoperative connectivity differences or postoperative connectivity changes were noted between patients with Engel I versus Engel II-III seizure outcome (p=0.2−0.9, unpaired t-test). Of note, changes in connectivity after surgery between CL and the medial occipital lobe, and between MR/PBC and CL, were similar in patients who were still on the same or similar antiepileptic medication regimens (N=9) compared with those who were off of medications or were on reduced medications (N=9, p=0.46−0.47, unpaired t-tests, uncorrected). Overall, these findings suggest partial ‘recovery’ of thalamic arousal network connectivity after epilepsy surgery in patients with seizures that are eliminated or reduced.
Figure 4.
Postoperative patients with TLE with improved seizures exhibit partial recovery of thalamic arousal network connectivity. (A) Cortical surface views are shown, demonstrating functional connectivity decreases in the medial occipital lobe in postoperative patients with TLE seeded from bilateral thalami. Data represent seed-to-voxel functional connectivity maps (bivariate correlation) comparing fMRI of postoperative versus preoperative patients with TLE (paired t-test, cluster threshold level p<0.05, FDR correction). Two-sided contrasts are shown. fMRIs are orientedwith respect to epileptogenic side for patients with TLE and matched controls were flipped accordingly. (B) Postoperative patients were foundto have some recovery of functional connectivity between CL and medial occipital lobe but connectivity values did not quite reach control subject values (left). However, postoperative patients’ functional connectivity between MR/PBC and CL are decreased compared with their preoperative connectivity with no difference found between postoperative and control subject connectivities (right). N=18 postoperative patients with TLE with improved seizures after surgery, the same 18 patients before surgery, and 18 matched control subjects. *p<0.01 analysis of variance with post-hoc Fisher’s least significant difference procedure. Centre bar shows median, bottom and top of box designate 25th and 75th percentiles, respectively, and whiskers indicate data extremes. A: anterior; C: contralateral; CL: central lateral; FDR: false discovery rate; I: ipsilateral; MR: median raphe; P: posterior; PBC: parabrachial complex; Post-Op: postoperative patients; Pre-Op: preoperative patients; TLE, temporal lobe epilepsy.
DISCUSSION
In this study, we observed disturbances in the thalamic arousal network in patients with TLE that may be associated with disease severity, and which may partially improve after seizures are reduced or eliminated with epilepsy surgery. For instance, the intralaminar thalamus is known to have dense projections to occipital lobe, and we observed a loss of normal connectivity between CL and medial occipital neocortex in TLE. We also found that connectivity between MR/PBC and CL was abnormal in patients with TLE compared with controls. CL is the largest rostral intralaminar thalamic nucleus,13 and it receives the greatest input from MR and PBC in the brainstem ARAS.15 While we previously demonstrated abnormal connectivity between ARAS nuclei and neocortex in TLE,5,9 brainstem– thalamic connectivity has not previously been examined in this disorder, to the best of our knowledge. Together, these results suggest abnormal thalamic arousal network connectivity in TLE.
The thalamus is a crucial hub for cortical synchrony and modulation of brain rhythms,12 such as the posterior dominant a-rhythm (8–13 Hz) in EEG.20 The a-rhythm was first discovered by Hans Berger and is considered a hallmark of the EEG of healthy resting-state eyes-closed adults.36 EEG and fMRI studies of resting-state healthy adults have shown negative correlations between the thalamus and occipital cortex during the resting-state a-rhythm,18,32,33 congruent with what we found in our healthy control participants. Patients with epilepsy may exhibit slowing of the a-rhythm,21,22 and studies have suggested that dysfunction of the a-rhythm is associated with various neuropsychiatric and neurological disease states.37 Furthermore, previous fMRI studies have demonstrated abnormal connectivity between the thalamus and the posterior neocortical quadrant in patients with TLE.38 EEG recordings were not performed in the present study, but correlating EEG patterns including the posterior dominant rhythm with thalamo-occipital connectivity may be interesting in future studies of TLE. Thalamic arousal networks connectivity changes in TLE may be related to the widespread functional changes typically seen in patients with TLE.
What are potential clinical implications of abnormal thalamic arousal network connectivity in TLE? We have previously hypothesised that aberrant connectivity of arousal networks may be associated with the broad extratemporal deleterious effects seen in TLE.6 For instance, patients with TLE demonstrate deficits that are not explained solely by temporal lobe and limbic network problems, such as impaired attention.39 Previous studies have suggested that projections of the intralaminar thalamic nuclei may play a role in attention and visual awareness.12,14 In this investigation, we show that connectivity between brainstem ARAS (MR/PBC) and thalamus (CL) is associated with worse performance on the trail-making test part A, which may reflect impaired visuospatial attention. In addition, the thalamus is also known to play a role in seizure propagation in TLE and prior studies have shown thalamic atrophy in TLE.23,35,40–42 In this study, we observed greater reductions in both bilateral thalamic volume and intrathalamic connectivity were related to a higher frequency of FACS, but not FICS. This differs from our previous study where we found that aberrant connectivity between ARAS and frontoparietal cortex was associated with increased frequency of FICS but not FACS.9 In general, this may suggest that these two seizure types have distinct influences on different arousal centres.
How does perturbed thalamic arousal connectivity respond to successful epilepsy surgery? Our present results suggest that postoperative thalamic network functional connectivity may partially recover after epilepsy surgery. We found that patients who experienced reduction or cessation of seizures demonstrated improvements in both thalamo-occipital connectivity and brainstem–thalamic connectivity, with connectivity values more closely resembling those in controls. These results are consistent with our recent work, in which we showed recovery of abnormal connectivity between ARAS and fronto-parieto-insular neocortex after successful epilepsy surgery.10 Next, how might studies of subcortical connectivity in TLE lead to novel treatment options? Identification of subcortical networks involved in TLE pathophysiology may allow the identification of novel targets for neuromodulation treatments, such as deep brain stimulation. Some work has already been done to study the effects of stimulating the CL across disorders of consciousness. Case studies have shown that deep brain stimulation of CL in minimally conscious traumatic brain injury patients may result in increased arousal.43 Also, in a rodent model of focal limbic seizures, others have shown that simultaneous stimulation of CL and pontine nucleus oralis during focal seizures prevented deleterious neocortical and behavioural effects seen without stimulation.24 Ultimately, neurostimulation of subcortical arousal networks may play a role in reducing morbidity in epilepsy when seizure-freedom cannot be achieved.
This study has limitations worth discussion. While preoperative connectivity patterns were associated with visuospatial attention problems, potential associations between postoperative connectivity improvement and changes in attention scores could not be evaluated, as our patients did not undergo long-term postoperative neuropsychological testing. As is typical in patients with TLE, our cohort is heterogeneous in various ways including: (i) patients underwent two different surgeries: selective amygdalohippocampectomy and standard anterior temporal lobectomy, (ii) not all of our patients displayed findings of MTS on either MRI or operative specimen pathology, and (iii) not all of our patients had a history of focal to bilateral tonic-clonic seizures. Unfortunately, our present patient cohort is too small to perform subgroup analyses testing for each of these variables. Future work should include a larger number of patients allowing multivariate subgroup analyses to be performed.
Next, in this work, we obtain one postoperative scan (ranging from 14 to 60 months after surgery) showing improved thalamic arousal network connectivity in patients with TLE with improved seizures. In future work, it would interesting to perform serial postoperative fMRI and observe any possible evolution of connectivity with time after surgery. Furthermore, we did not collect information regarding length of time since most recent seizure prior to fMRI, and in future studies the possible relationships between timing of the last seizure and connectivity should be investigated. Additionally, although all participants’ resting-state fMRI are acquired under identical conditions, in which they are told to remain awake, we do not directly measure level of arousal during the scans. This limitation could be addressed in future studies by incorporating quantitative measures of arousal such as simultaneous EEG-fMRI or eyes open resting state with tracking of eye movements. Finally, thalamic nuclei are impossible to visually identify on 3T anatomical imaging, which may affect the accuracy of our analyses. Nevertheless, we were able to obtain patient-specific maps of thalamic nuclei by using an active shape model that fit to each patient. Furthermore, in order to mitigate the susceptibility of small structures to motion and noise, our functional connectivity analyses were corrected for movement and physiological measures as a part of our preprocessing.
CONCLUSION
In conclusion, while others have studied the role of the thalamus in TLE, this work employs an innovative approach to this topic that merits highlighting, including (i) novel examination of the thalamic arousal network in epilepsy, (ii) investigation of connectivity changes after epilepsy surgery and (iii) utilisation of a custom subject-specific thalamic atlas. We observed significant perturbations of brainstem–thalamic and thalamo-occipital connectivity in patients with TLE, and certain connectivity patterns may be related to seizure frequency and visuospatial attention problems. Thalamic arousal network connectivity disturbances partially recovered in patients who achieved seizure-freedom or reduced seizure frequency with epilepsy surgery. Further study of thalamic arousal network disturbances in TLE may improve our understanding of broad neural and cognitive effects of chronic seizures, and aid in the identification of neuromodulation targets to treat this devastating disorder.
Funding
Supported by NIH R00 NS097618 (DJE), R01 NS095291 (BMD), R01 NS0757270 (VLM), R01 NS110130 (VLM), T32 EB021937 (HFJG), T32 GM07347 (HFJG), F31 NS106735 (HFJG), and the Vanderbilt Institute for Surgery and Engineering (VISE).
Footnotes
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval Vanderbilt University Institutional Review Board (IRB# 170560).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request.
REFERENCES
- 1.Engel J What can we do for people with drug-resistant epilepsy? The 2016 Wartenberg lecture. Neurology 2016;87:2483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Englot DJ, Chang EF. Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurg Rev 2014;37:389–405. discussion -5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witt JA, Helmstaedter C. Cognition in epilepsy: current clinical issues of interest. Curr Opin Neurol 2017;30:174–9. [DOI] [PubMed] [Google Scholar]
- 4.Aparicio J, Carreño M, Bargalló N, et al. Combined 18F-FDG-PET and diffusion tensor imaging in mesial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage Clin 2016;12:976–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Englot DJ, D’Haese P-F, Konrad PE, et al. Functional connectivity disturbances of the ascending reticular activating system in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 2017;88:925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Englot DJ, Konrad PE, Morgan VL. Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia 2016;57:1546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Englot DJ, Blumenfeld H. Consciousness and epilepsy: why are complex-partial seizures complex? Prog Brain Res 2009;177:147–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell B, Lin JJ, Seidenberg M, et al. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol 2011;7:154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Englot DJ, Gonzalez HFJ, Reynolds BB, et al. Relating structural and functional brainstem connectivity to disease measures in epilepsy. Neurology 2018;91:e67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González HFJ, Goodale SE, Jacobs ML, et al. Brainstem functional connectivity disturbances in epilepsy may recover after successful surgery. Neurosurgery. In Press;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelstyn NMJ, Mayes AR, Ellis SJ. Damage to the dorsomedial thalamic nucleus, central lateral intralaminar thalamic nucleus, and midline thalamic nuclei on the right-side impair executive function and attention under conditions of high demand but not low demand. Neurocase 2014;20:121–32. [DOI] [PubMed] [Google Scholar]
- 12.Saalmann YB. Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front Syst Neurosci 2014;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev 2002;39:107–40. [DOI] [PubMed] [Google Scholar]
- 14.Purpura KP, Schiff ND. The thalamic intralaminar nuclei: a role in visual awareness. Neuroscientist 1997;3:8–15. [Google Scholar]
- 15.Edlow BL, Takahashi E, Wu O, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol 2012;71:531–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benarroch EE. The midline and intralaminar thalamic nuclei: anatomic and functional specificity and implications in neurologic disease. Neurology 2008;71:944–9. [DOI] [PubMed] [Google Scholar]
- 17.Moosmann M, Ritter P, Krastel I, et al. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. Neuroimage 2003;20:145–58. [DOI] [PubMed] [Google Scholar]
- 18.Feige B, Scheffler K, Esposito F, et al. Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. J Neurophysiol 2005;93:2864–72. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, de Zwart JA, Yao B, et al. Finding thalamic BOLD correlates to posterior alpha EEG. Neuroimage 2012;63:1060–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist 2005;11:357–72. [DOI] [PubMed] [Google Scholar]
- 21.Stoller A Slowing of the alpha-rhythm of the electroencephalogram and its association with mental deterioration and epilepsy. J Ment Sci 1949;95:972–84. [DOI] [PubMed] [Google Scholar]
- 22.Pyrzowski J, Siemiński M, Sarnowska A, et al. Interval analysis of interictal EEG: pathology of the alpha rhythm in focal epilepsy. Sci Rep 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng L, Motelow JE, Ma C, et al. Seizures and sleep in the thalamus: focal limbic seizures show divergent activity patterns in different thalamic nuclei. J Neurosci 2017;37:11441–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kundishora AJ, Gummadavelli A, Ma C, et al. Restoring conscious arousal during focal limbic seizures with deep brain stimulation. Cereb Cortex 2017;27:1964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, D’Haese P-F, Newton AT, et al. , eds. Thalamic nuclei segmentation in clinical 3T T1-weighted Images using high-resolution 7T shape models. Medical Imaging 2015: Image-Guided Procedures, Robotic Interventions, and Modeling; Orlando, Fl, USA, 2015. [Google Scholar]
- 26.Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol 1997;387:588–630. [DOI] [PubMed] [Google Scholar]
- 27.Chakravorti S, Morgan VL, Trujillo Diaz P, et al. A structural connectivity approachto validate a model-based technique for the segmentation of the pulvinar complex.. In: Medical imaging 2018: biomedical applications in molecular, structural, and functional imaging.. International Society for Optics and Photonics, 2018: 10578 105780T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 2000;44:162–7. [DOI] [PubMed] [Google Scholar]
- 29.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2:125–41. [DOI] [PubMed] [Google Scholar]
- 30.Asman AJ, Landman BA. Non-local statistical label fusion for multi-atlas segmentation. Med Image Anal 2013;17:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engel J, Pedley TA. Epilepsy: a comprehensive textbook. Philadelphia, PA: Lippincott Williams & Wilkins, 2007. [Google Scholar]
- 32.Zou Q, Long X, Zuo X, et al. Functional connectivity between the thalamus and visual cortex under eyes closed and eyes open conditions: a resting-state fMRI study. Hum Brain Mapp 2009;30:3066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldman RI, Stern JM, Engel J, et al. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport 2002;13:2487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benarroch EE. Parabrachial nuclear complex: multiple functions and potential clinical implications. Neurology 2016;86:676–83. [DOI] [PubMed] [Google Scholar]
- 35.Gong G, Concha L, Beaulieu C, et al. Thalamic diffusion and volumetry intemporal lobe epilepsy with and without mesial temporal sclerosis. Epilepsy Res 2008;80:184–93. [DOI] [PubMed] [Google Scholar]
- 36.Berger H Über das Elektrenkephalogramm des Menschen. Archiv für Psychiatrie und Nervenkrankheiten 1929;87:527–70. [Google Scholar]
- 37.Niedermeyer E Alpha rhythms as physiological and abnormal phenomena. Int J Psychophysiol 1997;26:31–49. [DOI] [PubMed] [Google Scholar]
- 38.He X, Doucet GE, Sperling M, et al. Reduced thalamocortical functional connectivity in temporal lobe epilepsy. Epilepsia 2015;56:1571–9. [DOI] [PubMed] [Google Scholar]
- 39.Zhao F, Kang H, You L, et al. Neuropsychological deficits in temporal lobe epilepsy: a comprehensive review. Ann Indian Acad Neurol 2014;17:374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blumenfeld H The thalamus and seizures. Arch Neurol 2002;59:135–7. [DOI] [PubMed] [Google Scholar]
- 41.Natsume J, Bernasconi N, Andermann F, et al. MRI volumetry of the thalamus in temporal, extratemporal, and idiopathic generalized epilepsy. Neurology 2003;60:1296–300. [DOI] [PubMed] [Google Scholar]
- 42.Keller SS, Richardson MP, O’Muircheartaigh J, et al. Morphometric MRI alterations and postoperative seizure control in refractory temporal lobe epilepsy. Hum Brain Mapp 2015;36:1637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kundu B, Brock AA, Englot DJ, et al. Deep brain stimulation for the treatment of disorders of consciousness and cognition in traumatic brain injury patients: a review. Neurosurg Focus 2018;45. [DOI] [PMC free article] [PubMed] [Google Scholar]