Abstract
Pregnancy and lactation associated osteoporosis (PLO) is a rare, severe, early form of osteoporosis in which young women present with fractures, usually multiple vertebral fractures, during late pregnancy or lactation.
In studies of idiopathic osteoporosis (IOP) in premenopausal women, we enrolled 78 women with low trauma fractures and 40 healthy controls, all with normal menses and no secondary cause of bone loss. In 15 of the affected women, the PLO subgroup, fractures had occurred during late pregnancy or lactation. We hypothesized that clinical, bone structural and metabolic characteristics would differ between women with PLO and those with (non-PLO) IOP and controls. All were evaluated >12 months postpartum, when structural and remodeling characteristics would be expected to reflect baseline premenopausal status rather than transient postpartum changes.
As previously reported, affected subjects (PLO and IOP) had BMD and microarchitectural deficiencies compared to controls.
Women with PLO did not differ from those with IOP in terms of age, BMI, body fat, menarcheal age, parity, or age at first pregnancy. However, women with PLO had a more severe clinical presentation than those with IOP: more fractures (5.5±3.3 vs 2.6±2.1; p=0.005), more vertebral fractures (80% vs 17%; p<0.001) and higher prevalence of multiple fractures. BMD deficits were more profound and cortical width tended to be lower in PLO. PLO subjects also had significantly lower tissue level mineral apposition and bone formation rates (0.005±0.005 vs 0.011±0.010 mm2/mm/yr; p=0.006), as well as lower serum PINP (33±12 vs 44±18 µg/L; p=0.02) and CTX (257±102 vs 355±193 pg/mL; p=0.01) than IOP. The finding that women with PLO have a low bone remodeling state assessed more than a year postpartum increases our understanding of the pathogenic mechanism of PLO. We conclude that women with PLO may have underlying osteoblast functional deficits that could affect their therapeutic response to osteoanabolic medications.
Keywords: pregnancy and lactation associated osteoporosis, premenopausal osteoporosis, bone histomorphometry
INTRODUCTION:
Pregnancy and lactation are states of high calcium demand, associated with rapid physiologic and asymptomatic decreases in bone mineral density (BMD) that are followed by recovery both postpartum and also after the first 6 months of lactation1–8. Although trabecular bone is lost at a rate of 1–3% per month with lactation, fractures during this time period, and in premenopausal women in general, are very rare9.
Pregnancy and lactation associated osteoporosis (PLO) was first described in the 1940s and 1950s10,11. It is a rare, severe, early presentation of osteoporosis in which young women experience low trauma or spontaneous fractures, most commonly multiple vertebral fractures, during late pregnancy or lactation12–15. Prominent symptoms include severe back pain and height loss. Most women are otherwise healthy, with no known condition that would predispose them to fractures11,15–19. In two cohort studies (n=35 and 107)20,21, over 80% had no known cause20, 70% were primiparous20,21 and 89–93% presented with vertebral fractures20,21. BMD before pregnancy is usually not available since there would have been no indication to measure it. BMD by DXA at presentation is generally extremely low13,16–19,22–29 with reported Z–scores often below −3.0. Because of the rarity of this condition, very little is known about pathophysiology of PLO, its natural history and risk factors, and how best to treat it.
Our research program focuses on the clinical, hormonal and skeletal characteristics of premenopausal women with idiopathic osteoporosis (IOP), as well as treatment approaches for this condition. In the course of studies to investigate the pathophysiology of IOP, we assessed bone structural, hormonal and biochemical characteristics in 78 and performed tetracycline labeled transiliac crest bone biopsies in 75 normally menstruating, premenopausal women with unexplained fractures and no known secondary cause of osteoporosis. The women with IOP were compared to 40 previously described30–32 healthy controls without osteoporosis or fractures. Included among the IOP women were 15 whose fractures occurred the context of pregnancy or lactation, whom we designated as PLO. All were evaluated more than 12 months postpartum after bone recovery would normally have occurred so that the evaluations would reflect their baseline state of bone remodeling5 and density1–8. We hypothesized that clinical, bone structural, and bone metabolic characteristics would differ between premenopausal women with PLO, premenopausal women with (non-PLO) IOP, and controls.
MATERIALS AND METHODS:
Patient Population
Premenopausal women, aged 18–48, were recruited at Columbia University Medical Center (CUMC), New York, NY and Creighton University, Omaha, NE by advertisement, and self- or physician referral. The affected subjects included women with a documented low-trauma fracture after age 18, regardless of whether areal BMD (aBMD) by DXA was low. The original study also included women with low aBMD and no fractures, however we included only women with fractures in this analysis. Fractures were ascertained by review of radiographs or reports and categorized as low trauma (i.e., trauma equivalent to a fall from a standing height or less) after review by a physician panel (ES, AC, RRR). Skull and digit fractures were excluded. Subjects and controls were evaluated more than three months after their most recent fracture, and were normally menstruating/estrogen sufficient, more than 12 months postpartum and more than 6 months postweaning. Concurrently recruited controls had normal aBMD by DXA (T score ≥ −1.0 or Z score ≥ −1.0) and no history of adult low trauma fractures.
Affected subjects were enrolled in one of two studies: (1) AR049896, entitled “Idiopathic Osteoporosis in Premenopausal Women,” a cross-sectional comparison of IOP women with controls, initiated in 2005, or (2) FD003902, entitled, “Phase 2 Study of Teriparatide for Treatment of Osteoporosis in Premenopausal Women,” a randomized controlled study of teriparatide in premenopausal women with IOP, initiated in 2011. All data utilized from this study reflect baseline, untreated status. All control subjects were enrolled in AR049896.
Inclusion and exclusion criteria were as previously reported30–32. We defined premenopausal status as regular menses off hormonal contraception, and with early follicular phase follicle stimulating hormone (FSH) levels <20 mIU/mL. Secondary causes of osteoporosis were excluded by detailed history, physical and biochemical evaluation in subjects and controls32: estrogen deficiency, eating disorders associated with amenorrhea, endocrinopathies, celiac or other gastrointestinal diseases, abnormal mineral metabolism, marked hypercalciuria (>300 mg/gCr), and drug exposures. Women with serum 25-hydroxyvitamin D (25-OHD) levels below 20 ng/ml were excluded.
All subjects provided written informed consent. The Institutional Review Boards of both institutions approved these studies.
Laboratory Assessments
To exclude secondary causes of osteoporosis, fasting morning blood and a 24-hour urine were analyzed in a clinical laboratory (Quest Diagnostics, Madison, NJ). Serum and urine were stored at −80°C for batch analyses of serum calcium, 25-OHD, PTH, N-terminal propeptides of procollagen type 1 (P1NP); C-telopeptide (CTx), and 24 hour urinary calcium, as previously described32.
Areal bone mineral density (aBMD)
Areal BMD was measured by DXA (Hologic Inc., Walton, MA) at Columbia and Creighton University Medical Centers as previously described30,32.
Transiliac bone biopsy
For subjects/controls enrolled in AR049896, a transiliac crest bone biopsy was performed after double-labeling with tetracycline, using a Bordier-type trephine with an inner diameter of 7.5 mm33. The specimens were fixed and dehydrated in ethanol, subjected to micro-computed tomography (µCT), then embedded in polymethylmethacrylate for quantitative histomorphometry.
For subjects enrolled in FD003902, transiliac biopsies were performed as above after quadruple tetracycline labeling according to previously described techniques34,35. Two sets of tetracycline labels were administered, the first set before initiation of teriparatide or placebo and the second set after 2.5 months on teriparatide or placebo, followed by the biopsy procedure at 3 months on teriparatide or placebo. Two sets of bone remodeling measurements were then calculated on a single biopsy specimen that captured both baseline remodeling and remodeling after initiation of teriparatide or placebo. The remodeling data utilized for this analysis reflect baseline status, that is, the first set of tetracycline labels.
FD003902 was a placebo-controlled RCT with 2:1 randomization drug:placebo in the first 6 months. Because labeling medication was administered in all of the subjects prior to receiving drug/placebo, there are dynamic parameters calculated from the first set of labels available in all subjects, regardless of whether they were biopsied on drug or on placebo. In contrast to the dynamic histomorphometry parameters that have to be based on the first set of labels to exclude teriparatide treatment effects, the structural parameters could only be included for subjects who were biopsied on placebo. Since 2/3 of this study’s participants were biopsied on teriparatide, they were excluded from structural analyses, leading to smaller sample size for static histomorphetric/structural analyses (Table 3).
Table 3: Bone Structure.
Includes data only from those biopsied in the untreated state.
| CONTROL N=40 | ALL IOP N=54 | p* All IOP vs Controls | AMONG ALL IOP |
p* PLO vs Control | |||
|---|---|---|---|---|---|---|---|
| PLO N=7 | IOP (non-PLO) N=47 | p* PLO vs IOP | |||||
| Trabecular Structure based on 3D MicroCT | |||||||
| BV/TV (%) | 23.7 ± 7.7 | 19.9 ± 6.3 | 0.01 | 21.8 ± 8.2 | 19.7 ± 6.1 | 0.4 | 0.6 |
| Tb Thickness (µm) | 161 ± 38 | 166 ± 42 | 0.6 | 178 ± 61 | 164 ± 39 | 0.4 | 0.3 |
| Tb Number (1/mm) | 1.8 ± 0.4 | 1.5 ± 0.2 | <0.0001 | 1.5 ± 0.2 | 1.5 ± 0.2 | 0.8 | 0.02 |
| Tb Separation (µm) | 625 ± 61 | 703 ± 75 | <0.0001 | 697 ± 75 | 703 ± 76 | 0.8 | 0.008 |
| Trabecular Structure based on 2D Histomorphometry | |||||||
| BV/TV (%) | 21.6 ± 5.4 | 18.1 ± 4.5 | 0.001 | 17.5 ± 3.1 | 18.2 ± 4.7 | 0.7 | 0.06 |
| Tb Width (µm) | 133 ± 24 | 117 ± 27 | 0.004 | 123 ± 37 | 116 ± 25 | 0.5 | 0.4 |
| Tb Number (#/mm2) | 1.6 ± 0.3 | 1.6 ± 0.3 | 0.5 | 1.5 ± 0.3 | 1.6 ± 0.3 | 0.4 | 0.2 |
| Tb Separation (µm) | 621 ± 109 | 657 ± 171 | 0.2 | 693 ± 169 | 652 ± 172 | 0.6 | 0.1 |
| Cortical Structure based on Histomorphometry | |||||||
| Cortical Width (µm) | 866 ± 212 | 671 ± 232 | <0.001 | 547 ± 219 | 689 ± 230 | 0.1 | <0.001 |
| Cortical Pore Area (%) | 7.4 ± 3.1 | 5.8 ± 3.1 | 0.3 | 6.3 ± 3.9 | 5.8 ± 2.9 | 0.7 | 0.4 |
| Pore #/Cortical Area (#/mm2) | 5.4 ± 1.7 | 5.8 ± 2.3 | 0.07 | 5.7 ± 1.2 | 5.8 ± 2.4 | 0.9 | 0.6 |
| Cells/Cancellous Bone Surface | |||||||
| Osteoblast Number (#/mm) | 1.03 ± 0.57 | 1.38 ± 0.97 | 0.03 | 1.85 ± 1.27 | 1.31 ± 0.91 | 0.2 | 0.1 |
| Osteoclast Number (#/mm) | 0.03 ± 0.02 | 0.05 ± 0.04 | 0.02 | 0.04 ± 0.03 | 0.05 ± 0.04 | 0.8 | 0.2 |
Two sample t-tests used for comparisons between groups.
Micro-computed tomography (μCT)
Intact biopsies were scanned by µCT (µCT 40, Scanco Medical AG, Brüttisellen, Switzerland) with the long axis oriented along the rotation axis of the scanner at an isotropic, nominal resolution of 8 µm, as previously described in detail36,37. Trabecular indices were determined using a direct 3-dimensional (3D) approach38: bone volume density (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N). For subjects/controls enrolled in AR049896, all biopsies were performed in the untreated state. For subjects enrolled in FD003902, structural data was only included for those subjects biopsied while receiving placebo (n=11).
Bone histomorphometry
After µCT, biopsy specimens were embedded, sectioned (7 µm for stained, and 20 um for unstained sections) and stained (Goldner trichrome, toluidine blue and solochrome) according to established procedures37. Histomorphometry was performed with a digitizing image-analysis system (OsteoMeasure, Version 4.00C, OsteoMetrics, Inc, Atlanta, GA). All variables were calculated according to American Society for Bone and Mineral Research recommendations39. Cancellous BV/TV, trabecular width (Tb.Th), number (Tb.N) and separation (Tb.Sp) were derived from two-dimensional (2D) measurements of total tissue area, cancellous bone area, and perimeter39. Cortical width was the average distance between periosteal and endocortical surfaces for both cortices. Measurements of structural variables were performed at x20 magnification. Osteoblasts and osteoclasts were identified according to the criteria established by the American Society for Bone and Mineral Research39 and expressed as number per millimeter of cancellous bone surface(N.Ob and N.Oc, #/mm). For subjects enrolled in the treatment study FD003902, structural and cell count data are included only for those subjects biopsied prior to medical treatment (biopsied on placebo as part of FD003902 protocol).
Statistical analysis
Statistical analyses were performed using SAS software (SAS Institute, Cary NC, USA). For the main continuous variables of interest (BMD, bone turnover markers and tissue level bone remodeling), we used ANOVA models as an omnibus test to examine overall differences among the three groups (PLO, IOP, Controls). Unless noted, main between-groups comparisons are presented only for variables that showed significant overall differences among the three groups by ANOVA. Between groups comparisons were conducted using student’s t tests, and Chi Square statistics. All data in text, tables, and graphs are expressed as mean ± standard deviation (SD). Results were considered significant with p < 0.05.
RESULTS:
Included were 40 Controls, and 78 affected subjects with unexplained low trauma fracture(s). Of the 78 affected subjects, 15 were classified as PLO, while 63 had IOP with fractures that were temporally unrelated to pregnancy/lactation. We included subjects in all groups at a timepoint ≥12 months postpartum AND ≥6 months postweaning. The great majority were biopsied at a timepoint ≥12 months postpartum AND ≥ 12 months postweaning. Only 3 of 118 subjects/controls (all in the PLO group) were biopsied at a timepoint ≥12 months postpartum and between 6 and 12 months postweaning.
Clinical Characteristics
Consistent with our prior reports30,32, which included IOP subjects with fractures as well as those with very low BMD and no fractures, we found that, compared to Controls, affected fracture subjects (IOP and PLO) weighed less and had lower BMI (Table 1), lower aBMD by DXA (Figure 1) and poorer trabecular and cortical bone microstructure (Table 3). Controls had lower serum 25-OH-vitamin D level than affected subjects (Table 1). PLO subjects were younger than Controls (p=0.04) and tended to be younger than IOP subjects (p=0.1). PLO and IOP subjects did not differ in terms of height, weight, BMI or percent body fat by DXA. Indices of mineral metabolism were normal, as required for study inclusion. PLO subjects had slightly lower serum PTH and higher 25-OH vitamin D concentrations. Differences remained after age adjustment. Serum and urine calcium were similar.
Table 1:
Group Characteristics
| CONTROL N=40 | ALL IOP N=78 | p* All IOP vs Con-trols | AMONG ALL IOP | ||||
|---|---|---|---|---|---|---|---|
| PLO N=15 | IOP (nonPLO) N=63 | p* PLO vs IOP | |||||
| Age (yrs) | 37 ± 8 | 36 ± 8 | 0.5 | 33 ± 5 | 37 ± 8 | 0.1 | |
| Height (cm) | 165 ± 7 | 163 ± 7 | 0.1 | 162 ± 6 | 163 ± 7 | 0.5 | |
| Weight (kg) | 71 ± 15 | 61 ± 14 | <0.001 | 63 ± 10 | 61 ± 14 | 0.7 | |
| BMI (kg/m2) | 25.8 ± 4.7 | 23.0 ± 4.7 | 0.003 | 23.9 ± 3.7 | 22.8 ± 4.9 | 0.4 | |
| DXA whole body fat (%) | 35 ± 7 | 34 ± 8 | 0.3 | 37 ± 7 | 33 ± 8 | 0.06 | |
| DXA trunk fat (%) | 33 ± 9 | 30 ± 9 | 0.1 | 33 ± 8 | 29 ± 9 | 0.1 | |
| Serum Albumin-Corrected Calcium mg/dL | 9.0 ± 0.3 | 9.0 ± 0.5 | 0.3 | 9.0 ± 0.2 | 9.1 ± 0.5 | 0.2 | |
| Urinary Calcium mg/24h | 181 ± 92 | 175 ± 83 | 0.9 | 149 ± 79 | 180 ± 83 | 0.3 | |
| PTH (pg/mL) | 22 ± 9 | 24 ± 10 | 0.3 | 18 ± 9 | 26 ± 10 | 0.007 | |
| 25-OH Vitamin D (ng/mL) | 30 ± 13 | 39 ± 14 | 0.001 | 41 ± 11 | 39 ± 15 | 0.6 | |
Two sample t-tests used for comparisons between groups.
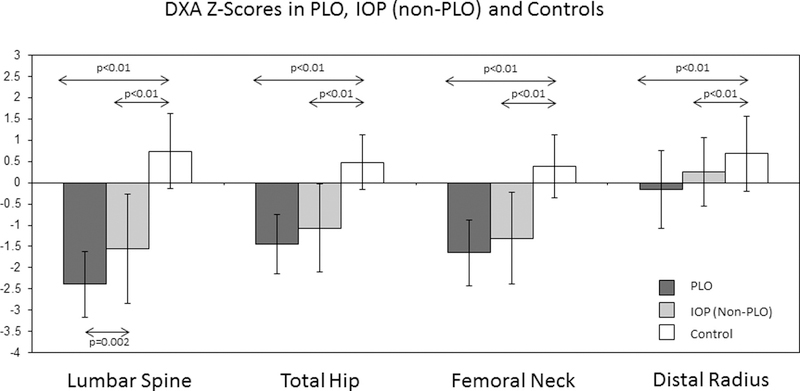
Figure 1.
Bone Mineral Density (DXA) Z-scores (± SD) at the lumbar spine, total hip, femoral neck and distal radius in premenopausal women with PLO, those with IOP but not PLO, and healthy premenopausal controls without osteoporosis. P values are shown for significant between groups comparisons; arrows designate groups compared via t test.
Reproductive factors such as age at menarche, parity, and age at first pregnancy did not differ between the groups. Among parous affected subjects, the PLO group was more likely to have ever breastfed.
Fracture History
Vertebral fractures were more common in PLO than IOP (80% versus 17%; p<0.001). Non-vertebral fractures were comparable; of the PLO subjects, 20% had rib, 20% had hip, 13% had pelvic and 73% had extremity fractures. Of the IOP subjects, 21% had rib, 18% had hip, 6% had pelvic and 56% had extremity fractures. History of childhood fractures was most common among PLO subjects, while reported family history of osteoporosis was similar in all participants.
Bone Density by DXA
Bone density by DXA, available in all subjects and controls, was significantly lower in affected subjects. Bone mineral density was lowest in the PLO group, and was significantly lower at the spine than in the IOP group (Fig 1). Results remained significant for all comparisons after excluding the 3 PLO subjects assessed between 6 and 12 months postweaning.
Serum Markers of Bone Remodeling
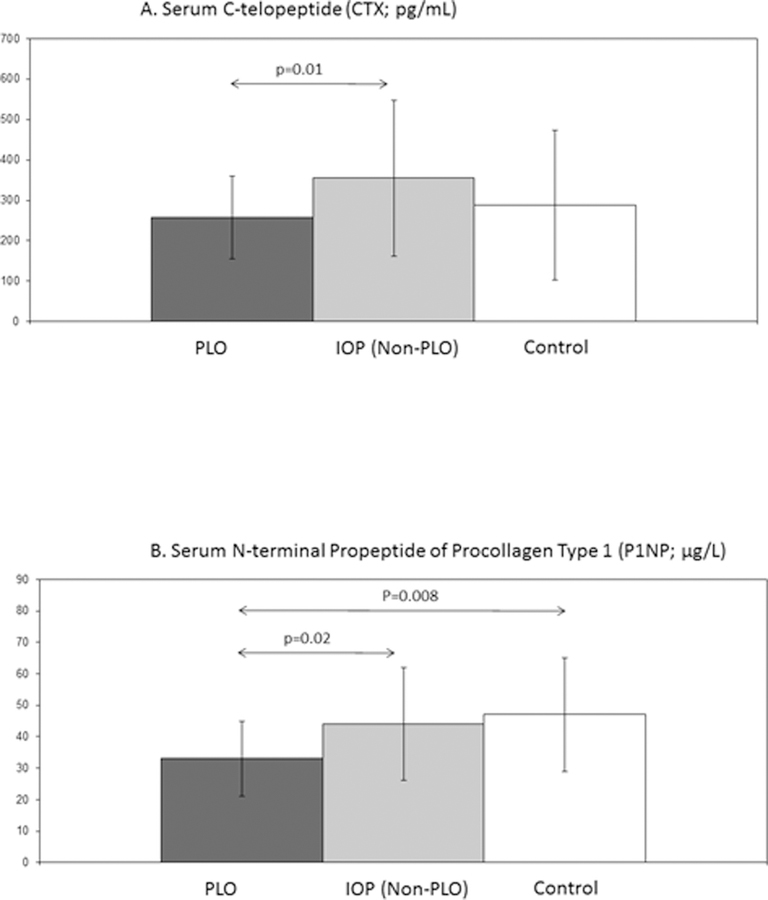
The serum marker of bone resorption, CTX, was lower in PLO than IOP, but was similar between PLO and Controls. In contrast, the serum marker of bone formation, PINP, was significantly lower among PLO subjects compared to both IOP and Controls (Fig 2). Results for PINP remained significant after adjustment for BMI or age. ANOVA models were significant for all main variables except CTX (p=0.07). Results remained significant or nearly significant (p=0.05 for PINP comparison of PLO vs IOP; p<0.05 for other comparisons) after exclusion of those assessed between 6 and 12 months postweaning.
Figure 2.
Serum Bone Turnover Markers: Mean (± SD) fasting morning serum C-telopeptide (CTX; pg/mL; Figure 2A) and serum N-terminal propeptide of procollagen type 1 (PINP; µg/L; Figure 2B) in premenopausal women with PLO, those with IOP (non-PLO), and healthy premenopausal controls. P values are shown for significant between groups comparisons; arrows designate groups compared via t test.
Bone Biopsy:
Because of the quadruple labelled biopsy protocol, remodeling indices from the untreated state are available in almost all participants (see below). However, structural indices were analyzed only in those biopsied in the untreated state, leading to a small sample size (n=7) for the PLO cohort for these parameters. The 7 PLO subjects with available biopsy structural data did not differ from the other PLO subjects in terms of age, height, weight, BMI, number of adult fractures, serum CTX, or cancellous bone formation rate. Those biopsied on placebo did have higher bone density at the spine (LS Z score −1.9±0.4 vs −2.8±0.7; p=0.01) but similar BMD at other sites. They also had higher serum PINP (39±12 vs 27±8 mcg/L; p=0.03).
Bone Structure, Osteoblast Number and Osteoclast Number:
Bone biopsies obtained in the untreated state were available in 7 PLO women, 47 with IOP (non-PLO) and 40 healthy controls. Consistent with our prior reports30,32, IOP subjects as a whole (PLO and non-PLO), and PLO subjects as a subset, had marked trabecular and cortical microarchitectural deficiencies compared to Controls (Table 3), measured both by 3-dimensional microCT and by 2-dimensional histomorphometry. Those with PLO were noted to have similar trabecular microarchitecture compared to IOP, and tended to have thinner cortices (p=0.1).
Osteoblast and osteoclast numbers were higher in affected subjects (PLO and non-PLO) than Controls. Although osteoblast number tended to be higher in the PLO subjects, cell numbers did not differ significantly between the small PLO cohort and the other groups (Controls or non-PLO IOP; Table 3).
Tissue level bone remodeling:
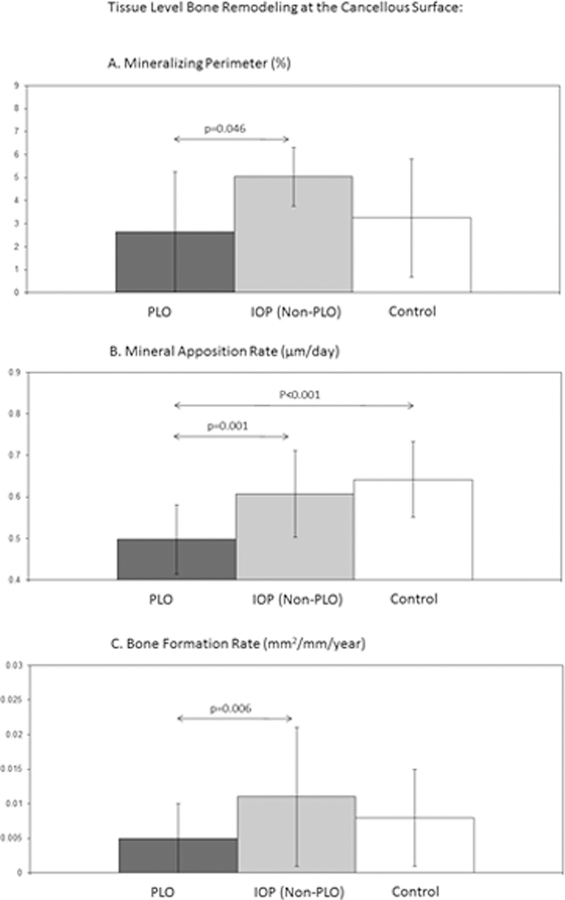
Bone biopsies with baseline remodeling indices were available in 14 PLO women, 61 with IOP (nonPLO) and 40 healthy controls. At the cancellous surface, PLO subjects had the lowest indices of bone formation. Mineral apposition rate was lower in PLO subjects than both IOP and Control groups (Fig. 3). This result remained significant after exclusion of the 3 PLO subjects assessed between 6 and 12 months postweaning. Results for tissue level remodeling indices were unchanged by adjustment for BMI or age. Of PLO subjects, 62% had a cancellous bone formation rate (BFR) < 0.006 mm2/mm/year, a cutoff we have previously used30 to define “low-turnover IOP.” Among the IOP (nonPLO) cohort, 30% had BFR below this cutoff (p=0.03). Supplementary figure shows scatter plots highlighting the difference in bone remodeling distribution.
Figure 3.
Tissue Level Bone Remodeling: Mean (± SD) mineralizing perimeter (Figure 3A), mineral apposition rate (Figure 3B) and bone formation rate (Figure 3C) at the cancellous surface on transiliac crest bone biopsy samples obtained after tetracycline labeling in the untreated state in premenopausal women with PLO, those with IOP (non-PLO), and healthy premenopausal controls. P values are shown for significant between groups comparisons; arrows designate groups compared via t test.
Although there were between groups differences in BFR and osteoblast number as noted above, there was a direct relationship between cancellous osteoblast number and cancellous BFR in Controls (r=0.7; p<0.001), Non-PLO IOP (r=0.6; p<0.001), and PLO (r=0.9; p=0.02).
There were no significant between groups differences at the endocortical or intracortical surfaces (data not shown). There was no evidence of osteomalacia on any of the biopsy samples.
PLO defined based on vertebral fractures:
We repeated the analyses after excluding those with PLO who did not present with vertebral fracture (n=3). These three subjects presented with rib and humerus, pubic ramus, and femoral fractures, respectively. Thus, including only those with PLO defined by vertebral fractures, between groups comparisons for BMD Z scores were unchanged. Results for PINP and CTX remained significant. Results for MAR were unchanged, and results for BFR remained directionally similar but not significant (p=0.1). Supplementary figure includes scatter plots with designation of the 3 PLO subjects defined based on non-vertebral fractures.
DISCUSSION:
In this, the largest bone biopsy study of PLO subjects to date, we compared clinical, bone structural and bone remodeling characteristics between PLO subjects and healthy Controls with normal BMD as well as with a cohort of premenopausal women with low trauma osteoporotic fractures that were unrelated to pregnancy or lactation (IOP). Compared to women with IOP unrelated to pregnancy and lactation, women with PLO had a clinical presentation that was substantially more severe. On average, they had more fractures and were more likely to have multiple fractures. The majority of PLO subjects presented with vertebral fractures, whereas this type of fracture was significantly less common among IOP subjects. Deficits in bone density were more profound in PLO subjects, and cortical width also tended to be lower in this group. PLO subjects also had evidence of a low bone turnover state, based both on serum remodeling markers and at the tissue level, suggesting the possibility of an underlying defect in osteoblast function.
Several case reports and small case series of PLO have included results of transiliac bone biopsies, some after double tetracycline labeling of bone-forming surfaces13,40,41. None of the biopsies revealed a specific etiology13,15,40,42 or evidence of osteomalacia13,15,40–42. Tissue-based bone remodeling activity varied, with reports of normal bone remodeling40, accelerated bone remodeling13,24,43,44, and slowed bone remodeling/reduced osteoblast activity15,24; however, there were no control biopsy data for comparison. In addition, the bone biopsies were obtained at varying times after delivery and lactation, and information on time of weaning and return of menses relative to time the biopsy was acquired was not provided.
The timing of bone biopsy in relation to delivery, lactation and weaning are critically important to the interpretation of the results. Studies of circulating bone turnover markers in the context of lactation in normal healthy women without PLO document dramatic changes. Bone turnover markers are elevated at early postpartum time-points, and decline thereafter5. Bone turnover marker levels correlate with duration of lactation and with concurrently measured changes in spine and femoral neck BMD5. Thus, in any postpartum subject, it is likely that biopsies obtained early in the postpartum period would show rapid bone remodeling, while those obtained at a time-point distant from pregnancy and lactation would be more likely to reflect that person’s baseline premenopausal bone remodeling state. Our study, in which biopsies were obtained a minimum of 12 months postpartum AND six months after weaning, allows us to test the hypothesis that women with PLO have an underlying abnormality of bone metabolism that could account for their vulnerability to the skeletal stresses of normal reproduction and lactation. The significant finding of low bone turnover, both in terms of serum markers and at the tissue level, should be viewed in this context. We acknowledge that we can never be certain that all women were assessed at their baseline bone remodeling status, and moreover, that it is possible that some women with PLO have a different or longer trajectory of bone remodeling and BMD change postpartum than normal healthy women45. However, if postpartum bone remodeling changes were extended in PLO women, we would expect them to have higher rather than lower bone remodeling parameters compared to other groups. We believe that our finding of low bone remodeling, unexpected at any postpartum timepoint, is of mechanistic interest.
The tissue level data available from our patients suggest that low bone formation contributes to the etiology of PLO, a finding with important mechanistic and therapeutic implications. Our data document lower bone formation in PLO women in the absence of lower osteoblast number, suggesting a functional osteoblast defect. In our previous publication that included both women with IOP and with PLO, we found that a subset had profoundly low cancellous BFR on biopsy and significantly lower wall width of completed bone modeling units, suggestive of decreased osteoblast productivity30. Although the group in the lowest tertile of BFR did not differ from the group in the highest tertile in terms of fractures, age, BMI or aBMD by DXA, they had the most profound microstructural defects on bone biopsy, with substantially lower trabecular bone volume and stiffness than the high bone formation group. Moreover, in a study of teriparatide treatment for IOP46,47, we identified a subgroup of women with very low bone formation (both on biopsies and serum bone turnover markers) and poor response to teriparatide, suggesting an underlying deficit in osteoblast responsiveness.
Thus, the very low bone remodeling state in women with PLO may have implications for both our understanding of disease mechanisms, and our expectations for responsiveness to therapeutic agents that stimulate bone formation. In this regard, a relatively large study of teriparatide in 27 women with PLO found that TPTD responsiveness was quite variable, with spine aBMD changes at 12 months ranging from 4.5% to 34.3%48. Thus, some women with PLO may be relatively unresponsive to teriparatide. Future studies will be required to determine whether lack of response to teriparatide is associated with low baseline bone formation rate at the tissue level.
This study has several limitations. As stated above, women were examined ≥ 12 months postpartum and at least 6 months postweaning, after bone recovery would normally have occurred so that the evaluations would reflect their baseline state of bone remodeling5 and density1–8. This assumption reflects data available from a great many studies of bone density and bone remodeling changes that occur with normal pregnancy, lactation and weaning in healthy women. As in healthy women, studies of PLO women document large postpartum and postweaning BMD increases45,48, however, it is not known whether PLO women experience the same trajectory of bone density and remodeling recovery as normal women. Thus, it is possible that some PLO women had further BMD increase after the study timepoint. Although 80% with PLO presented with vertebral fractures, not all PLO subjects presented with multiple vertebral fractures, which is the classical clinical presentation. The PLO subjects had similar BMI, but tended to have higher body fat than other IOPs. Bone remodeling differences between the PLO and IOP cohorts could be related to adiposity since we have previously documented inverse relationships between abdominal adiposity and tissue level bone remodeling rate in the healthy control subjects49. However, results were unchanged by adjustment for BMI. Additionally, the consistently lower PLO subject remodeling rate in comparison to Controls with similar body composition suggests that this finding is not solely related to body composition differences. In contrast to those available to investigate remodeling indices, the number of PLO biopsies available for analyses of structural indices was small, limiting conclusions that can be made from these data. Additionally, premenopausal women who volunteered for this study as controls may have done so because of a family history of osteoporosis, thus biasing our findings related to the presence of family history. Likewise, most of the women with IOP and PLO had already initiated vitamin D and calcium supplements before joining the study, which limited our ability to interpret the normal vitamin D levels and PTH levels in these subjects.
In conclusion, in a group of premenopausal women with unexplained low trauma fractures, those whose fractures occurred in the context of pregnancy and lactation have more fractures, substantially more vertebral fractures, lower bone density at the lumbar spine, and lower serum bone remodeling markers suggesting a low bone turnover state. In addition, we showed for the first time in women with PLO, particularly low tissue level bone remodeling at a time when postpartum bone remodeling would likely have returned to baseline. This finding has implications for our mechanistic understanding of the etiology of this type of bone fragility, and may also affect our expectations for therapeutic response to bone formation stimulating medications.
Supplementary Material
Supplementary Figure: Individual subjects within the 3 cohorts (PLO, IOP Non-PLO, Control) are plotted for serum and biopsy based bone remodeling parameters. The 3 subjects within the PLO cohort who presented with nonvertebral fractures are shown in black.
Table 2:
Reproductive and Fracture History
| CONTROL N=40 | ALL IOP N=78 | p* All IOP vs Controls | AMONG ALL IOP | |||
|---|---|---|---|---|---|---|
| PLO N=15 | IOP (nonPLO) N=63 | p* PLO vs IOP | ||||
| Reproductive History | ||||||
| Age at menarche | 12.6 ± 1.2 | 12.8 ± 1.2 | 0.4 | 12.6 ± 1.5 | 12.9 ± 1.2 | 0.4 |
| Parity | 1.4 ± 1.2 | 1.4 ± 1.2 | 0.9 | 1.7 ± 0.8 | 1.3 ± 1.2 | 0.3 |
| % Parous | 27/40=68% | 53/78=68% | 0.96 | 15/15=100% | 38/63 = 60% | NA |
| Reproductive History Among Parous Subjects Only | ||||||
| Age at First Pregnancy | 26 ± 6 | 27 ± 6 | 0.9 | 28 ± 4 | 26 ± 6 | 0.2 |
| % Ever Breastfed | 89% | 79% | 0.3 | 100% | 71% | 0.02 |
| Fracture History | ||||||
| Number of Fractures | NA | 3.2 ± 2.6 | NA | 5.5 ± 3.3 | 2.6 ± 2.1 | 0.005 |
| Vertebral Fractures | NA | 29% | NA | 80% | 17% | <0.0001 |
| History of Multiple Fractures | NA | 72% | NA | 100% | 65% | 0.007 |
| History of Childhood Fractures | 18% | 41% | 0.01 | 60% | 37% | 0.1 |
| Family History of Osteoporosis | 50% | 58% | 0.4 | 53% | 59% | 0.7 |
T-tests utilized for comparisons between groups for continuous variables. Chi-square statistic utilized for categorical variables.
Acknowledgments
These studies are supported by the following NIH and FDA funding sources: R01 AR049896 (ES), R01 FD003902 (ES and AC) and K23 AR054127 (AC)
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
REFERENCES
- 1.Karlsson MK, Ahlborg HG, Karlsson C. Maternity and bone mineral density. Acta Orthop February 2005;76(1):2–13. [DOI] [PubMed] [Google Scholar]
- 2.Karlsson C, Obrant KJ, Karlsson M. Pregnancy and lactation confer reversible bone loss in humans. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2001;12(10):828–834. [DOI] [PubMed] [Google Scholar]
- 3.Kolthoff N, Eiken P, Kristensen B, Nielsen SP. Bone mineral changes during pregnancy and lactation: a longitudinal cohort study. Clinical science April 1998;94(4):405–412. [DOI] [PubMed] [Google Scholar]
- 4.Sowers M, Corton G, Shapiro B, et al. Changes in bone density with lactation. Jama June 23–30 1993;269(24):3130–3135. [PubMed] [Google Scholar]
- 5.Sowers M, Eyre D, Hollis BW, et al. Biochemical markers of bone turnover in lactating and nonlactating postpartum women. The Journal of clinical endocrinology and metabolism July 1995;80(7):2210–2216. [DOI] [PubMed] [Google Scholar]
- 6.Kepley A, Boutroy S, Zhang C, et al. In breastfeeding women, trabecular bone loss at the radius, seen by high resolution peripheral quantitative CT, persists at 18 months postpartum. Paper presented at: American Society for Bone and Mineral Research 34th Annual Meeting2012. [Google Scholar]
- 7.Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M. The effect of calcium supplementation on bone density during lactation and after weaning. The New England journal of medicine August 21 1997;337(8):523–528. [DOI] [PubMed] [Google Scholar]
- 8.Polatti F, Capuzzo E, Viazzo F, Colleoni R, Klersy C. Bone mineral changes during and after lactation. Obstetrics and gynecology July 1999;94(1):52–56. [DOI] [PubMed] [Google Scholar]
- 9.Cohen A Premenopausal Osteoporosis. Endocrinology and metabolism clinics of North America March 2017;46(1):117–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordin BE, Roper A. Post-pregnancy osteoporosis; a syndrome? Lancet February 26 1955;268(6861):431–434. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs CS, Ralston SH. Presentation and management of osteoporosis presenting in association with pregnancy or lactation. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA September 2015;26(9):2223–2241. [DOI] [PubMed] [Google Scholar]
- 12.O’Sullivan SM, Grey AB, Singh R, Reid IR. Bisphosphonates in pregnancy and lactation-associated osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2006;17(7):1008–1012. [DOI] [PubMed] [Google Scholar]
- 13.Blanch J, Pacifici R, Chines A. Pregnancy-associated osteoporosis: report of two cases with long-term bone density follow-up. Br J Rheumatol March 1994;33(3):269–272. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs CS. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol Rev April 2016;96(2):449–547. [DOI] [PubMed] [Google Scholar]
- 15.Smith R, Athanasou NA, Ostlere SJ, Vipond SE. Pregnancy-associated osteoporosis. QJM : monthly journal of the Association of Physicians December 1995;88(12):865–878. [PubMed] [Google Scholar]
- 16.Hellmeyer L, Boekhoff J, Hadji P. Treatment with teriparatide in a patient with pregnancy-associated osteoporosis. Gynecol Endocrinol October 2010;26(10):725–728. [DOI] [PubMed] [Google Scholar]
- 17.Choe EY, Song JE, Park KH, et al. Effect of teriparatide on pregnancy and lactation-associated osteoporosis with multiple vertebral fractures. J Bone Miner Metab September 2012;30(5):596–601. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto J, Sato Y, Uzawa M, Matsumoto H. Five-year follow-up of a woman with pregnancy and lactation-associated osteoporosis and vertebral fractures. Therapeutics and clinical risk management 2012;8:195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura Y, Kamimura M, Ikegami S, et al. A case series of pregnancy- and lactation-associated osteoporosis and a review of the literature. Therapeutics and clinical risk management 2015;11:1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunne F, Walters B, Marshall T, Heath DA. Pregnancy associated osteoporosis. Clinical endocrinology October 1993;39(4):487–490. [DOI] [PubMed] [Google Scholar]
- 21.Kyvernitakis I, Reuter TC, Hellmeyer L, Hars O, Hadji P. Subsequent fracture risk of women with pregnancy and lactation-associated osteoporosis after a median of 6 years of follow-up. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA January 2018;29(1):135–142. [DOI] [PubMed] [Google Scholar]
- 22.Ozdemir D, Tam AA, Dirikoc A, Ersoy R, Cakir B. Postpartum osteoporosis and vertebral fractures in two patients treated with enoxaparin during pregnancy. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA January 2015;26(1):415–418. [DOI] [PubMed] [Google Scholar]
- 23.Lampropoulou-Adamidou K, Trovas G, Stathopoulos IP, Papaioannou NA. Case report: Teriparatide treatment in a case of severe pregnancy -and lactation- associated osteoporosis. Hormones (Athens) Oct-Dec 2012;11(4):495–500. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto N, Takahashi HE, Tanizawa T, Kawashima T, Endo N. Bone mineral density and bone histomorphometric assessments of postpregnancy osteoporosis: a report of five patients. Calcified tissue international January 1994;54(1):20–25. [DOI] [PubMed] [Google Scholar]
- 25.Phillips AJ, Ostlere SJ, Smith R. Pregnancy-associated osteoporosis: does the skeleton recover? Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2000;11(5):449–454. [DOI] [PubMed] [Google Scholar]
- 26.Vujasinovic-Stupar N, Pejnovic N, Markovic L, Zlatanovic M. Pregnancy-associated spinal osteoporosis treated with bisphosphonates: long-term follow-up of maternal and infants outcome. Rheumatology international March 2012;32(3):819–823. [DOI] [PubMed] [Google Scholar]
- 27.Sarikaya S, Ozdolap S, Acikgoz G, Erdem CZ. Pregnancy-associated osteoporosis with vertebral fractures and scoliosis. Joint, bone, spine : revue du rhumatisme January 2004;71(1):84–85. [DOI] [PubMed] [Google Scholar]
- 28.Ozturk C, Atamaz FC, Akkurt H, Akkoc Y. Pregnancy-associated osteoporosis presenting severe vertebral fractures. The journal of obstetrics and gynaecology research January 2014;40(1):288–292. [DOI] [PubMed] [Google Scholar]
- 29.Bonacker J, Janousek M, Krober M. Pregnancy-associated osteoporosis with eight fractures in the vertebral column treated with kyphoplasty and bracing: a case report. Archives of orthopaedic and trauma surgery February 2014;134(2):173–179. [DOI] [PubMed] [Google Scholar]
- 30.Cohen A, Dempster D, Recker R, et al. Abnormal Bone Microarchitecture and Evidence of Osteoblast Dysfunction in Premenopausal Women with Idiopathic Osteoporosis. The Journal of clinical endocrinology and metabolism 2011;96:3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen A, Liu XS, Stein EM, et al. Bone microarchitecture and stiffness in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab November 2009;94(11):4351–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen A, Recker RR, Lappe J, et al. Premenopausal women with idiopathic low-trauma fractures and/or low bone mineral density. Osteoporos Int January 2012;23(1):171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dempster DW, Shane E. Bone quantification and dynamics of bone turnover. In: Becker KL, ed. Principles and Practice of Endocrinology and Metabolism Philadelphia, PA: J.B. Lippincott Co; 2002:475–479. [Google Scholar]
- 34.Lindsay R, Cosman F, Zhou H, et al. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research March 2006;21(3):366–373. [DOI] [PubMed] [Google Scholar]
- 35.Rubin MR, Dempster DW, Sliney J Jr., et al. PTH(1–84) administration reverses abnormal bone-remodeling dynamics and structure in hypoparathyroidism. J Bone Miner Res November 2011;26(11):2727–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruegsegger P, Koller B, Muller R. A microtomographic system for the nondestructive evaluation of bone architecture. Calcified tissue international January 1996;58(1):24–29. [DOI] [PubMed] [Google Scholar]
- 37.Cohen A, Dempster DW, Muller R, et al. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos Int February 2010;21(2):263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hildebrand T, Laib A, Muller R, Dequeker J, Ruegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 1999;14(7):1167–1174. [DOI] [PubMed] [Google Scholar]
- 39.Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research January 2013;28(1):2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruber HE, Gutteridge DH, Baylink DJ. Osteoporosis associated with pregnancy and lactation: bone biopsy and skeletal features in three patients. Metabolic bone disease & related research 1984;5(4):159–165. [DOI] [PubMed] [Google Scholar]
- 41.Zimran A, Shilo S, Fisher D, Bab I. Histomorphometric evaluation of reversible heparin-induced osteoporosis in pregnancy. Archives of internal medicine February 1986;146(2):386–388. [PubMed] [Google Scholar]
- 42.Jensen J, Mortensen G. Pregnancy associated osteoporosis. Ugeskr Laeger 2000;162(27):3865–3866. [PubMed] [Google Scholar]
- 43.Megard M, Cuche M, Grapeloux A, Bojoly C, Meunier PJ. [Heparin osteoporosis : histomorphometric analysis of bone biopsy. One case (author’s transl)]. La Nouvelle presse medicale January 30 1982;11(4):261–264. [PubMed] [Google Scholar]
- 44.Grizzo FM, da Silva Martins J, Pinheiro MM, Jorgetti V, Carvalho MD, Pelloso SM. Pregnancy and Lactation-Associated Osteoporosis: Bone Histomorphometric Analysis and Response to Treatment with Zoledronic Acid. Calcified tissue international October 2015;97(4):421–425. [DOI] [PubMed] [Google Scholar]
- 45.Butscheidt S, Delsmann A, Rolvien T, et al. Mutational analysis uncovers monogenic bone disorders in women with pregnancy-associated osteoporosis: three novel mutations in LRP5, COL1A1, and COL1A2. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA July 2018;29(7):1643–1651. [DOI] [PubMed] [Google Scholar]
- 46.Cohen A, Manavalan JS, Kousteni S, et al. Circulating osteogenic progenitors (COPs) and COP surface IGF-1 receptor density correlate well with bone remodeling on biopsies and change markedly in response to teriparatide (TPTD) in premenopausal women with idiopathic osteoporosis (IOP). Paper presented at: American Society for Bone and Mineral Research Annual Meeting2015. [Google Scholar]
- 47.Cohen A, Stein EM, Recker RR, et al. Teriparatide for idiopathic osteoporosis in premenopausal women: a pilot study. J Clin Endocrinol Metab May 2013;98(5):1971–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong N, Kim JE, Lee SJ, Kim SH, Rhee Y. Changes in bone mineral density and bone turnover markers during treatment with teriparatide in pregnancy- and lactation-associated osteoporosis. Clinical endocrinology February 1 2018. [DOI] [PubMed]
- 49.Cohen A, Dempster DW, Recker RR, et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. The Journal of clinical endocrinology and metabolism June 2013;98(6):2562–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure: Individual subjects within the 3 cohorts (PLO, IOP Non-PLO, Control) are plotted for serum and biopsy based bone remodeling parameters. The 3 subjects within the PLO cohort who presented with nonvertebral fractures are shown in black.