Abstract
Intrahepatic cholangiocarcinoma has known histological heterogeneity. Mutations in IDH1 (mIDH1) define a molecular subclass of intrahepatic cholangiocarcinoma and IDH-targeted therapies are in development. Characterizing mIDH1 ICC histomorphology is of clinical interest for efficient identification. Resected ICCs with targeted next generation sequencing by MSK-IMPACT were selected. Clinical data were obtained. By slide review, blinded to IDH status, data were collected for histology type, mucin production, necrosis, fibrosis, cytoplasm cell shape (low cuboidal, plump cuboidal/polygonal, and columnar), and architectural pattern (anastomosing, tubular, compact tubular, and solid). A tumor was considered architecturally heterogeneous if no dominant pattern represented ≥75% of the tumor. Parameters were compared between mIDH1and IDH wild type controls. In the examined cohort (113 ICC: 29 mIDH1 and 84 IDH wild type), all IDH1 mutant tumors were of small duct type histology, thus analysis was limited to 101 small duct type tumors. mIDH1cases were more likely to have plump cuboidal/ polygonal shape (P=.014) and geographic-type fibrosis (P=.005) while IDH1 wild type were more likely to have low cuboidal shape (P=.005). Both groups were predominantly architecturally heterogeneous with no significant difference in the distribution of architectural patterns. Plump cuboidal/polygonal cell shape and a geographic-type pattern of intra-tumoral fibrosis are more often seen in mIDH1compared to IDH wild type tumors, however IDH1 mutation is not associated with a distinct histoarchitectural pattern.
Keywords: cholangiocarcinoma, histology, liver cancer, genomics, IDH1
1. Introduction
Biliary adenocarcinomas are classified as intrahepatic, peri-hilar and extrahepatic based on their anatomical location. Intrahepatic cholangiocarcinoma (ICC) is a rare tumor in western populations with an incidence of approximately 4–10 per 100,000 person years and a 5-year survival rate of around 15%.[1] Surgical resection with curative intent offers a 5-year survival of 20–40%.[2]
Recent genomic studies have shown that neomorphic mutations in isocitrate dehydrogenase 1 (IDH1) in cholangiocarcinoma is highly associated with intrahepatic origin, with a prevalence of approximately 20% in North American studies. [3, 4] IDH1 is an enzyme in the tricarboxylic acid cycle that catalyzes the oxidative decarboxylation of isocitrate to alpha-ketoglutarate. Neomorphic mutations in this enzyme, which also occur in acute myeloid leukemia, gliomas, and chondrosarcoma, typically lead to altered activity that results in the increased production of (R)-2-hydroxyglutarate, which interferes with histone and DNA demethylases, as well as several other alpha-ketoglutarate-consuming processes. [5, 6, 7, 8] An additional consequence of this mutation in myeloid neoplasms and glioma is interference with tumor differentiation.[9] Mutations in IDH1 tend to occur in hotspots such as R132, and in ICC the most common mutations are R132C, R132G, and R132L, which are distinct from the R132H mutation found in gliomas and not detected by commercially available immunohistochemical stains. [5, 10] Nonetheless, these activating mutations have been shown to increase the serum level of (R)-2-hydroxyglutarate in cholangiocarcinoma. [11] In cholangiocarcinoma, the effects of interference with phenotypic or functional differentiation are of great interest.
Given the singular metabolic disruption of IDH1-mutated neoplasms, it is logical to consider whether this molecular subtype produces a recognizable phenotype. Intrahepatic cholangiocarcinoma has been subclassified by both gross and histological configuration with varying acceptance. Grossly, ICC occurs in mass forming, periductal infiltrating, and intraductal subtypes. [12] Histologic subtyping has gained less widespread acceptance and clinical significance is less substantiated. On the one hand, the neoplasms have been separated into “peripheral” and “hilar” subtypes. [13, 14] Alternatively, the neoplasms have been divided into groups based on resemblance to large bile duct cells (large duct) versus cholangiolar cells (small duct type).[15] Additional patterns such as ductal plate malformation have been described. [16]
While the prognostic relevance of IDH1 mutation is uncertain, it is expected to be of therapeutic relevance. Survival analysis of IDH mutant ICC have shown conflicting results and are often limited in power because the mutation is present only in a subset of the populations studied.[3, 5, 10, 17, 18] Nonetheless, mutant IDH1 represents a promising actionable target for small molecule therapy in leukemia, glioma, and cholangiocarcinoma and clinical trials are in progress.[19, 20, 21] This study aims to comprehensively evaluate the cytological and architectural features of IDH1 mutated (mIDH1) ICC in comparison to ICC lacking hotspot mutations in IDH1 and IDH2 which could aid in the identification of IDH1 mutant tumors.
2. Materials and Methods
This study was approved by the Institutional Research Ethics Board of Memorial Sloan Kettering Cancer Center. Surgically resected ICCs from 1993 to 2017 which had undergone targeted next generation sequencing by MSK-IMPACT either on a clinical basis or as a part of retrospective, investigational testing (based on the availability of archived tumor and normal control tissue availability). MSK-IMPACT was clinically validated for the detection of mutations of IDH1 exon 4 (41–138) and IDH2 exon 4 (125–178) inclusive of hotspot mutations observed in intrahepatic cholangiocarcinoma. [22] Cases were stratified by the mutation status of IDH1. Tumors with IDH2 mutations were excluded, therefore the wild type IDH (wtIDH) was defined by lacking alterations in IDH1 and IDH2 as detected by MSK-IMPACT. The frequency of alterations was grouped by signaling pathways as determined using the Reactome open-source pathway database.
2.1. Clinical and pathologic variables
Clinical data including age at diagnosis, sex, ethnicity, history of autoimmune disease, chronic viral hepatitis, smoking, and clinical cirrhosis were obtained by review of electronic medical records. Pathology slides and reports were reviewed to assess characteristics of tumor size, grade, presence of perineural invasion, lymphovascular invasion, periductal infiltration, satellite nodules, presence of mucin, and intraductal precursor lesions. Small duct versus large duct types, results of albumin mRNA in situ hybridization, Arginase-1, and HepPar-1 immunohistochemistry were determined using methods and criteria from a prior publication.[15] Tumor grading was performed by applying the Bloom-Richardson system commonly used in breast carcinoma. [23] Clinical stage and lymph node status was assigned using the AJCC 8th edition using the available pathological and clinical information at the time of resection.[24]
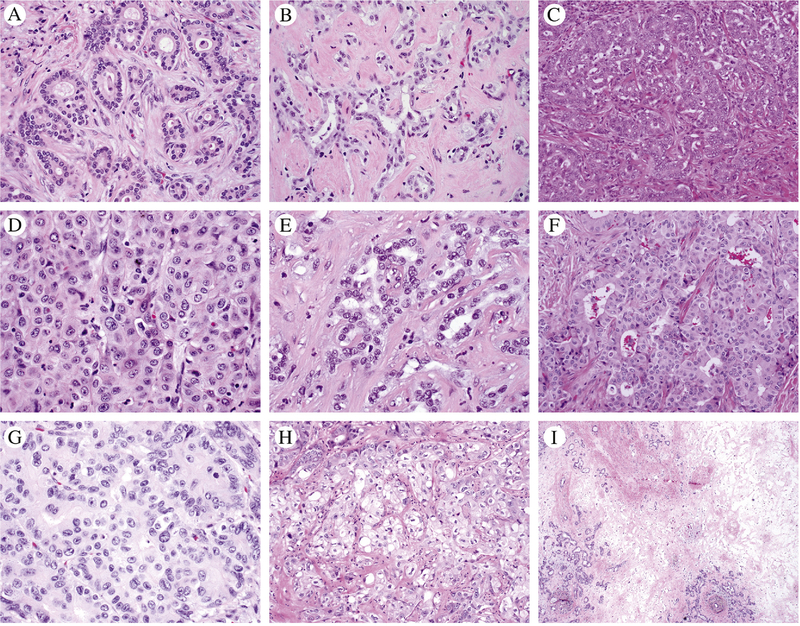
Tumor architecture was classified into four patterns as shown in Figure 1A–D. The anastomosing pattern consisted of anastomosing small ductular structures like the ductular reaction. The simple tubular pattern consisted of non-anastomosing tubules with stroma between glands. The compact tubular pattern had lumens or slit-like spaces, but the glands were compressed together without intervening stroma. The solid pattern was confluent with minimal lumens. Architecturally heterogeneous comprised no one pattern ≥ 75% of the tumor across all reviewed slides. Percent of necrosis and fibrosis were evaluated by semiquantitative estimation of volume involving tumor on each reviewed tumor slide with average across the aggregate of all tumor slides. Geographic-type fibrosis was defined as a discrete region of fibrosis without intervening viable tumor glands (Figure 1I). The cell shape was categorized as low cuboidal, plump cuboidal/polygonal, and columnar as illustrated in Figure 1. Low cuboidal had less than 1 nuclear diameter of apical cytoplasmic length, plump cuboidal/polygonal had between 1 to 2 nuclear diameters of apical cytoplasm or has cytoplasm which exceeds 2x the width of the nuclei, and columnar cells had more than 2 nuclear diameters of apical cytoplasm with or without mucin. Clear cell features were recorded as present if seen in >10% of tumor cells (Figure 1H)
Figure 1.
The various architectural patterns and cell shapes evaluated in this study are illustrated using IDH1 mutant intrahepatic cholangiocarcinomas. A) Simple tubular pattern; B) Anastomosing pattern; C) Compact tubular pattern; D) Solid pattern; E) Low cuboidal shape; F-G) Plump cuboidal shapes line lumens and polygonal cells are seen in crowded or solid areas; H) Clear cell features; I) Geographic fibrosis.
2.2. Statistical Methods
Statistical comparisons were performed by Fisher’s exact test for categorical variables. For histological comparisons, tumors that underwent neoadjuvant therapy were excluded from the analysis, and only small duct type tumors were included. For sensitivity and specificity, we tested IDH1 mutation predictions from logistic regression models against observed IDH1 mutation and used IDH1 mutation prevalence as the prediction threshold cut-off. We analyzed for associations between IDH1 mutation status for clinical and histological characteristics for all tumors, and then repeated the analysis for small duct type tumors only. Tumors with slides reviewed from recurrence were included in IDH1 morphology analysis. Statistical significance for all comparisons was set as P ≤ 0.05.
3. Results
We identified 29 mIDH1 ICC and a control population of 84 wtIDH ICC. The somatic alterations in IDH1 exon 4 consisted of p.R132C in n=23 (79.3%), p.R132L in n=3 (10.3%), and p.R132G in n=3 (10.3%). All mIDH1 were of small duct type whereas large duct (n=9) and indeterminate types (n=3) comprised a minority of the wtIDH group. Since large duct and indeterminate were exclusively in the wtIDH group, they were excluded from clinico-pathological analysis. For the clinical variables, only autoimmune disease was significantly associated with mIDH1 (Table 1). The six patients with autoimmune diseases in the mIDH1 group had Hashimoto’s thyroiditis (n=2), rheumatoid arthritis (n=2), scleroderma (n=1), and Crohn’s colitis (n=1). Patients with small duct type wtIDH small duct type had primary sclerosing cholangitis (n=1), Bell’s palsy (n=1), and multiple sclerosis (n=1).
Table 1:
Clinical characteristics of Patients with IDH1 Mutant and IDH Wild Type Intrahepatic Cholangiocarcinoma, small duct type
| Variable | Wild type IDH | Mutant IDH1 | p-value |
|---|---|---|---|
| N = 72 | N = 29 | ||
| Age (Median, IQR) | 67 (58, 73) | 73 (65, 78) | 0.052 |
| Sex | 0.7 | ||
| F | 42 (58%) | 15 (52%) | |
| M | 30 (42%) | 14 (48%) | |
| Ethnicity | 0.3 | ||
| Asian | 6 (8.3%) | 2 (6.9%) | |
| Black | 6 (8.3%) | 0 (0%) | |
| White | 60 (83%) | 27 (93%) | |
| Autoimmune disease | 3 (4.2%) | 6 (21%) | 0.016 |
| Chronic viral hepatitis B/C | 9 (19%) | 3 (14%) | 0.7 |
| Unknown | 25 | 8 | |
| Smoking | 29 (40%) | 14 (50%) | 0.5 |
| Unknown | 0 | 1 | |
| Clinical cirrhosis | 7 (9.7%) | 1 (3.6%) | 0.4 |
| Unknown | 0 | 1 | |
| Frequent alcohol | 3 (4.2%) | 3 (11%) | 0.3 |
| Unknown | 0 | 1 | |
| Steatosis | 4 (5.6%) | 3 (11%) | 0.4 |
| Unknown | 0 | 2 | |
| Neoadjuvant chemotherapy (systemic or intrahepatic) | >0.9 | ||
| Any chemo | 7 (9.7%) | 3 (11%) | |
| No chemo | 65 (90%) | 25 (89%) | |
| Unknown | 0 | 1 |
Significant differences in cell shape and fibrosis pattern were detected (Table 2). Plump cuboidal/polygonal shape and geographic fibrosis were significant associated with mIDH1 while low cuboidal shape was significantly associated with wtIDH1 while. The presence of plump cuboidal/polygonal shape had a sensitivity of 60% and specificity of 71% for identifying IDH1 mutation. Geographic-type fibrosis had a sensivitity 44% and specificity of 86% for identifying IDH1 mutation. The presence of both features (plump cuboidal/polygonal shape and geographic fibrosis) had a sensitivity of 72% and specificity of 60% for identifying IDH1 mutation.
Table 2:
Histopathological Characteristics of IDH1 Mutant and IDH Wild Type Intrahepatic Cholangiocarcinoma, small duct typea
| Variable | Wild type IDH N = 65 |
Mutant IDH1 N = 25 |
p-value |
|---|---|---|---|
| Median tumor size (cm, range) | 6.0 (4.2, 8.4) | 5.5 (3.8,6.5) | 0.14 |
| Unknown | 2 | 0 | |
| Heterogeneous histology | 44 (68%) | 18 (72%) | 0.9 |
| Homogeneous: Anastomosing | 6 (9.2%) | 0 | 0.2 |
| Homogeneous: Compact tubular | 11 (17%) | 4 (16%) | >0.9 |
| Homogeneous: Solid | 2 (3.1%) | 2 (8.0%) | 0.3 |
| Homogeneous: Simple tubular | 2 (3.1%) | 1 (4.0%) | >0.9 |
| % Anastomosing (Median, range) | 15 (0,35) | 18 (5,40) | 0.7 |
| % Tubular (Median, range) | 0 (0,20) | 10 (0,20) | 0.10 |
| % Compact (Median, range) | 40 (25,70) | 35 (23,64) | 0.5 |
| % Solid (Median, range) | 10 (0,30) | 8 (0,26) | 0.4 |
| Necrosis (mean % volume) | 3 (0, 0) | 2 (0,11) | 0.6 |
| Necrosis >25% | 7 (11%) | 0 | 0.2 |
| Necrosis (any) | 39 (60%) | 14 (56%) | >0.9 |
| Fibrosis (mean % volume) | 14 (10,20) | 20 (15,26) | 0.009 |
| Geographic-type fibrosis | 9 (14%) | 11 (44%) | 0.005 |
| Low cuboidal cell shape | 58 (89%) | 15 (60%) | 0.005 |
| Plump cuboidal/polygonal shape | 19 (29%) | 15 (60%) | 0.014 |
| Columnar cell shape | 2 (3.1%) | 0 | >0.9 |
| Variable cytoplasm pattern | 19 (29%) | 8 (32%) | >0.9 |
| Intracellular mucin | 8 (12%) | 1 (4.0%) | 0.4 |
| Extracellular mucin | 17 (26%) | 2 (8.0%) | 0.11 |
| Clear cell features | 24 (37%) | 15 (60%) | 0.082 |
| Grade | 0.8 | ||
| 1 | 32 (49%) | 14 (56%) | |
| 2 | 24 (37%) | 9 (36%) | |
| 3 | 9 (14%) | 2 (8.0%) | |
| Perineural invasion | 15 (24%) | 7 (30%) | 0.8 |
| Unknown | 3 | 2 | |
| Lymphovascular invasion | 16 (25%) | 4 (16%) | 0.6 |
| Periductal infiltration | 6 (9.2%) | 2 (8.0%) | >0.9 |
| Satellite nodules | 16 (25%) | 4 (16%) | 0.6 |
| AJCC 8th ed. Primary tumor Stage | 0.5 | ||
| 1 | 24 (37%) | 13 (54%) | |
| 2 | 32 (49%) | 10 (42%) | |
| 3 | 8 (12%) | 1 (4.2%) | |
| 4 | 1 (1.5%) | 0 (0%) | |
| Unknown | 0 | 1 | |
| Regional lymph nodes (at resection) | 0.4 | ||
| pN0 | 22 (34%) | 5 (20%) | |
| pNx | 8 (12%) | 5 (20%) | |
| pNl | 35 (54%) | 15 (60%) |
Tumors with neoadjuvant chemotherapy were excluded.
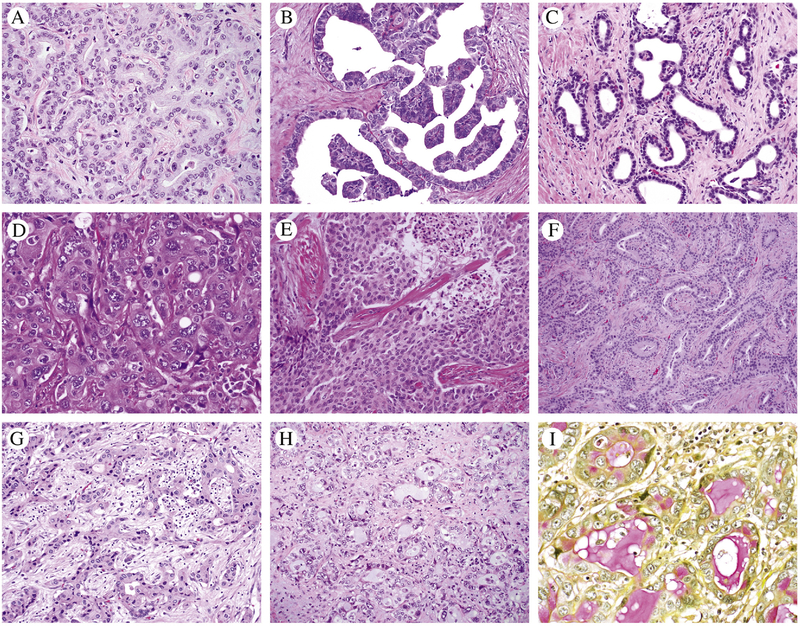
IDH mutation status was not associated with an architectural pattern (Table 2). Examples of IDH1 mutant histology are shown in Figure 2. The compact tubular pattern was the most common dominant pattern for both groups (14–15%). The volumes for the patterns were similar for both groups (Table 2).
Figure 2.
A-I) Diverse histology and cell shape patterns are seen in IDH1 mutants; A) Anastomosing glands with plump cuboidal cells; B) micropapillary; C) low cuboidal cells with dilated tubules; D) pleomorphic nuclei in polygonal cells, solid pattern; E) Solid pattern; F) Tubular and anastomosing glands, plump cuboidal; G) Anastomosing glnads plump cuboidal; H-I) tubules cuboidal cells and focal with intra and extracellular mucin highlighted by mucicarmine stain.
Necrosis and fibrosis were limited in all untreated tumors. The identification of mucin, intra- or extracellular, did not distinguish mIDH1 from wtIDH, but was notably present in both groups, albeit in a very low percentage of tumors and generally focal (Figure 2H–I).
Staining for albumin mRNA by in situ hybridization did not show a statistically significant difference in the groups. Of 26 mIDH1 tested, 17 (65%) were albumin positive and of the 56 wtIDH tested, 47 (83%) were albumin positive (P=0.084). The results for immunohistochemistry for markers of hepatocellular differentiation was: Arginase-1: positive in 0/23 tested mIDH1, 1/74 tested wtIDH; HepPar1: positive in 0/24 mIDH1, 1/74 tested wtIDH.
FGFR2 rearrangements, and the corresponding signaling pathway (MAPK), were the only two significant other genomic differences between the wtIDH and mIDH1 groups (Table 3).
Table 3:
Mutations and Genetic Pathways in IDH1 Mutant and IDH1 Wild Type Small Duct Intrahepatic Cholangiocarcinoma
| Variable | Wild type IDH | Mutant IDH1 | p-value |
|---|---|---|---|
| N = 72 | N = 29 | ||
| Gene | |||
| ARID1A | 18 (25%) | 6 (21%) | 0.8 |
| BAP1 | 11 (15%) | 7 (25%) | 0.3 |
| FGFR2 | 13 (18%) | 0 (0%) | 0.017 |
| KRAS | 7 (9.7%) | 0 (0%) | 0.2 |
| PBRM1 | 14 (19%) | 4 (14%) | 0.8 |
| TP53 | 11 (15%) | 5 (18%) | 0.8 |
| Pathway | |||
| SWI/SNFa | 32 (44%) | 9 (31%) | 0.3 |
| DNA damageb | 27 (38%) | 14 (48%) | 0.4 |
| MAPSc | 29 (40%) | 4 (14%) | 0.011 |
| PI3FCd | 2 (2.8%) | 3 (10%) | 0.14 |
SWI/SNF: ARID1A, PBRM1, ATRX
DNA Damage: BAP1, TP53, ATM, BRCA1, BARD1, KMT2A, KMT2C
MAPK: KRAS, FGFR2, NF1, ARAF, BRAF, RASA1, MAP2K1, MAPK3, PDGFRA, GRIN2A
PI3K: PTEN, PIK3R2, RPTOR
4. Discussion
IDH1 mutated intrahepatic cholangiocarcinoma has uniquely altered cellular biology known to affect cellular differentiation on a molecular level. Our study systematically classified the phenotype of a large cohort of ICC with prior targeted sequencing analysis and comparatively analyzed the histoarchitectural patterns of untreated tumors with the aim to detect phenotypic characteristics associated with this mutation. After our initial observation that all mIDH1 ICC were small duct type, subsequent analysis excluded large duct and indeterminate types in the wild type group. By comparing mIDH1 and wtIDH control groups, we determined that mIDH1 tumors have a significantly higher proportion of cells with plump cuboidal/polygonal shape (60% vs 29%, P=0.014) and geographic fibrosis (44% versus 14%, p=0.005).
Prior comparisons between mIDH1 and nonIDH mutant ICC have described a higher proportion of clear cell change and lower proportion of poor differentiation in mIDH tumors.[4, 10] We did not confirm these differences, however our methodology differed from other studies, for instance we included small duct histology only. There is no criteria-based system for grading differentiation for ICC and we experimentally applied a system borrowed from breast cancer grading to allow contributions to grade from mitotic activity and architecture. If we consider that our assessment of dominant (>75%) solid architecture is a surrogate marker of poor differentiation based on architecture only, there is no detectable difference between the groups.
Since complexity of histoarchitecture is a component of most criteria of tumor differentiation at various anatomic sites, another aim of this study was to determine whether mIDH1-related altered cellular differentiation would have an observable histoarchitectural association. Examining this question is of considerable interest, because intrahepatic cholangiocarcinoma has distinctive architectural patterns and several investigators have attempted to subclassify them based on the premise that the patterns may have potential clinical or biological significance.[25, 26] Recently, for example, the ductal plate malformation pattern was shown to have an association with ARID1A mutation [27]. We discovered that mIDH1 and wtIDH ICCs are equally architecturally heterogeneous with a strikingly similar distribution of dominant architectural patterns. As noted, instead of an architectural difference, a distinctive cytomorphological appearance was recognized. We find it especially interesting that the plump cuboidal/polygonal morphology resemble oncocytes and hepatocytes given metabolic and gene expression changes in these neoplasms. IDH1 mutants accumulate the metabolite (R)-2-hydroxyglutarate, show high expression of mitochondrial genes by integrative genomic analysis, and high mitochondrial DNA copy number.[5] Increased mitochondria are a feature of oncocytes, which typically have plump/polygonal shape. [5] In vitro models and mouse studies have also shown that mutant IDH blocks hepatocyte differentiation and IDH mutant ICC express a liver progenitor cell gene signature.[28].
Among the clinical variables we assessed, only autoimmune disease had a significant association with IDH1 mutation status. Notably, none of the mIDH1 patients had autoimmune diseases directly associated with biliary disease such as primary sclerosing cholangitis. Worldwide, there are regional differences in risk factors for cholangiocarcinoma and molecular subtypes have been shown to cluster with clinical factors such as liver fluke infection.[29] This study of ICC was performed in a population lacking liver fluke infection, hepatolithiasis, biliary cysts, and with a lower prevalence of chronic hepatitis (18%) compared to other published ICC cohorts.[13, 30]
A potentially confounding factor is the possibility of increased biologic heterogeneity in the wtIDH group. Removing the large duct and indeterminate types from our analysis was intended to mitigate biological heterogeneity within the wtIDH group because they are morphologically distinct from small duct type and there is some evidence that the large duct type has distinct biology and molecular associations.[13] We explored for potential genomic heterogeneity by comparing the mutation profiles of the two groups. Except for FGFR2 rearrangements, we found no significant differences between the two groups in the proportions of the most common mutations in ICC. Analysis that compared the detected mutations by grouping into signaling pathway only showed a significant difference in the MAPK pathway that includes FGFR2 rearrangements.
In summary, our findings indicate that mIDH1 intrahepatic cholangiocarcinoma are small duct type and have significant differences in cell shape (plump cuboidal/polygonal) and fibrosis (geographic) pattern compared to wtIDH. The tumor architectural patterns we studied do not distinguish mIDH1 and wtIDH1 intrahepatic cholangiocarcinoma.
Highlights.
IDH1 mutant intrahepatic cholangiocarcinomas are small duct histologic type
IDH1 mutant intrahepatic cholangiocarcinomas tend to have plump cuboidal/polygonal cell shape and geographic fibrosis
IDH1 and IDH wild type intrahepatic cholangiocarcinomas are predominantly architecturally heterogeneous with no significant difference in the distribution of tubular, anastomosing, compact tubular, and solid architectural patterns
Acknowledgements
We gratefully acknowledge the administrative support of Shanna Guercio, Sarah King, Marco Gonzalez, and Rebecca Andrade, laboratory support from Dr. Achim Jungbluth and Denise Frosina, and members of the Molecular Diagnostics Service in the Department of Pathology.
Funding disclosure and Conflict of Interest statement
This work was funded in part by the Marie-Josée and Henry R. Kravis Center for Molecular Oncology, the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute [P30CA008748], and Cycle for Survival. Carlie Sigel received reimbursement for transportation to attend one meeting from Agios Pharmaceuticals. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The manuscript is not under consideration for publication elsewhere. Preliminary data was presented as a poster at USCAP 2018 in Vancouver, BC by Dr. Tao Wang. This manuscript has not been published in whole or in part elsewhere. We attest to the fact that all authors have read and approved the manuscript and agree to its submission to this journal.
5. References
- [1].Flemming JA, Zhang-Salomons J, Nanji S, Booth CM, Increased incidence but improved median overall survival for biliary tract cancers diagnosed in ontario from 1994 through 2012: A population-based study. Cancer 2016;122:2534–43. 10.1002/cncr.30074 [DOI] [PubMed] [Google Scholar]
- [2].Meza-Junco J, Montano-Loza AJ, Ma M, Wong W, Sawyer MB, Bain VG, Cholangiocarcinoma: Has there been any progress? Can J Gastroenterol 2010;24:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, et al. , Exome sequencing identifies frequent inactivating mutations in bap1, arid1a and pbrm1 in intrahepatic cholangiocarcinomas. Nat Genet 2013;45:1470–3. 10.1038/ng.2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kipp BR, Voss JS, Kerr SE, Barr Fritcher EG, Graham RP, Zhang L, et al. , Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol 2012;43:1552–8. 10.1016/j.humpath.2011.12.007 [DOI] [PubMed] [Google Scholar]
- [5].Farshidfar F, Zheng S, Gingras MC, Newton Y, Shih J, Robertson AG, et al. , Integrative genomic analysis of cholangiocarcinoma identifies distinct idh-mutant molecular profiles. Cell Rep 2017;19:2878–80. 10.1016/j.celrep.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fu X, Chin RM, Vergnes L, Hwang H, Deng G, Xing Y, et al. , 2-hydroxyglutarate inhibits atp synthase and mtor signaling. Cell Metab 2015;22:508–15. 10.1016/j.cmet.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Parker SJ, Metallo CM, Metabolic consequences of oncogenic idh mutations. Pharmacol Ther 2015;152:54–62. 10.1016/j.pharmthera.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Losman JA, Kaelin WG Jr., What a difference a hydroxyl makes: Mutant idh, (r)-2-hydroxyglutarate, and cancer. Genes Dev 2013;27:836–52. 10.1101/gad.217406.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, et al. , Idh mutation impairs histone demethylation and results in a block to cell differentiation. Nature 2012;483:474–8. 10.1038/nature10860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Goyal L, Govindan A, Sheth RA, Nardi V, Blaszkowsky LS, Faris JE, et al. , Prognosis and clinicopathologic features of patients with advanced stage isocitrate dehydrogenase (idh) mutant and idh wild-type intrahepatic cholangiocarcinoma. Oncologist 2015;20:1019–27. 10.1634/theoncologist.2015-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Delahousse J, Verlingue L, Broutin S, Legoupil C, Touat M, Doucet L, et al. , Circulating oncometabolite d-2-hydroxyglutarate enantiomer is a surrogate marker of isocitrate dehydrogenase-mutated intrahepatic cholangiocarcinomas. Eur J Cancer 2018;90:83–91. 10.1016/j.ejca.2017.11.024 [DOI] [PubMed] [Google Scholar]
- [12].Aloia TPT, Taouli B, Rubbia-Brandt L, Vauthy J-N, Intrahepatic bile ducts, In: Amin MB (Ed.).Ajcc cancer staging manual, Chicago IL: American Joint Committee on Cancer, Springer; 2017, pp. 295–302. [Google Scholar]
- [13].Aishima S, Kuroda Y, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, et al. , Proposal of progression model for intrahepatic cholangiocarcinoma: Clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol 2007;31:1059–67. 10.1097/PAS.0b013e31802b34b6 [DOI] [PubMed] [Google Scholar]
- [14].Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H, Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol 2010;2:419–27. 10.4254/wjh.v2.i12.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sigel CS, Drill E, Zhou Y, Basturk O, Askan G, Pak LM, et al. , Intrahepatic cholangiocarcinomas have histologically and immunophenotypically distinct small and large duct patterns. Am J Surg Pathol 2018;42:1334–45. 10.1097/PAS.0000000000001118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nakanuma Y, Sato Y, Ikeda H, Harada K, Kobayashi M, Sano K, et al. , Intrahepatic cholangiocarcinoma with predominant “ductal plate malformation” pattern: A new subtype. Am J Surg Pathol 2012;36:1629–35. 10.1097/PAS.0b013e31826e0249 [DOI] [PubMed] [Google Scholar]
- [17].Wang P, Dong Q, Zhang C, Kuan PF, Liu Y, Jeck WR, et al. , Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene 2013;32:3091–100. 10.1038/onc.2012.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhu AX, Borger DR, Kim Y, Cosgrove D, Ejaz A, Alexandrescu S, et al. , Genomic profiling of intrahepatic cholangiocarcinoma: Refining prognosis and identifying therapeutic targets. Ann Surg Oncol 2014;21:3827–34. 10.1245/s10434-014-3828-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fujiwara H, Tateishi K, Kato H, Nakatsuka T, Yamamoto K, Tanaka Y, et al. , Isocitrate dehydrogenase 1 mutation sensitizes intrahepatic cholangiocarcinoma to the bet inhibitor jq1. Cancer Sci 2018;109:3602–10. 10.1111/cas.13784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Saha SK, Gordan JD, Kleinstiver BP, Vu P, Najem MS, Yeo JC, et al. , Isocitrate dehydrogenase mutations confer dasatinib hypersensitivity and src dependence in intrahepatic cholangiocarcinoma. Cancer Discov 2016;6:727–39. 10.1158/2159-8290.CD-15-1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dang L, Yen K, Attar EC, Idh mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol 2016;27:599–608. 10.1093/annonc/mdw013 [DOI] [PubMed] [Google Scholar]
- [22].Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. , Memorial sloan ketteringintegrated mutation profiling of actionable cancer targets (msk-impact): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 2015;17:25164 10.1016/j.jmoldx.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bloom HJ, Richardson WW, Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer 1957;11:359–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Amin MB, American Joint Committee on Cancer., American Cancer Society., Ajcc cancer staging manual, Eighth edition / editor-in-chief, Amin M ed., Chicago IL: American Joint Committee on Cancer, Springer, 2017. [Google Scholar]
- [25].Nakanuma Y, Kakuda Y, Pathologic classification of cholangiocarcinoma: New concepts. Best Pract Res Clin Gastroenterol 2015;29:277–93. 10.1016/j.bpg.2015.02.006 [DOI] [PubMed] [Google Scholar]
- [26].Sempoux C, Jibara G, Ward SC, Fan C, Qin L, Roayaie S, et al. , Intrahepatic cholangiocarcinoma: New insights in pathology. Semin Liver Dis 2011;31:49–60. 10.1055/s-0031-1272839 [DOI] [PubMed] [Google Scholar]
- [27].Sasaki M, Sato Y, Nakanuma Y, Cholangiolocellular carcinoma with “ductal plate malformation” pattern may be characterized by arid1a genetic alterations. Am J Surg Pathol 2019;43:352–60. 10.1097/PAS.0000000000001201 [DOI] [PubMed] [Google Scholar]
- [28].Saha SK, Parachoniak CA, Bardeesy N, Idh mutations in liver cell plasticity and biliary cancer. Cell Cycle 2014;13:3176–82. 10.4161/15384101.2014.965054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, et al. , Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov 2017;7:1116–35. 10.1158/2159-8290.CD-17-0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yu TH, Yuan RH, Chen YL, Yang WC, Hsu HC, Jeng YM, Viral hepatitis is associated with intrahepatic cholangiocarcinoma with cholangiolar differentiation and n-cadherin expression. Mod Pathol 2011;24:810–9. 10.1038/modpathol.2011.41 [DOI] [PubMed] [Google Scholar]