Abstract
Introduction
Minor salivary gland carcinomas of the head and neck are rare cancers with variable clinical behavior. We explore the incidence, pathology, clinical behavior and the factors predictive of outcome in a large cohort of patients treated at Memorial Sloan Kettering Cancer Center (MSK) over a 30-year period (1985 to 2015).
Methods
Clinical, pathological, treatment and outcome data was collected. Unadjusted and adjusted hazard ratios for each variable were calculated using univariate and multivariable Cox regression for survival and recurrence outcomes.
Results
450 patients were included. 55% were female, 56% were less than 60 years old and there was a median follow up of 74 months (range 1–364 months). The most common site was the oral cavity with 306 tumors (68%), followed by oropharynx with 96 (21%), sinonasal cavity 38 (8%), trachea 7 (2%), and larynx 4 (1%). The most common histological types were mucoepidermoid carcinoma (180 tumors, 40%), adenoid cystic carcinoma (141 tumors, 31%) and polymorphous low grade adenocarcinoma (PLGA) (54 tumors, 12%). The 5-year predicted overall survival was 86% and the disease specific survival was 94% at 5 years. Pathology and tumor stage were significant variables on multivariate analysis for overall survival, disease specific survival, recurrence free survival, local recurrence free survival, regional recurrence free survival and distant recurrence free survival.
Conclusion
AJCC stage and pathology were the most predictive variables across all outcomes. Tumor site, post-operative radiotherapy and margin status were not statistically significant variables after tumor stage and pathology were controlled for in most outcomes.
Keywords: Minor Salivary Carcinoma, Outcomes, Prediction, Head and Neck cancer, Survival
Precis:
Large retrospective study of 450 minor salivary gland tumors of the Head and Neck. Tumor stage and histological type are the most predictive variables of survival and recurrence.
Introduction
Minor salivary gland carcinomas of the head and neck are a group of rare cancers with significant heterogeneity in histological types and ultimate clinical behavior. The incidence of salivary malignancy is estimated to be 4–135 cases per million population per year(1) with only 10–15% arising in the minor salivary glands(2). The annual incidence of minor salivary gland malignancies has been estimated to be 0.16–0.4/100,000 population(3). Salivary gland carcinomas demonstrate an unparalleled histological diversity when compared to any other organ systems(4). The most common histological types reported in retrospective studies varies depending on the population, geographic location and reporting center’s expertise(5). However, the two most common histological types are reported as adenoid cystic carcinoma and mucoepidermoid carcinoma(2).
The majority of the literature on minor salivary tumors is limited due to the rarity and diversity of these tumors(2). Previous reports describe these tumors within an anatomical subsite(6) or by histological type in which major and minor tumors are reported together(7). Few centers have enough volume of cases to report on meaningful outcomes.
In this study, we aim to explore the incidence, pathology, clinical behavior and the factors predictive of outcome in a large cohort of patients with minor salivary gland cancers treated at Memorial Sloan Kettering Cancer Center (MSK) over a 30-year period from 1985 to 2015.
Methods
Institutional Research Board approval was granted to perform a retrospective study examining patients with malignancies of the minor salivary glands.
Patients
Patients that received primary treatment of minor salivary gland malignancy at MSK from 1985 to 2015 were identified. Only patients that received primary surgery at MSK, with curative intent at the local site were included. Patients that had received previous treatments or surgery at an outside institution for the primary treatment of the malignancy were excluded.
Data collection
The electronic patient record was accessed to record clinical and pathological characteristics, treatments and outcomes. Staging was recorded according to the American Joint Committee on Cancer Staging Manual 7th Edition(8). Minor salivary glands are staged, by convention, using the mucosal tumor staging classification, according to the anatomical site of the tumor. Patient data was stored on an institutional network drive using the oncological database software, Caisis (Biodigital), with access available only to authors.
Definitions
The anatomical site was determined using clinical examination and radiological imaging. Tumors were categorized into oral cavity, nasal cavity and paranasal sinuses, oropharynx, larynx and trachea. The oral cavity consisted of the subsites buccal mucosa, floor of the mouth, hard palate, mandible, lip, retromolar trigone, tongue and upper gum. The oropharynx included the subsites of base of tongue, soft palate and tonsil. The nasal cavity included the subsites maxillary sinus, nasal cavity, nasal septum and nasopharynx.
The histopathology of minor salivary glands is complex with multiple different histopathological types. The WHO classification of Head and Neck tumors was used for histological classification and for definitions of grade within pathological groups(9). Tumors were categorized into low, intermediate and high histology risk groups to allow for analysis because of the large number of histological types. Tumors categorized as high risk were salivary duct carcinoma, high grade mucoepidermoid carcinoma (MEC), high grade carcinoma ex-pleomorphic adenoma (CEPA), high grade adenocarcinoma, high grade myoepithelial, high grade adenoid cystic carcinoma (ACC), and high grade acinic cell carcinoma. Intermediate risk tumors included ACC. Tumors classified as low risk included low grade acinic cell carcinoma, low and intermediate grade MEC, low grade adenocarcinoma, low grade CEPA, low grade myoepithelial carcinoma and all polymorphous adenocarcinoma (PLGA) and epithelial-myoepithelial carcinomas.
In tumors such as adenoid cystic carcinoma, mucoepidermoid carcinoma and myoepithelial carcinoma, accepted histological convention was used to classify these tumors(10). Lymphovascular invasion (LVI) was defined as the presence of malignant cells in lymphatic or vascular vessels on histological examination. Perineural invasion (PNI) was defined as tumor cells invading or spreading around the space surrounding nerves(11).
The margin status was determined by expert head and neck pathologists. A positive margin was defined as tumor at a cut tumor surface and a negative margin was a specimen with 0.5cm or more normal tissue between the cut surface and the tumor. A close margin was anything less than 0.5cm.
Definitions of outcomes
Overall survival was calculated from the day of definitive surgery to the last known hospital follow up date or date of death found in the hospital records or social security index. Disease specific survival was calculated from the day of surgery until last known follow up or death from minor salivary cancer reported in the patient record. Recurrence was calculated from the day of surgery until the first local, regional or distant recurrence reported in the patient record. The patient’s disease status had to be confirmed in the patient’s chart by a member of the head and neck departmental treatment team.
Statistical analysis
The Kaplan-Meier method was used to calculate estimates of survival and to compare the different strata of a variable and describe the impact on the outcome. Unadjusted and adjusted hazard ratios for each variable were calculated using univariate and multivariable Cox regression respectively for overall survival, disease specific survival, recurrence free survival, local recurrence free survival, regional recurrence free survival and distant recurrence free survival. Variables identified in univariate analysis were considered for inclusion in the multivariable model. A step-down reduction approach was used to remove noncontributory variables, keeping those that have previously been shown to be important and remained significant on analysis. Five and ten year predicted results for all the outcomes were also calculated. Excel (Microsoft, 2015) was used to organize the data and SPSS (IBM, Version 25) was used for statistical analysis.
Results
Demographics
There were 450 patients included in the study. 56% were less than 60 years old, with 25 (6%) at least 80 years old and 9 patients (2%) less than 20 years old. 55% were female and most were without comorbidity (58%). 50% had a history of tobacco use and 60% had a history of alcohol use. The median follow-up time was 74 months (range 1–364 months) (Table 1).
Table 1.
Clinical Characteristics
| Characteristic | Freq (n= 450) | % | |
|---|---|---|---|
| Age Group | 0–19 | 10 | 2 |
| 20–39 | 75 | 17 | |
| 40–59 | 167 | 37 | |
| 60–79 | 173 | 38 | |
| ≥ 80 | 25 | 6 | |
| Age Group | < 60 | 252 | 56 |
| ≥ 60 | 198 | 44 | |
| Gender | Male | 202 | 45 |
| Female | 248 | 55 | |
| Comorbidities | Yes | 188 | 42 |
| No | 262 | 58 | |
| Tobacco | Yes | 222 | 49 |
| No | 214 | 48 | |
| NK | 14 | 3 | |
| Alcohol | Yes | 272 | 60 |
| No | 161 | 36 | |
| NK | 17 | 4 | |
| cT | cTx | 1 | 0 |
| cT1 | 219 | 49 | |
| cT2 | 114 | 25 | |
| cT3 | 29 | 6 | |
| cT4 | 87 | 19 | |
| cN | cN0 | 400 | 89 |
| cN1 | 22 | 5 | |
| cN2 | 27 | 6 | |
| cN3 | 1 | 0 | |
| M | M0 | 446 | 99 |
| M1 | 4 | 1 |
(NK Not known)
Treatment
All patients were treated with surgical resection, of whom 130 (29%) had a neck dissection. 280 (62%) received surgery alone, while 35% received adjuvant radiation and 2% received adjuvant chemo-radiotherapy. The indications for adjuvant treatment were high grade tumors, high stage disease and positive margins.
Salivary tumors site and histology
The most common site for minor salivary cancer was the oral cavity with 306 tumors (68%), followed by oropharynx with 96 (21%), sinonasal cavity 38 (8%), trachea 7 (2%), and larynx 4 (1%) (Figure 1A).
Figure 1A.
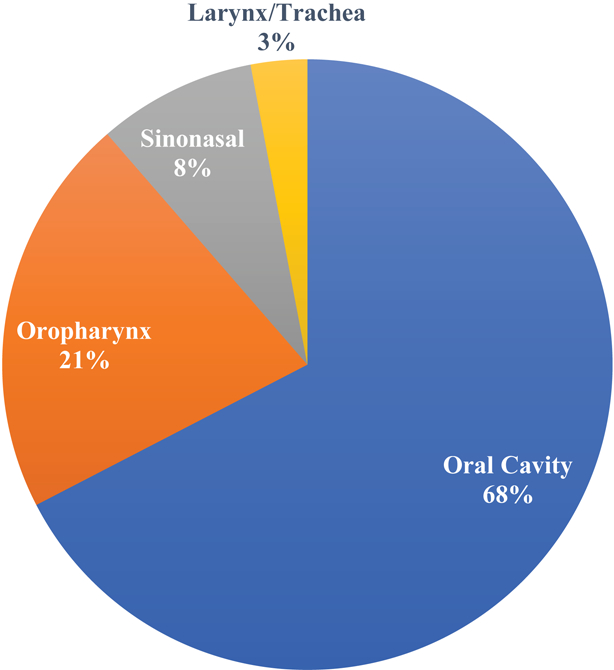
Proportion of minor salivary glands at different subsites
The most common histological types were mucoepidermoid carcinoma (180 tumors, 40%), adenoid cystic carcinoma (141 tumors, 31%), polymorphous low grade adenocarcinoma (PLGA) (54 tumors, 12%), adenocarcinoma (28 tumors, 6%), myoepithelial carcinoma (10 tumors, 2%), acinic cell carcinoma (10 tumors, 2%), and 21 (5%) were other rare histological types (Figure 1B).
Figure 1B.
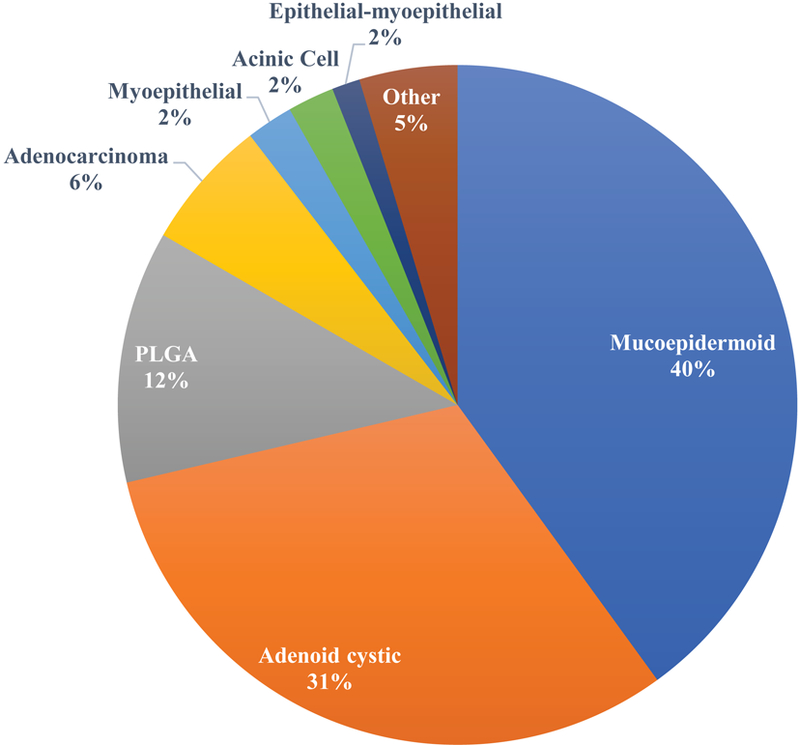
Histological types of different minor salivary carcinoma
Figure 1C shows the most common histology types classified by site. Most tumors in the oral cavity and oropharynx were mucoepidermoid tumors (45% and 40%, respectively). However, in the other sites adenoid cystic carcinoma was the most frequent pathology seen, accounting for 63% of sinonasal tumors, 75% of larynx tumors and 57% of tracheal tumors.
Figure 1C.
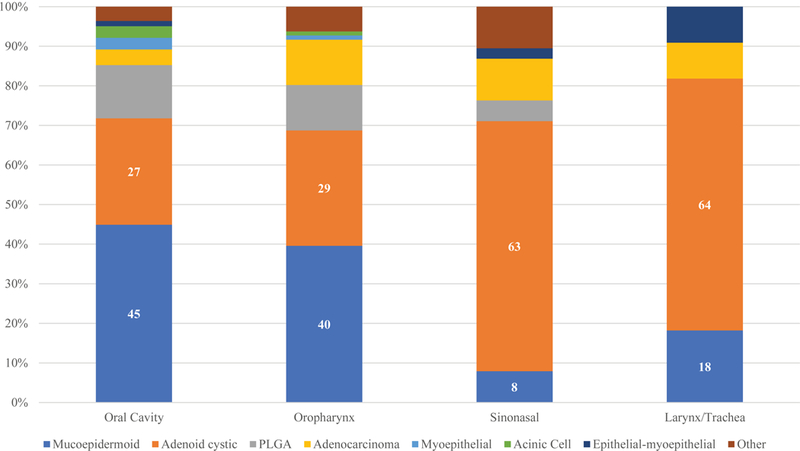
Proportion of histology type by anatomical subsite
Minor salivary tumors are staged according to the AJCC system for the primary site. The majority of the tumors presented with an early T stage, with 49% cT1 and 25% cT2. 89% had a clinically negative neck at presentation (Table 1).
Of the 130 patients that had a neck dissection as part of their treatment, 64 (49%) had pathologically negative necks, while 24 (18%) were pN1, and 41 (9%) were pN2, and 1 was pN3 (Table 2).
Table 2.
Pathological features of minor salivary tumors
| Variable | Frequency | % | |
|---|---|---|---|
| pT | pTx | 2 | 0 |
| pT1 | 246 | 55 | |
| pT2 | 94 | 21 | |
| pT3 | 20 | 4 | |
| pT4 | 88 | 20 | |
| pN | pNx | 320 | 71 |
| pN0 | 64 | 14 | |
| pN1 | 24 | 5 | |
| pN2 | 41 | 9 | |
| pN3 | 1 | 0 | |
| Margin | Positive | 126 | 28 |
| Close | 103 | 23 | |
| Negative | 214 | 48 | |
| NK | 7 | 2 | |
| PNI | Present | 167 | 37 |
| Absent | 123 | 27 | |
| NK | 160 | 36 | |
| LVI | Present | 53 | 12 |
| Absent | 214 | 48 | |
| NK | 183 | 41 | |
| Pathology risk group | Low | 233 | 52 |
| Intermediate | 113 | 25 | |
| High | 101 | 22 | |
| NK | 3 | 1 | |
| Pathological AJCC stage | I | 223 | 50 |
| II | 74 | 16 | |
| III | 33 | 7 | |
| IV | 118 | 26 | |
| NK | 2 | 0 | |
| Max size primary tumor (cm) | ≤ 1.9 | 219 | 49 |
| 2.0 – 3.9 | 144 | 32 | |
| 4.0 – 5.9 | 39 | 9 | |
| ≥ 6 | 10 | 2 | |
| NK | 38 | 8 | |
| Histology | Mucoepidermoid | 180 | 40 |
| Adenoid cystic | 141 | 31 | |
| PLGA | 54 | 12 | |
| Adenocarcinoma | 28 | 6 | |
| Myoepithelial | 10 | 2 | |
| Acinic Cell | 10 | 2 | |
| Epithelial-myoepithelial | 6 | 1 | |
| Other | 21 | 5 |
(NK Not known)
Four patients (1%) presented with metastatic disease and received local treatment to the primary.
The majority of tumors were less than 2cm in size, 219 (49%). There were 144 (32%) between 2.0–3.9cm, 39 (9%) between 4.0–5.9cm and 10 (2%) at least 6cm. The size was not known in 38 (8%) tumors. For an accurate AJCC stage the pathological T stage and pathological N stage were used. The majority of patients were stage I (223, 50%) or stage II (74, 16%). On margin assessment, 126 (28%) were positive, 103 (23%) were close and 214 (48%) were negative. Seven (2%) margin statuses were not reported.
PNI was present in 167 tumors (37%) and absent in 123 (27%). It was not known in 160 (36%) of tumors. LVI was present in 53 (11.8%) of tumors and absent in 213 (47.2%) of tumors. It was not known in 185 (41%) of the tumors.
Regarding pathologic risk group, there were 233 (52%) patients in the low risk group, 113 (25%) in the intermediate risk group and 101 (22%) high risk group. Grading was not known in 3 (1%) of the tumors, so risk could not be determined.
Overall Survival
The 5-year and 10-year predicted overall survival rates were 86% and 69%, respectively (Figure 2). Factors predictive of failure in overall survival on univariate analysis were age greater or equal to 60 years, male gender, history of tobacco, history of serious comorbidity, PNI, LVI, close/positive margins, histological risk group, postoperative radiation, pathological T and N stage as well as AJCC oncological overall stage (Table 3). Due to the difficulty in margin assessment for these complex three dimension tumors it can be hard to be confident of a wide resection. Therefore, we grouped positive and close margin results together. On univariate analysis of margins, in all outcomes this grouping was supported as positive and close margin groups had similar behaviors.
Figure 2.
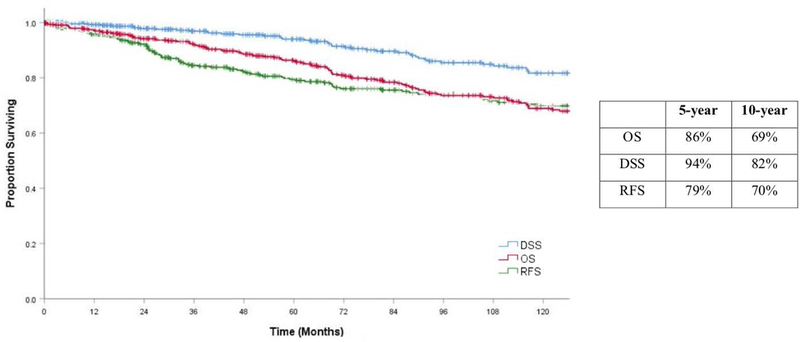
Kaplan Meier estimates of Overall Survival (OS), Recurrence Free Survival (RFS) and Disease Specific Survival (DSS) with 5 and 10 year percentage estimates
Table 3.
Predictors of Overall Survival (OS)
| OS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | |||||
| Covariate | Covariate Grp | Patients | 5y OS (%) | 10y OS (%) | ||||
| Age | < 60 | 252 | 92 | 83 | Ref | < 0.001 | Ref | <0.001 |
| ≥ 60 | 198 | 79 | 52 | 3.75 (2.59 – 5.45) | 3.62 (2.42 – 5.43) | |||
| Gender | Male | 202 | 85 | 62 | Ref | 0.003 | Ref | 0.004 |
| Female | 248 | 88 | 75 | 0.59 (0.42 – 0.84) | 0.58 (0.40 – 0.84) | |||
| Alcohol | No | 161 | 87 | 70 | Ref | 0.794 | ||
| Yes | 272 | 85 | 68 | 1.05 (0.73 – 1.51) | ||||
| Tobacco | No | 214 | 90 | 76 | Ref | 0.027 | ||
| Yes | 222 | 82 | 63 | 1.50 (1.05 – 2.16) | ||||
| Comorbidities | No | 262 | 92 | 77 | Ref | < 0.001 | ||
| Yes | 188 | 79 | 59 | 2.08 (1.48 – 2.94) | ||||
| Site | Oral Cavity | 305 | 86 | 74 | Ref | 0.633 | Ref | 0.063 |
| Oropharynx | 96 | 89 | 59 | 1.25 (0.84 – 1.88) | 0.87 (0.56 – 1.34) | |||
| Sinonasal | 38 | 84 | 57 | 1.19 (0.64 – 2.24) | 0.56 (0.29 – 1.08) | |||
| Larynx/ Trachea | 11 | 100 | 80 | 0.71 (0.18 – 2.89) | 0.20 (0.05 – 0.83) | |||
| PNI | No | 123 | 93 | 79 | Ref | < 0.001 | ||
| Yes | 167 | 80 | 55 | 2.76 (1.55 – 4.89) | ||||
| LVI | No | 214 | 90 | 71 | Ref | 0.001 | ||
| Yes | 53 | 71 | 47 | 2.23 (1.34 – 3.71) | ||||
| Margin | Negative | 214 | 90 | 77 | Ref | 0.015 | Ref | 0.042 |
| Close/Positive | 229 | 83 | 61 | 1.54 (1.09 – 2.19) | 1.56 (1.02 – 2.39) | |||
| Pathology risk group | Low | 233 | 93 | 84 | Ref | < 0.001 | Ref | 0.001 |
| Intermediate | 113 | 90 | 60 | 2.06 (1.31 – 3.22) | 1.33 (0.76 – 2.31) | |||
| High | 101 | 68 | 48 | 3.72 (2.46 – 5.63) | 2.40 (1.51 – 3.82) | |||
| pT stage | T1 | 246 | 92 | 82 | Ref | < 0.001 | ||
| T2 | 94 | 82 | 60 | 2.24 (1.42 – 3.52) | ||||
| T3 | 20 | 95 | 24 | 4.04 (2.09 – 7.82) | ||||
| T4 | 88 | 73 | 53 | 2.73 (1.79 – 4.16) | ||||
| pN stage | N0 | 384 | 90 | 75 | Ref | < 0.001 | ||
| N1 | 24 | 62 | 40 | 2.64 (1.49 – 4.67) | ||||
| N2 | 41 | 67 | 33 | 4.21 (2.62 – 6.78) | ||||
| AJCC stage | Stage I | 223 | 94 | 85 | Ref | < 0.001 | Ref | < 0.001 |
| Stage II | 74 | 87 | 68 | 1.96 (1.11 – 3.44) | 1.60 (0.86 – 2.99) | |||
| Stage III | 33 | 82 | 35 | 4.15 (2.24 – 7.24) | 6.01 (3.08 – 11.74) | |||
| Stage IV | 118 | 73 | 49 | 3.94 (2.61 – 5.97) | 3.86 (2.34 – 6.36) | |||
| Radiation | No | 280 | 87 | 77 | Ref | 0.011 | Ref | 0.068 |
| Yes | 170 | 85 | 56 | 1.56 (1.10 – 2.19) | 0.64 (0.39 – 1.03) | |||
Perineural invasion (PNI), Lymphovascular invasion (LVI), American Joint Cancer Committee (AJCC), Hazard ratio (HR), Reference (Ref)
On multivariable analysis, age 60 or greater was significant and was associated with a 3.62 (95% CI 2.42 – 5.43) increased risk of death, p<0.001. Female gender was associated with a lower risk of death, HR 0.59 (95% CI 0.42 – 0.84, p=0.003). Compared to tumors in the oral cavity site, tumors of the larynx/ trachea sites had a decreased risk of death HR 0.20 (95% CI 0.05 – 0.83). Tumor histology is known to be an important predictor of overall survival and in the adjusted model there was not a significant different in survival between low and intermediate histology groups. The high-risk histology group had a 2.40 (95% CI 1.51 – 3.82, p<0.001) increased risk of death compared to the low risk histology group.
Univariate analysis of margins showed that the positive margin and the close margin groups had similar outcomes compared to the negative margins group. Positive and close margins were therefore combined for analysis. Positive/close margins were associated with an increased risk of death, HR 1.56 (95% CI, 1.02 – 2.39, p=0.042) compared to a negative margin. Overall stage was an important predictor in the multivariable model. AJCC Stage II tumors had a 1.60 (95% CI 0.86 – 2.99) increased risk compared to stage I and stage III and IV had HRs of 6.01 (95% CI 3.08 – 11.74) and 4.73 (95% CI 2.34 – 6.36), respectively compared to stage I. In the multivariable model PORT was associated with reduced risk of death, HR 0.64 (95% CI 0.39 – 1.03), but this did not reach significance (p= 0.068).
Disease Specific Survival
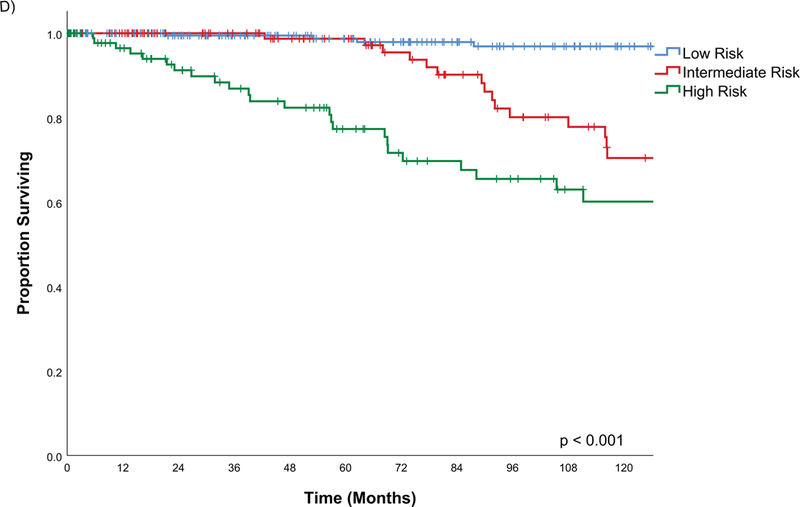
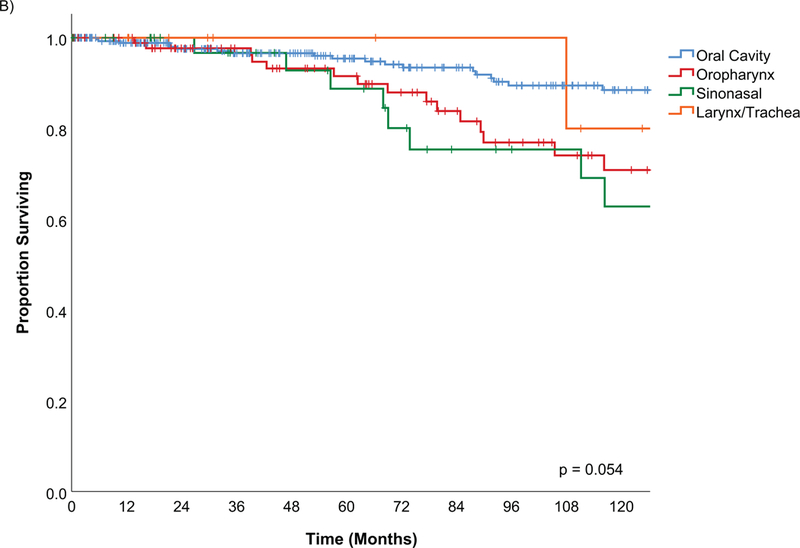
The estimated disease specific survival (DSS) at 5-years and 10-years was 94% and 82% (Figure 2). Factors predictive of DSS on univariate analysis were male gender (Figure 3A), history of tobacco, PNI, LVI, positive margins, histological risk group (Figure 3C), postoperative radiation, pathological T and N stage as well as AJCC oncological overall stage (Figure 3D) (Table 4).
Figure 3A.
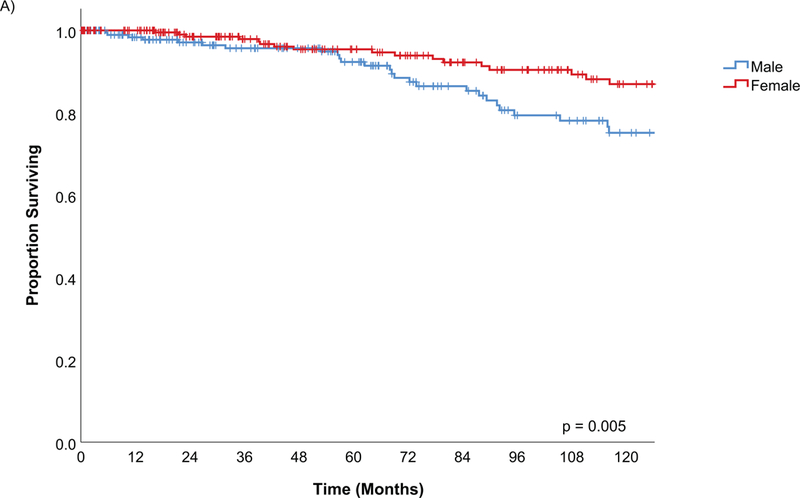
Kaplan Meier estimates of Disease Specific Survival by gender
Figure 3C.
Kaplan Meier estimates of Disease Specific Survival by AJCC Stage
Figure 3D.
Kaplan Meier estimates of Disease Specific Survival by pathological risk group
Table 4.
Predictors of Disease Specific Survival (DSS)
| DSS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Covariate Grp | Patients | 5y DSS (%) | 10y DSS (%) | Unadjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value |
| Age | < 60 | 252 | 94 | 85 | Ref | 0.168 | ||
| ≥ 60 | 198 | 93 | 75 | 1.49 (0.84 – 2.61) | ||||
| Gender | Male | 202 | 92 | 75 | Ref | 0.005 | Ref | 0.021 |
| Female | 248 | 95 | 87 | 0.67 (0.50 – 0.89) | 0.49 (0.26 – 0.90) | |||
| Alcohol | No | 161 | 96 | 86 | Ref | 0.204 | ||
| Yes | 272 | 92 | 79 | 1.49 (0.80 – 2.79) | ||||
| Tobacco | No | 214 | 96 | 87 | Ref | 0.048 | ||
| Yes | 222 | 92 | 77 | 1.82 (1.00 – 3.32) | ||||
| Comorbidities | No | 262 | 95 | 82 | Ref | 0.762 | ||
| Yes | 188 | 92 | 82 | 1.09 (0.61 – 1.95) | ||||
| Site | Oral Cavity | 305 | 95 | 89 | Ref | 0.054 | Ref | 0.336 |
| Oropharynx | 96 | 92 | 71 | 1.91 (1.01 – 3.62) | 1.00 (0.50 – 2.01) | |||
| Sinonasal | 38 | 89 | 63 | 2.52 (1.14 – 5.60) | 0.64 (0.28 – 1.49) | |||
| Larynx/ Trachea | 11 | 100 | 80 | 2.04 (0.48 – 8.62) | 0.31 (0.07 – 1.39) | |||
| PNI | No | 123 | 98 | 88 | Ref | 0.009 | ||
| Yes | 167 | 91 | 71 | 2.90 (1.25 – 6.71) | ||||
| LVI | No | 214 | 98 | 85 | Ref | 0.001 | ||
| Yes | 53 | 79 | 58 | 3.24 (1.60 – 6.53) | ||||
| Margin | Negative | 214 | 96 | 88 | Ref | 0.008 | ||
| Close/Positive | 229 | 92 | 74 | 2.14 (1.20 – 3.83) | ||||
| Pathology risk group | Low | 233 | 99 | 97 | Ref | < 0.001 | Ref | < 0.001 |
| Intermediate | 113 | 99 | 70 | 8.39 (3.15 – 22.35) | 2.76 (0.93 – 8.19) | |||
| High | 101 | 77 | 60 | 14.69 (5.62 – 38.38) | 7.24 (2.57 – 20.44) | |||
| pT Stage | T1 | 246 | 97 | 95 | Ref | < 0.001 | ||
| T2 | 94 | 90 | 73 | 5.30 (2.23 – 12.30) | ||||
| T3 | 20 | 100 | 28 | 15.20 (5.98 – 38.62) | ||||
| T4 | 88 | 89 | 69 | 7.10 (3.18 – 15.81) | ||||
| pN stage | N0 | 384 | 97 | 88 | Ref | < 0.001 | ||
| N1 | 24 | 80 | 48 | 4.35 (1.91 – 9.93) | ||||
| N2 | 41 | 77 | 40 | 8.07 (4.13 – 15.76) | ||||
| AJCC stage | Stage I | 223 | 98 | 98 | Ref | < 0.001 | Ref | < 0.001 |
| Stage II | 74 | 95 | 85 | 7.58 (1.89 – 30.37) | 5.10 (1.20 – 21.74) | |||
| Stage III | 33 | 96 | 40 | 32.49 (9.16 – 115.29) | 31.26 (7.75 – 126.11) | |||
| Stage IV | 118 | 85 | 61 | 26.37 (8.02 – 86.74) | 13.46 (3.58 – 50.69) | |||
| Radiation | No | 280 | 96 | 94 | Ref | < 0.001 | Ref | 0.903 |
| Yes | 170 | 91 | 65 | 5.14 (2.68 – 9.84) | 0.95 (0.44 – 2.07) | |||
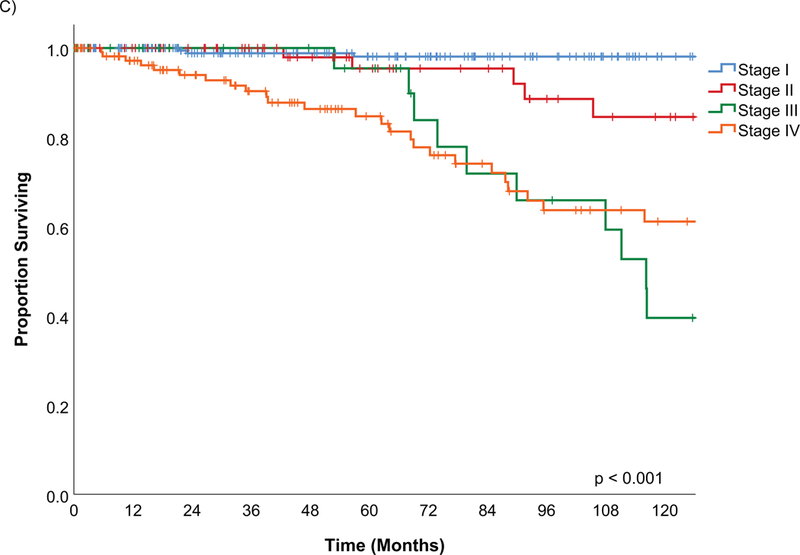
On univariate analysis, tumors of the sinonasal site had a higher risk of disease related death compared to the oral cavity site, HR 2.52 (95% CI 1.14 – 5.60) (Figure 3B). Oropharynx tumors also had a 1.91 (95% CI 1.01 – 3.62) times higher rate of disease-specific death.
Figure 3B.
Kaplan Meier estimates of Disease Specific Survival by tumor site
A multivariable model calculating adjusted hazard ratios using the variables gender, tumor site, pathology risk group, AJCC stage and post-operative radiation showed that gender, high risk pathology group and AJCC stage remained significant predictors of disease related death. Female gender had a lower risk of death in the adjusted model, HR 0.49 (0.26 – 0.90, p=0.021). On multivariable analysis, the tumor sites predictive on univariate analysis were no longer significant. Similarly, post-operative radiation was no longer significant for disease specific survival. The high-risk pathology group had an increased risk of disease related death, HR 7.24 (2.57 – 20.44, p <0.001). All the AJCC stages remained significant on multivariable analysis (Table 5).
Table 5.
Summary of predictive factors for outcomes in minor salivary tumors: results of multivariate analysis (Statistically significant variables highlighted in grey)
| OS | DSS | RFS | LRFS | RRFS | DRFS | ||
|---|---|---|---|---|---|---|---|
| Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | ||
| Covariate | Covariate Grp | ||||||
| Age | < 60 | Ref | |||||
| ≥ 60 | 3.62 (2.42 – 5.43) | ||||||
| Gender | Male | Ref | Ref | ||||
| Female | 0.58 (0.40 – 0.84) | 0.49 (0.26 – 0.90) | |||||
| Alcohol | No | ||||||
| Yes | |||||||
| Tobacco | No | ||||||
| Yes | |||||||
| Comorbidities | No | ||||||
| Yes | |||||||
| Site | Oral Cavity | Ref | Ref | Ref | Ref | Ref | |
| Oropharynx | 0.87 (0.56 – 1.34) | 1.00 (0.50 – 2.01) | 1.59 (0.97 – 2.62) | 1.10 (0.44 – 2.76) | 1.78 (0.68 – 4.66) | ||
| Sinonasal | 0.56 (0.29 – 1.08) | 0.64 (0.28 – 1.49) | 1.01 (0.56 – 1.82) | 1.58 (0.67 – 3.72) | 0.46 (0.06 – 3.71) | ||
| Larynx/ Trachea | 0.20 (0.05 – 0.83) | 0.31 (0.07 – 1.39) | 0.75 (0.29 – 1.93) | 0.38 (0.05 – 3.00) | - | ||
| PNI | No | ||||||
| Yes | |||||||
| LVI | No | ||||||
| Yes | |||||||
| Margin | Negative | Ref | Ref | Ref | |||
| Close/Positive | 1.56 (1.02 – 2.39) | 1.52 (0.91 – 2.52) | 1.72 (0.74 – 3.99) | ||||
| Pathology risk group | Low | Ref | Ref | Ref | Ref | Ref | Ref |
| Intermediate | 1.33 (0.76 – 2.31) | 2.76 (0.93 – 8.19) | 3.31 (1.65 – 6.65) | 1.51 (0.48 – 4.81) | 2.79 (0.72 – 10.81) | 8.18 (2.79 – 23.99) | |
| High | 2.40 (1.51 – 3.82) | 7.24 (2.57 – 20.44) | 4.83 (2.47 – 9.45) | 4.78 (1.71 – 13.36) | 6.87 (2.19 – 21.50) | 8.60 (2.87 – 25.79) | |
| pT stage | T1 | Ref | |||||
| T2 | 4.23 (1.27 – 14.12) | ||||||
| T3 | 16.27 (3.61 – 73.30) | ||||||
| T4 | 9.57 (3.10 – 29.58) | ||||||
| pN stage | N0 | Ref | |||||
| N1 | 1.13 (0.14 – 9.25) | ||||||
| N2 | 3.09 (0.79 – 12.12) | ||||||
| AJCC stage | Stage I | Ref | Ref | Ref | Ref | ||
| Stage II | 1.60 (0.86 – 2.99) | 5.10 (1.20 – 21.74) | 3.21 (1.43 – 7.22) | 3.98 (1.29 – 12.32) | |||
| Stage III | 6.01 (3.08 – 11.74) | 31.26 (7.75 – 126.11) | 7.35 (3.10– 17.41) | 11.05 (3.70 – 32.96) | |||
| Stage IV | 3.86 (2.34 – 6.36) | 13.46 (3.58 – 50.69) | 7.11 (3.51 – 14.41) | 9.28 (3.52 – 24.44) | |||
| Radiation | No | Ref | Ref | Ref | Ref | ||
| Yes | 0.64 (0.39 – 1.03) | 0.95 (0.44 – 2.07) | 0.63 (0.27 – 1.46) | 0.64 (0.20 – 2.05) |
Treatment failure
There were 97 recurrences within the 450 patients with minor salivary tumors (Figure 4). The most common site for recurrence was at a distant site, occurring in 41 patients. 21 patients had a local failure with recurrence at the primary site. There were 12 patients with a regional failure. 13 patients had both a local and distant failure, 3 patients had a local and regional failure and 6 patients had a regional and distant failure. 1 patient had a local, regional and distant failure.
Figure 4.
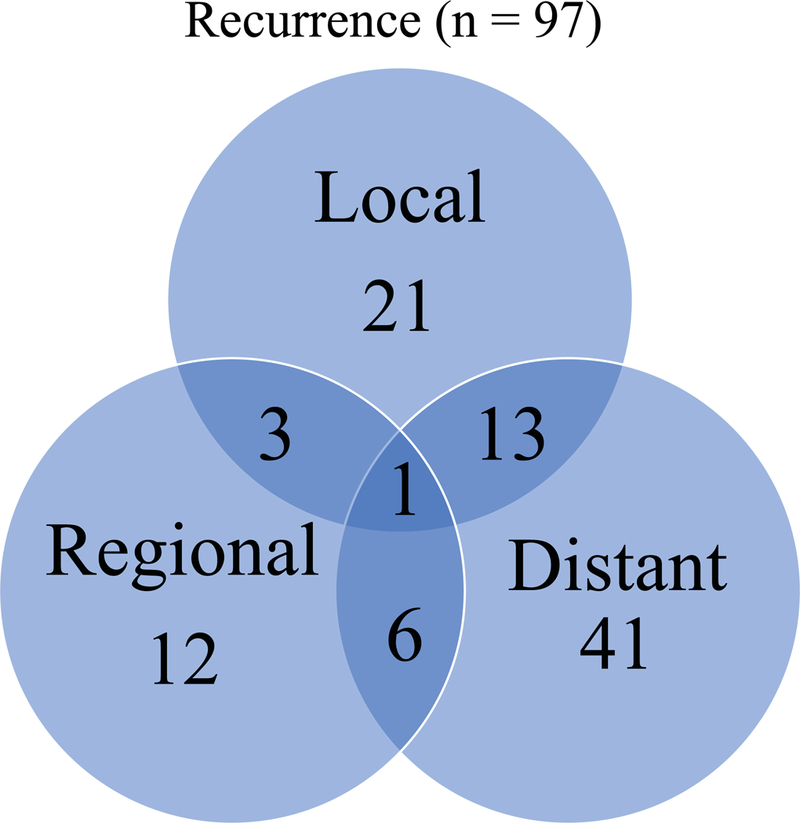
Pattern of recurrence of minor salivary carcinoma
Recurrence free survival
The estimated 5-year and 10-year recurrence free survival (RFS) was 79% and 69% (Figure 2). The factors that were significant on univariate analysis were gender, tumor site, PNI, LVI, margin status, pathology risk group, pT stage, pN stage, AJCC stage and post-operative radiation. Age, alcohol, tobacco and comorbidities were not significant predictors of recurrence (Supplemental 2).
On multivariable analysis, the factors that remained statistically significant predictors of recurrence were the pathological risk group and AJCC stage. The site of tumor and margin status were not significant factors. Compared to the low risk histology group, intermediate risk pathologies had an increased risk of recurrence, HR 3.31 (95% CI 1.65 – 6.65). High risk pathologies were associated with a HR 4.83 (95% CI 2.47 – 9.45) compared to the low risk group. Higher AJCC stage was also associated with increased risk of recurrence. Stage II patients had a 3.21 times increased risk of recurrence compared to stage I (95% CI 1.43– 7.22). Stage III and IV patients had 7.35 (95% CI 3.10 – 17.41) and 7.11 (95% CI 3.51 – 14.41) times increased risk of recurrence compared to stage I, respectively.
Local recurrence free survival
The estimated 5-year and 10-year local recurrence free survivals were 92% and 88%, respectively (Figure 5). The factors on univariate analysis that were significant for local recurrence were tumor site (sinonasal), LVI, margin status, pathology risk group, pT stage, pN stage, AJCC stage and post-operative radiation (Supplemental 3).
Figure 5.
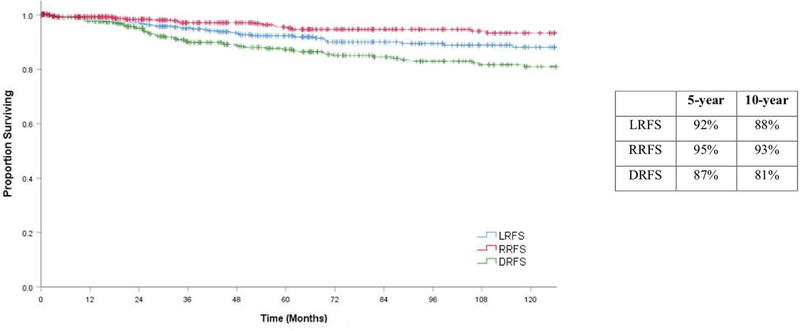
Kaplan Meier estimates of Regional Recurrence Free Survival (RRFS), Local Recurrence Free Survival (LRFS), Distant Recurrence Free Survival (DSS) with 5 and 10 year percentage estimates
In the multivariable model using tumor site, margin status, pathology risk group, pT stage and post-operative radiation the factors that remained significant predictors of local recurrence were the pathology risk group and pT stage. The intermediate pathology risk group had an increased risk of local recurrence compared to the low risk group, HR 1.51 (95% CI 0.48 – 4.81) and the high-risk pathology group had a HR 4.78 (95% CI 1.71 – 13.36). The risk of local recurrence for pT2 tumors was greater than pT1 tumors, HR 4.23 (95% CI 1.27 – 14.12). pT3 and pT4 tumor also had an increased risk of local recurrence in the multivariable model, HR 16.27 (95% CI 3.61 – 73.30) and HR 9.57 (95% CI 3.10 – 29.58.0). Although the sinonasal site had an increased risk of local recurrence on univariate analysis, this was not significant after controlling for the other variables.
Regional recurrence free survival
5-year and 10-year estimated regional recurrence free survival was 95% and 93%, respectively. On univariate analysis, the factors predictive of regional recurrence were the oropharynx subsite, margin status, high risk pathology group, pN stage and AJCC stage (Supplemental 4).
On multivariable analysis using site, pathology risk group, pN stage and post-operative radiation the variables that remained significant predictors of regional failure were the high-risk pathology group and pN2 stage. Although the oropharynx subsite had a higher risk for regional failure compared to oral cavity subsite, this was not significant after controlling for the other variables.
Distant recurrence free survival
At 5 and 10 years, the distant recurrence free survival was 87% and 81%, respectively. Factors on univariate analysis that were associated with distant failure included gender, tumor site, PNI, LVI, margin status, pathology risk groups, pT stage, pN stage, AJCC stage, and post-operative radiation (Supplemental 5).
On a multivariable model, the variables that were predictive of distant failure were pathology risk group and AJCC stage. Tumors of intermediate risk pathologies had a 8.18 times higher risk of distant failure compare to low risk pathologies (95% CI 2.79 – 23.99). High risk pathologies had an 8.60 times increased risk of distant failure (95%CI 2.87 – 25.79) compared to low risk pathologies. The risk of distant recurrence in AJCC stage II patients was 3.98 times greater (95% CI 1.29 – 12.32,) compared to AJCC stage I. AJCC stage III and IV also had a higher risk of distant failure, 11.05 (95% CI 3.70– 32.96) and 9.28 (3.52 – 24.44), respectively.
Of the 61 distant failures, the most common histology type was ACC (69%) (Figure 6). Within the histology types, 30% of ACC patients and 21% of adenocarcinoma patients developed a distant metastasis (Supplemental 1).
Figure 6.
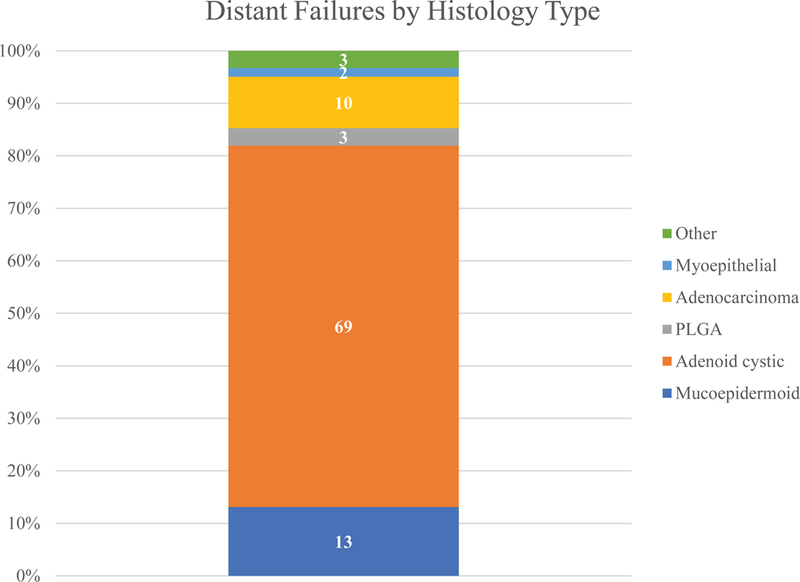
Proportion of distant failure in minor salivary gland carcinoma by histopathology
In the 4 patients with M1 disease included in the study, all had tumors in the oral cavity with ACC histology. Two were still alive at the end of the study with OS of 38 and 30 months, with 1 developing progression with liver metastasis. There were deaths from disease at 64 months and 155 months. Both died of progressive disease, but not local disease.
Discussion
Minor salivary gland carcinoma is a rare and heterogeneous group of tumors. This study presents a large modern experience of 450 patients treated between 1985 and 2015 with primary surgery at MSK. It includes all pathologies and subsites of minor salivary glands in the head and neck.
Clinical series have often shown an increased incidence in females(9), similar to this study with 55% of the cohort being female. However, a more recent SEER report suggested the incidence is more equal between male and females, 49.9% versus 50.1%(12). Typically, the most common ages to present with salivary carcinoma are the 6th and 7th decade (9). The SEER data and this study support this finding for minor salivary carcinomas. However, these tumors also do occur at the extremes of age.
A majority of patients presented with early stage tumors, with 49% cT1 and 89% with a cN0 neck. This has shown an interesting change from an historic series from our institution in which only 27% had early stage tumors(13). Staging information was absent from the SEER report of outcomes(12) but describes 39.8% presenting with localized disease. A number of recent studies have reported on patients following post-operative radiation and had a lower incidence of early stage tumors, 20% had T1 tumors in the Cianchetti study and 23.4% were T1 in a paper by Salgado et al (14, 15). There is a paucity in the description of presenting stage for these tumors including all subsites and pathologies. This series represents surgical treated patients, with only 1% presenting with M1 disease. The SEER data study reported 8.3% distant metastasis rate at presentation in all new diagnoses(12). In this study, the number of T3 tumors was low, 6% were cT3 and 4% were pT3. However, the number of clinical and pathological T4 tumors was 19% and 20% respectively. This may be due to the staging system in which the smaller anatomical areas of the head and neck that minor salivary carcinomas originate from will not allow a tumor over 4cm without it invading a major structure to become upstaged to T4.
The most frequent site for minor salivary carcinoma was the oral cavity, accounting for 68% of the tumors and then the oropharynx representing 21% of the tumors. This has been reported in the SEER data report, with 58.7% in the oral cavity and 21.2% in the pharynx(12). These likely correlates with the greatest density of minor salivary glands.
The most common pathological type in this series was mucoepidermoid carcinoma (40%) followed by adenoid cystic carcinoma (31%). However, this did vary by subsite with mucoepidermoid carcinoma more frequently found in the oral cavity and oropharynx and adenoid cystic carcinoma being the more common histological type in the sinonasal tract and in the larynx and trachea. Other studies reporting individual subsites have shown mucoepidermoid to be more frequent in the oral cavity(16), however adenoid cystic carcinoma has been reported to be the most frequent pathology in other reports (17). This report from a Canadian institution had a high percentage of sinonasal tumors (40.4%) than most of the literature. The mucosa at the anatomical site also appears to have a correlation with the histological type of tumor (Fig 1A). In the squamous cell predominate mucosa of the oral cavity and oropharynx, the proportion of MEC was higher than that of ACC (45% and 40% versus 27% and 29%). In contrast, the proportion of ACC in the respiratory epithelium lined sites of the sinonasal tract and larynx/ trachea group was higher compared to MEC, (MEC 8% and 18% versus ACC 63% and 64%).
The variation in histology and different grading systems for the different pathologies provides difficultly in summarizing the effect of tumor grade on outcome for minor salivary carcinoma. In this series, we grouped pathologies into high, intermediate and low risk groups. 22% of this cohort were regarded as high risk pathologies. Other series assigned a grade to each individual pathology and then reported all the grades individually(17). Therefore, the proportion of patient with high grade pathology varied between 20– 43%.
The 5-year predicted overall survival in this study was 86%. This is similar to previous reports, Spiro et al reported a 5-year survival rate of 75%(13), Vander Poorten et al reported 68%(18) and Loh et al reported 73.8%(17).
The DSS in this study was 94% at 5 years and 82% at 10 years. After 10-years patients continued to die from their cancer. Therefore, there is a long natural history for patients with minor salivary carcinoma. Examining the Kaplan Meir plot of recurrence free survival (Figure 5), distant recurrence occurs more frequently than local and regional recurrences and can occur at any time interval following diagnosis. There were 61 distant recurrences compared to 38 local failures and 22 regional failures. The DSS and RFS curves after 24 months are similar in their shape indicating most of the late recurrence is distant metastasis. Therefore, the continued mortality of this disease in the long term is the effect of distant disease. This has been shown previously in a Netherland registry report that showed distant recurrences occurring late and after 5 years(18). ACC accounted for 69% of all the distant failures. Late failure did occur with other pathologies but this was very uncommon. After 24 months of follow up there were 44 distant recurrences, with ACC accounting for 32 of these. There were 5 late distant metastases from adenocarcinomas, 1 mixed carcinoma, 5 MEC carcinoma and 1 PLGA. This could suggest that long term follow up beyond 5 years is only indicated in patients with ACC.
A detailed analysis of predictive factors for all survival and recurrence outcomes was performed with a multivariable model being possible due to the large cohort and individual patient data being available. There were a number of significant factors predictive of overall survival on both univariate and multivariable analysis.
Age was important for overall survival but had no effect on the other survival or recurrence outcomes in multivariable analysis. This has been seen in other studies(6, 18–20) and age should not impact the optimal treatment plan(2), although it may affect the practical considerations in choosing appropriate therapy. Females had better overall survival and disease specific outcome compared to men on multivariable analysis. This implies that while gender is an important predictor of mortality, there is also an interaction between gender and the disease process.
Disease site has previously been shown to be an important prognostic factor on univariate analysis with sinonasal tumors having a worse outcome(17, 21). However, in our study, when tumor stage and grade are controlled for with multivariable analysis, site was no longer significant(17). In this cohort, patients with oral cavity tumors had a worse overall survival when age, gender, margin, pathology, stage and adjuvant radiation were controlled for. Further multivariable analysis did not show tumor site had a statistically significant effect on disease specific survival or the different recurrence free survivals. Crude survival of larynx and tracheal tumors appears positive and may represent a smaller volume of tumor but there were no statistically different results.
The role of a negative resection margin on outcome has previously been shown to be correlated with better outcomes(16), but local and regional control is usually good in most tumors with the long-term mortality attributed to distant failure(2). In this study, when age, gender, site, pathology risk group, AJCC stage and PORT were controlled for, patients with a positive or close margin had a 56% increased chance of death. However, there was no statistically significant effect on recurrence free survival or local recurrence free survival.
The histology has been reported to an important predictor of outcomes for salivary malignancy(6, 22–25). In this study, pathology risk group was a significant predictor on univariate analysis for all outcomes and for recurrence free survivals. This highlights the importance of good pathological analysis. The pathology of the carcinoma, incorporating histological type and high risk factors, is a major driver of the outcomes of this disease. PNI and LVI were both significant predictors on univariate analysis, however, due to missing information on older cases multivariable analysis was not possible. Often this was incorporated into the overall report of grade and would have overlapping covariates.
The stage of the tumor, including T or N stage or combining these into the AJCC overall stage has been shown to have important prognostic significance in other studies(13, 18). The AJCC stage was a statistically significant predictor for all outcomes and for all recurrence outcomes. Together with pathological grade, this study shows these independent predictors of outcome are the strongest covariates affecting survival and recurrence outcomes.
Radiation is not considered a curative primary treatment for salivary malignancy, but it has been used extensively as an adjuvant treatment. The indications for post-operative radiotherapy (PORT) are usually high grade and high stage salivary gland tumors. Margin status, tumor location, neck disease and the presence of extra nodal extension (ENE) have also been considered indications(15, 26). Local control and survival benefits have been reported with adjuvant radiotherapy in retrospective cohorts(15, 27). In our study, univariate analysis showed patients receiving PORT had worse outcomes but when other variables were controlled for in multivariable analyses PORT was associated with improved OS, DSS, LRFS and RRFS, but was not statistically significant.
This study is not without its limitations. This study used retrospectively collected data and the results should be interpreted with caution when making treatment decisions. Selection bias and treatment bias occurs in non-randomized patient cohorts, and these biases cannot be controlled for in the statistical analysis. Despite this, it is a study with a large number of rare tumors treated at a single institution presented in an inclusive fashion to give a broad experience of the behavior of these tumor types.
In summary, this study addressed minor salivary malignancy with an inclusive method to incorporate all anatomical locations and histological types from a tertiary center with a consistent management philosophy during the period of the study. AJCC stage and pathology risk group were the most predictive variables across all survival and recurrence outcomes. Tumor site, post-operative radiotherapy and margin status were not statistically significant variables after tumor stage and pathology were controlled for in most outcomes, suggesting that the most important predictors of outcome are related to biology of the tumor and stage at diagnosis. This information is useful when considering treatment and adjuvant strategies in this rare group of tumors and can be applied across all the anatomical subsites.
Supplementary Material
Acknowledgments
Support Grant: P30 CA008748
Funding: None
Footnotes
Conflicts of interest: All the authors declare that they have no conflicts of interest.
References
- 1.Albeck H, Nielsen NH, Hansen HE, Bentzen J, Ockelmann HH, Bretlau P, et al. Epidemiology of nasopharyngeal and salivary gland carcinoma in Greenland. Arctic Med Res 1992;51(4):189–95. [PubMed] [Google Scholar]
- 2.Vander Poorten V, Hunt J, Bradley PJ, Haigentz M Jr., Rinaldo A, Mendenhall WM, et al. Recent trends in the management of minor salivary gland carcinoma. Head Neck 2014;36(3):444–55. [DOI] [PubMed] [Google Scholar]
- 3.Bradley PJ, McGurk M. Incidence of salivary gland neoplasms in a defined UK population. Br J Oral Maxillofac Surg 2013;51(5):399–403. [DOI] [PubMed] [Google Scholar]
- 4.Seethala RR, Stenman G. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Salivary Gland. Head Neck Pathol 2017;11(1):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers EN, Ferris RL. Salivary gland disorders Berlin; [London]: Springer; 2007. xv, 517 p. : ill. (chiefly col.); 25 cm. p. [Google Scholar]
- 6.Lopes MA, Santos GC, Kowalski LP. Multivariate survival analysis of 128 cases of oral cavity minor salivary gland carcinomas. Head Neck 1998;20(8):699–706. [DOI] [PubMed] [Google Scholar]
- 7.Ko JJ, Siever JE, Hao D, Simpson R, Lau HY. Adenoid cystic carcinoma of head and neck: clinical predictors of outcome from a Canadian centre. Curr Oncol 2016;23(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours 8th ed: Wiley-Blackwell; 2016. 1 online resource (272 pages). p. [Google Scholar]
- 9.Barnes L, International Academy Of P, University Hospital ZDoP, World Health O. World Health Organization Classification of tumours : pathology and genetics of head and neck tumours Lyon: IARC Press; 2005. 430 p. : ill ; 27 cm. p. [Google Scholar]
- 10.Barnes L, Chiosea SI, Seethala RR. Head and neck pathology New York: Demos Medical Publishing; 2011. xi, 199 p. : col. ill. ; 29 cm. p. [Google Scholar]
- 11.Batsakis JG. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol 1985;94(4 Pt 1):426–7. [PubMed] [Google Scholar]
- 12.Baddour HM Jr., Fedewa SA, Chen AY. Five- and 10-Year Cause-Specific Survival Rates in Carcinoma of the Minor Salivary Gland. JAMA Otolaryngol Head Neck Surg 2016;142(1):67–73. [DOI] [PubMed] [Google Scholar]
- 13.Spiro RH, Thaler HT, Hicks WF, Kher UA, Huvos AH, Strong EW. The importance of clinical staging of minor salivary gland carcinoma. Am J Surg 1991;162(4):330–6. [DOI] [PubMed] [Google Scholar]
- 14.Cianchetti M, Sandow PS, Scarborough LD, Morris CG, Kirwan J, Werning JW, et al. Radiation therapy for minor salivary gland carcinoma. Laryngoscope 2009;119(7):1334–8. [DOI] [PubMed] [Google Scholar]
- 15.Salgado LR, Spratt DE, Riaz N, Romesser PB, Wolden S, Rao S, et al. Radiation therapy in the treatment of minor salivary gland tumors. Am J Clin Oncol 2014;37(5):492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer NG, Kim L, Nixon IJ, Palmer F, Kraus D, Shaha AR, et al. Factors predicting outcome in malignant minor salivary gland tumors of the oropharynx. Arch Otolaryngol Head Neck Surg 2010;136(12):1240–7. [DOI] [PubMed] [Google Scholar]
- 17.Loh KS, Barker E, Bruch G, O’Sullivan B, Brown DH, Goldstein DP, et al. Prognostic factors in malignancy of the minor salivary glands. Head Neck 2009;31(1):58–63. [DOI] [PubMed] [Google Scholar]
- 18.Vander Poorten VL, Balm AJ, Hilgers FJ, Tan IB, Keus RB, Hart AA. Stage as major long term outcome predictor in minor salivary gland carcinoma. Cancer 2000;89(6):1195–204. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JN Jr., Beenken SW, Crowe R, Soong SJ, Peters G, Maddox WA, et al. Prognostic factors in minor salivary gland cancer. Head Neck 1995;17(6):480–6. [DOI] [PubMed] [Google Scholar]
- 20.Kakarala K, Bhattacharyya N. Survival in oral cavity minor salivary gland carcinoma. Otolaryngol Head Neck Surg 2010;143(1):122–6. [DOI] [PubMed] [Google Scholar]
- 21.Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg 1986;8(3):177–84. [DOI] [PubMed] [Google Scholar]
- 22.Auclair PL, Goode RK, Ellis GL. Mucoepidermoid carcinoma of intraoral salivary glands. Evaluation and application of grading criteria in 143 cases. Cancer 1992;69(8):2021–30. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Zhang XR, Liu XK, Liu ZM, Liu WW, Li H, et al. Long-term treatment outcome of minor salivary gland carcinoma of the hard palate. Oral Oncol 2012;48(5):456–62. [DOI] [PubMed] [Google Scholar]
- 24.Spiro RH, Koss LG, Hajdu SI, Strong EW. Tumors of minor salivary origin. A clinicopathologic study of 492 cases. Cancer 1973;31(1):117–29. [DOI] [PubMed] [Google Scholar]
- 25.van der Wal JE, Snow GB, van der Waal I. Histological reclassification of 101 intraoral salivary gland tumours (new WHO classification). J Clin Pathol 1992;45(9):834–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd S, Yu JB, Ross DA, Wilson LD, Decker RH. A prognostic index for predicting lymph node metastasis in minor salivary gland cancer. Int J Radiat Oncol Biol Phys 2010;76(1):169–75. [DOI] [PubMed] [Google Scholar]
- 27.Terhaard CH, Lubsen H, Rasch CR, Levendag PC, Kaanders HH, Tjho-Heslinga RE, et al. The role of radiotherapy in the treatment of malignant salivary gland tumors. Int J Radiat Oncol Biol Phys 2005;61(1):103–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


