Abstract
Background and aims.
Increased uric acid levels correlate with cardiovascular disease and cardiovascular/overall mortality. To identify an uric acid threshold above which cardiovascular mortality rises, we studied the relationship between uric acid concentration and overall/cardiovascular mortality.
Methods and results.
We analyzed data from the InCHIANTI study, a cohort study of Italian community-dwelling people with 9 years of follow-up. We selected a sample of 947 individuals over 64 years of age, free from cardio-cerebrovascular disease and with an available uric acid measurement at baseline. The sample was divided according to plasma uric acid tertiles. The Hazard ratio (HR) for mortality was calculated by multivariate Cox proportional hazard model. Mean age of participants was 75.3±7.3 years; the mean value of uric acid was 5.1±1.4 mg/dl. Over 9-years of follow-up, 342 (36.1%) participants had died, 143 deaths (15.1%) were due to cardiovascular disease. Subjects with higher uric acid concentrations presented a higher cardiovascular mortality [II (4.6–5.5 mg/dl) vs I (1.8–4.5 mg/dl) tertile HR: 1.98, 95%C.I. 1.22–3.23; III (≥5.6 mg/dl) vs I tertile HR: 1.87, 95%C.I. 1.13–3.09]. We found a non-linear association between uric acid concentrations and cardiovascular mortality with the lowest mortality for values of about 4.1 mg/dl and a significant risk increment for values above 4.3 mg/dl.
Conclusion.
In community-dwelling older individuals free from cardio-cerebrovascular events, the lowest 9-year cardiovascular mortality was observed for uric acid values far below current target values. If confirmed, these data might represent the background for investigating the efficacy of uric acid levels reduction in similar populations.
Keywords: uric acid, mortality, cardiovascular mortality, elderly, community-dwelling, InChianti study
INTRODUCTION
Uric acid (UA) is an end-product of purine metabolism. Normal UA blood concentration is commonly reported as 3.5–7 mg/dl (0.5–1 mg/dl less in women), but its normal range varies sensibly in agreement with different population-based observations [1]. It is worthy of note that UA normal range has been established only on an observational basis, without considering accurately its prognostic value. The worldwide increase of mean plasma UA levels and gout prevalence observed in the last decades [2] seems to be related to Western diet (high consumption of animal products, alcohol and fructose intake) [3], and increasing medicalization (drugs impairing UA clearance).
Epidemiological data have shown a consistent association between UA and vascular alterations (hypertension, coronary heart disease - CHD, congestive heart failure, and stroke) [4–7]. UA induced endothelial dysfunction results in impaired nitric oxide production and boosted incretion of vasoconstrictors [8] giving (at least partial) explanation for the associations observed between UA, hypertension, and cardiovascular diseases (CVD). Preclinical studies found that inflammation, a well-known effect of urate crystals, seems to occur even in presence of soluble UA [9–11], and might play a role in promoting atherogenesis and thrombosis [12,13]. However, although several mechanisms related to high UA levels might contribute to vascular damage [1,8], it is still debated whether UA might be just a marker of raised cardiovascular risk, without a direct cause-effect relationship on CVD. Also the role of UA in redox reaction in vivo is still not completely understood; in fact UA represents the main plasma antioxidant, but in certain conditions it can become a potent pro-oxidant [14].
A robust evidence supports the hypothesis that UA is associated with both CVD incidence and mortality [15,16]. The concept that UA plasma concentration could be related with CVD, and that detrimental effects might occur even under the classical precipitation threshold of about 7 mg/dl, seems to be enough to question the actual laboratory ranges of UA normality. Indeed, some authors already tried to infer which concentration of UA could be clinically relevant. For example, Zhang et al. tried to find the optimal UA cut-off able to predict the development of metabolic syndrome and identified this value in 6.3 mg/dl in men and 4.9 mg/dl in women [17].
The aim of this study was to investigate the relationship between UA concentration and CVD mortality in a sample of elderly individuals free from CVD, with the aim of identifying a possible threshold that might represent an UA cut-off useful for future clinical studies.
METHODS
Study cohort
Data are from subjects enrolled in the InCHIANTI (Invecchiare in Chianti; Aging in the Chianti area) Study, a prospective population-based epidemiological study with a 9-year follow-up conducted on a representative sample of the population living in two towns of the Chianti area (Greve in Chianti and Bagno a Ripoli, Tuscany, Italy). The study was developed to investigate factors affecting mobility in late life. Methodology of the InCHIANTI study has been described in detail elsewhere [18].
Briefly, in August 1998, 1270 persons ≥65 years and 30 men and women in each decade of age between 20 and 60 years and in the age group 61–64 were randomly selected from the population registry. Of the 1530 persons originally sampled, 1453 (94%) agreed to participate in the study. Of these, 1343 accepted to donate a blood sample and 1325 (86% of those originally sampled eligible) completed the baseline data collection which started in September 1998 and ended in March 2000. The Italian National Research Council on Aging (INRCA) Ethical Committee ratified the study protocol and participants provided written consent to participate. The study cohort used for the analyses presented here consisted of 947 persons of at least 65 years of age in which UA determination was not missing and who did not have a previous cardio-cerebrovascular disease (defined as the presence of previous myocardial infarction and/or angina and/or stroke).
Biochemical Measures
At baseline, blood samples were obtained from participants after a 12-h fast. Aliquots of serum and plasma were stored at −80°C and were not thawed until analyzed. Plasma UA (mg/dl) was measured using an enzymatic-colorimetric method (Roche Diagnostics, GmbH, Germany). The lower limits of detection were 0.2 mg/dl, range 0.2–25.0 mg/dl, intra-assay and inter-assay coefficients of variation (CV) were 0.5 and 1.7%, respectively.
Hemoglobin levels were analyzed using the hematology automated autoanalyzer DASIT SE 9000 (Sysmex Corporation, Kobe, Japan) and white blood cells using the automatic analyzer Coulter LH 750 (BECKMAN Coulter, Instrumentation Laboratory, Milano). Creatinine levels were determined by commercial assays (Roche Diagnostics, Mannheim, Germany) and creatinine clearance was calculated according to Cockcroft-Gault equation [19]. Commercial enzymatic tests (Roche Diagnostics) were used for determining serum total cholesterol, high-density lipoprotein cholesterol (HDL-C) and triglycerides. Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald’s formula. High sensitivity C-reactive protein (hs-CRP) levels were measured by ELISA using purified protein and polyclonal anti-CRP antibodies (Roche Diagnostics, GmbH, Mannheim, Germany), sensitivity was 0.03 mg/l and inter-assay CV was 5%.
Other Measures
Socio-demographic characteristics included age and gender. History of smoking, disability in basic activities of daily living (BADL, defined as a lack of autonomy in at least two of these activities) [20], diuretics use (in particular thiazide and loop diuretics) and antigout agents assumption were ascertained from baseline interview. Daily alcohol intake was estimated by the European Prospective Investigation into Cancer and Nutrition Food Frequency Questionnaire [21]. The prevalence of specific medical conditions considered in the present study (hypertension and type 2 diabetes) was established using standardized criteria that combined information from self-reported history, medical records and a clinical medical examination.
Weight and height were measured using standard techniques. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m). Waist circumference was measured to the nearest 0.5 cm using a non-elastic plastic tape, at the level of the smallest area of the waist between the lower rib margin and the iliac crest.
Mortality follow-up
Participants were evaluated for the 3-year (2001–2003), 6-year (2004–2006) and 9-year (2007–2009) follow-up visits. Mortality data of the original InCHIANTI cohort were collected using data from the Mortality General Registry maintained by the Tuscany Region, and the death certificates that are deposited immediately after death at the Registry office of the municipality of residence. Cardiovascular mortality, based on underlying cause of death, was defined as any cardiovascular mortality and coded using the International Classification of Diseases, 9th Revision (ICD-9 codes: 390–459). Subjects lost at follow-up were 102 (10.8%) of whom 79 during the last year.
Statistical analysis
The study cohort was divided according to tertiles of UA levels (I. 1.8–4.5 mg/dl, n=328; II. 4.6–5.5 mg/dl, n=315; III. ≥5.6 mg/dl, n=304). Based on this classification, all subjects with elevated UA concentrations, by the current definition of hyperuricemia, were included in the III tertile. Clinical, metabolic, and biochemical characteristics of the study cohort were compared according to tertiles of UA levels, using a χ2 test and ANOVA model for categorical and continuous variables, respectively. Continuous variables with skewed distribution were log-transformed in order to approximate a normal distribution.
Death rates (for all-cause and for cardiovascular mortality) per 1000-person years were calculated and Hazard ratios (HR) for each tertile of UA were estimated by Cox proportional hazard regression adjusting for potential confounders, with the I tertile as the reference category. We adjusted the results for variables found to be associated with UA in univariate analysis, as well as other known risk factors for total and CVD mortality in older persons. First (Model 1), we estimated HR and 95% confidence interval (95%CI) adjusting for age and gender. Then (Model 2), we adjusted for sex, age, smoke, alcohol, waist circumference, hypertension, type 2 diabetes, use of diuretics, BADL disability, creatinine clearance, LDL-cholesterol, and hs-CRP. Proportional hazard (PH) assumption of Cox model was assessed calculating Schoenfeld residuals and test; an interaction term with time was added to the model in case of variables that do not respect the PH assumption. Subsequently, to better understand the behavior of CVD mortality within the range of UA I tertile values, we re-calculated the HR by using only two categories (i.e. greater and lower the specific cut-off). Finally, a Restricted Cubic Spline (RCS) regression model (i.e. a continuous function consisting of k cubic polynomial segments, where k in this case was set = 5) has been used in Cox regression to examine the non-linear relationship between UA and the logarithm of HR, using UA levels as continuous data (taking UA as a categorical variable, it is unlikely that the HR within each category was constant). In order to exclude the outliers (extremely low and high values of UA), we estimated the RCS Cox regression model on a subset composed of the central 95% of all values, that is taking into account those observations with UA concentration between 3 and 8 mg/dl. All analyses were performed using Stata 11.0 for Windows (College Station, TX: Stata Corporation).
RESULTS
Mean age of study participants was 75.3±7.3 years and 549 (58.0%) were female. The mean value of UA was 5.1±1.4 mg/dl. In Table 1 are presented the socio-demographic, anthropometric, clinical, and biochemical characteristics of the study cohort according to UA tertiles. Subjects with higher UA levels were older, more often males and smokers, and had a greater alcohol intake. As expected, they had greater BMI and waist circumference, and higher prevalence of hypertension and use of diuretics. UA levels were positively associated with hemoglobin levels and with triglycerides, and negatively with HDL-C. No significant association between UA and total cholesterol or LDL-C was found. As regards inflammatory markers, subjects with higher UA concentrations had significantly higher white blood cells and hs-CRP. Subjects belonging to the I and III UA tertiles both displayed higher BADL disability and lower creatinine clearance compared with those belonging to II tertile.
Table 1.
Socio-demographic, anthropometric, clinical, and biochemical characteristics of study participants according to uric acid tertiles.
| Characteristics | Entire Sample (n=947) |
Uric Acid Tertiles (mg/dl) | p-value | ||
|---|---|---|---|---|---|
| UA 1 (n=328) |
UA 2 (n=315) |
UA 3 (n=304) |
|||
| Female, N (%) | 549 (58.0) | 249 (75.9) | 169 (53.7) | 131 (43.1) | <0.001 |
| Age, mean ± SD | 75.3 ± 7.3 | 74.7 ± 7.2 | 75.0 ± 7.0 | 76.1 ± 7.7 | 0.049 |
| Smoking, N (%) | |||||
| Former | 274 (28.9) | 68 (20.7) | 101 (32.1) | 105 (34.5) | |
| Current | 106 (11.2) | 34 (10.4) | 38 (12.1) | 34 (11.2) | 0.001 |
| Alcohol >1 drink per day, N (%) | 294 (31.1) | 76 (23.2) | 100 (31.8) | 118 (38.8) | <0.001 |
| Anthropometric indices, mean ± SD | |||||
| -Waist circumference, cm | 92.5 ± 10.3 | 88.6 ± 10.3 | 93.2 ± 9.7 | 96.1 ± 9.4 | <0.001 |
| -Body mass index, Kg/m2 | 27.4 ± 4.1 | 26.0 ± 3.6 | 27.9 ± 4.1 | 28.5 ± 4.2 | <0.001 |
| Medical conditions, N (%) | |||||
| -Hypertension | 588 (62.1) | 183 (55.8) | 191 (60.6) | 214 (70.4) | 0.001 |
| -Type 2 diabetes | 49 (5.2) | 22 (6.7) | 12 (3.8) | 15 (4.9) | 0.246 |
| -Use of diuretics | 100 (10.6) | 17 (5.2) | 24 (7.6) | 59 (19.4) | <0.001 |
| BADL disability, N (%) | 77 (8.2) | 26 (7.9) | 15 (4.8) | 36 (11.8) | 0.005 |
| Biochemical parameters: | |||||
| -Hemoglobin, g/dl, mean ± SD | 13.7 ± 1.4 | 13.4 ± 1.2 | 13.9 ± 1.3 | 13.9 ± 1.6 | <0.001 |
| -White blood cells, Kμl, mean ± SD | 6.1 ± 1.5 | 5.9 ± 1.6 | 6.0 ± 1.5 | 6.4 ± 1.5 | <0.001 |
| -Creatinine clear, ml/min, mean ± SD | 63.3 ± 19.3 | 61.8 ± 17.5 | 66.2 ± 19.0 | 61.8 ± 21.1 | 0.005 |
| -Total Cholesterol, mg/dl, mean ± SD | 217.6 ± 39.9 | 219.8 ± 38.4 | 216.4 ± 38.1 | 216.4 ± 43.1 | 0.453 |
| -HDL-Cholesterol, mg/dl, mean ± SD | 56.2 ± 15.1 | 60.6 ± 14.5 | 56.2 ± 14.5 | 51.4 ± 14.9 | <0.001 |
| -LDL-Cholesterol, mg/dl, mean ± SD | 136.1 ± 34.5 | 137.2 ± 33.0 | 136.2 ± 32.5 | 134.7 ± 38.1 | 0.665 |
| -Triglycerides, median [IQR] | 110 [83; 147] | 100 [76; 130] | 105 [78; 141] | 132 [98; 182] | <0.001 |
| -HS-CRP (ln), μg/ml, median [IQR] | 1.00 [0.26;1.73] | 0.79 [0.09;1.69] | 0.92 [0.23;1.63] | 1.27 [0.55; 1.83] | <0.001 |
Over 9 years of follow-up, 342 (36.1%) participants had died with the following division for tertiles: I. 100 events (37.9 events/1000 person-year), II. 111 events (44.3 events/1000 person-year), III. 131 events (58.6 events/1000 person-year). A positive trend between UA concentrations and mortality was found, but it was not statistically significant (Table 2).
Table 2.
Event rates for all-cause mortality according to uric acid tertiles, adjusted for potential confounders in 947 older subjects without cardio-cerebrovascular disease.
| Uric Acid (mg/dl) | No. of events | Events for 1000 person-years | Model 1 - HR (CI) | Model 2 - HR (CI) |
|---|---|---|---|---|
| I. 1.8–4.5 | 100 | 37.9 | - | - |
| II. 4.6–5.5 | 111 | 44.3 | 1.00 (0.76–1.33) | 1.05 (0.79–1.40) |
| III. ≥ 5.6 | 131 | 58.6 | 1.18 (0.90–1.54) | 1.16 (0.87–1.55) |
Model 1 adjusted for sex and age. Model 2 adjusted for sex, age, smoke, alcohol, hypertension, type 2 diabetes, use of diuretics, BADL disability, creatinine clearance, LDL-cholesterol and C-reactive protein.
Of the total number of deaths, 143 (15.1% of the study cohort, 41.8% of the deaths for all causes) were due to CVD with the following rates: I. 26 events (9.9 events/1000 person-year), II. 53 events (21.1 events/1000 person-year), III. 64 events (28.6 events/1000 person-year). As shown in Table 3, participants in the II and III tertiles had a greater CVD mortality. In the final model, adjusted for age, gender, smoking, alcohol, waist circumference, hypertension, type 2 diabetes, use of diuretics, BADL disability, creatinine clearance, LDL-cholesterol, and hs-CRP, subjects with higher UA concentrations still presented a higher mortality (II vs I tertile HR: 1.98, 95%C.I. 1.22–3.23; III vs I tertile HR: 1.87, 95%C.I. 1.13–3.09). These results were unchanged after exclusion of few subjects taking antigout therapy.
Table 3.
Event rates for cardiovascular mortality according to uric acid tertiles, adjusted for potential confounders in 947 older subjects without cardio-cerebrovascular disease.
| Uric Acid (mg/dl) | No. of events | Events for 1000 person-years | Model 1 - HR (CI) | Model 2 - HR (CI) |
|---|---|---|---|---|
| I. 1.8–4.5 | 26 | 9.9 | - | - |
| II. 4.6–5.5 | 53 | 21.1 | 1.99 (1.24–3.20) | 1.98 (1.22–3.23) |
| III. ≥ 5.6 | 64 | 28.6 | 2.27 (1.42–3.61) | 1.87 (1.13–3.09) |
Model 1 adjusted for sex and age. Model 2 adjusted for sex, age, smoke, alcohol, hypertension, type 2 diabetes, use of diuretics, BADL disability, creatinine clearance, LDL-cholesterol and C-reactive protein.
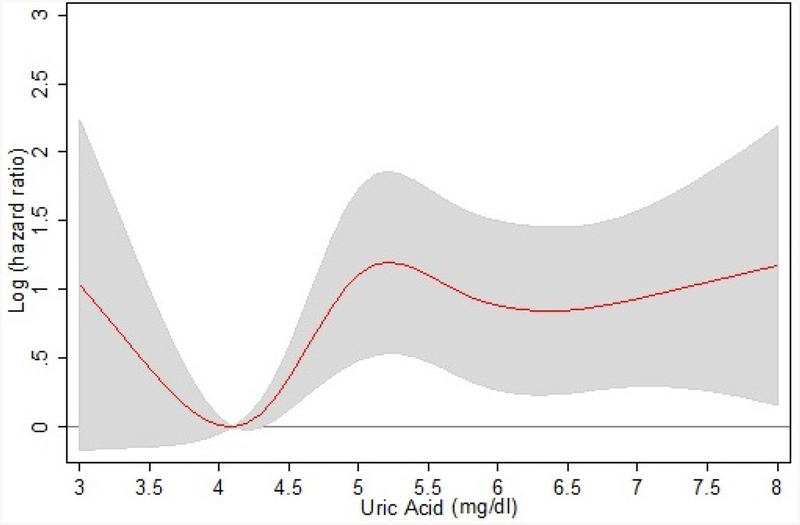
Successively, we analyzed the relationship between UA levels and 9-year CVD mortality by a RCS regression model (Figure 1); the risk at UA value of 4.1 mg/dl (lowest risk) was taken as reference for the HR. A non-linear association between UA concentrations and CVD mortality risk was found. CVD mortality was high for lower UA values but progressively decreased with UA rising, with the lowest mortality for UA values of about 4.1 mg/dl; then, CVD mortality gradually and proportionally increased with UA concentrations, becoming stable for values around 5 mg/dl and over. The boundaries of the 95% CI indicated that the decrease in log(HR) was not statistically significant. A significantly higher than 0 log(HR) was reported for UA values greater than 4.3 mg/dL. Data about all-cause and cardiovascular mortality are also shown in Supplementary Figure S1 as Kaplan-Meier survival analysis and event rates after 9 years of follow-up. Finally, in order to identify the true critical threshold for CVD mortality within the range of UA values belonging to the I tertile, we considered different cut-off points and calculated the HR of two UA categories (greater and lower the fixed cut-off values). Table 4 shows, for each cut-off of UA value, the HR for the higher UA class compared to the lower one (reference); the last cut-off point after which the significance disappears was 4.3 mg/dl, thus confirming this value as the cut-off point beyond which the risk of CVD mortality became significant.
Figure 1.
Relationship between serum uric acid levels and 9-year cardiovascular mortality (data shown are adjusted for sex, age, smoke, alcohol, hypertension, type 2 diabetes, use of diuretics, BADL disability, creatinine clearance, LDL-cholesterol and C-reactive protein). Supplementary Figure S1.Kaplan-Meier survival analysis and event rates after 9 years of follow-up for all-cause and cardiovascular mortality.
Table 4.
Hazard ratios estimated by Cox regression model by using two categories of uric acid levels based on different cut-off values, all belonging to the I tertile. All reported HR refers to values greater than the indicated cut-off.
| Uric Acid cut-off (mg/dl) | HR | 95% CI | p-value |
|---|---|---|---|
| 4.5 | 1.93 | 1.23 – 3.04 | 0.004 |
| 4.4 | 2.11 | 1.31 – 3.39 | 0.002 |
| 4.3 | 1.82 | 1.13 – 2.93 | 0.013 |
| 4.2 | 1.61 | 0.99 – 2.61 | 0.054 |
| 4.1 | 1.57 | 0.96 – 2.57 | 0.075 |
Models are adjusted for age, gender, smoke, alcohol, hypertension, type 2 diabetes, use of diuretics, BADL disability, creatinine clearance, LDL-cholesterol, and C-reactive protein.
DISCUSSION
We investigated the relationship between UA levels and 9-year mortality in a large sample of community dwelling elderly individuals free from previous cardio-cerebrovascular events.
The first very interesting finding of the study was that subjects with UA greater than 4.3 mg/dl, a UA value currently considered as absolutely “normal”, displayed a significant increase in 9-year CVD mortality risk; the relationship was strong (about twofold HR) and independent of important confounders. Several studies have previously found an association between hyperuricemia and CVD mortality. Indeed, Kim et al demonstrated an increased CHD mortality in hyperuricemic patients [15] and Zhao et al found an association between CVD mortality and UA levels, while a significant correlation with all-cause mortality emerged only in men [16]. The two major limitations of the studies analyzed in these meta-analyses are the broad variability in hyperuricemia definition, and the lack of adequate correction for strong confounders (e.g. medications and previous CVD events). Another study conducted on a very large number of subjects enrolled in the third NHANES found, besides the association between gout and CVD mortality, that the adjusted HR per 1 mg/dl increase in UA was 1.16 (95% CI 1.10–1.22) for total and CVD mortality [22]. A recent meta-analysis demonstrated a 15% increase in CVD mortality for an increase of 1 mg/dl in UA levels; the definition of hyperuricemia ranged from 5.6 to 7.7 mg/dl in males, and from 4.7 to 7.0 mg/dl in females [23]. Wu et al. demonstrated that high UA levels were associated with greater risk of all cause and CVD mortality in older adults; in this case the cut-off points were set a priori at 7.0 mg/dl for men and 6.0 mg/dl for women [24]. However, as recently underlined by Borghi et al., although many studies have been published on this topic (all unanimous in demonstrating the association between hyperuricemia and CVD) they are really heterogeneous as regards study population, time of follow-up, and UA cut-off points (i.e. tertiles, quartiles, predefined threshold of 7 mg/dl) [25]. Based on the available data, these authors identified in 6 mg/dl a possible clinically meaningful UA cut-off, above which CVD risk would significantly increase.
A second finding of our study was the association between lower UA levels and increased CVD mortality. A “J-shaped” relationship between all-cause mortality and serum UA concentrations had already been observed in hemodialysis patients; both patients in the highest UA quintile (≥9 mg/dl) and in the lowest one (≤6.5 mg/dl) had higher risk of mortality compared with the middle three quintiles [26]. Another study, performed on patients with high CVD risk, demonstrated an increased overall/CVD mortality risk in patients in the I (<4.5 mg/dl) and III (>8.2 mg/dl) UA tertile compared with the second one [27]. Interestingly, we found that BADL disability and creatinine clearance worsened in subjects with low UA concentrations; similarly, previous studies pointed out a non-linear association between UA values and functional status. Huang et al., by analyzing the relationship between UA levels and muscle strength, found a J-shaped relation, with the worse performance in subjects belonging to the I and IV UA quartiles compared to the central ones [28]. We do not have a convincing explanation for this kind of association. Since it is known that UA has also antioxidant properties, it is possible that very low UA levels might be associated with a lower ability to counteract oxidative stress and damage.
On the whole, our study expands previous literature on the relationship between UA and CVD mortality for two main reasons. First, individuals with previous cardio/cerebrovascular events were excluded in order to reduce the possible confounding effects of CVD and to understand the possible interest of UA level modification in primary CVD prevention. Another study, similarly performed in older subjects free from CVD, renal dysfunction or diuretic use, found UA concentrations greater than 7.0 mg/dl to be associated with higher CVD mortality; but once again, this cut-off was chosen just because it is commonly used to define hyperuricemia [29]. Second, unlike previous studies, we specifically searched for a possible UA cut-off value, and found that CVD mortality risk significantly increased for values of UA over 4.3 mg/dl, a threshold that is much lower than previously reported.
Based on our results, we could hypothesize a new and much more clinically relevant definition of “hyperuricemia”, based not on the risk of developing gout or on population distribution, but on the evidence that CVD mortality significantly increases over a specific cut-off. If confirmed by further observations, our results might support the possibility of a future change from “normal” UA levels (actual definition) to “desirable” UA levels; indeed, UA concentrations previously regarded as normal might be considered as a CVD risk factor with possible therapeutic implications.
With regards to this crucial point, there are no studies showing the efficacy of UA lowering on reducing mortality. For both allopurinol and febuxostat, instead, some studies demonstrated the efficacy on some cardiovascular endpoints [30]. In asymptomatic hyperuricemic patients, allopurinol improved systolic blood pressure and endothelial dysfunction, two important CVD risk factors [31]. Among the beneficial effects of allopurinol, it is noteworthy the increase of myocardial efficiency in patients with dilated cardiomyopathy [32], the improvement of endothelium-dependent vasodilation, and the reduction in vascular oxidative stress in patients with stable coronary artery disease [33]. Febuxostat has shown positive cardiovascular effects too [34]; in particular, in patients with severe tophaceous gout, febuxostat, but not allopurinol, prevented the increase of carotidfemoral pulse wave velocity [35]. Moreover, Sezai at al. found that febuxostat had reno-protective effect, inhibited oxidative stress, showed anti-atherogenic activity, had anti-hypertensive effects, preventing vascular endothelial damage in high-risk cardiac surgery patients with hyperuricemia [36].
Finally, some limitations of our study should be acknowledged. First, although our data were longitudinal (9 years follow-up) and adjusted for several important confounders, we cannot rule out the possibility that our results were influenced by factors not considered in our analysis. As a consequence, we cannot infer for certain a causal relation between high UA levels and increased CVD mortality. Second, the total number of CVD deaths was relatively limited, thus precluding the possibility of further stratifying the sample, e.g. by gender.
In conclusion, we found that in community dwelling older individuals free from previous cardiocerebrovascular events, the lowest 9-year CVD mortality was observed for UA values of about 4.1 md/dl. Moreover, UA concentrations >4.3 mg/dl, a value currently considered “normal”, were associated with a significant and independent increase in the risk of CVD death. These findings, if confirmed, might represent the background for investigating the efficacy of UA levels reduction in CVD primary prevention in high risk individuals.
Supplementary Material
Subjects with higher uric acid levels present a higher cardiovascular mortality
The association between uric acid levels and cardiovascular mortality is non-linear
The lowest mortality is seen for uric acid values of about 4.1 mg/dl
There is a significant increase in mortality risk for uric acid values >4.3 mg/dl
The lowest cardiovascular mortality is for uric acid values far below current target
ACKNOLEDGEMENTS
We would like to thank Dr. Elisa Maietti for her contributions for advanced statistical analysis.
FUNDING
The InCHIANTI Study (Invecchiare in Chianti, aging in the Chianti area) is currently supported by a grant from the National Institute on Aging (NIH, NIA, Bethesda, USA) and is coordinated by the Tuscany Regional Health Agency in a partnership with the Florence Health Care Agency, the local Administrators and the primary care physicians of Greve in Chianti and Bagno a Ripoli, the two small towns in the countryside of the Tuscany were the study is conducted. The Study was initially managed by the National Institute on Research and Care of the Elderly (INRCA, Ancona, Italy) and it was funded by Italian Health Ministry and by a NIH contract. In particular, the InCHIANTI study baseline (1998–2000) was supported as a “targeted project” (ICS110.1/RF97.71) by the Italian Ministry of Health, and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336). The Follow-up 1 (2001–2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1–1 and N.1-AG-1–2111); the Follow-up 2 and 3 studies (2004–2010) were financed by the U.S. National Institute on Aging (Contract: N01-AG-5–0002). Supported in part by the Intramural research program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland.
ABBREVIATIONS:
- UA
uric acid
- CHD
coronary heart disease
- CVD
cardiovascular diseases
- CV
coefficients of variation
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- hs-CRP
high sensitivity C-reactive protein
- BADL
basic activities of daily living
- BMI
body mass index
- HR
Hazard ratio
- 95%CI
95% confidence interval
- RCS
Restricted Cubic Spline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS
The Authors declare no conflict of interest
REFERENCES
- 1-.Gustafsson D, Unwin R. The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC Nephrol 2013;14:164 10.1186/1471-2369-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2-.Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther 2010;12(6):223 10.1186/ar3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3-.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ 2008;336:309–12. 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4-.Brand FN, McGee DL, Kannel WB, Stokes J 3rd, Castelli WP. Hyperuricemia as a risk factor of coronary heart disease: The Framingham Study. Am J Epidemiol 1985;121:11–8. 10.1093/oxfordjournals.aje.a113972. [DOI] [PubMed] [Google Scholar]
- 5-.Høieggen A, Alderman MH, Kjeldsen SE, Julius S, Devereux RB, De Faire U, et al. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int 2004;65(3):1041–9. 10.1111/j.1523-1755.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 6-.Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: The Rotterdam Study. Stroke 2006;37(6):1503–7. 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 7-.Baker J, Schumacher H, Krishnan E. Serum uric acid level and risk for peripheral arterial disease: analysis of data from the multiple risk factor intervention trial. Angiology 2007;58:450–7. 10.1177/0003319707303444. [DOI] [PubMed] [Google Scholar]
- 8-.Kanbay M, Segal M, Afsar B, Kang DH, Rodriguez-Iturbe B, Johnson RJ. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart 2013;99:759–66. 10.1136/heartjnl-2012-302535. [DOI] [PubMed] [Google Scholar]
- 9-.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol 2005;16:3553–62. 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 10-.Crisan TO, Cleophas MC, Oosting M, Lemmers H, Toenhake-Dijkstra H, Netea MG, et al. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann Rheum Dis 2016;75(4):755–62. 10.1136/annrheumdis-2014-206564. [DOI] [PubMed] [Google Scholar]
- 11-.Xiao J, Zhang XL, Fu C, Han R, Chen W, Lu Y, et al. Soluble uric acid increases NALP3 inflammasome and interleukin-1β expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. Int J Mol Med 2015;35(5):1347–54. 10.3892/ijmm.2015.2148. [DOI] [PubMed] [Google Scholar]
- 12-.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J 2010;74(2):213–20. 10.1253/circj.CJ-09-0706. [DOI] [PubMed] [Google Scholar]
- 13-.Marini MG, Sonnino C, Previtero M, Biasucci LM. Targeting inflammation: impact on atherothrombosis. J Cardiovasc Transl Res 2014;7(1):9–18. 10.1007/s12265-013-9523-7. [DOI] [PubMed] [Google Scholar]
- 14-.Kang DH, Ha SK. Uric Acid Puzzle: Dual Role as Anti-oxidant and Pro-oxidant. Electrolyte Blood Press 2014;12(1):1–6. 10.5049/EBP.2014.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15-.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2010;62(2):170–80. 10.1002/acr.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16-.Zhao G, Huang L, Song M, Song Y. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: a meta-analysis of prospective studies. Atherosclerosis 2013;231(1):61–8. 10.1016/j.atherosclerosis.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 17-.Zhang ML, Gao YX, Wang X, Chang H, Huang GW. Serum uric acid and appropriate cutoff for prediction of metabolic syndrome among Chinese adults. J Clin Biochem Nutr 2013;52(1):38–42. 10.3164/jcbn.12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18-.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 2000;48:1618–25. 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 19-.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16(1):31–41. 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 20-.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963;185:914–9. 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 21-.Kaaks R, Riboli E. Validation and calibration of dietary intake measurements in the EPIC project: methodological considerations. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol 1997;26:S15–25. 10.1093/ije/26.suppl_1.S15. [DOI] [PubMed] [Google Scholar]
- 22-.Stack AG, Hanley A, Casserly LF, Cronin CJ, Abdalla AA, Kiernan TJ, et al. Independent and conjoint associations of gout and hyperuricaemia with total and cardiovascular mortality. Q J Med 2013;106(7):647–58. 10.1093/qjmed/hct083. [DOI] [PubMed] [Google Scholar]
- 23-.Li M, Hu X, Fan Y, Li K, Zhang X, Hou W, et al. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci Rep 2016;6:19520 10.1038/srep19520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24-.Wu CY, Hu HY, Chou YJ, Huang N, Chou YC, Lee MS, et al. High Serum Uric Acid Levels Are Associated with All-Cause and Cardiovascular, but Not Cancer, Mortality in Elderly Adults. J Am Geriatr Soc 2015;63(9):1829–36. 10.1111/jgs.13607. [DOI] [PubMed] [Google Scholar]
- 25-.Borghi C, Rosei EA, Bardin T, Dawson J, Dominiczak A, Kielstein JT, et al. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens 2015;33(9):1729–41. 10.1097/HJH.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 26-.Hsu SP, Pai MF, Peng YS, Chiang CK, Ho TI, Hung KY. Serum uric acid levels show a ‘J-shaped’ association with all-cause mortality in haemodialysis patients. Nephrol Dial Transplant 2004;19(2):457–62. 10.1093/ndt/gfg563. [DOI] [PubMed] [Google Scholar]
- 27-.Wu Y, Li M, Li J, Luo Y, Xing Y, Hu D. Elevated serum uric acid level as a predictor for cardiovascular and all-cause mortality in Chinese patients with high cardiovascular risk. J Geriatr Cardiol 2008;5:15–20. [Google Scholar]
- 28-.Huang C, Niu K, Kobayashi Y, Guan L, Momma H, Cui Y, et al. An inverted J-shaped association of serum uric acid with muscle strength among Japanese adult men: a cross-sectional study. BMC Musculoskelet Disord 2013;14:258 10.1186/1471-2474-14-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29-.Dutta A, Henley W, Pilling LC, Wallace RB, Melzer D. Uric acid measurement improves prediction of cardiovascular mortality in later life. J Am Geriatr Soc 2013;61(3):319–26. 10.1111/jgs.12149. [DOI] [PubMed] [Google Scholar]
- 30-.Volterrani M, Iellamo F, Sposato B, Romeo F. Uric acid lowering therapy in cardiovascular diseases. Int J Cardiol 2016;213:20–2. 10.1016/j.ijcard.2015.08.088. [DOI] [PubMed] [Google Scholar]
- 31-.Kanbay M, Huddam B, Azak A, Solak Y, Kadioglu GK, Kirbas I, et al. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol 2011;6(8):1887–94. 10.2215/CJN.11451210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32-.Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, et al. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation 2001;104(20):2407–11. 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- 33-.Rajendra NS, Ireland S, George J, Belch JJ, Lang CC, Struthers AD. Mechanistic insights into the therapeutic use of high-dose allopurinol in angina pectoris. J Am Coll Cardiol 2011;58(8):820–8. 10.1016/j.jacc.2010.12.052. [DOI] [PubMed] [Google Scholar]
- 34-.Malik UZ, Hundley NJ, Romero G, Radi R, Freeman BA, Tarpey MM, et al. Febuxostat inhibition of endothelial-bound XO: implications for targeting vascular ROS production. Free Radic Biol Med 2011;51(1):179–84. 10.1016/j.freeradbiomed.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35-.Tausche AK, Christoph M, Forkmann M, Richter U, Kopprasch S, Bielitz C, et al. As compared to allopurinol, urate-lowering therapy with febuxostat has superior effects on oxidative stress and pulse wave velocity in patients with severe chronic tophaceous gout. Rheumatol Int 2014;34(1):101–9. 10.1007/s00296-013-2857-2. [DOI] [PubMed] [Google Scholar]
- 36-.Sezai A, Soma M, Nakata K, Hata M, Yoshitake I, Wakui S, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients (NU-FLASH Trial). Circ J 2013;77(8):2043–9. 10.1253/circj.CJ-13-0082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.