Abstract
Priapism is a serious, but episodic, complication of sickle cell disease (SCD). We had previously reported that subjects with SCD had variable red blood cell (RBC) adhesion to the immobilized sub-endothelial protein laminin (LN). We examined adhesion to LN in a microfluidic device, of RBCs from men with homozygous sickle cell anemia. Adhesion under hypoxic, but not ambient, conditions was greater in men with a history of priapism, with median adhesion of 529 RBCs per 32 mm2/unit area (range 5–5248) rising to 3268 RBCs per 32 mm2/unit area (range 49–18368), P=0.004), under ambient and hypoxic conditions, respectively (n=14). This was not seen in RBCs from men without a history of priapism (median 402 (range 14–785) and 122 (range 31–4112) RBCs per 32 mm2/unit area, ambient and hypoxic conditions, respectively (P=N.S., N=12). We also observed an association between hypoxia-enhanced RBC adhesion in vitro and a history of hemoglobin desaturation in vivo independent of priapism. Prolonged Hb desaturation may increase sickle polymer formation and RBC damage, resulting in enhanced RBC adhesion, hemolysis, and endothelial dysfunction. The identification of distinct RBC phenotypes could prompt clinical evaluation for suitability for novel or under-used therapies, like oxygen.
Introduction:
Sickle cell disease (SCD) arises from an inherited mutation in the beta globin geneThe most common form of SCD is homozygous sickle cell disease (HbSS), or sickle cell anemia. The population of people with HbSS is expanding worldwide, as are novel therapies for its treatment1–3. It is predicted that the number of newborns with SCD who are born per annum in Sub-Saharan Africa and India will increase from approximately 350,000 in 2010 to 445,000 in 20504. Although the disease is caused by only a single amino acid substitution, this deceptively minor molecular change has a profound physiological impact, resulting in lifelong anemia, progressive endothelial dysfunction, and acute and chronic organ damage. People with SCD suffer from pain and co-morbidities, often contributing to a shorter and pain-filled life. One serious complication of this disease is priapism, an episodic, prolonged, painful penile erection, which over time may result in impotence. Pripaism was first linked with SCD in 1934, and has since been recognized as a common complication, afflicting one-quarter to one-third of men with SCD over their lifespan.5 Intravascular hemolysis, NO scavenging, and resultant endothelial damage and vasculopathy are plausible pathophysiological underpinnings to priapism in SCD6–8.
Utilizing a microfluidic adhesion assay, we had previously reported that subjects with SCD had variable red blood cell (RBC) adhesion to the immobilized sub-endothelial protein laminin (LN) under hypoxia in vitro, depending on clinical phenotype, extent of hemolysis, and disease severity9–12. Here, we asked whether RBC adhesion to LN, under ambient or hypoxic conditions, differed between men with or without a history of priapism. Anecdotally, many of patients with SCD report awakening with priapism. Nocturnal Hb desaturation, in which Hb saturation (SpO2) decreases during sleep, is relatively common in people with SCD, and also associates with HbSS, hemolysis, and decreased NO availability.7,13,14 We examined this clinical association with RBC adhesion as well.
RBC adhesion to LN, under ambient and hypoxic conditions, was quantitated in samples taken at clinical baseline from men with HbSS, with or without a history of priapism. We utilized an in vitro microfluidic platform (SCD Biochip) to measure RBC adhesion under physiologic flow conditions12. For hypoxia experiments, we integrated this platform with a micro-gas exchanger that exposed the blood to low oxygen conditions prior to being flown across the SCD biochip, resulting in desaturation of Hb in the sample (to an SpO2 of 83%)9.
Materials & Methods:
Surplus blood draw samples, collected in ethylenediaminetetraacetic acid (EDTA), were obtained from people with SCD during non-crisis clinic visits at the Adult Sickle Cell Disease Clinic at University Hospitals Seidman Cancer Center/ CWRU in Cleveland, OH. All de-identified samples were processed within 24 hours. Clinical information, including treatment, medical history, and laboratory tests, was obtained from the electronic medical records system. We recorded a history of priapism, at any time, and any evaluation for hemoglobin desaturation sufficient to warrant prescription of oxygen within 5 years, e.g. 6-minute walk distance test, sleep study, or overnight oximetry. Laboratory tests, obtained at non-crisis clinic visits, included a complete blood count (CBC), comprising white blood cell count (WBC, 109/ L), platelet count (109/ L), and absolute neutrophil count (ANC, 106/ L), as well as reticulocyte count (109/ L), lactate dehydrogenase levels (LDH, U/L), ferritin levels, a renal panel, liver function tests, hemoglobin S (HbS) %, hemoglobin F (HbF) %, and total hemoglobin (g/dL). Pain level data, on a numerical scale from 0 (no pain) to 10 (highest pain), were routinely collected at each clinic visit. Concomitant treatments such as hydroxyurea or transfusions were recorded. All studies were performed with institutional review board approval and written informed consent from subjects.
The SCD Biochip was designed to recapitulate volume and flow of post-capillary venules. It was functionalized with LN and blocked with 2% BSA to prevent non-specific adhesion9–12. The microchannels were connected with inlet tubing which was non-permeable (ambient conditions), or gas permeable within non-permeable tubing, which contained 5% CO2 and 95% N2. As previously reported, 15 µl of undiluted EDTA-anticoagulated blood sample was injected into the channels through the inlet tubing at an approximate shear stress of 0.1 Pa, and non-adherent cells were rinsed out by flowing a wash buffer solution. Phase-contrast images of the microchannels with adherent RBCs were recorded with an inverted microscope, and adherent RBCs were manually quantified with Adobe Photoshop software (San Jose, CA) in a 32 mm2 window9–12.
A test of normality was performed on relevant variables. Non-normally and normally distributed variables were described with medians and ranges. Appropriate two-sample tests were used to compare groups, t-tests for normally distributed values and Mann-Whitney for non-normally distributed variables. Appropriate paired t-tests were used to compare the change from normoxic and hypoxic states. Analyses were performed with MiniTab (MiniTab, Inc.; State College, PA) and OriginLab (OriginLab Corporation; Northampton, MA) software. When more than one baseline value was available for a single subject, median values for all data (adhesion and lab values) were used for analysis. We performed the k-means clustering method to analyze univariate models and determined 2 distinct groups of subjects with respect to their lactate dehydrogenase levels and absolute reticulocyte counts. A custom written code in Matlab (Mathworks, MA, USA) was utilized to partition the subject group into two clusters.
Results:
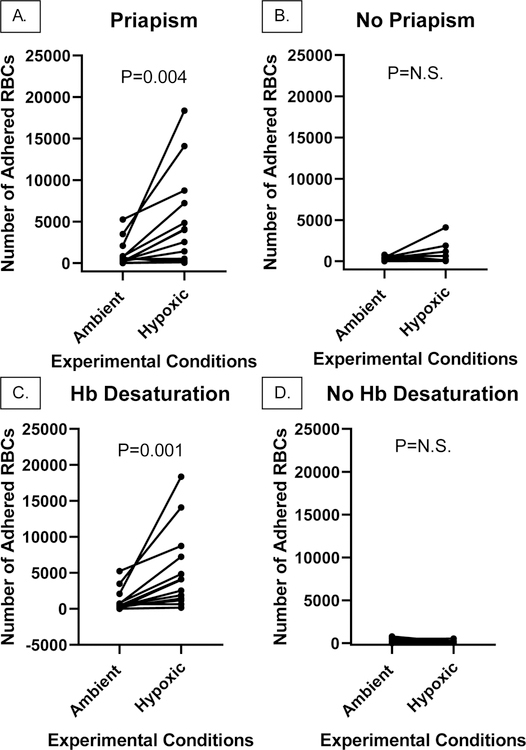
Paired RBC samples from men with a history of priapism showed significantly hypoxia-enhanced adhesion to LN, rising from a median of 529 RBCs/32 mm2/unit area (range 5–5248) under ambient conditions to 3268 RBCs/32 mm2/unit area under hypoxic conditions (range 49–18,368, P=0.004, N=14). This was not seen in men without a history of priapism (402 (range 14–785) and 122 (31–4112, N=12) RBCs/32 mm2/unit area, under ambient and hypoxic conditions, respectively, (P=N.S., Fig. 1). Of note, there was no significant difference in baseline adhesion between these groups under ambient conditions (P=N.S., not shown).
Figure 1. Adhesion of RBCs, under ambient or hypoxic conditions, from men with HbSS with or without a history of priapism, and with or without a known history of Hb desaturation in vivo.
Shown is median adhesion of RBCs from men with HbSS, and A. a history of priapism, under ambient (529, range 5–5248) or hypoxic (3268, range 49–18368, P=0.004, N=14) conditions in vitro, or B. without a history of priapism, under ambient (402, range 14–785) or hypoxic (122, range 31–4112, P=NS, N=12) in vitro. Shown also is adhesion of RBCs in this population, analyzed according to history of Hb desaturation in vivo, C. under ambient (440, range 16–5248) or hypoxic (4000, range 171–21576, P=0.001, N=15) conditions in vitro, or D. no known history of Hb desaturation, under normoxic (494, range 5–785) or hypoxic (102, range 31–521, N=11, P=N.S.) conditions in vitro.
Although there was a trend towards Hb desaturation in vivo in men with a history of priapism (Table 1), its presence, analyzed primarily, was highly associated with hypoxia enhanced RBC adhesion in vitro, regardless of priapism history. Men with a clinical history of Hb desaturation in vivo showed hypoxia-enhanced adhesion to LN, rising from a median 440 (range 16–5248) to a median 4000 (range 16–5248) RBCs/32 mm2/unit area, under ambient and hypoxic conditions, respectively (N=15, P<0.001, Fig. 1). This effect of hypoxia was not seen in men who had not been tested or did not have Hb desaturation in vivo, whose adhesion to LN was a median 494 (range 5–785) to a median 102 (range 31–521) RBCs/32 mm2/unit area, under ambient and hypoxic conditions, respectively (N=11, P=N.S., Fig. 1).
Table 1.
Clinical associations and RBC adhesion in adult men with HbSS, with or without a history of priapism
| Clinical Data | History of Priapism (n=14, median, range) | No History of Priapism (n=10, median, range) | P Value | |||
|---|---|---|---|---|---|---|
| Age* (years) | 34 (24–63) | 29 (21–45) | 0.078 | |||
| Hgb (g/dL) | 7.85 (6.1–11.9) | 9.1 (7–10.6) | 0.105 | |||
| Reticulocyte Count (109/L) | 275 (65–770) | 245 (115–749) | 0.487 | |||
| Total Bilirubin (mg/dL)* | 3.6 (0.5–11.5) | 2.2 (0.7–6.8) | 0.178 | |||
| WBC (109/L) | 11.48 (7.1–16.6) | 8.65 (5.5–17.2) | 0.022 | |||
| ANC (x106/L) | 7160 (2190–11300) | 4275 (2710–10460) | 0.045 | |||
| LDH (U/L) | 474 (217–1114) | 278 (122–360) | 0.008 | |||
| Ferritin (ug/L)* | 2433 (60–7680) | 360 (11–8900) | 0.471 | |||
| Hemoglobin Desaturation in vivo | 71% of subjects (10/14) | 46% of subjects (5/12) | 0.126 (χ2) | |||
| RBC Adhesion* | Ambient 529 (5–5248) | Hypoxic 3268 (49–18368) | Ambient 402 (14–785) | Hypoxic 122 (31–4112) | Priapism 0.004 | No Priapism 0.635 |
non-normal distribution.
Men with a history of priapism had evidence for chronic inflammation (elevated WBC and neutrophil counts and elevated ferritin levels) and hemolysis (elevated LDH levels, Table 1). In separate K-means clustering analyses, men with HbSS and a ‘hemolytic’ phenotype (elevated LDH levels and high absolute reticulocyte counts) were more likely to have a history of priapism than were men with a less hemolytic phenotype (Fig. 1 supplemental).
Conclusions:
Previous studies have indicated that a history of priapism is associated with greater disease severity.8 Our research describes an association between hemolysis and priapism, which has been highlighted by others. However, our data show for the first time that a history of priapism associates with RBC phenotype in vitro, i.e. specifically hypoxia-enhanced adhesion to LN, rather than adhesion under ambient oxygen concentrations at baseline. This suggests a subtle but detectable and persistent RBC characteristic that associates with high-risk, priapism-associated disease.
Many men in this study had both a history of priapism and a history of Hb desaturation, which itself has been associated with more severe disease and pain.15,16 Strikingly, hypoxia-enhanced adhesion was more likely in men with a history of Hb desaturation to LN in vivo, regardless of priapism history. The identification of hypoxia enhanced RBC adhesion in vitro could highlight those people with SCD who are at risk for Hb desaturation in vivo, and who could potentially benefit from oxygen therapy. This is currently being tested prospectively in both men and women with homozygous SCD.
Supplementary Material
Figure 1 Supplemental. LDH and Absolute Reticulocyte Count (ARC) in men with HbSS Men with HbSS and a hemolytic lab profile (Group 1, elevated LDH, median 697 (range 316–1114) and elevated ARC, median 580 (range 198–749), n=9) compared to men with a less hemolytic lab profile (Group 2, lesser elevations of LDH, median 290 (range 122–363) and ARC, median 237 (range 29–407), N=17, defined by K-means clustering), were more likely to have a history of priapism (8/9 vs. 6/17, respectively, Chi-square P=0.009).
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the generous participation of people with SCD who are followed at the University Hospitals Cleveland Medical Center Adult Sickle Cell Disease clinic, and their providers in this clinic.
FUNDING SOURCE
This research was supported by the National Science Foundation CAREER Award 1552782, Doris Duke Charitable Foundation 2013126, and National Heart Lung and Blood Institute R01HL133574 grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Telen MJ, Malik P & Vercellotti GM Therapeutic strategies for sickle cell disease: towards a multi-agent approach. Nat Rev Drug Discov 18, 139–158, 10.1038/s41573-018-0003-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoppe C & Neumayr L Sickle Cell Disease: Monitoring, Current Treatment, and Therapeutics Under Development. Hematol Oncol Clin North Am 33, 355–371, 10.1016/j.hoc.2019.01.014 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Rees DC, Williams TN & Gladwin MT Sickle-cell disease. Lancet 376, 2018–2031, 10.1016/S0140-6736(10)61029-X (2010). [DOI] [PubMed] [Google Scholar]
- 4.Piel FB, Hay SI, Gupta S, Weatherall DJ & Williams TN Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Med 10, e1001484, 10.1371/journal.pmed.1001484 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottsch HP, Berger RE & Yang CC Priapism: comorbid factors and treatment outcomes in a contemporary series. Advances in urology 2012, 672624, 10.1155/2012/672624 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nolan VG, Wyszynski DF, Farrer LA & Steinberg MH Hemolysis-associated priapism in sickle cell disease. Blood 106, 3264–3267 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anele UA, Morrison BF & Burnett AL Molecular pathophysiology of priapism: emerging targets. Curr Drug Targets 16, 474–483 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madu AJ et al. Priapism in homozygous sickle cell patients: important clinical and laboratory associations. Medical principles and practice : international journal of the Kuwait University, Health Science Centre 23, 259–263, 10.1159/000360608 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim M, Alapan Y, Adhikari A, Little JA & Gurkan UA Hypoxia enhanced adhesion of red blood cells in microscale flow. Microcirculation, 10.1111/micc.12374 (2017). [DOI] [PMC free article] [PubMed]
- 10.Alapan Y et al. Sickle cell disease biochip: a functional red blood cell adhesion assay for monitoring sickle cell disease. Transl Res 173, 74–91 e78, 10.1016/j.trsl.2016.03.008 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucukal E, Little JA & Gurkan UA Shear dependent red blood cell adhesion in microscale flow. Integr Biol (Camb) 10, 194–206, 10.1039/C8IB00004B (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alapan Y, Little JA & Gurkan UA Heterogeneous red blood cell adhesion and deformability in sickle cell disease. Sci Rep 4, 7173, 10.1038/srep07173 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagoda G, Sezen SF, Cabrini MR, Musicki B & Burnett AL Molecular analysis of erection regulatory factors in sickle cell disease associated priapism in the human penis. J Urol 189, 762–768, 10.1016/j.juro.2012.08.198 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rotz SJ et al. Nocturnal hemoglobin desaturation is associated with reticulocytosis in adults with sickle cell disease and is independent of obstructive sleep apnea. American journal of hematology 91, E355–356, 10.1002/ajh.24432 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Setty BN, Stuart MJ, Dampier C, Brodecki D & Allen JL Hypoxaemia in sickle cell disease: biomarker modulation and relevance to pathophysiology. Lancet 362, 1450–1455, 10.1016/S0140-6736(03)14689-2 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Hargrave DR, Wade A, Evans JP, Hewes DK & Kirkham FJ Nocturnal oxygen saturation and painful sickle cell crises in children. Blood 101, 846–848, 10.1182/blood-2002-05-1392 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1 Supplemental. LDH and Absolute Reticulocyte Count (ARC) in men with HbSS Men with HbSS and a hemolytic lab profile (Group 1, elevated LDH, median 697 (range 316–1114) and elevated ARC, median 580 (range 198–749), n=9) compared to men with a less hemolytic lab profile (Group 2, lesser elevations of LDH, median 290 (range 122–363) and ARC, median 237 (range 29–407), N=17, defined by K-means clustering), were more likely to have a history of priapism (8/9 vs. 6/17, respectively, Chi-square P=0.009).