Abstract
Background and Purpose
Previously, we showed that internal cues (such as singing) produce similar motor benefits as external cues (such as listening to music) for people with Parkinson disease (PD). This study takes that research further by exploring how singing—either aloud or mentally—at different tempos can ameliorate gait, and it offers insight into how internal cueing techniques may enhance motor performance for older adults and people with PD.
Methods
Sixty participants aged ≥50 years (30 female) were recruited; 30 had PD and 30 were healthy age-matched controls. Participants completed walking trials involving internal and external cueing techniques at 90, 100, and 110% of preferred cadence. The effects of different cue types and rates were assessed in a repeated-measures cross-sectional study by comparing gait characteristics (velocity, cadence, stride length) and variabilities (coefficients of variation of stride length, stride time, single support time).
Results
All participants modified their cadence and stride length during cued conditions, resulting in changes in gait velocity closely reflecting expected changes based upon cue rate. External cues resulted in increased gait variability, whereas internal cues decreased gait variability relative to uncued walking. Variability decreases were more substantial during mental singing at tempos at or above preferred cadence.
Discussion and Conclusions
Matching movement to one’s own voice improves gait characteristics while reducing gait variability for older adults and people with PD. Optimizing the use of internal cues to facilitate movement is an important step towards more effectively meeting the needs of people with gait disorders related to aging or neurological disease.
INTRODUCTION
Parkinson disease (PD), the second most common neurodegenerative disorder, can cause debilitating effects on gait that may contribute to increased falls and decreased quality of life.1 Dopamine depletion within the substantia nigra of the basal ganglia leads to malfunctioning of temporal control mechanisms, which disrupts both movement timing and amplitude.2,3 This affects walking ability; people with PD tend to walk slower and with less stability. Reductions in gait speed are typically attributed to a combination of shorter step lengths and decreased step frequency and indicate a decline in overall health in both aging and patient populations.4
External auditory cueing through metronome beats or music is a well-established method of normalizing gait speed for people with PD. By creating an external template to which people can align their footfalls, auditory cues impose a walking cadence that, presumably, reduces reliance on defective internal timing mechanisms and increases motivation, thereby increasing walking speed.5 Externally-imposed cues can induce immediate effects on gait at a wide range of stimulation rates from 80% to 125% of preferred cadence, with optimal frequencies generally considered to be within 10% above or below.6 However, little is known about the effects of rhythmic auditory cueing on gait variability, although recent evidence suggests this may be an important yet often overlooked byproduct of rhythmic auditory cueing.
Gait variability is a quantifiable measure of altered walking performance that is strongly indicative of overall stability. In aging populations, increased gait variability is characterized by inconsistent step timing and reduced step symmetry and is considered a measure of dyscontrol, arrythmicity, and instability.7 For people with PD, fluctuations between strides are even more pronounced. Both temporal and spatial measures of variability are associated with functional status and clinical outcomes and are highly predictive of falls in the elderly8 and people with PD.9 Hence, slower, more variable gait may contribute to diminished stability and increase the risk of falls.10
Although external cues are commonly considered a “pacemaker” which act to restore gait rhythmicity and thereby reduce gait variability, increasing numbers of studies reporting gait variability reveal that external cues can, instead, increase measures of variability in both healthy older adults11–17 and in people with PD.13,18–22 Detrimental effects on gait variability could reflect the difficulty of synchronizing to an outside source. Recent work from our lab explored singing as an alternative to externally imposed musical cueing. Singing might be considered an internal cue that utilizes vocal-motor coupling to match one’s movement to one’s own voice. Previously, we saw that this form of cueing particularly aids gait variability, as evidenced by reductions in measures of both spatial and temporal variability as compared to external cueing.23
In this study, we extended our past research to explore the use of mental singing, or singing in one’s head. Several reports suggest that people with PD use this technique in their daily lives, however, only one prior study we know of has tested the technique, in which improvements in motor timing were shown, but precise gait characteristics were not measured.24 We hypothesized that mental singing would be as effective as singing aloud at improving gait for all participants. We also expected to see greater effects with increased cue rates, based upon a recent meta-analysis,25 although optimal cue rates vary widely across individuals, so we included slower cue rates as well. We tested both people with PD and healthy controls to better understand how disrupted rhythmic processing in PD might hinder the efficacy of internal cueing techniques.
METHODS
Participants
A total of 60 participants, thirty (15 female) in each of two groups – healthy controls and people with Parkinson disease (PD) – took part in this study (Table 1). Group size was determined by power analysis based on preliminary data.23 Participants with PD were recruited from the Movement Disorders Center at Washington University School of Medicine. Healthy controls were recruited via the Research Participant Registry through the Volunteers for Health database managed by Washington University School of Medicine and via emails, social media, and flyers in and around the Washington University School of Medicine campus. All participants were ≥ 50 years of age, and participants with PD had a neurological diagnosis of “definite PD”, as previously described and based upon established criteria.26,27
Table 1.
Participant Demographics.
| Controls | PD | |
|---|---|---|
| N (male) | 30(15) | 30(15) |
| Age, yrs | 64.9(±7.2) | 65.8(±6.5) |
| MDS-UPDRS-III | - | 24.9(±10.27) |
| MMSE, median (range) | 30(27,30) | 29(24,30) |
| Years since dx | - | 5.77(±3.79) |
| LEDD, mg | - | 933(±658) |
| Musical experience, yrs | 4.42(6.02) | 7.77(11.45) |
| BQMI | 1.68(0.57) | 2.12(0.68) |
Values represent mean ±SD, except where noted.
MDS-UPDRS, Movement Disorder Society Unified Parkinson Disease Rating Scale. MMSE, Mini Mental Status Examination. LEDD, Levodopa Equivalent Daily Dose. BQMI, Betts’ Questionnaire upon Mental Imagery (auditory portion only).
All participants were able to stand independently for at least 30 minutes and had no evidence of dementia (Mini Mental Status Examination (MMSE) ≥ 26). We excluded people with history of neurological deficit (aside from PD), orthostatic hypotension, or deep brain stimulation surgery. One healthy control was excluded for cognition (MMSE < 26) and an additional participant was recruited.
All participants provided written informed consent prior to testing and were compensated for their time. The protocol was approved by the Human Research Protection Office at Washington University School of Medicine. The Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) was used to assess disease severity. Sub-sections I (non-motor symptoms), II (motor aspects of daily living), and III (motor sign severity) were administered and scored by certified staff. Additional questionnaires included the New Freezing of Gait Questionnaire (nFOGq) and the Fall History questionnaire. Auditory imagery was assessed using the Betts’ Questionnaire upon Mental Imagery (BQMI), which uses a 7-point vividness scale, with 1 indicating high imagery ability and 7 indicating low imagery ability.28 We collected only the auditory imagery portion of the test and calculated an average for each participant. We defined musical experience as any form of musical training, practice, and/or performance and quantified it as the total number of years.
Experimental Protocol
Participants with PD were tested in the “on” state as determined by self-report during the Movement Disorder Society Unified Parkinson Disease Rating Scale (MDS-UPDRS) Part III evaluation to capture their normal walking condition. A 5 m instrumented, computerized walkway (GAITRite Walkway, CIR Systems, Inc., Franklin, NJ) recorded walking trials. Three baseline trials (UNCUED) were used to assess each participant’s comfortable walking characteristics. All participants then completed three blocks of cued trials trials at 90%, 100% and 110% of preferred walking cadence. The block of trials cued at 100% of preferred cadence was always completed first followed by blocks at either 90% or 110% of preferred cadence, the order of which was randomized and counterbalanced. Within each block, the randomized conditions were:
MUSIC: Music was playing and participants were asked to walk to the beat of the song. This represents typical external cueing techniques. Participants listened to one verse of the song and began walking when they were ready, similar to a beat-synchronization paradigm. The song looped throughout the duration of the trial.
SING: Participants were asked to sing aloud while walking. In this condition, no external source provided a cue while they walked, so participants generated the cue themselves. Participants listened to one verse of the song and then began walking and singing as soon as the music stopped.
MENTAL: Participants were asked to sing in their heads without moving their lips or producing overt sound. As in the SING condition, participants listened to one verse of the song and then began walking when the music stopped.
All conditions were cued using an instrumental version of “Row, Row, Row your Boat” designed with a salient beat that could be readily detected by participants. Everyone was familiar with the lyrics and melody of the song and able to sing it without difficulty. The musical cue was administered from a laptop connected to speakers no farther than 10 m from the participant during walking and at an audible volume. Song tempo was adjusted based upon each individual’s preferred walking cadence while maintaining key consistency using open source audio editing software (Audacity, The Audacity Team, audacity.sourceforge.net/).
Statistical Analysis
Statistical analyses were done using commercial software (SPSS Statistics 24, IBM ____). For each participant, data were averaged across the three trials of each condition. Gait characteristics (velocity, cadence, and stride length) and variability (coefficients of variation for stride length, stride time, and single support time) were compared in three separate analyses, one for each cue tempo. Coefficients of variation (CV) were calculated as the ((standard deviation/mean) x 100) for each person in each condition. Gait asymmetry (GA) was calculated for each condition at each tempo based on previous reports as: GA= 100 x ln (swing ratio).16 Swing ratio was defined as the ratio of the mean left and right swing times with the larger value in the numerator. Mixed model repeated measures ANOVAs with between-subject factor of group and within-subject factor of condition were used to assess differences, and Tukey-corrected post-hoc pairwise comparisons were used as appropriate. When Mauchley’s test of sphericity was not met, adjusted multivariate and univariate (Greenhouse-Geisser) statistics were reported. Differences between groups in auditory imagery ability were assessed via independent t-test. Statistical significance was set at α=0.05.
RESULTS
Gait Characteristics
Velocity
Differences between conditions
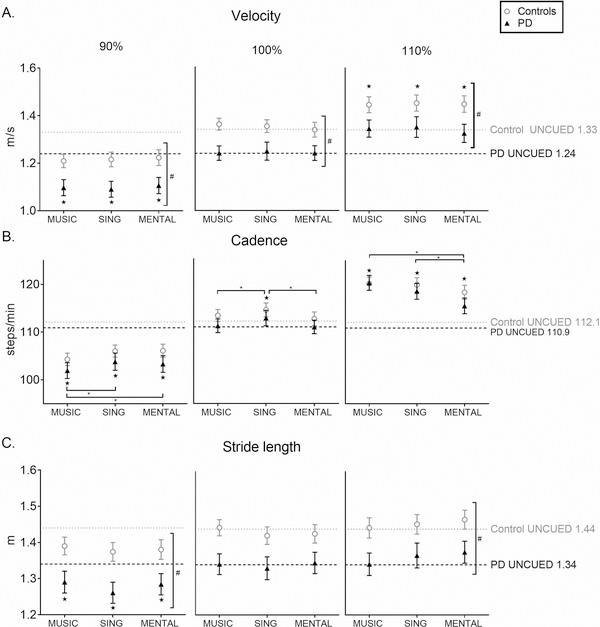
There was a within-subject main effect of condition at 90% (F(2.03,117.97)=51.06, p<0.001) and at 110% of preferred cadence (F(1.71, 99.42)=27.28, p<0.001) with pairwise comparisons indicating that velocity was significantly different for these cued conditions as compared to UNCUED (all p<0.001) (Figure 1, Supplemental Table 1). At 100% of preferred cadence, there was no effect of condition on velocity.
Figure 1.
Gait Characteristics
Gait characteristics of velocity (A), cadence (B), and stride length (C) across conditions as compared to UNCUED walking at three different tempos: 90%, 100%, and 110% of preferred walking cadence. All bars represent means ± SEM. The gray and black dotted lines represent the Control group and PD group baseline mean values, respectively. Horizontal significance bars indicate an overall effect of condition. ★ indicates significant difference from UNCUED across groups. * indicates between-condition difference across groups. # indicates between-group difference across conditions.
Differences between groups
There were between-group differences showing that healthy controls walked faster than participants with PD at 90% (F(1,58)=7.70, p=0.007), 100% (F(1,58)=7.301, p=0.009), and 110% (F(1,58)=5.47, p=0.023) of preferred cadence. There were no significant interactions.
Cadence
Differences between conditions
At 90%, there was a within-subject main effect of condition (F(2.64,153.30)=91.29, p<0.001) with pairwise comparisons indicating that cadence was significantly lower for all cued conditions than UNCUED. Cadence was also significantly lower for MUSIC than SING (p=0.04) and MENTAL (p=0.01). At 100%, there was a within-subject main effect of condition (F(3, 174)=7.696, p<.001) with pairwise comparisons indicating that cadence was higher in SING than UNCUED (p=0.002), MUSIC (p=0.05), and MENTAL (p=0.004). At 110%, there was a within-subject main effect of condition (F(3,174)=97.75, p<0.001) with pairwise comparisons indicating that cadence was higher for all cued conditions than UNCUED (p<.001) and that MUSIC and SING were higher than MENTAL (all p<0.001).
Differences between groups
There were no significant differences between groups and no interactions.
Stride length
Differences between conditions
At 90%, there was a within-subject main effect of condition (F(1.69, 98.03)=14.21, p<0.001) with pairwise comparisons indicating that stride lengths were shorter in all cued conditions than UNCUED (all p<0.014). There were no differences at 100%. At 110%, there was a trend toward a within-subject main effect of condition (F(1.61, 93.45)=3.09, p=0.06) with pairwise comparisons indicating that strides were longer for MENTAL than MUSIC (p=0.002).
Differences between groups
At 90%, there was a between-group difference showing that controls took longer strides than participants with PD (F(1,58)=7.38, p=0.009), regardless of condition. At 110%, there was a between-group difference (F(1,58)=5.71, p=0.02) showing that controls took longer steps than participants with PD, regardless of condition. There were no significant interactions.
Gait variabilities
Stride length variability
Differences between conditions
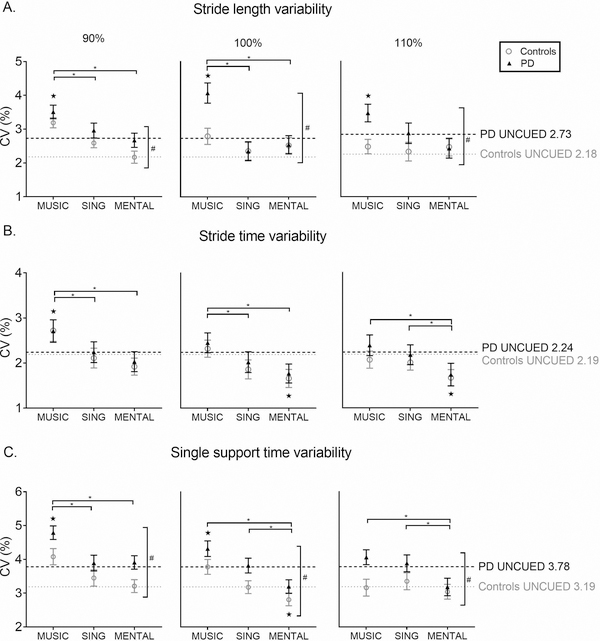
At 90%, there was a within-subject main effect of condition (F(2.62, 151.88)=9.77, p<0.001) with pairwise comparisons indicating higher variability in MUSIC than UNCUED (p<0.001), SING (p=0.048), and MENTAL (p<0.001). At 100%, there was a within-subject main effect of condition (F(2.10, 121.89)=7.35, p=0.001) with pairwise comparisons indicating higher variability in MUSIC than UNCUED (p=0.02), SING (p=0.004), and MENTAL (p=0.02). At 110%, there was a within-subject main effect of condition (F(2.59, 150.07)=2.98, p=0.04) with pairwise comparisons indicating higher variability in MUSIC than MENTAL (p=0.006).
Differences between groups
At 90%, there was a between-subject main effect of group (F(1,58)=4.63, p=0.036) indicating that participants with PD had higher variability than controls. At 100%, there was a between-subject main effect of group (F(1,58)=4.41, p=0.04) indicating that participants with PD had higher variability than controls. At 110%, there was a between-subject main effect of group (F(1,58)=5.58, p=0.022) indicating that participants with PD had higher variability than controls. There were no significant interactions.
Stride time variability
Differences between conditions
At 90%, there was a within-subject main effect of condition (F(2.61, 151.28)=9.10, p<0.001) with pairwise comparisons indicating higher variability in MUSIC than UNCUED (p=0.009), SING (p=0.01), and MENTAL (p<0.001). At 100%, there was a within-subject main effect of condition (F(2.61, 151.32)=11.01, p<0.001) with pairwise comparisons indicating that MUSIC was higher than SING (p=0.004) and MENTAL (p<0.001). MENTAL was also significantly lower than UNCUED (p=0.002). At 110%, there was a within-subject main effect of condition (F(3, 174)=5.67, p=0.001) with pairwise comparisons indicating lower variability in MENTAL than UNCUED (p=0.006), MUSIC (p<0.001), and SING (p<0.029).
Differences between groups
There were no significant group differences and no interactions.
Single support time variability
Differences between conditions
At 90%, there was a within-subject main effect of condition (F(3, 178)=12.35, p<0.001) with pairwise comparisons indicating higher variability in MUSIC than UNCUED (p<0.001), SING (p=0.001), and MENTAL (p<0.001). At 100%, there was a within-subject main effect of condition (F(2.31, 133.81)=11.46, p<0.001) with pairwise comparisons indicating higher variability in MUSIC than UNCUED (p=0.047). MENTAL had lower variability than UNCUED (p=0.016), MUSIC (p<0.001), and SING (p=0.003). At 110%, there was a within-subject main effect of condition (F(3, 174)=4.06, p=0.008) with pairwise comparisons indicating lower variability in MENTAL than MUSIC (p=0.013) and SING (p=0.038).
Differences between groups
There was a between-subject effect of group at 90% (F(1,58)=6.87, p=0.011), at 100% (F(1,58)=5.35, p=0.024), and at 110% (F(1,58)=5.82, p=0.019) indicating that participants with PD had higher variability than controls at every tempo regardless of condition. There were no significant interactions.
Gait Asymmetry
Univariate tests showed a main effect of group at each tempo: 90% (F(1,58)=26.42, p<0.001), at 100% (F(1,58)=15.59, p<0.001), and at 110% (F(1,58)=20.00, p<0.001)(Supplemental Table 1). There were no differences between conditions at any tempo.
Auditory Imagery Ability
Controls ranked their auditory imagery abilities lower (better) than participants with PD (F(2,58)=2.579, p=0.013) (Table 1). Bivariate correlations of auditory imagery and changes in gait variabilities during MENTAL were not significant.
DISCUSSION
The goal of this study was to determine if internal cueing in the form of singing or mental singing could elicit similar gait improvement as external cueing techniques such as listening to music. While external cueing is commonly used to improve gait characteristics in people with PD, little is known about the effects of internal cueing, in spite of evidence that it may be more beneficial to gait variability than external cueing.23 Our results showed that both healthy controls and participants with PD were able to utilize internal cues and garner similar improvements in gait performance as with external cues. However, only internal cues elicited improvements in gait variability as well. Benefits observed during mental singing render internal cueing techniques more ecologically relevant for people who would not be comfortable walking down the street while singing aloud. At tempos faster than preferred cadence, participants were able to significantly improve velocity and variability, both markers of stability.
Confirming previous work, we saw several differences in baseline gait characteristics, as people with PD walked slower, with shorter strides, and higher levels of gait variability and asymmetry. Lower auditory imagery ability, in spite of more years of musical experience, on average, did not impede ability to modify walking cadence. Changes in cadence during different cue rates suggest that both groups were able to adapt their cadence to match the external cue. As velocity is a by-product of both cadence and stride length, altering either one can translate to changes in gait speed. Here, the slower cue rate elicited significant reductions in all three gait characteristics of velocity, cadence, and stride length. Although detrimental effects on gait of experimentally imposed slower cadences have been noted previously in both healthy populations29 and people with PD,18,30–32 some have suggested that slower auditory stimulation allows for longer step lengths, particularly in people with higher disease severity19 and those who experience freezing of gait.29 Thus, the lack of a discernable benefit at the slower rate in our study may relate to less impaired baseline gait among our participants. At the 100% cue rate, we saw minimal effects on gait, which is consistent with a meta-analysis revealing small effects of unmodulated external cues without training.25 The faster cue rate elicited the most benefit, allowing people to increase velocity and cadence while stride length increases were non-significant.
In this study, we extend research on external cueing to show that internal cueing, can also elicit significant changes in gait characteristics from UNCUED walking. Changes in velocity, cadence, and stride length that were significantly different during external cueing were also significant for internal cueing. That is, regardless of cueing condition, the slower cue rate decreased velocity, cadence, and stride length from UNCUED, and the faster cue rate increased velocity and cadence from UNCUED. Notably, at the 110% cue rate, increases in stride length were non-significant, which could indicate that rhythmic cueing is less conducive than attentional strategies for lengthening strides. We also noted that, at 110% cue rate, velocity changes during mental singing appear to be driven more by increases in stride length than cadence (which was significantly lower than the other cued conditions) indicating that mental singing may be more beneficial as a tool to increase velocity while also counteracting debilitating tendencies to festinate in people with PD. These results suggest that external stimulation may not be necessary and, instead, people may gain similar benefit by cueing themselves through their own voices.
It is notable that, while external and internal cues elicited similar effects on gait characteristics as compared to UNCUED, only internal cues also improved gait variability. A number of previous studies suggest that gait improvements with external rhythmic auditory cues come at the cost of increasing gait variability.14,29,33,34 For healthy older adults, external cues commonly degrade gait variability, presumably because they interfere with normally-functioning internal timing mechanisms.35 In people with PD, external cues are thought to act as an external pacemaker that stabilizes the defective internal timing mechanisms and restores rhythmicity, thereby reducing gait variability, but mounting evidence suggests this is not always the case.
Inconsistent effects of external cues have led researchers to explore a range of cue rates in order to glean more benefit. The majority of studies show that slow paced cues tend to negatively affect gait variability.18,36,37 Our results support this, as increases were most apparent at the slower cue rate, in which MUSIC increased all measures of variability from UNCUED. In contrast, Hausdorff et al33 found no effect on gait variability until the cue rate was raised to 110%. This was only true of people with PD and not of healthy controls, leading the authors to suggest that effects of external cues on PD gait may be rate-dependent. Others, however, show that increasing walking speed alone does not reduce variability,38–40 supporting a dissociation between gait speed and gait variability that is more in line with our results, as we observed that external cueing did not benefit gait variability for either group at any tempo.
In contrast to external cues, internal cues did not cause similar increases in variability. Compared to externally-generated cues, internal cues generally reduced variability measures, as we showed in a previous study for singing aloud.23 In the present study, however, mental singing elicited even greater reductions in variability than overt singing. Compared to UNCUED, MENTAL significantly reduced temporal variability of stride time at 100% and 110% and single support time at 100% whereas decreases during SING were not significant.
In light of reductions of gait variability during mental singing, we wondered if gait asymmetry, a known marker of gait rhythmicity, would also improve. Calculated as a measure of swing time variability, gait asymmetry may be a more reliable assay of impaired gait automaticity independent of gait speed.16,41 As expected, gait asymmetry was higher in participants with PD, presumably due to impaired rhythmic processing mechanisms in the basal ganglia. Cueing did not significantly improve asymmetry in either group although some differences during cued conditions (from 3.87 to 3.04 in MENTAL at 110%, for instance) fell within a range reported as sufficient to reduce risk of freezing of gait and falls.16,42
From a theortical perspective, degradation of gait variability with the use of external cues in people with PD is problematic to explain because rhythmic auditory stimulation is presumed to replace malfunctioning basal ganglia-related timing mechanisms. Others have pointed out that differential effects between groups and at different rates of cueing also present difficulties to this theory.33,36 Perhaps the simplest explanation for increases in gait variability during external cueing is that it is difficult to synchronize to an outside source. External cues require constant adjustment to match the auditory stimulus, which may further degrade gait rhythmicity and lead to less consistent step patterns.
Internal cues may pose less of a challenge compared to external cues. Potentially, matching one’s movement to one’s own voice through vocal-motor coupling enables more accurate motor entrainment. In this study, even greater reductions in variability during mental singing imply that it may not be necessary to overtly produce the vocal component in order to benefit gait. Perhaps, by eliminating the need to create and monitor sound, participants were able to direct more attentional resources to walking.22 Elements of vocalization such as respiratory kinematics, word formation, and monitoring aural feedback, unnecessary when mental singing, potentially simplified task demands and enabled more efficient movement.
Another benefit of mental singing is that it may facilitate greater integration of motor, kinesthetic, and auditory imagery capabilities.43,44 This distinction could make it more accessible than overt singing for neurologic populations. The preservation of motor imagery ability in people with PD suggests that auditory imagery may also remain vivid and accurate. While participants with PD in our sample reported higher (worse) auditory imagery vividness than controls, both groups reported better than normative averages. Thus, auditory imagery impairment in people with PD may not be sufficient to erode sensorimotor synchronization capabilities during imagined, or mental, singing, though future work may shed more light on this.
Limitations
A few limitations should be considered. During the mental singing condition, we monitored lip movement and audible vocalizations but not laryngeal movements, so small sub-glottal movements may have contributed to motor output. Although participants verbally confirmed that they were, in fact, singing in their heads, we did not otherwise validate this. Also, in order to compare conditions within different tempos, participants heard the music play before all walking trials, even those considered “internal”. This external pacing before each trial may account for improvements during internal cueing that may not translate to daily walking outside of the laboratory. Although external auditory stimuli can establish a temporal structure that can be continued in silence within the mind of the listener even after the cue is removed, basal ganglia involvement in this process may impede people with PD from generating the cue themselves, which would necessitate the use of a pre-cue. The propensity of healthy adults to retrieve familiar songs at previously-encoded absolute tempos when singing aloud or imagining well-known songs suggests that the pre-cue may not be necessary in order to initiate internal cueing techniques, but work should address the feasibility of this in people with PD as well.45,46
Interpretation of our results is also limited by our testing only short bouts of walking. During internal cueing, we observed less extreme differences in cadence than during external cueing, which suggests that, without a cue present, people may exhibit a tendency to drift back towards their preferred walking cadence. Though slight, this reversion suggests the possibility that, over longer time courses, internal cues may allow gait to regress toward baseline rates. Lastly, up to 40 footfalls may be necessary to capture reliable estimates of gait variability and asymmetry47 so future studies should assess gait over longer periods of time in order to assess how well internal cueing techniques would transfer to real world situations for people with PD. Finally, we did not assess whether responsiveness to cuing was influenced by disease severity in our participants with PD, as has been suggested by prior work.50
Conclusions
The results of this research indicate that older adults and people with PD may gain greater benefit from internal versus external cueing techniques, the latter of which are commonly prescribed and seemingly detrimental to gait variability. In contrast, internal cues allow people to increase gait velocity while simultaneously reducing gait variability, which may ultimately contribute to overall gait stability and reduced fall risk. Furthermore, although we saw the greatest benefit to gait at tempos above preferred cadence, this does not preclude the possibility that some participants may benefit more from slower cue rates. Optimal cue rate is highly variable and should likely be determined on an individual basis. Internal cues may also be useful for improving gait in other populations, as a recent study showed improvements in velocity, cadence, and stride length after a single session of mental singing in patients with post-stroke hemiplegia. Here, we showed that mental singing provides more benefit to gait variability than singing aloud which makes internal cueing more practical for everyday use.
Supplementary Material
Figure 2.
Gait Variability
Coefficients of variation of stride length (A), stride time (B), and single support time (C) across conditions as compared to UNCUED walking at three different tempos: 90%, 100%, and 110% of preferred walking cadence. All bars represent means ± SEM. The gray and black dotted lines represent the Control group and PD group baseline mean values, respectively. Horizontal significance bars indicate an overall effect of condition. ★ indicates significant difference from UNCUED across groups. * indicates between-condition difference across groups. # indicates between-group difference across conditions.
Acknowledgements
This work was supported by the GRAMMY Museum Grant Program (EH and GE) and the National Institutes of Health [T32HD007434] (AH). The authors gratefully acknowledge Martha Hessler and Richard Nagel for assistance with data collection.
Conflicts of Interest and Sources of Funding:
Authors A and C have received a grant from the GRAMMY Museum Grant Awards Program to fund this research. Author B is supported by National Institutes of Health [T32HD007434].The authors declare no conflicts of interest. The work represented in the manuscript was previously presented at Movement: Brain Body Cognition, Harvard Medical School, Boston, MA.
Footnotes
List of Supplemental Digital Content
Supplemental Digital Content 1. Video Abstract.
Video Abstract available for more insights from authors (see Video, Supplemental Digital Content 1)
REFERENCES
- 1.Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The evolution of disability in Parkinson disease. Movement disorders : official journal of the Movement Disorder Society. 2008;23:790–796. doi: 10.1002/mds.21879 [DOI] [PubMed] [Google Scholar]
- 2.Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. The basal ganglia network mediates the planning of movement amplitude. The European journal of neuroscience. 2004;19:2871–2880. doi: 10.1111/j.0953-816X.2004.03395.x [DOI] [PubMed] [Google Scholar]
- 3.Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Movement disorders : official journal of the Movement Disorder Society. 1998;13:428–437. doi: 10.1002/mds.870130310 [DOI] [PubMed] [Google Scholar]
- 4.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x [DOI] [PubMed] [Google Scholar]
- 5.Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease normalization strategies and underlying mechanisms. Brain : a journal of neurology. 1996;119:551–568. doi:DOI 10.1093/brain/119.2.551 [DOI] [PubMed] [Google Scholar]
- 6.Ghai S, Ghai I, Effenberg AO. Effect of rhythmic auditory cueing on gait in cerebral palsy: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2018;14:43–59. doi: 10.2147/NDT.S148053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson’s disease. Journal of geriatric psychiatry and neurology. 2003;16:53–58. [DOI] [PubMed] [Google Scholar]
- 8.Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997;78(3):278–283. [DOI] [PubMed] [Google Scholar]
- 9.Pickering RM, Grimbergen YAM, Rigney U, et al. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Movement Disorders. 22(13):1892–1900. doi: 10.1002/mds.21598 [DOI] [PubMed] [Google Scholar]
- 10.Hausdorff JM. Gait dynamics in Parkinson’s disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos. 2009;19:026113. doi: 10.1063/1.3147408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamacher D, Hamacher D, Herold F, Schega L. Effect of dual tasks on gait variability in walking to auditory cues in older and young individuals. Experimental brain research. 2016;234:3555–3563. doi: 10.1007/s00221-016-4754-x [DOI] [PubMed] [Google Scholar]
- 12.Hausdorff JM, Nelson ME, Kaliton D, et al. Etiology and modification of gait instability in older adults: a randomized controlled trial of exercise. J Appl Physiol (1985). 2001;90:2117–2129. doi: 10.1152/jappl.2001.90.6.2117 [DOI] [PubMed] [Google Scholar]
- 13.Brodie MAD, Beijer TR, Lord SR, et al. Auditory cues at person-specific asymmetry and cadence improve gait stability only in people with Parkinson’s disease (PD). Movement Disord. 2013;28:S277–S278. [Google Scholar]
- 14.Wittwer JE, Webster KE, Hill K. Music and metronome cues produce different effects on gait spatiotemporal measures but not gait variability in healthy older adults. Gait posture. 2013;37:219–222. doi: 10.1016/j.gaitpost.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 15.Arias P, Cudeiro J. Effect of Rhythmic Auditory Stimulation on Gait in Parkinsonian Patients with and without Freezing of Gait. PLOS ONE. 2010;5(3):e9675. doi: 10.1371/journal.pone.0009675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yogev G, Plotnik M, Peretz C, Giladi N, Hausdorff JM. Gait asymmetry in patients with Parkinson’s disease and elderly fallers: when does the bilateral coordination of gait require attention? Exp Brain Res. 2007;177(3):336–346. doi: 10.1007/s00221-006-0676-3 [DOI] [PubMed] [Google Scholar]
- 17.Peper CL, Oorthuizen JK, Roerdink M. Attentional demands of cued walking in healthy young and elderly adults. Gait Posture. 2012;36(3):378–382. doi: 10.1016/j.gaitpost.2012.03.032 [DOI] [PubMed] [Google Scholar]
- 18.Ebersbach G, Heijmenberg M, Kindermann L, Trottenberg T, Wissel J, Poewe W. Interference of rhythmic constraint on gait in healthy subjects and patients with early Parkinson’s disease: evidence for impaired locomotor pattern generation in early Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 1999;14:619–625. [DOI] [PubMed] [Google Scholar]
- 19.Arias P, Cudeiro J. Effects of rhythmic sensory stimulation (auditory, visual) on gait in Parkinson’s disease patients. Experimental brain research. 2008;186:589–601. doi: 10.1007/s00221-007-1263-y [DOI] [PubMed] [Google Scholar]
- 20.Young WR, Rodger MWM, Craig CM. Auditory observation of stepping actions can cue both spatial and temporal components of gait in Parkinson’s disease patients. Neuropsychologia. 2014;57:140–153. doi: 10.1016/j.neuropsychologia.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 21.Cochen De Cock V, Dotov DG, Ihalainen P, et al. Rhythmic abilities and musical training in Parkinson’s disease: do they help? npj Parkinson’s Disease. 2018;4:8. doi: 10.1038/s41531-018-0043-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalla Bella S, Benoit CE, Farrugia N, et al. Gait improvement via rhythmic stimulation in Parkinson’s disease is linked to rhythmic skills. Scientific reports. 2017;7. doi:ARTN 42005 10.1038/srep42005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison EC, McNeely ME, Earhart GM. The feasibility of singing to improve gait in Parkinson disease. Gait posture 2017;53:224–229. doi: 10.1016/j.gaitpost.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satoh M, Kuzuhara S. Training in mental singing while walking improves gait disturbance in Parkinson’s disease patients. European neurology. 2008;60:237–243. doi: 10.1159/000151699 [DOI] [PubMed] [Google Scholar]
- 25.Ghai S, Ghai I, Schmitz G, Effenberg AO. Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Scientific reports. 2018;8:506. doi: 10.1038/s41598-017-16232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson’s disease. Annals of neurology. 1992;32 Suppl:S125–7. [DOI] [PubMed] [Google Scholar]
- 27.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson’s disease. American journal of medical genetics. 1999;88:539–543. [PubMed] [Google Scholar]
- 28.Sheehan PW. A shortened form of Betts’ questionnaire upon mental imagery. J Clin Psychol. 1967;23(3):386–389. [DOI] [PubMed] [Google Scholar]
- 29.Willems AM, Nieuwboer A, Chavret F, et al. The use of rhythmic auditory cues to influence gait in patients with Parkinson’s disease, the differential effect for freezers and non-freezers, an explorative study. Disability and rehabilitation. 2006;28:721–728. doi: 10.1080/09638280500386569 [DOI] [PubMed] [Google Scholar]
- 30.Baker K, Rochester L, Nieuwboer A. The immediate effect of attentional, auditory, and a combined cue strategy on gait during single and dual tasks in Parkinson’s disease. Archives of physical medicine and rehabilitation. 2007;88:1593–1600. doi: 10.1016/j.apmr.2007.07.026 [DOI] [PubMed] [Google Scholar]
- 31.Lohnes CA, Earhart GM. The impact of attentional, auditory, and combined cues on walking during single and cognitive dual tasks in Parkinson disease. Gait posture. 2011;33:478–483. doi: 10.1016/j.gaitpost.2010.12.029 [DOI] [PubMed] [Google Scholar]
- 32.Chester EL, Turnbull GI, Kozey J. The Effect of Auditory Cues on Gait at Different Stages of Parkinson’s Disease and During “On”/”Off” Fluctuations: A Preliminary Study. Topics in Geriatric Rehabilitation The Older Driver, Part 2. 2006;22(2):187–195. [Google Scholar]
- 33.Hausdorff JM, Lowenthal J, Herman T, Gruendlinger L, Peretz C, Giladi N. Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease. The European journal of neuroscience. 2007;26:2369–2375. doi: 10.1111/j.1460-9568.2007.05810.x [DOI] [PubMed] [Google Scholar]
- 34.Dotov DG, Bayard S, Cochen de Cock V, et al. Biologically-variable rhythmic auditory cues are superior to isochronous cues in fostering natural gait variability in Parkinson’s disease. Gait posture. 2017;51:64–69. doi: 10.1016/j.gaitpost.2016.09.020 [DOI] [PubMed] [Google Scholar]
- 35.Shashank Ghai IG, Shashank Ghai IG. Effect of Rhythmic Auditory Cueing on Aging Gait: A Systematic Review and Meta-Analysis. Aging and disease. 2017;9(5):901–923. doi: 10.14336/AD.2017.1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.del Olmo MF, Cudeiro J. Temporal variability of gait in Parkinson disease: effectsof a rehabilitation programme based on rhythmic sound cues. Parkinsonism & Related Disorders. 2005;11(1):25–33. doi: 10.1016/j.parkreldis.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 37.Almeida QJ, Frank JS, Roy EA, Patla AE, Jog MS. Dopaminergic modulation of timing control and variability in the gait of Parkinson’s disease. Movement Disorders. 2007;22(12):1735–1742. doi: 10.1002/mds.21603 [DOI] [PubMed] [Google Scholar]
- 38.Baker K, Rochester L, Nieuwboer A. The effect of cues on gait variability--reducing the attentional cost of walking in people with Parkinson’s disease. Parkinsonism & related disorders. 2008;14:314–320. doi: 10.1016/j.parkreldis.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 39.Bryant MS, Rintala DH, Hou JG, et al. GAIT VARIABILITY IN PARKINSON’S DISEASE: INFLUENCE OF WALKING SPEED AND DOPAMINERGIC TREATMENT. Neurol Res. 2011;33(9):959–964. doi: 10.1179/1743132811Y.0000000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jordan K, Challis JH, Newell KM. Walking speed influences on gait cycle variability. Gait Posture. 2007;26(1):128–134. doi: 10.1016/j.gaitpost.2006.08.010 [DOI] [PubMed] [Google Scholar]
- 41.Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM. Effect of gait speed on gait rhythmicity in Parkinson’s disease: variability of stride time and swing time respond differently. Journal of NeuroEngineering and Rehabilitation. 2005;2(1):23. doi: 10.1186/1743-0003-2-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM. Is freezing of gait in Parkinson’s disease related to asymmetric motor function? Annals of Neurology. 2005;57(5):656–663. doi: 10.1002/ana.20452 [DOI] [PubMed] [Google Scholar]
- 43.Zatorre RJ, Halpern AR, Perry DW, Meyer E, Evans AC. Hearing in the Mind’s Ear: A PET Investigation of Musical Imagery and Perception. Journal of Cognitive Neuroscience. 1996;8(1):29–46. doi: 10.1162/jocn.1996.8.1.29 [DOI] [PubMed] [Google Scholar]
- 44.Kleber B, Birbaumer N, Veit R, Trevorrow T, Lotze M. Overt and imagined singing of an Italian aria. NeuroImage. 2007;36:889–900. doi: 10.1016/j.neuroimage.2007.02.053 [DOI] [PubMed] [Google Scholar]
- 45.Halpern AR. Mental scanning in auditory imagery for songs. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1988;14(3):434–443. doi: 10.1037//0278-7393.14.3.434 [DOI] [PubMed] [Google Scholar]
- 46.Levitin DJ, Cook PR. Memory for musical tempo: additional evidence that auditory memory is absolute. Percept Psychophys. 1996;58:927–935. [DOI] [PubMed] [Google Scholar]
- 47.Rennie L, Löfgren N, Moe-Nilssen R, Opheim A, Dietrichs E, Franzén E. The reliability of gait variability measures for individuals with Parkinson’s disease and healthy older adults - The effect of gait speed. Gait Posture. 2018;62:505–509. doi: 10.1016/j.gaitpost.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 48.Brach JS, Perera S, Studenski S, Katz M, Hall C, Verghese J. Meaningful change in measures of gait variability in older adults. Gait Posture. 2010;31(2):175–179. doi: 10.1016/j.gaitpost.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SY, Seok H, Kim S-H, Park M, Kim J. Immediate Effects of Mental Singing While Walking on Gait Disturbance in Hemiplegic Stroke Patients: A Feasibility Study. Ann Rehabil Med. 2018;42(1):1–7. doi: 10.5535/arm.2018.42.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lirani-Silva E, Lord S, Moat D, Rochester L, Morris R. Auditory cueing for gait impairment in persons with Parkinson disease: a pilot study of changes in response with disease progression. J Neurol Phys Ther 2019;43: 50–55 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.