Abstract
Background:
Individuals with Mal de Debarquement Syndrome (MdDS) experience persistent oscillating vertigo lasting for months or years. Transcranial magnetic stimulation (TMS) can modulate the motion perception of MdDS.
Materials and Methods:
Twenty-six TMS naïve individuals received single administrations of continuous theta burst stimulation (cTBS) over the occipital cortex, cerebellar vermis, and lateral cerebellar hemisphere, in randomized order. A 0-100 point Visual Analogue Scale was used to assess acute changes in oscillating vertigo severity after each session. Repeated treatments were given over the target that led to the most acute reduction in symptoms. All treatments were performed with neuronavigation using the participant’s own brain MRI. The Dizziness Handicap Inventory (DHI), MdDS Balance Rating Scale (MBRS), and Hospital Anxiety and Depression Scale (HADS) were assessed weekly at four pre-treatment and six post-treatment time points.
Results:
Twenty participants chose either the occipital cortex (11) or cerebellar vermis (9) targets as most effective in reducing the oscillating vertigo; one chose lateral cerebellar hemisphere; five chose none. After 10-12 sessions of 1200 pulses over the target of choice, 19/25 treatment completers noted ≥ 25% reduction, 12/25 ≥50% reduction, and 8/25 ≥75% reduction in oscillating vertigo intensity. A one-way repeated measures ANOVA of DHI, MBRS, and HADS scores before and after treatment showed significant reductions in DHI, MBRS, and the HADS Anxiety sub-score immediately after treatment with most improvement lasting through post-treatment week 6. There were no significant Depression sub-score changes. Participants who had chosen vermis stimulation had comparatively worse balance at baseline than those who had chosen occipital cortex stimulation.
Conclusion:
cTBS over either the occipital cortex or cerebellar vermis is effective in reducing the oscillating vertigo of MdDS acutely and may confer long-term benefits. Sustained improvement requires more frequent treatments.
Keywords: Mal de Debarquement Syndrome, Theta Burst Stimulation, Cerebellar Vermis, Occipital Cortex, Oscillating Vertigo
Introduction:
Mal de debarquement Syndrome (MdDS) is a motion perceptual disorder of persistent oscillating vertigo caused by entrainment to periodic motion such as occurs during prolonged water, air, or land travel (1-3). Though brief periods of post-motion oscillating vertigo described as a feeling of “rocking,” “bobbing,’ or “swaying,” occurs commonly in healthy individuals, prolonged symptoms lasting more than one-month are pathological and can lead to high levels of morbidity (4-6).
Treatment options for persistent MdDS are limited with longer symptom duration associated with decreasing chance of resolution (7). Benzodiazepines and antidepressants in the selective serotonin reuptake inhibitor (SSRI) class can ameliorate some symptoms but incomplete control is typical, especially for episodes that last for years (7,8). Investigational treatments for MdDS using non-invasive brain stimulation with transcranial magnetic stimulation (TMS) have shown that the motion perception can be acutely altered with therapies that modulate functional connectivity (9-11). Though sustained improvement requires long-term treatment, these studies indicate that the underlying biology of MdDS may involve functional transitions between metastable brain states that can be entrained by external periodic input.
Initial studies using repetitive TMS (rTMS) of the dorsolateral prefrontal cortex (DLPFC) have shown that both high-frequency (10Hz) left DLPFC and low-frequency (1Hz) right DLPFC stimulation are able to drive down motion perception acutely after single sessions and for months after multiple sessions (12-14). Functional connectivity measurements with both functional MRI and high-density EEG implicate a reduction in long-range intrinsic functional connectivity (IFC) correlating with symptom improvement after DLPFC stimulation (9). Additionally, connectivity between the posterior default mode network and the left entorhinal cortex, a region previously found to be hypermetabolic in MdDS, decreases in treatment responders (10,15).
Functional MRI and EEG studies in MdDS to date indicate that additional neuromodulation targets might be used more directly and perhaps more effectively than DLPFC (16-18). Potential targets suggested by IFC differences between individuals with MdDS and healthy controls or in pre vs. post scans of individuals with MdDS who respond to neuromodulation, include: 1] primary visual cortex, 2] visual association areas, 3] default mode network, and 4] fronto-parietal attention network (2,9-11,15,19).
On the basis of these prior studies, we explored whether cortical targets suggested by neuroimaging markers would be more effective than DLPFC in reducing the oscillating vertigo of MdDS (10,12,13). For this study, we chose three targets: 1] visual association area 2] default mode network, and 3] fronto-parietal attention network (20-22). Primary visual cortex was not chosen because of the induction of phosphenes that would prevent adequate blinding between targets.
In order to perform all stimulations over a similar region of the head, we chose the cerebellar representations of the default mode network (lateral cerebellar hemisphere over the horizontal fissure) and fronto-parietal network (cerebellar vermis lobule VII) (22,23). The visual association area just below the parieto-occipital sulcus in the occipital lobe was the final target. This strategy limited the surface area of potential stimulation and created a similar level of discomfort between stimulation sites. After administering this protocol in 12 participants and finding only one person with minimal improvement to the lateral (right) cerebellar hemisphere target, we shifted this target down to the right cerebellar lobule VIII, which is functionally connected with the precuneus another node of the default mode network (24).
Given the posterior location of the treatment targets and the lower threshold for pain in stimulating these regions, we chose a continuous theta burst stimulation protocol (cTBS) over conventional rTMS. cTBS paradigms are given as trains of three pulses every 20ms (50Hz) which are repeated every 200ms for a total of 600 pulses delivered in about 40 seconds (25,26). cTBS paradigms have the additional advantage of yielding more durable motor suppression than conventional rTMS given at 1Hz and can potentially provide more durable clinical responses in other targets (27-29). We chose cTBS over the functionally opposite intermittent TBS (iTBS) paradigm because iTBS had previously been shown to enhance functional connectivity in the default mode network, whereas our goal was to decrease functional connectivity (23).
Because MdDS is a relatively uncommon disorder and all participants in this study had to travel in from other States to participate, we used an ‘n-of-1’ design to rapidly test the acute efficacy of each target site by serially, but in randomized order, treating each participant over each target. These designs add statistical power, have high utility in the study of rare disorders, and are a step toward the personalization of treatment protocols (30-32). This method allowed us to test several different targets in the same session while also allowing each participant to be treated with their self-rated most effective protocol. Though prior imaging data were the basis of the treatment targets for this study and pre and post treatment imaging was performed as part of this study, this report will focus on the clinical outcomes, only.
Materials and Methods:
Informed Consent
Study procedures were completed according to Declaration of Helsinki guidelines and approved by Western IRB (www.wirb.com). Participants provided written informed consent and were recruited under ClinicalTrials.gov study . This study used rTMS in an off-label manner. The study was completed between August 2015 and July 2017.
Inclusion and exclusion criteria
Inclusion criteria included: 1. Chronic perception of oscillating vertigo that started within 48-hours after disembarking from sea, air, or land based motion exposure; 2: symptoms lasting at least six months; 3: no other cause for symptoms after evaluation by a neurologist or otolaryngologist with appropriate testing for peripheral inner ear or other central nervous system cause for symptoms. Exclusion criteria included: 1. An unstable medical or psychiatric condition, including a history of bipolar disorder or psychosis; 2: pregnant or planning to become pregnant during the study; 3: contraindications to receiving rTMS or MRI, including medications known to reduce seizure threshold; 4: an unclear history of the onset of symptoms; 5: an inability to complete all study related testing. The CONSORT diagram of the recruitment pathway for this study is shown in Supplemental Fig.1. In order to recruit a medically refractory cohort who had the greatest benefit to risk ratio for participating, this study group had tried and failed at least one benzodiazepine, a selective serotonin reuptake inhibitor (SSRI) or a SSRI/selective norepinephrine reuptake inhibitor (SNRI), and physical therapy before entering the study (8).
Reporting
Participants reported daily symptom scores on an encrypted SurveyMonkey® weblink that was individualized with their participant codes starting from Day −24 and ending on Day +48 relative to their first on-site study date. Additionally, for each of 11 weekends (10 weeks inclusive of the weekend that lead into and ended the participation period), participants completed a series of questionnaires on a separate SurveyMonkey® weblink. These included the Dizziness Handicap Inventory (DHI) (33) scored from 0–100; MdDS Balance Rating Scale (MBRS) (13) scored from 1–10; and the Hospital Anxiety and Depression Scale (HADS) with each component scored from 0–21 (34). In each of these scales, higher scores represent worse symptoms. Participants were compensated at our standard institutional rates for each activity (interviews, MRI, EEG, rTMS) as well as for the online diaries. They were not compensated for travel expenses.
MRI for navigation
All participants underwent structural brain MRI imaging for use in neuronavigation during the rTMS sessions. A T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence with SENSE using the following parameters was acquired: FOV = 240 mm, axial slices per slab = 190, slice thickness = 0.9 mm, image matrix = 256×256, TR/TE = 5/2.012 ms, acceleration factor R = 2, flip angle = 8°, inversion time TI = 725 ms, sampling band-width = 31.2 kHz.
rTMS procedures
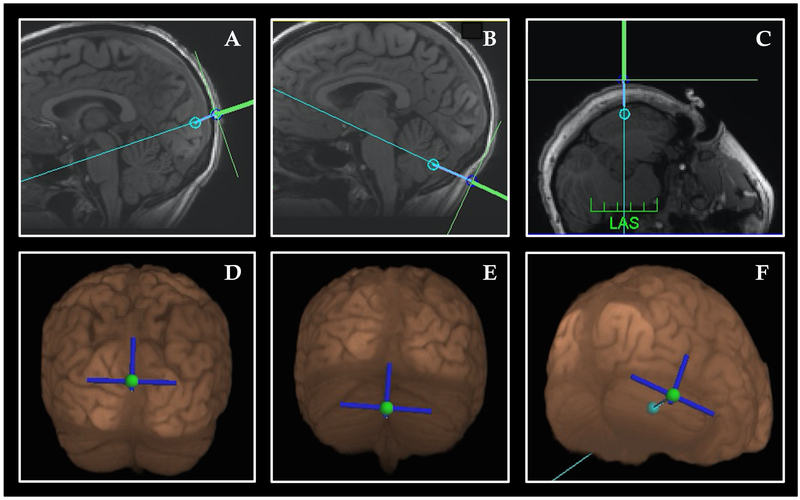
rTMS was performed with the Magventure MagPro X100 stimulator with a cooled angled double-cone coil in biphasic mode (Cool D-B80, Magventure). The Localite TMS Navigator (Localite GmBH, Germany) frameless stereotaxy system was used for neuronavigation to identify each target (occipital cortex, cerebellar vermis, lateral cerebellar hemisphere). In preliminary trials of this paradigm, stimulation at the active motor thresholds of the abductor pollicis brevis were not well tolerated in these participants due to neck muscle contraction and variation between hair and neck thickness between subjects that made a standard motor threshold an unreliable measure on which to base an initial stimulation intensity. Therefore we started with standard stimulation intensities across participants (45% for the occipital target, 42% for the cerebellar vermis target, and 40% for the lateral cerebellar target) to compensate for the relative degrees of discomfort induced in each region. Once tolerance was assessed and the participant habituated to the stimulation, we raised the intensity to the maximum tolerated level that would not elicit pain for each site. Right-handed subjects received right lateral cerebellar stimulation. Since our prior DLPFC study suggested that left-handed participants required an oppositely lateralized paradigm from right-handed participants, the two left-handed participants received left lateral cerebellar stimulation (12). The occipital and vermis targets were midline in all participants. A representative image of the targeting used for each site is shown in Fig. 1. Participants wore earplugs and sat with their face down on a portable massage chair with the neck slightly flexed during stimulation.
Figure 1: Neuronavigation targets.
(A, D) Sagittal T1-structural image (A) and 3-D reconstruction (D) of visual association area target below the parieto-occipital sulcus. The path of stimulation was angled to pass between the midbrain-pontine junction, angled downward; (B, E) Sagittal T1-structural image (B) and 3-D reconstruction (E) cerebellar vermis target. The path of stimulation was angled to pass between the midbrain-pontine junction, angled upward (C, F) Axial T1-structural image (C) and 3-D reconstruction (F) of lateral cerebellar hemisphere. Twelve subjects received stimulation over the right horizontal fissure shown here; 12 received stimulation over lobule VIII just below (not shown).
Trial design:
On Day 1, participants underwent baseline imaging and symptom assessments. On Day 2, the participants received one session of 600 pulses over each of the three cTBS targets in randomized order between participants. Participants sat for 5-minutes following the end of each stimulation session before they were allowed to move. Each session was followed by a 60-minute reporting period of symptom change on a visual analogue scale of 0–100. Since a 3-item list can be permuted in six ways (3×2×1), each order of stimulation was given to four right-handed participants (6×4=24). Not enough left-handers could be recruited to make a full panel of six. The specific order of treatment received was determined by the participants’ study order entry. The stimulation target that most optimally lowered the participant’s symptoms on the VAS was chosen for repetitive treatment sessions.
Postural sway was assessed with the modified Balance Error Scoring System (mBESS) using the Sway Balance® mobile app (35). Scores are from 0 to 100 with 100 indicating better balance. Because there is a practice effect with balance testing, balance was measured on Day 1 and Day 2 before any TMS was performed. Day 2 scores were used as the baseline measurements.
Four treatment sessions consisting of 1200 pulses divided into two blocks (600 pulses x 2 separated by a 20 second break) were given on Days 3 and 4 and typically two to four treatment sessions were given on Day 5, as time allowed. Repeated sessions were separated by about 30-minutes. The participants traveled home, typically by flying, the day after the last treatment. Participants were required to avoid any medications changes or any other travel lasting more than two-hours for the duration of the study.
Statistical analysis
Data were analyzed with STATA IC version 14.2 (www.stata.com). Changes in VAS scores used simple raw data with differences measured between Day 1 and Day 5 and percent change measured with the Day 1 score as the denominator. For the weekly questionnaires (DHI, MBRS, HADS), the median of the four scores obtained pre-treatment was used as the baseline for each participant. This was to account for the inherent variability in symptoms. The mean was not used because it is sensitive to outlier effects. The difference between this median value and scores after TMS and the six weeks post TMS were entered into a one-way repeated-measures ANOVA analysis. Greenhouse-Geisser correction was made to account for the non-independence of within subject data. Linear prediction models with 95% confidence intervals were calculated for the DHI, MBRS, HADS Anxiety, and HADS Depression scores and are presented graphically.
Participant characteristics
Twenty-four right-handed and two left-handed rTMS naïve women with MdDS were recruited. The study was open to either sex, but only women completed all pre-study requirements. Mean age at the time of the study was 51.3± 12.4 years (range: 30–70 years, median: 53.5 years); mean age of onset for MdDS symptoms= 48.7± 12.4 years (range: 25–69 years, median: 49.5 years); mean duration of illness= 30.0±31.1 months (range: 7–132 months, median: 17.0 months); triggers included 17 water-based travel, six air travel, and three land travel (Table 1).
Table 1: Demographic data of study participants.
Under the SSRI column, participants who were using a mixed SNRI/SSRI are indicated with an *. The distance column indicates the number of miles between the participant’s place of origin and the study site.
| Subject no. | Age at study | Handedness | Duration (mo) | Trigger | Benzodiazepine | SNRI/SSRI | Distance (mi) |
|---|---|---|---|---|---|---|---|
| 1 | 57 | R | 72 | Plane | Yes* | Yes* | 428 |
| 2 | 58 | R | 18 | Cruise | No | No | 893 |
| 3 | 63 | R | 40 | Cruise | No | Yes* | 1449 |
| 4 | 34 | R | 52 | Car | Yes | No | 371 |
| 5 | 31 | R | 29 | Car | No | No | 829 |
| 6 | 33 | R | 10 | Plane | No | Yes | 2134 |
| 7 | 51 | R | 28 | Plane | Yes* | No | 270 |
| 8 | 39 | R | 21 | Boat | No | Yes* | 750 |
| 9 | 55 | R | 7 | Boat | No | No | 1276 |
| 10 | 65 | R | 9 | Plane | Yes* | Yes | 1217 |
| 11 | 31 | R | 18 | Cruise | No | No | 918 |
| 12 | 65 | R | 25 | Plane | Yes | Yes | 752 |
| 13 | 44 | R | 32 | Cruise | Yes* | Yes* | 266 |
| 14 | 55 | R | 104 | Cruise | No | No | 956 |
| 15 | 30 | R | 60 | Boat | Yes* | No | 1454 |
| 16 | 70 | R | 8 | Cruise | Yes* | Yes* | 288 |
| 17 | 55 | R | 15 | Cruise | Yes | No | 1503 |
| 18 | 67 | R | 15 | Cruise | No | Yes* | 1064 |
| 19 | 48 | R | 15 | Cruise | No | No | 712 |
| 20 | 45 | R | 12 | Plane | No | Yes | 670 |
| 21 | 48 | R | 12 | Cruise | Yes* | Yes* | 315 |
| 22 | 62 | R | 16 | Cruise | No | No | 1505 |
| 23 | 52 | R | 11 | Cruise | Yes* | No | 1108 |
| 24 | 61 | R | 22 | Cruise | No | Yes* | 1430 |
| 25 | 66 | L | 132 | Cruise | No | No | 893 |
| 26 | 50 | L | 8 | Car | Yes | No | 258 |
Results
Twenty-five of 26 total participants completed 10–12 sessions of cTBS given over three days. One participant did not finish the entire week due to developing a concurrent medical illness. Her data were not included in the long-term response analysis. Twenty participants favored one treatment target over the others with 11 choosing the occipital target, nine choosing the cerebellar vermis target, and one choosing the cerebellar hemisphere target based on reduction in vertigo intensity. One participant worsened with all study targets and was treated with a slightly more dorsal target (precuneus), which was well tolerated. Her data were included in the long-term response analysis. The remaining three participants who did not report any benefit were randomly assigned to one of the three treatment targets (Supplemental Fig.2).
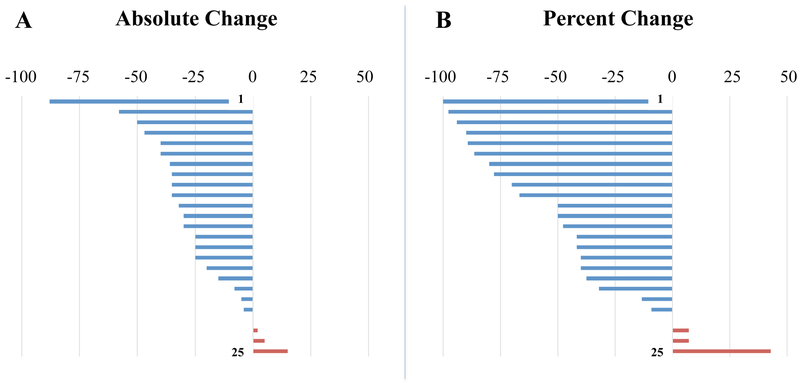
In total, 19/25 (76%) reported ≥25% reduction, 12/25 (48%) reported ≥50% reduction and 8/25 (32%) reported ≥75% reduction in vertigo intensity based on a 0–100 VAS from Day 1 to Day 5 (Fig. 2). This was much higher than the previously reported response to DLPFC stimulation, in which only 30% had a positive response of any magnitude (10). A small number of participants worsened within the week; the subject who worsened the most did so after the initial testing day and not after repeated treatment. Though she gradually improved, she did not return to baseline during the week.
Figure 2: Change in VAS daily score pre to post TMS.
Absolute changes on a 0-100 VAS scale (A) and percent changes (B) based on individual baselines in the total daily symptom score before and after treatment. Deflections to the left in blue represent a reduction of oscillating vertigo; deflections to the right in red represent a worsening of oscillating vertigo.
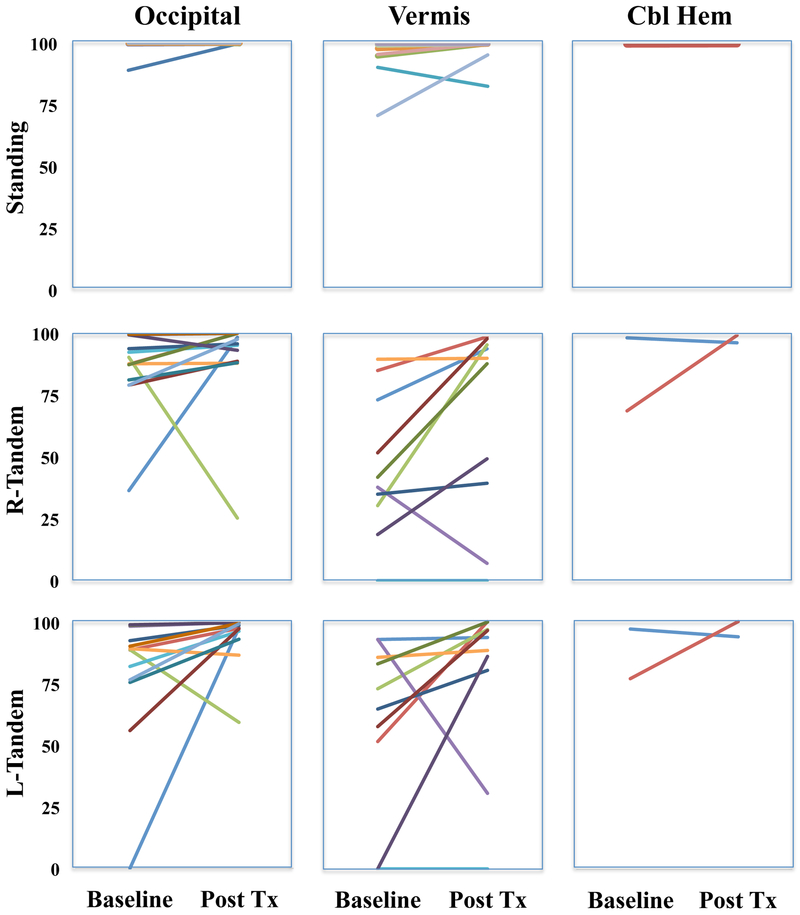
The change in balance measured by mBESS from Day 2 to Day 5 for each treatment group is presented in Fig 3.
Figure 3: mBESS sway baseline to post treatment.
Baseline and post treatment changes in posture scores on the modified balance error scoring system (mBESS). Higher scores represent better balance. The best score is 100. Cbl Hem= Cerebellar Hemisphere.
Baseline differences in responder groups
One-way ANOVA with one factor and three levels were performed for response groups (occipital target, vermis target, no-response) in order to determine any baseline factors that could predict which target an individual preferred. Since only one person chose the lateral cerebellar target, this was not included as a separate group. There were no differences in age of onset, age at time of study, duration, DHI, MBRS, or HADS scores that were related to target choice (Table 2). Although there was a trend for a difference in age of onset and duration of symptoms, there were no significant differences in post hoc analyses.
Table 2: Baseline differences between response groups.
Mean and standard deviations of demographic features, ratings, and balance testing tabulated according to best treatment selection.
| Occipital | Vermis | Non- Responder |
ANOVA | Post hoc Bonferroni test | ||||
|---|---|---|---|---|---|---|---|---|
| Clinical | F (2,22) | p value | Occ vs Vermis | Occ vs NR | Vermis vs NR | |||
| Age of onset | 52.5 [12.1] | 52.3 [11.6] | 40.2 [9.2] | 2.68 | 0.0909 | |||
| Age at study | 55.1 [11.5] | 53.6 [11.5] | 44.7 [13.5] | 1.57 | 0.2297 | |||
| Duration (mon) | 25.2 [23.7] | 18.9 [7.2] | 52.8 [51.7] | 2.6 | 0.0966 | |||
| Ratings | ||||||||
| DHIT* | 46.2 [13.7] | 51.1 [13.0] | 52.0 [11.3] | 0.51 | 0.6095 | |||
| MBRS* | 4.6 [1.3] | 5.2 [0.83] | 4.7 [1.5] | 0.73 | 0.4941 | |||
| HADA* | 6.2 [3.3] | 6.3 [3.7] | 7.8 [4.8] | 0.43 | 0.6565 | |||
| HADD* | 7.0 [3.4] | 8.0 [3.2] | 8.5 [4.3] | 0.37 | 0.6931 | |||
| Balance | ||||||||
| Feet together^ | 98.8 [3.5] | 94.9 [9.6] | 99.1 [2.0] | 1.19 | 0.3219 | |||
| Tandem left^ | 77.7 [30.3] | 58.0 [36.0] | 83.7 [10.0] | 1.68 | 0.2093 | |||
| Tandem right^ | 85.1 [18.7] | 48.7 [30.5] | 76.4 [24.8] | 5.34 | 0.0129 | (−36.4) p=0.013 | (−8.7) p=1.0 | (+27.7) p=0.139 |
NR= Non-responder; mon= months; DHIT= Dizziness Handicap Inventory; MBRS= Mal de Debarquement Balance Rating Scale; HADA= Hospital Anxiety and Depression Scale Anxiety subscale; HADD=Hospital Anxiety and Depression Scale Depression subscale
higher scores represent better balance
There was a ceiling effect on the feet together eyes closed condition at baseline with mean scores in the high 90’s in all groups (Table 2), indicating fairly good unstressed balance. There were, however, significant differences in the three groups in the right foot forward stance condition F(2,22)=5.34, p=0.0129. Post hoc Bonferroni analysis showed that there was a significant difference between the occipital cortex target group (score 85.1+/−18.7) compared to the vermis target group (48.7+/−30.5) p=0.013, suggesting worse baseline balance in individuals who chose the vermis target. There were no significant differences between the non-responders and either responder group (Table 2). This may indicate that balancing on the non-dominant foot (left, for most participants) is an appropriate level of difficulty to bring out differences between groups. Kruskal-Wallis equality of populations rank test showed no difference in the proportion of participants in each group with a history of migraine or concurrent use of an SSRI, SSRI/SNRI, or a benzodiazepine.
Response durability
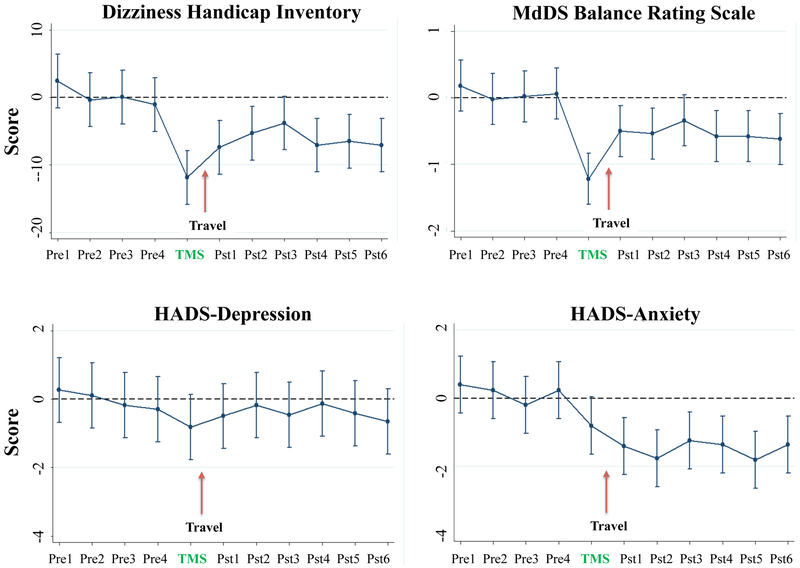
Repeated measures ANOVA using the four-week median scores for DHI, MBRS, and HADS as the baseline, showed significant decreases in DHI [F(10,24)= 4.38, p<0.005], MBRS [F(10,24)=4.44, p<0.05], and the HADS Anxiety sub-score [F(10,24)=3.92, p<0.05] during the course of the study. HADS Depression sub-scores did not change significantly [F(10,24)=0.43, p=ns]. Linear prediction models with 95% confidence intervals showed score changes all decreasing significantly (p<0.05) for six weeks post TMS except at post week 3 in which there was a trend for significance (Fig 4). The correlation between the DHI and MBRS was R2=0.4867; for MBRS and HADS Anxiety R2=0.2002, and MBRS and HADS Depression R2=0.1959.
Figure 4: Longitudinal cTBS effects on DHI, MBRS, and HAD scores.
Linear prediction model of repeated measures ANOVA with 95% confidence intervals presented for four baseline measurements, post TMS week, and six weeks post treatment for the DHI, MBRS, and the HADS Anxiety and Depression components. Pre= pre TMS scores, Pst= post TMS scores.
Discussion:
We report results of an open-label “n-of-1” design trial of cTBS over occipital cortex and cerebellum for MdDS. This trial design allows for rapid testing of multiple targets and individualization of treatment protocols. Acute improvement was noted when treatments were given 3–4 times per day, stacked every 30-minutes. Participant choice of occipital cortex and cerebellar vermis were evenly divided with rare choice of the lateral cerebellar hemisphere. Responders to vermis stimulation had worse baseline balance than occipital cortex responders.
Selection of targets
The occipital cortex target was chosen because of a prior study that showed that individuals with MdDS exhibit higher baseline functional connectivity between the entorhinal cortex and posterior visual processing areas compared to healthy controls (15). In order to maintain adequate blinding in the study across conditions, we chose a region dorsal to primary visual cortex in order to avoid inducing phosphenes. This region would roughly fall within visual area V3, which is bilaterally connected to human motion sensitive area V5 (20), becomes activated during imagined motion (21) and has previously been shown to have greater cortical thickness in individuals with MdDS compared to healthy controls (19).
Our second target was cerebellar vermis lobule VII. This region is functionally connected to bilateral fronto-parietal networks (22,23,36). Given the huge swath of cortex represented by this network, a cortically based target would have been impractical. We hypothesized that a cerebellar target may represent a smaller node of entry into these networks. Targeting cerebellar vermis lobule VII was previously shown to access the fronto-parietal attention network (23,37).
The lateral cerebellar hemisphere around the region of the horizontal fissure was chosen because of its functional connectivity to the default mode network, but there were no treatment successes with this target. Only one participant chose it as the best site but the reduction in her symptoms stayed within her normal range of fluctuation. For the second half of participants, we chose a slightly more caudal target, cerebellar lobule VIII, which is functionally connected to the precuneus, another major hub of the default mode network (24). There were again no treatment successes with this target. One participant who had worsened with all three targets did respond to direct stimulation over the precuneus however, which may be considered a future stimulation target to access the default mode network more directly.
Theta burst vs conventional rTMS
The improved response to rTMS in this paradigm compared to DLPFC stimulation could be attributed to several factors. Primarily, the target choice was more likely to be optimal given the prior connectivity changes seen both at baseline and as a change with improved symptom status (9,10,15). Second, about twice the number of treatments was administered (10–12 sessions) under the current protocol than in the DLPFC protocol (5 sessions) suggesting a dose effect (13,14). Third, theta burst stimulation induces longer neuroplasticity changes compared to conventional rTMS (25,26). Finally, because of the shorter duration of stimulation time period (total 100 seconds with intervening rest) compared to the DLPFC protocol (total ~ 45 minutes), more sessions could be given in a shorter time period allowing for potentially cumulative effects.
Stimulation intensities
The minimum distance between the scalp and the surface of the cerebellum has been measured to be in the range of 2–3cm but it is unknown what stimulus intensity is required for adequate cerebellar modulation of non-motor areas (38). Because there are trade-offs between depth of penetration, focality, and tolerability, we titrated the intensity of the stimulation to the individual’s clinical response rather than staying with the same intensity. One issue, of course, is whether stimulation at one site could inadvertently stimulate one of the other sites given the relatively small head surface area over which we were working. Hardwick et.al have calculated that at the locations we used, which included the midline region below the inion by 2–3cm, the chances of inadvertent occipital stimulation was 10% or less (38). In the lateral cerebellar hemisphere target (about 3–4cm from the midline), the risk of stimulating the occipital cortex was about 3% (38).
A possibility remains, however, that midline vermis stimulation did stimulate both lateral hemispheres and potentially affect the default mode network in a way that unilateral cerebellar hemisphere stimulation could not. This is particularly possible since we used a double-cone coil,which induces a deeper magnetic field that is wider at the surface (39). If this were the case, it would indicate that clinical effects were induced with stimulation of both lateral hemispheres but not with one hemisphere. The lateral cerebellar hemisphere target may not have been as effective as expected because the cerebellar representation of the default mode network is itself large and simultaneous intervention on both hemispheres may be required to adequately penetrate this network. This effect is not determinable with our current clinical outcomes but may be indirectly deducible with pre and post treatment imaging with fMRI and EEG.
Limitations
Because there was a risk of worsening symptoms simply by traveling to and from our site, we selected participants who had exhausted all medical options and had an optimal benefit to risk ratio of participating. Less than one-third of potential participants who were screened for the study eventually participated. Additionally, a limitation to working with a rare disorder is in obtaining adequate sample size when traditional clinical trial methods are used to create a separate control group. These considerations informed our decision to use an ‘n-of-1’ design to allow all participants to have an opportunity to receive all possible treatments. Since it is well known that additional travel can exacerbate MdDS symptoms, we could not ethically have a true placebo arm for repeated sessions. Response to the lateral cerebellar stimulation was extremely rare so functionally this target did serve as an active control, though it was not intentionally considered one at the outset. An active control is particularly helpful in TMS studies because it can recapitulate the sound, discomfort, and other non-specific effects of true stimulation.
We do advise our participants to take travel precautions such as getting adequate rest and using a one-time dose of a benzodiazepine prior to flying (both to and from the site) in order to reduce the chance of a relapse. However, exacerbations after travel are an inherent challenge in studying a travel-related disorder. A notable elevation in symptoms relative to the immediate post treatment improvement was seen after travel home, an expected outcome. Group level statistically significant improvements were observed out to post week 6 but in order to sustain the same magnitude of symptom reduction observed immediately after completion of treatment, sustained therapy would be required.
Despite these limitations, we note that this protocol was much more effective than rTMS over DLPFC when using the same metrics for symptom change (10,11,13). The current study protocol was developed through measuring IFC markers that correlated with symptom improvement after DLPFC stimulation. Similarly, the fMRI and EEG markers collected in the current study will be used to develop more precise treatment iterations going forward. In this way, neuroimaging with fMRI and EEG may inform more effective treatment protocols for MdDS and potentially other chronic balance disorders.
Supplementary Material
Supplemental Figure 1 CONSORT diagram for recruitment.
Supplemental Figure 2: Target selection choice by each participant based on best response
Participant choice of most effective target based on acute response.
Cbl hemi= lateral cerebellar hemisphere
Acknowledgments
The authors thank the many individuals who traveled long distances to participate in this study.
Financial Support: This work was supported by the Laureate Institute for Brain Research, the William K. Warren Foundation, an equipment and study grant from the MdDS Balance Disorders Foundation, the Springbank Foundation, an award through NSF EPSCoR RII Track-2 #1539068, and NIH/NIGMS grant P20 GM121312.
Footnotes
Conflict of Interest Statement: The authors report no financial or ethical conflicts of interest in the execution of this study
References
- 1.Brown JJ, Baloh RW. Persistent mal de debarquement syndrome: A motion-induced subjective disorder of balance. Am J Otolaryngol 1987;8(4):219–22. [DOI] [PubMed] [Google Scholar]
- 2.Cha YH. Mal de debarquement. Semin Neurol 2009, November;29(5):520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hain TC, Cherchi M. Mal de débarquement syndrome. Handb Clin Neurol 2016;137:391–5. [DOI] [PubMed] [Google Scholar]
- 4.Macke A, Leporte A, Clark BC. Social, societal, and economic burden of mal de debarquement syndrome. J Neurol 2012, January 10;259(7):1326–30. [DOI] [PubMed] [Google Scholar]
- 5.Clark BC, Leporte A, Clark S, Hoffman RL, Quick A, Wilson TE, Thomas JS. Effects of persistent mal de debarquement syndrome on balance, psychological traits, and motor cortex exctiability. J Clin Neurosci 2013, March;20(3):446–50. [DOI] [PubMed] [Google Scholar]
- 6.Arroll M, Attree E, Cha Y, Dancey C. The relationship between symptom severity, stigma, illness intrusiveness and depression in mal de debarquement syndrome (mdds). Journal of Health Psychology 2014, October 20:1–12. [DOI] [PubMed] [Google Scholar]
- 7.Cha YH, Brodsky J, Ishiyama G, Sabatti C, Baloh RW. Clinical features and associated syndromes of mal de debarquement. J Neurol 2008, July;255(7):1038–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cha YH, Cui YY, Baloh RW. Comprehensive clinical profile of mal de debarquement syndrome. Front Neurol 2018;9:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding L, Shou G, Yuan H, Urbano D, Cha YH. Lasting modulation effects of rtms on neural activity and connectivity as revealed by resting state EEG. IEEE Trans Biomed Eng 2014, March 25;61(7):2070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan H, Shou G, Urbano D, Ding L, Cha YH. Resting state functional connectivity signature of treatment effects of rtms in mal de debarquement syndrome. Brain Connect 2017, November 1;7(9):617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha YH, Shou G, Gleghorn D, Doudican BC, Yuan H, Ding L. Electrophysiological signatures of intrinsic functional connectivity related to rtms treatment for mal de debarquement syndrome. Brain Topogr 2018, November;31(6):1047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cha YH, Cui Y, Baloh RW. Repetitive transcranial magnetic stimulation for mal de debarquement syndrome. Otol Neurotol 2013, January;34(1):175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cha Y-H, Urbano D, Pariseau N. Randomized single blind sham controlled trial of adjunctive home-based tdcs after rtms for mal de debarquement syndrome: Safety, efficacy, and participant satisfaction assessment. Brain Stimul 2016;9(4):537–44. [DOI] [PubMed] [Google Scholar]
- 14.Cha Y, Deblieck C, Wu A. Double-blind sham-controlled cross-over trial of repetitive transcranial magnetic stimulation for mal de debarquement syndrome. Otol Neurotol 2016;37(6):805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cha YH, Chakrapani S, Craig A, Baloh RW. Metabolic and functional connectivity changes in mal de debarquement syndrome. PLoS One 2012;7(11):e49560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan H, Urbano D, Ding L, Cha YH. Impact of rTMS on resting state functional connectivity in mal de debarquement syndrome. 21st Annual Meeting of the Organization for Human Brain Mapping 2015. [Google Scholar]
- 17.Shou G, Yuan H, Urbano D, Cha YH, Ding L. Optimizing rTMS treatment of a balance disorder with EEG neural synchrony and functional connectivity. 38th Annual Intl Conf of the IEEE EMBC, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Li C, Shou G, Urbano D, Cha YH, Ding L, Yuan H. 440 Assessing rTMS effects in MdDS: Cross-modal comparison between resting state EEG and fMRI connectivity. Conf Proc IEEE Eng Med Biol Soc 2017;2017:1950–3. [DOI] [PubMed] [Google Scholar]
- 19.Cha YH, Chakrapani S. Voxel based morphometry alterations in mal de debarquement syndrome. PLoS One 2015;10(8):e0135021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci 1983, December;3(12):2563–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goebel R, Khorram-Sefat D, Muckli L, Hacker H, Singer W. The constructive nature of vision: Direct evidence from functional magnetic resonance imaging studies of apparent motion and motion imagery. Eur J Neurosci 1998, May;10(5):1563–73. [DOI] [PubMed] [Google Scholar]
- 22.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 2011, November;106(5):2322–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A. Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci 2014, September 3;34(36):12049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, Li C-SR. Functional connectivity mapping of the human precuneus by resting state fmri. Neuroimage 2012;59(4):3548–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YZ, Sommer M, Thickbroom G, Hamada M, Pascual-Leonne A, Paulus W, et al. Consensus: New methodologies for brain stimulation. Brain Stimul 2009, January;2(1):2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 2005, January 20;45(2):201–6. [DOI] [PubMed] [Google Scholar]
- 27.Cárdenas-Morales L, Nowak DA, Kammer T, Wolf RC, Schönfeldt-Lecuona C. Mechanisms and applications of theta-burst rtms on the human motor cortex. Brain Topogr 2010, January;22(4):294–306. [DOI] [PubMed] [Google Scholar]
- 28.Di Lazzaro V, Dileone M, Pilato F, Capone F, Musumeci G, Ranieri F, et al. Modulation of motor cortex neuronal networks by rtms: Comparison of local and remote effects of six different protocols of stimulation. J Neurophysiol 2011, May;105(5):2150–6. [DOI] [PubMed] [Google Scholar]
- 29.Wischnewski M, Schutter DJ. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimul 2015, March 26;8(4). [DOI] [PubMed] [Google Scholar]
- 30.Vieira R, McDonald S, Araújo-Soares V, Sniehotta FF, Henderson R. Dynamic modelling of n-of-1 data: Powerful and flexible data analytics applied to individualised studies. Health Psychol Rev 2017;11(3):222–34. [DOI] [PubMed] [Google Scholar]
- 31.Mirza RD, Punja S, Vohra S, Guyatt G. The history and development of n-of-1 trials. J R Soc Med 2017, August;110(8):330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kempf L, Goldsmith JC, Temple R. Challenges of developing and conducting clinical trials in rare disorders. Am J Med Genet A 2018, April;176(4):773–83. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Archives of Otolaryngology--Head & Neck Surgery 1990;116(4):424–7. [DOI] [PubMed] [Google Scholar]
- 34.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 35.Patterson JA, Amick RZ, Thummar T, Rogers ME. Validation of measures from the smartphone sway balance application: A pilot study. Int J Sports Phys Ther 2014, April;9(2):135–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, et al. Resting state cortico-cerebellar functional connectivity networks: A comparison of anatomical and self-organizing map approaches. Front Neuroanat 2012;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proceedings of the National Academy of Sciences 2011;108(52):21229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardwick RM, Lesage E, Miall RC. Cerebellar transcranial magnetic stimulation: The role of coil geometry and tissue depth. Brain Stimul 2014;7(5):643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng ZD, Lisanby SH, Peterchev AV. Coil design considerations for deep transcranial magnetic stimulation. Clin Neurophysiol 2014, June;125(6):1202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 CONSORT diagram for recruitment.
Supplemental Figure 2: Target selection choice by each participant based on best response
Participant choice of most effective target based on acute response.
Cbl hemi= lateral cerebellar hemisphere