Abstract
Background
The identification of extrauterine disease is critical in the management of high risk endometrial cancer. The purpose of this study was to determine the accuracy in the detection of extrauterine disease on preoperative PET/CT.
Methods
Women with high risk endometrial cancer were prospectively enrolled. They underwent preoperative PET/CT followed by surgery including sentinel lymph node biopsy and lymphadenectomy. Primary tumor factors on PET/CT were correlated with lymph node pathology. Sensitivity, specificity, positive predictive value and negative predictive value were calculated for the detection of lymphadenopathy and peritoneal disease by PET/CT.
Results
A total of 112 patients were enrolled and had a PET/CT between April 2013 and May 2016; 108 were evaluable On PET/CT, 21 patients (19.4%) had extrauterine disease; 18 (17%) had positive lymph nodes and 8 (7%) had peritoneal disease. 108 patients underwent surgery, 103 (95%) underwent lymphadenectomy. The sensitivity of PET/CT to detect positive nodes was 45.8%, specificity 91.1%, positive predictive value 61.1%, and negative predictive value 84.7%. The false negative rate was 54.2%. There was no difference in primary tumor characteristics on imaging between patients with positive and negative lymph nodes. The sensitivity of PET/CT to detect peritoneal disease was 37.5%, specificity 97.8%, positive predictive value 75%, and negative predictive value 90.0%. The false negative rate was 62.5%.
Conclusions
Preoperative PET/CT did not reliably predict the presence of extrauterine disease in women with high risk endometrial cancer. Given the high false negative rates, PET/CT should not be used in the preoperative treatment planning of these patients.
Keywords: endometrial cancer, PET/CT, peritoneal disease, extrauterine disease, lymph nodes, lymphadenectomy
Precis:
Preoperative PET/CT does not reliably predict extrauterine disease in women with high risk endometrial cancer. PET/CT should not be used in the preoperative treatment planning of patients with high risk endometrial cancer.
Introduction
Endometrial cancer is the most commonly diagnosed gynecologic cancer in the United States with an estimated 63,230 new cases and 11,350 deaths in 20181. In 1988, the International Federation of Gynecology and Obstetrics (FIGO) moved from a clinical to a surgical staging system for endometrial cancer. This decision was based on prospective data published by the Gynecologic Oncology Group (GOG) that established a relationship between disease prognosis and surgically determined risk factors2,3. The current 2010 FIGO recommendation for surgical staging of endometrial cancer includes hysterectomy, bilateral salpingo-oophorectomy, pelvic and paraaortic lymphadenectomy (LAD) and biopsy of any suspicious lesions. While complete lymphadenectomy is included in the FIGO staging of endometrial cancer, it remains controversial, especially in those patients with presumed early stage disease4. The accurate determination of lymph node status and the extent of metastatic disease is crucial in determining the most appropriate adjuvant therapy. The use of sentinel lymph node mapping has emerged as an effective diagnostic tool in lieu of a full lymphadenectomy and has been validated in prospective studies with a more favorable morbidity profile even in patients with high risk histology5,6.
In an effort to predict extrauterine disease preoperatively and optimize surgical planning, a multitude of non-invasive techniques have been evaluated. Endometrial biopsy, transvaginal ultrasound, computed tomography scan (CT) and preoperative CA-125 have all been shown to be ineffective in the detection of lymph node or metastatic disease7–10. The use of PET/CT in high risk endometrial cancer to identify positive lymph nodes and extrauterine disease has also been evaluated, but studies have shown an unacceptably low sensitivity to be considered a recommended standard preoperative test. A meta-analysis of 16 studies published in 2013 demonstrated pooled sensitivity and specificity of 72.3% and 92.9% for nodal metastasis and 95.7% and 95.4% for metastatic disease using PET/CT, respectively11. Preoperative MRI, PET/CT and ultrasound have also been prospectively compared and correlated with pathology findings and none of these modalities were shown to be accurate enough to eliminate the need for surgical staging12.
The primary objective of this prospective study was to estimate the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and false negative rate (FNR) in the detection of positive lymph nodes and peritoneal disease on preoperative PET/CT when compared to pathological findings in high risk EC patients.
Methods
This study was conducted after approval by the Institutional Review Board at the University of Texas MD Anderson Cancer Center. All newly diagnosed endometrial cancer patients were consecutively screened for eligibility and prospectively enrolled in this single arm study (). Patients were recruited from both MD Anderson Cancer Center and Lyndon B. Johnson Hospital and deemed eligible if they had a preoperative diagnosis of a high-risk endometrial cancer and were also a candidate for full surgical staging. High-risk endometrial cancer was defined as grade 3 endometrioid, serous, clear cell, carcinosarcoma or any mixed tumor containing one of these cell types. Patients with grade 1 or 2 endometrioid tumors with evidence of deep myometrial invasion or biopsy proven cervical involvement were also categorized as having high risk endometrial cancer and were also eligible for enrollment in the study. Patients were excluded if they had a positive pregnancy test, received any preoperative treatment for endometrial cancer including radiation or chemotherapy. Patients who had evidence of extensive peritoneal or distant metastatic disease on preoperative PET/CT who did not undergo surgery were excluded from the final analysis. Written informed consent was obtained prior to imaging.
Preoperative PET/CT was performed in all patients using18 F-fluorodeoxyglucose (FDG) per standard clinical protocol. Patients were required to fast for at least 6 hours and the serum blood glucose was measured and confirmed to be ≤200 mg/dl at the time of injection of the radiotracer. The PET/CT scan was then performed at least 60 minutes after the radiotracer injection to allow adequate distribution and localization. PET/CT scanning was from the level of the orbits to the proximal thighs, although the precise scan extent was allowed to vary slightly based on individual patient parameters. PET acquisition parameters were based on body mass index, and CT was performed using tube current modulation for dose reduction purposes. FDG-PET/CT scans were interpreted clinically.
For the study, two nuclear medicine physicians (BC, FW) who were blinded to the surgical results, independently reviewed each scan. They reported the presence or absence of pelvic and/or retroperitoneal nodal hypermetabolism using a confidence scale of 1–4 for each site (1=no disease, 2=suspected no disease, 3=suspected disease, 4=definite disease). Visible lymph nodes were assessed using bi-dimensional measurements and intensity was assessed using maximum SUV. SUV quantifies the distribution of the tracer uptake within a region of interest normalized to the administered amount and patient weight. The primary endometrial tumor was analyzed separately to include tumor intensity (maximum and peak SUV), and MTV (metabolic tumor volume) obtained at a threshold of 40% of maximum and at a threshold of SUV=3.
All patients underwent comprehensive surgical staging. This included hysterectomy, bilateral salpingo-oophorectomy, pelvic cytology and sentinel lymph node mapping followed by a pelvic and paraaortic lymphadenectomy up to the level of the renal vessels. If peritoneal disease was encountered during surgery, sentinel lymph node mapping and lymphadenectomy were performed at the discretion of the surgeon. A minimally invasive surgical approach was offered to all appropriate candidates and the procedure was performed using traditional laparoscopy or with robotic assistance according to surgeon preference. The technique used for sentinel lymph node mapping has been previously described and validated5. The sentinel lymph nodes removed underwent ultrastaging. All sentinel lymph nodes <5mm were bi-valved and those >5mm were serially sectioned every 2mm. An H&E was performed on each section. If negative, an additional wide H&E stained slide and two unstained slides were obtained 250 μm into the tissue block. When the deeper H&E level was negative, a pan-cytokeratin stain was performed. It was ultimately considered negative if the pan-cytokeratin stain was negative or if it was positive and no tumor cells were identified. All macroscopic (>2mm), microscopic (0.2–2mm) and isolated tumor cells (<0.02mm) were considered positive for metastatic disease.All of the final pathologic specimens were reviewed by a gynecologic pathologist and were compared to both the preoperative PET/CT and to the pathologic results of the sentinel lymph nodes.
The primary outcome was to determine the false negative rate of PET/CT in the detection of positive lymph nodes and peritoneal disease in women with high risk endometrial cancer as compared to final histology. We also determined the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of PET/CT. Sample size was determined to be 100 patients based on an estimated 25% node positivity rate.
We used descriptive statistics to summarize age, body mass index (BMI), histology, grade, FIGO (2010) stage, maximum SUV, peak SUV, MTV SUV3, and MTV 40% overall and stratified by pathology result (positive/negative). We compared categorical variables using Fisher’s exact test, and we compared medians of continuous variables using the Wilcoxon rank sum test. We estimated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and false negative rate (FNR) with 95% exact binomial confidence intervals.
Study data were collected and managed using REDCap electronic data capture tools hosted at MD Anderson. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources. All statistical analyses were performed using SAS 9.3 for Windows (Copyright © 2002–2010 by SAS Institute Inc., Cary, NC) and StatXact-7© for Windows (Copyright © 2005, 1989–2005, Cytel Software Corporation, Cambridge, Massachusetts)13.
Results
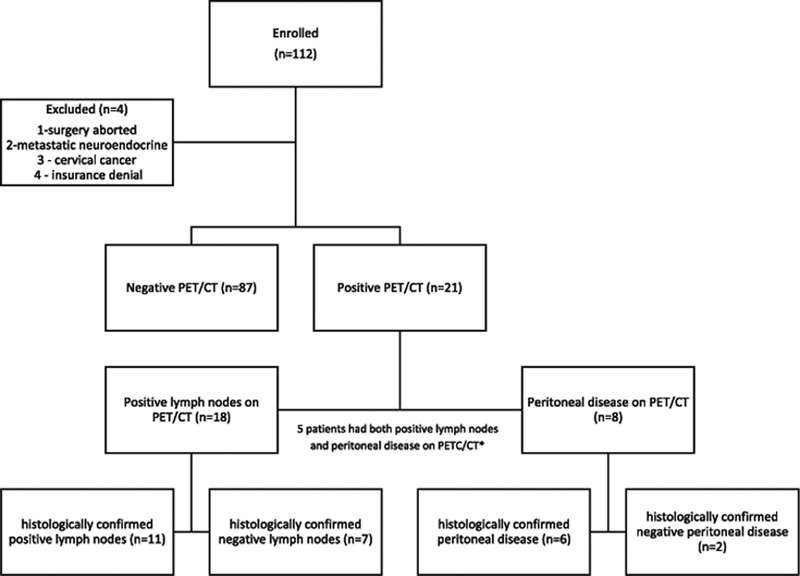
There were 123 patients enrolled in the study between April 2013 and May 2016. Eleven patients were excluded because they did not have a preoperative PET/CT completed. Of the 112 patients with preoperative PET/CT data available, four additional patients were excluded because: 1) surgery was aborted due to widespread disease and only vaginal biopsies were completed 2) biopsy demonstrated metastatic neuroendocrine tumor 3) final pathology demonstrated cervical cancer and 4) taken off study due to insurance denial. The remaining 108 patients were included in the final analysis (Figure 1).
Figure 1.
Consort diagram of preoperative PET/CT results
*These patients were included in both categories of PET/CT disease positivity for subsequent analysis.
The demographic characteristics of the included patients are shown in Table 1. Median age was 62 years (range 29 – 86). Median body mass index (BMI) was 32.1 kg/m2 (range 15.8 – 64.3). The most common histology was endometrioid (37%) followed by papillary serous (27%) and clear cell carcinoma (19%). Two patients had no residual cancer on final pathology.Most patients (72%) had stage I-II disease on final pathology. A total of 90 patients (83%) underwent minimally invasive surgery (laparoscopy 44%, robotic 37%, combined 3%). In the combined cases, a retroperitoneal paraaortic lymph node dissection was done via a laparoscopic approach and the hysterectomy and bilateral pelvic lymphadenectomy was performed using the robotics platform. All PET/CT scans were performed within 5 weeks of surgery, except for one patient had a PET/CT done 7 weeks prior to surgery.
Table 1.
Demographic Characteristics
| N=108 | |
|---|---|
| Median Age | 62 years (29 – 86) |
| Median BMI | 32.1 kg/m2 (15.8 – 64.3) |
| Histology | |
| Endometrioid | 40 (37%) |
| Papillary Serous | 29 (27%) |
| Clear Cell | 20 (19%) |
| MMMT | 10 (9%) |
| Mixed high grade | 7 (6%) |
| No residual cancer | 2 (2%) |
| Surgical Approach | |
| Laparoscopy | 47 (44%) |
| Robotic | 40 (37%) |
| Open | 18 (17%) |
| Combined laparoscopy and robotic | 3 (3%) |
| FIGO Stage | |
| IA | 48 (44%) |
| IB | 15 (14%) |
| II | 15 (14%) |
| IIIA | 3 (3%) |
| IIIC1 | 12 (11%) |
| IIIC2 | 10 (9%) |
BMI = Body mass index; MMMT = malignant mixed mullerian tumor; FIGO = International Federation of Gynecology and Obstetrics
On PET/CT review of the 108 patients, a total of 21 patients (19.4%) had extrauterine disease; 18 (17%) had positive lymph nodes and 8 (7%) had peritoneal disease. Of those with peritoneal disease on PET/CT, 5 of 8 also had positive lymph nodes on PET/CT. All patients underwent primary surgery and 103 (95%) underwent LAD. On final pathology, 24 patients had positive lymph nodes and 16 patients had peritoneal disease. In the node positive group, 8/24 patients were determined to be lymph node positive on the basis of sentinel lymph node involvement only. Additionally, 2 of these 8 patients were determined to have positive lymph nodes by the presence of isolated tumor cells only. On PET/CT, there were no differences in the primary tumor characteristics between those patients with and without confirmed lymph node metastasis on final pathology as shown in Table 2. Median maximum SUV was 18.2 and 16.4 for patients with negative and positive lymph nodes, respectively (p=0.92). Median peak SUV was 15.9 vs 12.5 (p=0.38), median MTV SUV3 was 25.5 vs 31.8 (p=0.28), and median MTV 40% was 9.6 vs 19.6 (p=0.05) for patients with negative and positive lymph nodes, respectively.
Table 2.
Imaging Characteristics of Primary Tumor
| Lymph Node Pathology | |||
|---|---|---|---|
| Negative | Positive | p-value | |
| Maximum SUV | 18.2 | 16.4 | 0.9194 |
| Peak SUV | 15.9 | 12.5 | 0.3761 |
| MTV SUV3 | 25.5 | 31.8 | 0.2826 |
| MTV 40% | 9.6 | 19.6 | 0.0526 |
Median values are shown. SUV = standardized uptake value; MTV = Metabolic tumor volume obtained at a threshold of 40% of maximum and at a threshold of SUV=3.
The efficacy of PET/CT in the detection of histologically confirmed positive lymph nodes is shown in Table 3. The sensitivity of PET/CT to detect positive nodes was 45.8%, specificity 91.1%, PPV 61.1%, and NPV 84.7%. The FNR was 54.2%. The efficacy of PET/CT in the detection of pathologically confirmed peritoneal disease is shown in Table 4. The sensitivity of PET/CT to detect peritoneal disease was 37.5% and specificity was 97.8%. The PPV and NPV was 75% and 90.0%, respectively. The FNR was 62.5%.
Table 3.
Detection Rates for Positive Lymph Nodes
| Lymph Node Status | ||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| PET/CT | Positive | 11 | 7 | 18 |
| Negative | 13 | 72 | 85 | |
| Total | 24 | 79 | 103 | |
| 95% Confidence Interval | ||||
| Sensitivity | 45.8% | 26.2% | 66.8% | |
| Specificity | 91.1% | 82.0% | 96.1% | |
| PPV | 61.1% | 36.1% | 81.7% | |
| NPV | 84.7% | 74.9% | 91.3% | |
PET/CT = positron emission tomography-computed tomography; PPV = positive predictive value; NPV = negative predictive value
Table 4.
Detection Rates for Peritoneal Disease
| Peritoneal Disease | ||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| PET/CT | Positive | 6 | 2 | 8 |
| Negative | 10 | 90 | 100 | |
| Total | 16 | 92 | 108 | |
| 95% Confidence Interval | ||||
| Sensitivity | 37.5% | 16.3% | 64.1% | |
| Specificity | 97.8% | 91.6% | 99.6% | |
| PPV | 75.0% | 35.6% | 95.5% | |
| NPV | 90.0% | 82.0% | 94.8% | |
PET/CT = positron emission tomography-computed tomography; PPV = positive predictive value; NPV = negative predictive value
Discussion
In this prospective study, preoperative PET/CT in high risk endometrial cancer patients failed to identify patients with positive lymph nodes and metastatic disease 54.2% and 62.5% of the time, respectively. These findings demonstrate that PET/CT should not be utilized as a primary tool in the initial preoperative evaluation, staging or surgical planning for high risk endometrial cancer patients. Surgical staging including intraoperative lymph node assessment should still be completed in all patients with high risk endometrial cancer given the unreliability of preoperative imaging in ruling out extrauterine disease.
In review of the literature, PET/CT has been reported to be marginally better than a standard diagnostic CT in the detection of lymph node involvement and metastatic disease in the recently published ACRIN 6671/GOG 0233 trial. The sensitivity, however, was only 65% for PET/CT versus 48% for CT alone in the detection of positive pelvic lymph node metastasis14,15. The authors of that study concluded that given the high specificity (86.1%) and PPV (95.4%) in the detection of distant metastasis, PET/CT should be included in the staging evaluation. However, based on our own prospective data, a FNR of 54.2% is too high to consider omitting a lymph node assessment during surgical staging. This could lead to under treatment of those patients with positive lymph node involvement not detected on PET/CT during adjuvant therapy planning. In addition, if extrauterine disease is detected on PET/CT, surgical treatment will often still be warranted as complete resection of macroscopic disease has been associated with improved survival16. Another multicenter prospective study comparing PET/CT and MRI reported sensitivities of 74.2% and 58.8% in the detection of lymph node involvement12. Surgical staging remains superior in the detection of lymph node involvement and preoperative PET/CT, MRI or CT should not be used to justify omission of intraoperative lymph node assessment. In addition, we found a false positive rate of 8.9% in the detection of positive lymph nodes using PET/CT demonstrating that histologic confirmation should be obtained prior to treatment planning.
In order to eliminate the requirement for surgical staging with lymph node evaluation, a preoperative imaging study must be as reliable as surgical nodal evaluation in the detection of lymph node metastasis. A retrospective study of 300 patients with endometrial cancer investigated the use of six preoperative criteria to predict lymph node metastasis. The criteria included: age > 55 years, CA-125 level, non-endometrioid histology, grade 3 tumors, metastatic lymph node disease assessed by MRI or CT and deep myometrial invasion assessed by pelvic MRI only. The sensitivity and specificity of combined CA-125 with CT or MRI in the detection of lymph node metastasis was 86.7% and 71.4% with corresponding false positive/false negative rates of 68.7% and 2.7%. The use of all six criteria resulted in a sensitivity of 100% but a specificity of only 28.9% and a false positive rate of 84.6% in the accurate prediction of lymph node involvement10. In an effort to validate preoperative noninvasive nodal staging using novel imaging modalities, there is also an ongoing phase II/III multicenter UK-based trial, assessing the detection rates of FDG PET/CT, fluoro-ethyl-choline (FEC) PET-CT and diffusion weighted imaging (DWI)-MRI in the detection of nodal metastasis in surgically staged cervical and endometrial cancer (MAPPING TRIAL; clinicaltrials.gov identifier: ). In comparison, sentinel lymph node biopsy has been reported to have a sensitivity of 97.2% and FNR of 3% in the prospective, multicenter FIRES trial. Based on the FIRES trial as well as a prospective single center validation study from MD Anderson, lymphadenectomy can safely be omitted when bilateral sentinel lymph node mapping is successful5,6. This single procedure decreases the surgical burden and obviates the need to perform multiple, expensive preoperative tests that do not adequately predict lymph node status.
Historically, preoperative tests have been used to determine which patients may not need surgical staging due to the concern for increased surgical morbidity from potentially unnecessary procedures. In GOG 244, the LEG study, reported a lymphedema rate of 34% in 733 endometrial cancer patients who underwent a lymph node dissection17. Data from the prospective randomized study SENTICOL-II demonstrated a reduction in surgical and postoperative morbidity associated with sentinel lymph node biopsy as compared to comprehensive lymphadenectomy in cervical cancer patients18. In endometrial cancer, however, there is not yet similar high quality evidence establishing the incidence of morbidity after sentinel lymph node biopsy. We must extrapolate based on data from other disease sites until future studies are published.
At this time PET/CT has no established role in the initial staging of endometrial cancer. Surgical determination of lymph node status is the most important prognostic indicator and the FNR of PET/CT is too high to serve as a surrogate to lymph node sampling. Several studies have investigated the prognostic value of a preoperative PET/CT in endometrial cancer and not surprisingly, PET/CT characteristics of the primary tumor correlate to surgical findings. Tumor maximum and mean SUV, MTV and total lesion glycolysis were significantly related to high histological grade, deep myometrial invasion and the presence of lymph node metastases in a prospective study of 129 patients. However, the authors reported a sensitivity of only 77–85% in the detection of positive lymph nodes on preoperative PET/CT19. Interestingly, MTV has also been shown to correlate with FIGO stage in a retrospective study of 76 patients20. In this study, MTV 40% approached statistical significance, but MTV SUV3 did not. Future studies should include more thorough metabolic imaging parameters to determine the most appropriate SUV threshold to obtain MTV.
Strengths of this study are the prospective evaluation of PET/CT in high risk endometrial cancer patients as compared to the gold standard of histologically confirmed disease. We were able to then accurately calculate sensitivity, specificity, PPV, NPV and FNR of PET/CT detection rates. The PET/CT scans were independently reviewed by two expert nuclear medicine physicians who were blinded to the surgical outcomes, thereby eliminating bias.
The main limitation of this study is that it was performed at a single, large tertiary care center, therefore, the results may not be generalizable to all settings. The ACRIN 6671/GOG 0233 multicenter trial demonstrated a significant difference in PET/CT detection rates of distant metastatic disease between local institutional readers and blinded central readers in both cervical and endometrial cancers. Notably, the sensitivity remained low in both groups14. The use of a sentinel lymph node protocol with ultrastaging in this study could have contributed to the lower sensitivity of PET/CT to detect nodal metastases as compared to ACRIN 6671/GOG 0233 where ultrastaging was not utilized. There were two patients who were considered node positive based on the presence of isolated tumor cells only who had corresponding negative PET/CT scans. Lastly, PET/CT scans are typically performed at many different centers with varying protocols, thus, we would expect the overall predictive capability of the PET/CT to remain poor and we would not expect the sensitivity to improve in a real world setting.
In conclusion, given the importance of lymph node status in guiding adjuvant therapy and prognosis, preoperative PET/CT should be used with caution given the low sensitivity and high false negative rate. Based on the inaccuracy of preoperative PET/CT, this test alone cannot replace surgical staging. The use of validated sentinel lymph node protocols should decrease the requirement for a complete lymphadenectomy and its associated morbidities.
Acknowledgement of Research Support:
This research was supported in part by the NIH T32 Training Grant for Gynecologic Oncologists (CA101642), Cancer Center Support Grant (NCI Grant P30 CA016672), and NCI SPORE in Uterine Cancer (2P50 CA098258–06).
Conflict of interest disclosures: Stewart: none. Chasen: none. Erwin: grants from FUJIFILM Radiopharmaceuticals USA, Inc., grants from Alfasigma S.p.A, grants from Oncosil Medical, Ltd., grants from Rita Foundation, grants from Radiomedix, grants from NIH/NCI outside the submitted work. Fleming: none. Westin: grants from NIH during the conduct of the study; personal fees and other from AstraZeneca, personal fees and other from Clovis, personal fees and other from Tesaro, personal fees and other from Roche/Genentech, other from Cotinga Pharmaceuticals, personal fees from Merck, personal fees from Pfizer, other from Novartis outside the submitted work;. Dioun: none. Frumovitz: grants and personal fees from Novadaq/Stryker, grants from Navidea, personal fees from Johnson and Johnson, personal fees from Genetech, personal fees from Ipsen outside the submitted work. Ramirez: none. Lu: none. Wong: None. Aloia: None. Soliman: grants from Novartis, personal fees from Clovis Oncology.
Bibliography
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ CK. Cancer Statistics Review, 1975–2014 - SEER Statistics. SEER Cancer Statistics Review, 1975–2014, National Cancer Institute; Bethesda, MD: https://seer.cancer.gov/csr/1975_2014/. Published 2016. Accessed December 1, 2017. [Google Scholar]
- 2.Benedet JL, Bender H, Jones H, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70(2):209–262. http://www.ncbi.nlm.nih.gov/pubmed/11041682. Accessed December 21, 2017. [PubMed] [Google Scholar]
- 3.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(8 Suppl):2035–2041. http://www.ncbi.nlm.nih.gov/pubmed/3652025. Accessed December 21, 2017. [DOI] [PubMed] [Google Scholar]
- 4.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 5.Soliman PT, Westin SN, Dioun S, et al. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer. Gynecol Oncol. 2017;146(2):234–239. doi: 10.1016/j.ygyno.2017.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3). doi: 10.1016/S1470-2045(17)30068-2 [DOI] [PubMed] [Google Scholar]
- 7.Arko D, Takac I. High frequency transvaginal ultrasonography in preoperative assessment of myometrial invasion in endometrial cancer. J Ultrasound Med. 2000;19(9):639–643. doi: 10.7863/jum.2000.19.9.639 [DOI] [PubMed] [Google Scholar]
- 8.Connor JP, Andrews JI, Anderson B, Buller RE. Computed tomography in endometrial carcinoma. Obstet Gynecol. 2000;95(5):692–696. http://www.ncbi.nlm.nih.gov/pubmed/10775731. Accessed April 24, 2018. [DOI] [PubMed] [Google Scholar]
- 9.Sood AK, Buller RE, Burger RA, Dawson JD, Sorosky JI, Berman M. Value of preoperative CA 125 level in the management of uterine cancer and prediction of clinical outcome. Obstet Gynecol. 1997;90(3):441–447. http://www.ncbi.nlm.nih.gov/pubmed/9277659. Accessed April 24, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Han S-S, Lee SH, Kim DH, et al. Evaluation of preoperative criteria used to predict lymph node metastasis in endometrial cancer. Acta Obstet Gynecol Scand. 2010;89(2):168–174. doi: 10.3109/00016340903370114 [DOI] [PubMed] [Google Scholar]
- 11.Kakhki VRD, Shahriari S, Treglia G, et al. Diagnostic performance of fluorine 18 fluorodeoxyglucose positron emission tomography imaging for detection of primary lesion and staging of endometrial cancer patients: systematic review and meta-analysis of the literature. Int J Gynecol Cancer. 2013;23(9):1536–1543. doi: 10.1097/IGC.0000000000000003 [DOI] [PubMed] [Google Scholar]
- 12.Antonsen SL, Jensen LN, Loft A, et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer — A multicenter prospective comparative study. Gynecol Oncol. 2013;128(2):300–308. doi: 10.1016/J.YGYNO.2012.11.025 [DOI] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gee MS, Atri M, Bandos AI, Mannel RS, Gold MA, Lee SI. Identification of Distant Metastatic Disease in Uterine Cervical and Endometrial Cancers with FDG PET/CT: Analysis from the ACRIN 6671/GOG 0233 Multicenter Trial.Radiology. November 2017:170963. doi: 10.1148/radiol.2017170963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atri M, Zhang Z, Dehdashti F, et al. Utility of PET/CT to Evaluate Retroperitoneal Lymph Node Metastasis in High-Risk Endometrial Cancer: Results of ACRIN 6671/GOG 0233 Trial. Radiology. 2017;283(2):450–459. doi: 10.1148/radiol.2016160200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bristow RE, Zahurak ML, Alexander CJ, Zellars RC, Montz FJ. FIGO stage IIIC endometrial carcinoma: resection of macroscopic nodal disease and other determinants of survival. Int J Gynecol Cancer. 13(5):664–672. http://www.ncbi.nlm.nih.gov/pubmed/14675352. Accessed May 22, 2018. [DOI] [PubMed] [Google Scholar]
- 17.Carter J, Huang H, Armer JA, Carlson JW, Lockwood S, Nolte S, Stewart BR, Wenzel L, Kauderer J, Hutson A, Walker JL, Fleury AC, Soper JT, Mathews CA, Zivanovic O RW, Albertsm DS and Barakat RR GOG 244, The lymphedema and gynecologic cancer (LEG) study: The association between the gynecologic cancer lymphedema questionnaire (GCLQ) and lower extremity lymphedema. In: Abstracts of the 49th Annual Meeting of the Society of Gynecologic Oncology. New Orleans: Society of Gynecologic Oncology; 2018:40 https://www.sgo.org/wp-content/uploads/2018/03/Abstracts-2018-SGO-Annual-Meeting-on-Womens-Cancer-1.pdf. Accessed May 16, 2018. [Google Scholar]
- 18.Mathevet Patrice FL. Effect of sentinel lymph-node biopsy alone on the morbidity of the surgical treatment of early cervical cancer: Results from the prospective randomized study Senticol2. J Clin Oncol. 2015;33(15_suppl (May 20 2015)):5521–5521. doi: 10.1200/jco.2015.33.15_suppl.5521 [DOI] [Google Scholar]
- 19.Husby JA, Reitan BC, Biermann M, et al. Metabolic Tumor Volume on 18F-FDG PET/CT Improves Preoperative Identification of High-Risk Endometrial Carcinoma Patients. J Nucl Med. 2015;56(8):1191–1198. doi: 10.2967/jnumed.115.159913 [DOI] [PubMed] [Google Scholar]
- 20.Chung HH, Lee I, Kim HS, et al. Prognostic value of preoperative metabolic tumor volume measured by 18F-FDG PET/CT and MRI in patients with endometrial cancer. Gynecol Oncol. 2013;130(3):446–451. doi: 10.1016/j.ygyno.2013.06.021 [DOI] [PubMed] [Google Scholar]